Introduction
A chronic wound results in an ulcer of the skin
area, and is clinically characterized as requiring a long period of
healing, skin tissue infection and defects or necrosis.
Consequently, the wound is not able to repair in a timely manner
through normal processes, eventually leading to skin tissue
dysfunction and anatomical defects (1,2).
Diabetic foot is a serious complication of diabetes, which is
caused by peripheral nerve dysfunction and leads to a decline in
the defensive function of the lower limbs. Furthermore, peripheral
nerve dysfunction may occur, and is accompanied by peripheral
artery disease, resulting in poor blood circulation to the
extremities, finally causing ulcer and gangrene (3). Many factors contribute to the
development of diabetic foot, which negatively affects wound
healing. When normal skin tissue is injured, a local inflammatory
response is required to initiate the wound healing process. A
moderate local inflammatory response helps to improve immunity and
promote wound healing (4,5). However, patients with diabetic foot
usually present an excessive local inflammatory response in the
wound tissue (6). The injury
related to an excessive local inflammatory reaction contributes to
inflammation imbalance, and is a major contributor to the
development of chronic skin ulcer (7). Bone marrow-derived mesenchymal stem
cells (BMSCs) have the ability for self-renewal and
omni-directional transformation into specific cell populations, and
are highly effective for repairing skin wounds (8,9).
Previous studies have indicated that stem cells possess
immunoregulation properties (10,11).
Therefore, the authors speculated that stem cells may be able to
regulate the excessive inflammatory response in the diabetic
refractory wound healing process, in order to promote wound
healing. In the current study, a hyperglycemic full-thickness skin
wound mouse model was established, and the mice were intravenously
injected with BMSCs to assess their effects on the hyperglycemia
refractory wound healing process. Furthermore, the expression of
the inflammatory cytokines interleukin (IL)-6 and tumor necrosis
factor (TNF)-α was evaluated to explore the mechanism underlying
the MSC-induced promotion of refractory wound healing.
Materials and methods
Isolation, culture and identification
of mice BMSCs
Mouse BMSCs were cultured using the tissue-explants
adherent method (12). BALB/c mice
at 8 weeks of age were anesthetized with 10% chloral hydrate
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and cuts were made
in the femur and tibia. The bone marrow was flushed out from the
femur and tibia punctures with Dulbecco's Modified Eagle's medium
(DMEM; Promega Corporation, Madison, WI, USA), and centrifuged at
37°C to separate the cells (2,250 × g, 5 min). The cells were
re-suspended in DMEM containing 10% FBS (Sigma-Aldrich; Merck
KGaA), and maintained in a saturated humidified incubator at 37°C,
in a 5% CO2 atmosphere, to observe their growth. When
the cells reached 80% confluence, they were digested, centrifuged
at 37°C and 2,250 × g, and subcultured in DMEM without serum. To
identify BMSCs, flow cytometry was used to detect MSC-specific
markers: CD29, CD44, Sca-1, CD45 and CD34. When the BMSCs at the
third passage grew to 80% confluence, the cells were harvested with
trypsin digest solution. Following washing the cells with 4°C PBS
three times, the cell density was adjusted to 1.0×105/ml
with 4°C PBS containing 0.2% BSA (Promega Corporation) and fixed in
4% formalin (Sigma-Aldrich; Merck KGaA). Then the cells were
centrifuged at 350 × g for 5 min. Prior to antibody treatment, the
cells were blocked with goat serum at room temperature for 10 min
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Following this, the supernatant was discarded, and the remaining
cells were stained with primary monoclonal antibodies against mice
CD29 (catalog no. 46-0291-82), CD44 (catalog no. 46-3476-87), Sca-1
(catalog no. 47-0544-36), CD45 (catalog no. 46-2217-44; 1:25;
Invitrogen; Thermo Fisher Scientific, Inc.) and secondary CD34
(catalog no. 46-5562-33; 1:50; Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 2 h, and then conjugated
with fluorescein isothiocyanate, respectively. The cells were
visualized with propodium (PI) staining. Finally, the expressions
of these molecules on cells were detected by flow cytometry
(FACScan; BD Biosciences, Franklin Lakes, NJ, USA).
Preparation of hyperglycemia mice
A total of 24 BALB/c mice, with a male/female ratio
of 1:2, weighing 20 to 25 g, (provided by the Xinxiang Medical
University Laboratory Animal Center, Xinxiang, China) were
accustomed to a 24 h light/dark cycle and had free access to food
and water prior to fasting for 24 h, and then received a peritoneal
injection of 70 mg/kg body weight streptozotocin (Sigma-Aldrich;
Merck KGaA). The weight and blood glucose level changes were
monitored every day, and mice with a blood glucose level of
>16.7 mmol/l were selected as the hyperglycemia mice group. The
mice were sacrificed following anesthesia with diethyl ether. The
study was approved by the ethics committee of Xinxiang Medical
University (Xinxiang, China).
Establishment of the full-thickness
skin wound mouse model
The dorsi of the mice were shaved, and the mice were
anesthetized via intramuscular injection of 10% chloral hydrate,
followed by disinfection with 70% alcohol. Circular full-thickness
sections of the skin, 1 cm in diameter, were excised from both
sides of the back, and the wounds were stanched with sterile
gauze.
Animal experiments and grouping
The mice of the full-thickness skin wound model were
randomly divided into three groups, with eight mice per group: A
blank control group (no treatment); hyperglycemic mice with a
full-thickness skin wound and no further treatment; and
hyperglycemic mice with a full-thickness skin wound treated with
BMSCs at a dose of 6×107 cells/kg body weight.
Wound-healing rate
Photographs of the wounds of the three groups of
mice were captured with a digital camera at 3, 6, 9 and 12 days
following treatment, and analyzed using Image-Pro Plus software
(version, 6.0; Media Cybernetics, Inc., Rockville, MD, USA). The
edge of the wound was marked and the wound area was measured. Then,
the rate of wound healing was calculated three times, as follows:
wound healing rate = [(initial wound area - wound area) / initial
wound area]×100%.
Conventional hematoxylin and eosin
staining
A total of 15 days following treatment, new skin
tissue samples were collected from the wounds of mice, fixed in 4%
formaldehyde, paraffin-embedded, sliced and stained with
hematoxylin and eosin for observation of the skin organization
structure under inverted microscope.
Enzyme-linked immunosorbent assay
(ELISA) for IL-6 and TNF-α expression
Skin tissue samples around the wound were collected
from each group before and at 1–6 days once daily following
treatment, weighed and crushed with liquid nitrogen.
Radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA)
was added to the crushed samples to obtain the lysate, which was
centrifuged (3,913 × g, 10 min) to collect the supernatant for
determination of IL-6 and TNF-α protein concentrations with ELISA
kits according to the manufacturer's protocol (catalog no. KHO0411;
Elabscience Biotechnology Co., Ltd., Wuhan, China).
Statistical analysis
All statistical analyses were performed using SPSS
software version 19 (IBM SPSS, Armonk, NY, USA). Data are expressed
as the mean ± standard error of the mean. The differences between
groups were tested using Student's t-test or one-way analysis of
variance, followed by Fisher's least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Culture and identification of stem
cells
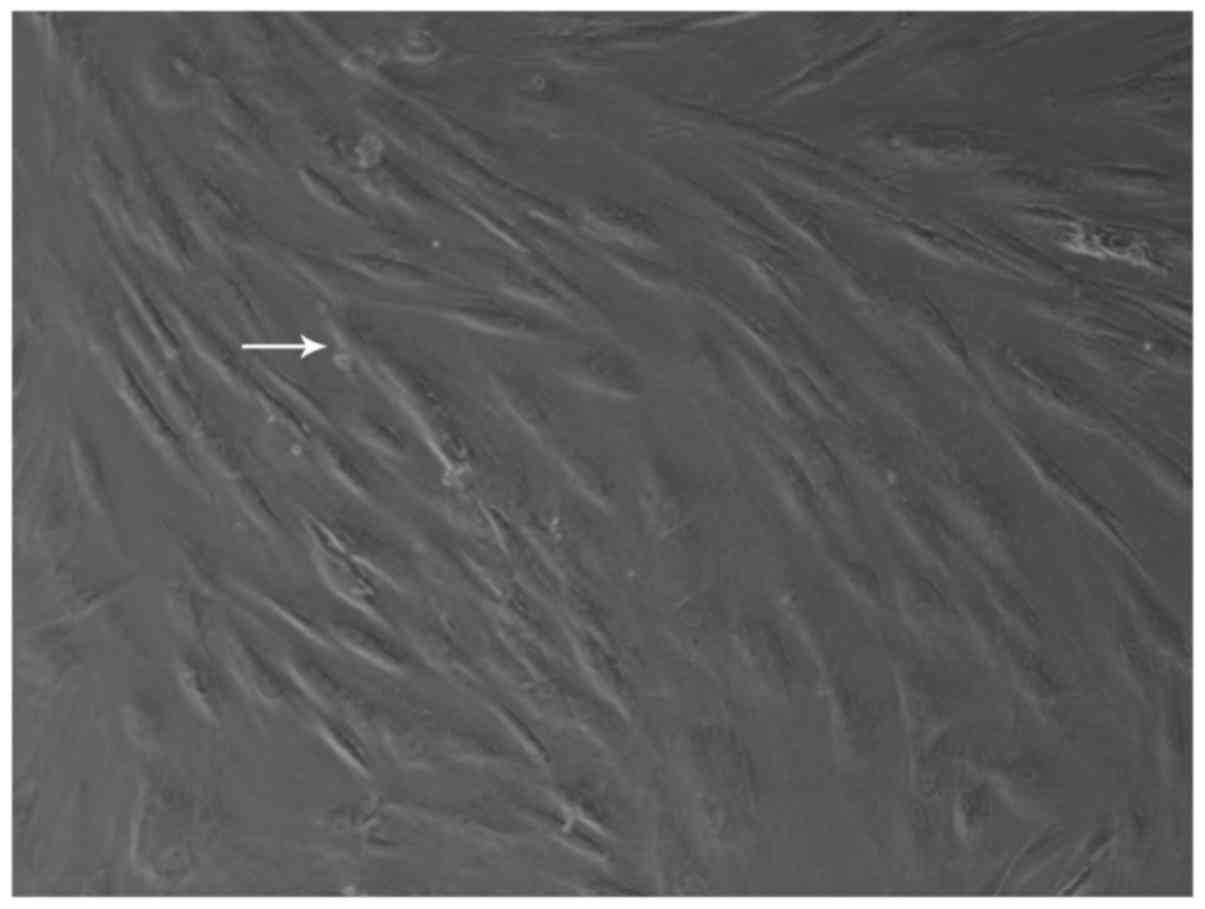
Newly generated cells could be seen after 5–7 days.
The cells grew, adhering to the wall, and were shuttle-shaped or
polygonal; their distribution was mostly non-uniform (Fig. 1). Examination by flow cytometry
demonstrated that the phenotype of the cells conformed with the
typical phenotypic characteristics of BMSCs: CD29(+), CD44(+),
Sca-1(+), CD45(−) and CD34(−) (Fig.
2). Specifically, CD29, CD44 and Sca-1 expression constituted
>95% of antigen expression, and expression of CD45 and CD34
expression accounted for <2%.
Healing of the full-thickness skin
wound
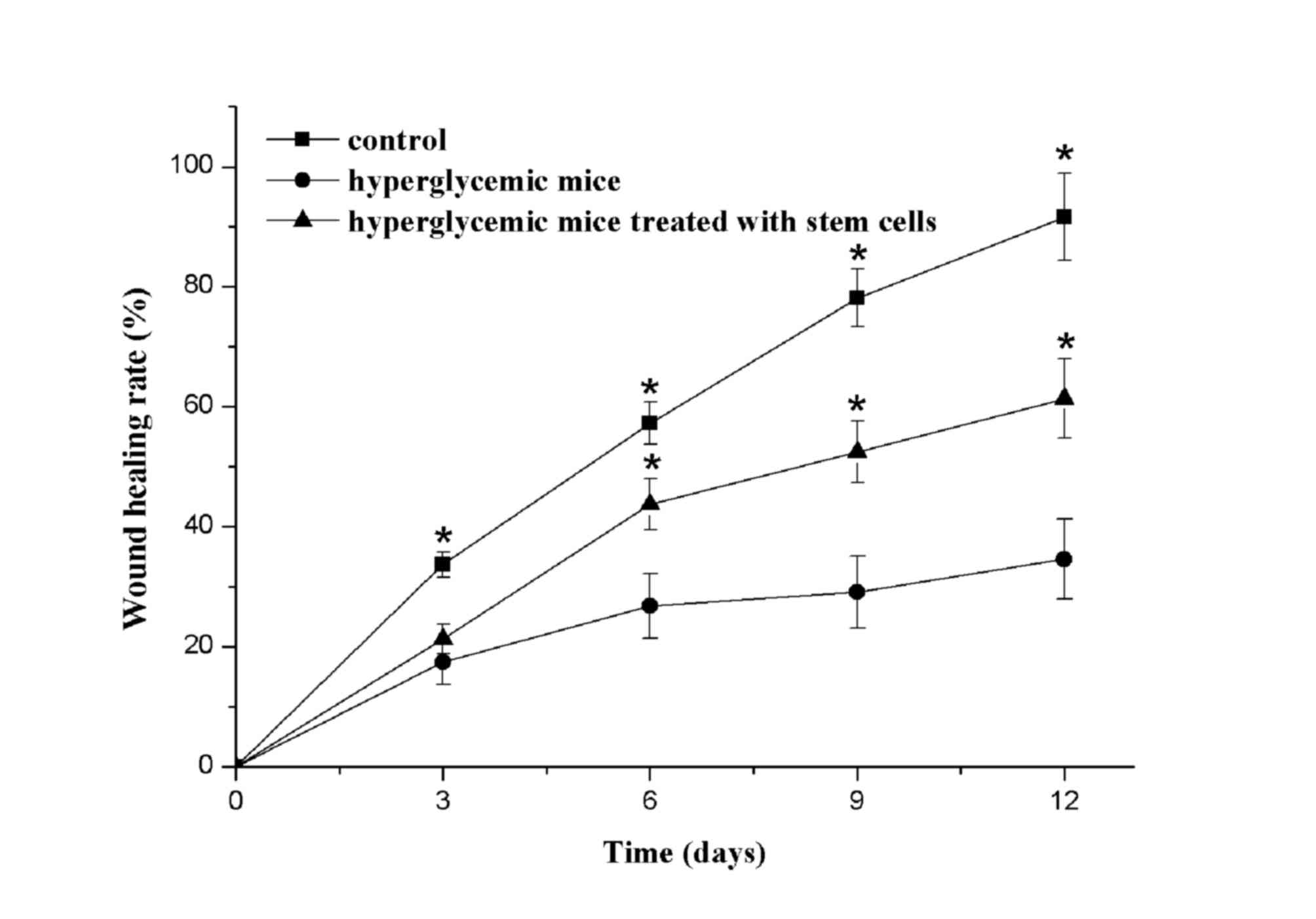
Fig. 3 presented
the wound healing rates of the three groups. Obvious scar tissue
appeared around the wounds of the blank control group at 3 days
following treatment, whereas the hyperglycemia group and
hyperglycemia + stem cells group presented no scar tissue. At 6
days following treatment, obvious scar tissue was still visible in
the blank control group, however, there were clear signs of healing
in the blank group and hyperglycemia + stem cells group. At 9 days,
the wound healing percentages of the blank control group, and the
hyperglycemia + stem cells groups, demonstrated significant
differences, when compared with the hyperglycemia group. At 12
days, the wounds of the blank control group were almost completely
healed. In addition, the hyperglycemic mice treated with stem cells
demonstrated good wound healing; yet the hyperglycemia group still
had a large area that had not healed.
Histological examination of
full-thickness skin wounds
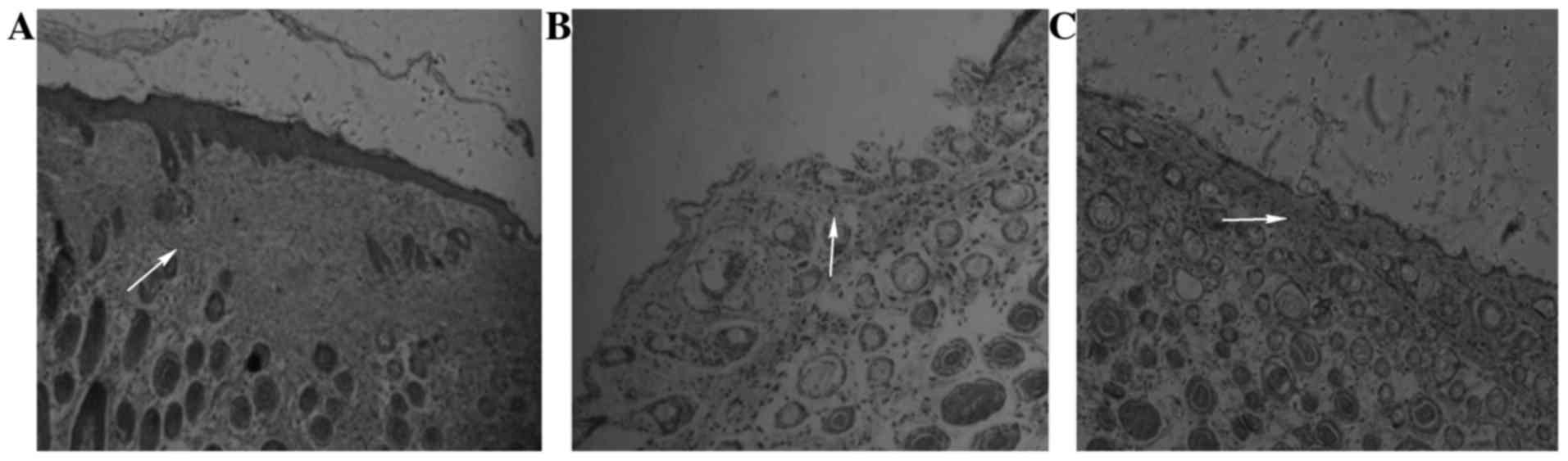
A total of 15 days following treatment, hematoxylin
and eosin staining was applied to the new skin tissue of the wounds
of the three groups, which were observed with an inverted
microscope. In the blank control group, the basal layer of new skin
tissue was uniform, the horny and granular layers were thick,
demonstrating good fusion with the wound edge (Fig. 4A). In the hyperglycemia group, the
new skin wound tissue basal layer was flat, and the prickle cell
layer, granular layer, and hierarchical structure of the horny
layer were visible, but the horny layer and granular layer were
thinner and incomplete, presenting poor fusion with the wound edge
(Fig. 4B). In the hyperglycemic
mice treated with stem cells, the new skin tissue basal layer was
uniform, the horny layer and granular layer were thinner, and
fusion with the wound edge was better (Fig. 4C).
IL-6 and TNF-α expression
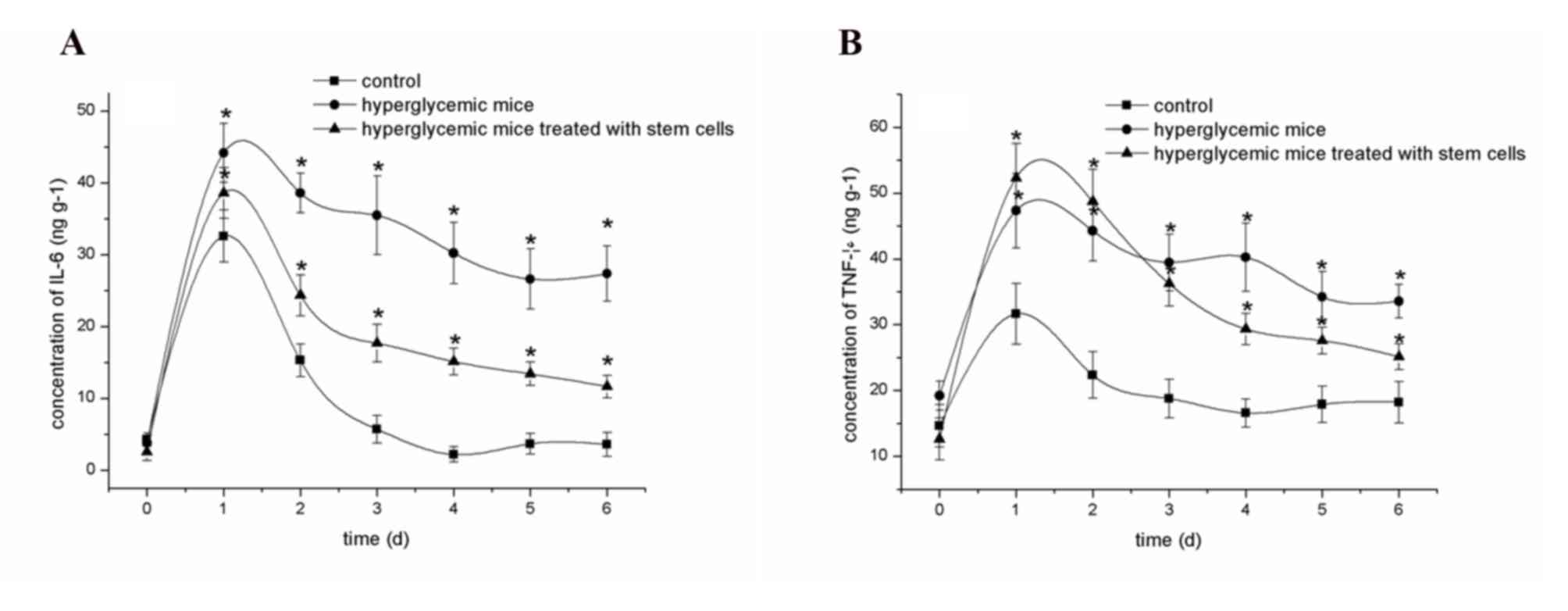
Fig. 5A
demonstrated the concentration of IL-6 in the skin tissue
homogenate of mice. Prior to trauma, the IL-6 concentration in the
skin tissue of normal mice and hyperglycemic mice were very low
(<5 ng/g), which dramatically increased in all three groups
following trauma. Over time, the concentration of IL-6 in the blank
control group gradually reduced to a visibly consistent level at 3
days following trauma. In the hyperglycemic group, the
concentration of IL-6 decreased slightly from day 1 to day 6 within
the scope of 45–28 ng/g. In the hyperglycemic mice treated with
stem cells, the concentration of IL-6 decreased which was slightly
higher than that of the blank group (P=0.521).
Fig. 5B presents
the concentrations of TNF-α in the skin tissue homogenates of the
mice. Prior to trauma, the concentrations of TNF-α in the skin
tissue of normal mice and hyperglycemic mice were 10–20 ng/g, and
demonstrated a dramatic increase in all three groups following
trauma. As time increased, the concentration of TNF-α in the blank
group gradually reduced to approximating the normal level at 3 days
following trauma. In the hyperglycemic group, the concentration of
TNF-α remained higher than 30 ng/g throughout the study period. In
the hyperglycemic mice treated with stem cells, the concentration
of TNF-α significantly decreased, which was slightly higher than
that of the blank group.
Discussion
Chronic wounds result in ulceration to the skin area
and tend to take a long time to heal, frequently becoming infected,
leading to defects or necrosis. Furthermore, the wound is often not
repaired through the normal processes, leading to skin tissue
dysfunction and anatomic defects (11,12).
In addition, the chronic wound area is characterized by long-term
high expression levels of inflammatory cytokines, indicating an
inflammation imbalance, which is an important contributor to the
development of chronic skin ulcers (7). Owing to their characteristic of
multipotent differentiation, stem cells have been previously
demonstrated to accelerate wound healing in a number of studies
(13–15). Therefore, in the present study, the
authors evaluated the potential of intravenous BMSCs on refractory
wound healing in a hyperglycemia full-thickness skin wound mouse
model. In addition, the expression levels of inflammatory cytokines
were measured to explore the mechanism by which BMSCs may promote
refractory wound healing.
The results indicated that the wound healing of the
hyperglycemia group was slower than that of the blank group, and
the hyperglycemic mice treated with stem cells presented faster
healing than the hyperglycemia group. The horny layer and granular
layer were thinner and incomplete in the new skin tissue of the
hyperglycemic group, whereas the new skin wound tissue basal layer
was flat and demonstrated better fusion with the wound edge in the
other two groups. These results suggested that BMSCs could
accelerate the wound healing process in hyperglycemic mice. To
further explore the mechanism by which stem cells promote the
process of refractory wound healing, the authors detected the
expression of inflammatory cytokines (IL-6 and TNF-α) during the
wound healing process. The expression of inflammatory cytokines
(IL-6 and TNF-α) was increased in all three groups, with
continuously higher expression in the hyperglycemic group and
decreased expression in the other two groups over time. These
results suggested that BMSCs could effectively alleviate the
excessive inflammatory reaction in hyperglycemia, thus accelerating
the wound healing process.
Inflammation is a normal physiological response to
stimuli such as trauma, hemorrhage or pathogen infection in
biological tissue (14). Following
damage to normal skin tissue, a local inflammatory reaction is
required to initiate wound healing: Neutrophils enter the wound
area and secrete a large number of chemokines (15), inducing the mononuclear cells in
the blood into the wound tissue to become macrophages. In addition,
neutrophils secrete a large number of enzymes with antimicrobial
effects, and then the neutrophils disappear through an apoptotic
process (16,17). Macrophages can promote repair
through inducing cell proliferation and migration in autocrine and
paracrine manners (18) through
the secretion of various chemokines and growth factors (19,20)
and promotion of various cytokines into the wound area. A local
inflammatory reaction that is not only excessive, but also
long-lasting in a chronic wound, is generally referred to as a
local excessive inflammatory reaction (21) and is one factor contributing to the
difficulty in wound healing (22).
Stem cells can promote rehabilitation and reconstruction during
injury repair in a variety of tissues and organs, largely due to
their immune regulation effects (23,24).
Many studies have demonstrated the involvement of BMSCs in the
immune inflammatory response, which help to accelerate tissue
regeneration. Specifically, BMSCs from the bone marrow were
indicated to effectively reduce the inflammatory reaction (25,26).
The current experiments confirmed that BMSCs can accelerate the
healing process of a skin wound in hyperglycemia mice, and could
effectively curb the inflammatory reaction during the process of
wound healing. Therefore, the authors speculate that the difficulty
of wound healing in hyperglycemia is related to an excessive
inflammatory reaction, and that administration of BMSCs may
effectively alleviate the excessive inflammatory reaction to
accelerate the wound healing process.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. U1304819 and 81401519) and
the Scientific Research Fund of Xinxiang Medical University (grant
no. 2014QN137).
References
|
1
|
Kucisec-Tepes N: Prevention of chronic
wound infection. Acta Med Croatica. 67:(Suppl 1). S51–S58. 2013.(In
Croatian).
|
|
2
|
Park NJ, Allen L and Driver VR: Updating
on understanding and managing chronic wound. Dermatol Ther.
26:236–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yazdanpanah L, Nasiri M and Adarvishi S:
Literature review on the management of diabetic foot ulcer. World J
Diabetes. 6:37–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gourevitch D, Kossenkov AV, Zhang Y, Clark
L, Chang C, Showe LC and Heber-Katz E: Inflammation and its
correlates in regenerative wound healing: An alternate perspective.
Adv Wound Care (New Rochelle). 3:592–603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oberyszyn TM: Inflammation and wound
healing. Front Biosci. 12:2993–2999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gyurko R, Siqueira CC, Caldon N, Gao L,
Kantarci A and Van Dyke TE: Chronic hyperglycemia predisposes to
exaggerated inflammatory response and leukocyte dysfunction in
Akita mice. J Immunol. 177:7250–7256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen KT, Seth AK, Hong SJ, Geringer MR,
Xie P, Leung KP, Mustoe TA and Galiano RD: Deficient cytokine
expression and neutrophil oxidative burst contribute to impaired
cutaneous wound healing in diabetic, biofilm-containing chronic
wounds. Wound Repair Regen. 21:833–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jackson WM, Nesti LJ and Tuan RS:
Mesenchymal stem cell therapy for attenuation of scar formation
during wound healing. Stem Cell Res Ther. 3:202012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin L and Peterson DA: Human mesenchymal
stem cell grafts enhance normal and impaired wound healing by
recruiting existing endogenous tissue stem/progenitor cells. Stem
Cells Transl Med. 2:33–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng L, Chu J, Tan L, Shi Y and Han Y:
Immunoregulation of autologous peripheral blood stem cell
transplantation in patients with hepatitis B-related end-stage
liver disease. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 29:407–410.
2013.(In Chinese). PubMed/NCBI
|
|
11
|
Akiyama K, Chen C, Wang D, Xu X, Qu C,
Yamaza T, Cai T, Chen W, Sun L and Shi S:
Mesenchymal-stem-cell-induced immunoregulation involves
FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell.
10:544–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Short BJ, Brouard N and Simmons PJ:
Prospective isolation of mesenchymal stem cells from mouse compact
bone. Methods Mol Biol. 482:259–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blanpain C: Stem cells: Skin regeneration
and repair. Nature. 464:686–687. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morin C, Roumegous A, Carpentier G,
Barbier-Chassefière V, Garrigue-Antar L, Caredda S and Courty J:
Modulation of inflammation by Cicaderma ointment accelerates skin
wound healing. J Pharmacol Exp Ther. 343:115–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Minton K: Chemokines: Neutrophils leave a
trail for T cells. Nat Rev Immunol. 15:5972015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ariel A, Fredman G, Sun YP, Kantarci A,
Van Dyke TE, Luster AD and Serhan CN: Apoptotic neutrophils and T
cells sequester chemokines during immune response resolution
through modulation of CCR5 expression. Nat Immunol. 7:1209–1216.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian M, Qing C, Niu Y, Dong J, Cao X, Song
F, Ji X and Lu S: The relationship between inflammation and
impaired wound healing in a diabetic rat burn model. J Burn Care
Res. 37:e115–e124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koh TJ and DiPietro LA: Inflammation and
wound healing: The role of the macrophage. Expert Rev Mol Med.
13:e232011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gardian K, Janczewska S, Olszewski WL and
Durlik M: Analysis of pancreatic cancer microenvironment: Role of
macrophage infiltrates and growth factors expression. J Cancer.
3:285–291. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Owen JL and Mohamadzadeh M: Macrophages
and chemokines as mediators of angiogenesis. Front Physiol.
4:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosas-Ballina M, Valdés-Ferrer SI, Dancho
ME, Ochani M, Katz D, Cheng KF, Olofsson PS, Chavan SS, Al-Abed Y,
Tracey KJ and Pavlov VA: Xanomeline suppresses excessive
pro-inflammatory cytokine responses through neural signal-mediated
pathways and improves survival in lethal inflammation. Brain Behav
Immun. 44:19–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li HP, Chen X and Li MQ: Gestational
diabetes induces chronic hypoxia stress and excessive inflammatory
response in murine placenta. Int J Clin Exp Pathol. 6:650–659.
2013.PubMed/NCBI
|
|
23
|
Wang J, Zhou J, Zheng HF and Fu ZZ: Effect
of decitabine on immune regulation in patients with acute myeloid
leukemia after allogeneic hematopoietic stem cell transplantation.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 22:1448–1452. 2014.(In
Chinese). PubMed/NCBI
|
|
24
|
Zhao Y, Huang Z, Qi M, Lazzarini P and
Mazzone T: Immune regulation of T lymphocyte by a newly
characterized human umbilical cord blood stem cell. Immunol Lett.
108:78–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen YT, Sun CK, Lin YC, Chang LT, Chen
YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, et al:
Adipose-derived mesenchymal stem cell protects kidneys against
ischemia-reperfusion injury through suppressing oxidative stress
and inflammatory reaction. J Transl Med. 9:512011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moslem M, Eberle I, Weber I, Henschler R
and Cantz T: Mesenchymal stem/stromal cells derived from induced
pluripotent stem cells support CD34(pos) hematopoietic stem cell
propagation and suppress inflammatory reaction. Stem Cells Int.
2015:8430582015. View Article : Google Scholar : PubMed/NCBI
|