Introduction
Human induced pluripotent stem (iPS) cells are
established with the introduction of reprogramming factors
(1). iPS cells have the potential
to differentiate into hepatocyte-like cells by stimulation with
growth factors or by introduction of transcription factors
(2,3). It is hypothesized that hepatocytes
generated from iPS cells could be used in the future to treat liver
failure, a fatal condition due to major loss of hepatocytes, by
transplantation into patients (4).
Current protocols, however, have many limitations, including that
the hepatocytes generated from iPS cells remain at an immature
state (5). In addition, current
protocols require several weeks to obtain hepatocyte-like
cells.
Growth factors and transcription factors are
important for differentiation of iPS cells to hepatocyte lineage.
Transcription factor protocols efficacy depends on the efficiency
of the method used for the introduction of the gene of interest to
target cells. This problem has been, partly, overcome by the use of
adenovirus vectors (6).
Transcription factors have also been introduced into iPS cells with
conventional reagents (7,8). Alternatively, a combination of growth
factors and media has been demonstrated to affect the
differentiation of iPS cells to hepatocyte lineage. A recent study
reported that William's E medium (WE) is suitable for hepatocyte
differentiation of iPS cells when followed by culturing in
hepatocyte differentiation inducer medium (9). WE medium was originally formulated to
culture primary hepatocytes, and it has been demonstrated to
maintain hepatocyte-specific drug metabolism (10,11).
However, whether growth factor supplementation in WE medium is
sufficient to initiate hepatocyte differentiation of iPS cells
remains unkown.
Oncostatin M, a member of interleukin 6 family, is
expressed in fetal liver, and its expression decreases after birth
(12). Oncostatin M promotes
maturation of hepatocyte differentiation from fetal hepatocytes
in vitro (13). The
resulting hepatocytes express hepatocyte-specific genes, accumulate
glycogen, and remove ammonia (13). Based on these properties, it is
hypothesized that oncostatin M may promote hepatocyte
differentiation of iPS cells, but its effect as a supplement in WE
medium remains unknown.
The present study, therefore, investigated whether
supplementation of WE medium with various growth factors, including
oncostatin M, was a suitable method for hepatocyte differentiation
of iPS cells. The results may provide a novel method for the
successful differentiation of iPS cells to hepatocytes, which could
be potentially useful in the future for therapeutic transplantation
into patients.
Materials and methods
Cell culture
Human iPS 201B7 cells (RIKEN BioResource Center,
Tsukuba, Japan) were cultured in ReproFF (Reprocell, Inc.,
Yokohama, Japan) medium in 6-well plates coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) under feeder-free conditions.
The cells were then harvested using Accutase (Innovative Cell
Technologies, Inc., San Diego, CA, USA) and centrifuged at 100 × g
for 3 min at 4°C. The cells were spread onto new 6-well plates or
96-well plates coated with Matrigel. For experiments, cells were
cultured in ReproFF or in WE (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) media supplemented with nicotinamide (1.2 mg/ml;
Wako Pure Chemical Industries, Ltd., Osaka, Japan), proline (30
ng/ml; Wako Pure Chemical Industries, Ltd.) and 10% Knockout serum
replacement (Thermo Fisher Scientific, Inc.).
Growth factors
The growth factors used in the present study were:
Basic fibroblast growth factor (5 ng/ml; Wako Pure Chemical
Industries, Ltd.), bone morphogenetic protein 4 (20 ng/ml; Wako
Pure Chemical Industries, Ltd.), oncostatin M (20 ng/mg; Wako Pure
Chemical Industries, Ltd.), epidermal growth factor (20 ng/ml; Wako
Pure Chemical Industries, Ltd.), β-nerve growth factor (100 ng/ml;
R&D Systems, Inc., Minneapolis, MN, USA), all-trans retinoic
acid (1 µM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
transforming growth factor-β1 (2 ng/ml; R&D Systems, Inc.),
hepatocyte growth factor (20 ng/ml; Wako Pure Chemical Industries,
Ltd.), dexamethasone (10−7 M; Wako Pure Chemical
Industries, Ltd.), and insulin-transferrin-sodium selenite media
supplement (100x; Sigma-Aldrich; Merck KGaA).
Plasmid construction
The EcoR1-Sal1 fragment of the human α-fetoprotein
(AFP) promoter (Switchgear Genomics, Carlsbad, CA, USA) was
subcloned into the pMetLuc2-reporter plasmid (Clontech
Laboratories, Inc., Mountainview, CA, USA) to produce the
pMetLuc2/AFP promoter reporter plasmid. Similarly, the
Sac1-Hind3 fragment of the human albumin promoter
(Switchgear Genomics) was subcloned into the pMetLuc2-reporter
plasmid to produce the pMetLuc2/albumin promoter reporter
plasmid.
Transfection and luciferase assay
201B7 cells were cultured in 96-well plates coated
with Matrigel. Cells in each well (5×105 cells/well)
were transfected with 100 ng pMetLuc2/AFP or pMetLuc2/albumin
plasmid using FuGENE HD transfection reagent (Promega Corporation,
Madison, WI, USA), according to the manufacturer's instructions. As
a control, 10 ng pSEAP2-control plasmid (Clontech Laboratories,
Inc.), which expresses secreted alkaline phosphatase (SEAP), was
transfected into the cells in parallel to monitor the transfection
efficiency. The transfected cells were then cultured in ReproFF
(FF) or WE media, with or without growth factors. Following two
days of culture, media samples were evaluated by a Ready-To-Glow
secreted luciferase assay (Clontech Laboratories, Inc.) and a SEAP
assay (Clontech Laboratories, Inc.). The luciferase measurement was
divided by the SEAP measurement to calculate the relative gene
promoter activity.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (~5 µg) was isolated from cultured cells
using Isogen (Nippon Gene Co., Ltd., Tokyo, Japan) and was utilized
for the synthesis of first-strand cDNA using SuperScript III
reverse transcriptase and oligo (dT) primers (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Total RNA from human fetal liver was purchased from Clontech
Laboratories, Inc. RT-qPCR was performed using Fast SYBR-Green
Master Mix (Thermo Fisher Scientific, Inc.) and the results were
analyzed using the MiniOpticon Real-Time PCR System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). qPCR was performed in a
volume of 20 µl for 40 cycles, with thermocyclining conditions
according to the Fast SYBR-Green Master Mix suggested protocol.
Primer sequences are listed in Table
I. RPL19 was used as an endogenous reference control because it
is a constitutively expressed housekeeping gene (14). The gene expression levels were
analyzed automatically using the MiniOpticon System, based on the
2−ΔΔCq method (15).
The relative expression was calculated as the expression level of a
specific gene divided by that of RPL19.
 | Table I.Primer sequences used for quantitative
polymerase chain reaction. |
Table I.
Primer sequences used for quantitative
polymerase chain reaction.
| Gene | Primer | Sequence (5′-3′) | Product size
(bp) | GenBank accession
number |
|---|
| AFP |
|
|
|
|
|
| F |
ACACAAAAAGCCCACTCCAG | 147 | NM_001134 |
|
| R |
GGTGCATACAGGAAGGGATG |
|
|
| RPL19 |
|
|
|
|
|
| F |
CGAATGCCAGAGAAGGTCAC | 157 | BC095445 |
|
| R |
CCATGAGAATCCGCTTGTTT |
|
|
| Albumin |
|
|
|
|
|
| F |
GCTCGTGAAACACAAGCCCAAG | 114 | NM_000477 |
|
| R |
GCAAAGCAGGTCTCCTTATCGTC |
Statistical analysis
Data are presented as means ± standard deviation of
three independent repeats. Results were analyzed for statistical
significance by one-way analysis of variance followed by Turkey's
post hoc test, using JMP version 10.0.2 software (SAS Institute,
Inc., Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Human iPS 201B7 cells were cultured in WE medium
supplemented with various growth factors for 7 days and then mRNA
expression levels of AFP and albumin were analyzed by RT-qPCR. As a
control, cells were also cultured in WE medium alone and in
ReproFF, a medium that maintains pluripotency. The mRNA expression
levels of AFP were significantly increased in cells cultured in WE
with most of the growth factors tested, except all-trans retinoic
acid, compared with cells cultured in ReproFF (Fig. 1A). Out of all the factors tested,
Oncostatin M supplementation resulted in the highest increase in
AFP mRNA (Fig. 1A). The mRNA
expression levels of albumin was increased in cells cultured in WE
medium supplemented with all-trans retinoic acid and
insulin-transferrin-selenium compared with cells cultured in
ReproFF (Fig. 1B).
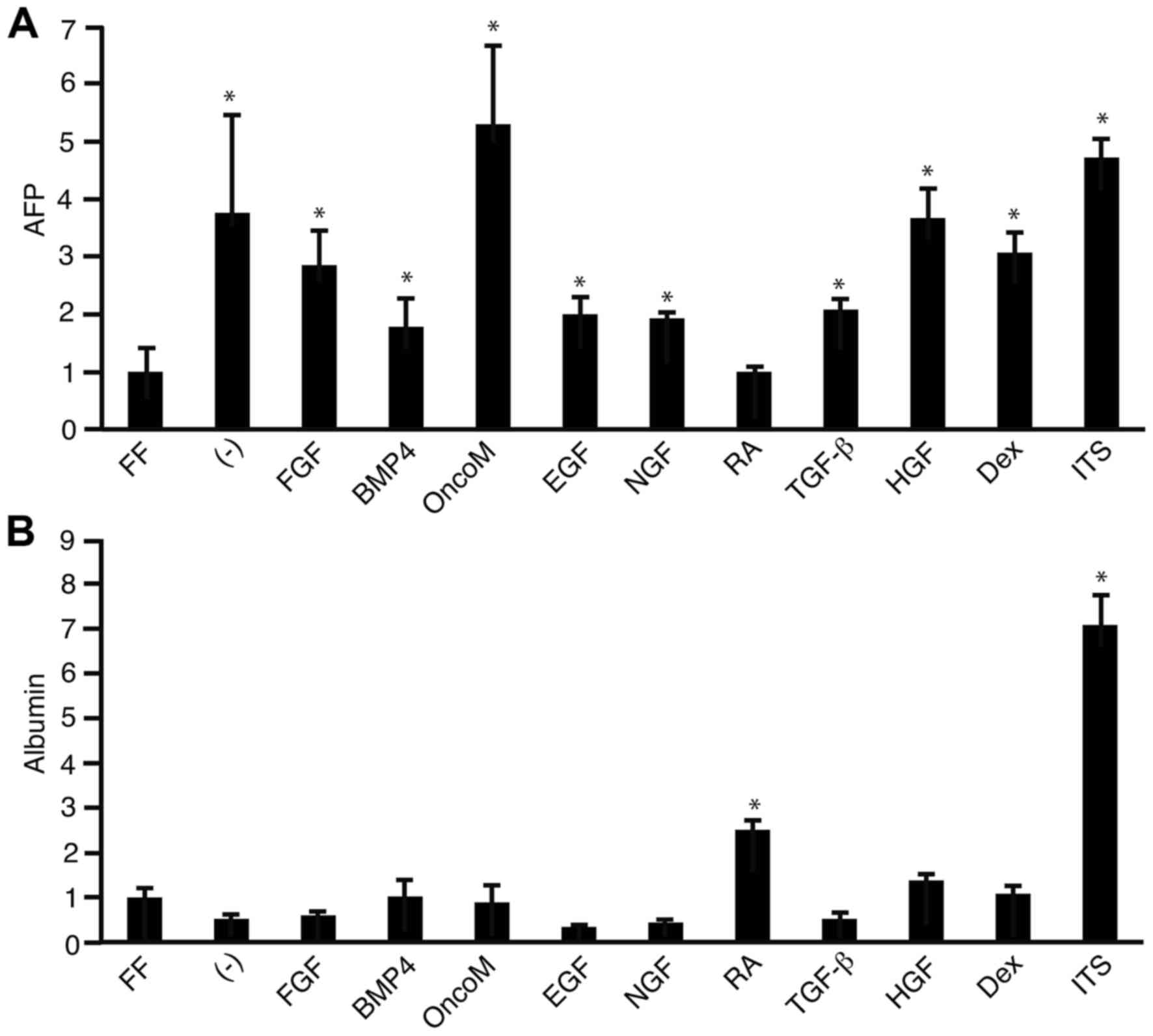 | Figure 1.Effect of growth factors on AFP and
albumin mRNA expression. 201B7 cells were cultured in FF medium or
in WE medium with or without (−) growth factors for 7 days. Reverse
transcription-quantitative polymerase chain reaction was then
performed to analyze the mRNA expression levels of (A) AFP and (B)
albumin. *P<0.05 vs. FF. AFP; α-fetoprotein; FF, ReproFF; WE,
William's E; FGF, fibroblast growth factor; BMP4, bone
morphogenetic protein 4; OncoM, oncostatin M; EGF, epidermal growth
factor; NGF, nerve growth factor; RA, retinoic acid; TGF-β,
transforming growth factor β; HGF, hepatocyte growth factor; Dex,
dexamethasone; ITS, insulin-transferrin-selenium. |
A luciferase reporter assay was performed to
investigate the effect of the growth factors on the promoter
activity of the AFP and albumin genes. 201B7 cells were transfected
with either the pMetLuc2/AFP (Fig.
2A) or pMetLuc2/albumin (Fig.
2B) promoter reporter plasmid, in parallel with pSEAP2-control
plasmid, which expresses alkaline phosphatase, as a control for
transfection efficiency. Following 2 days of culturing the
transfected cells in ReproFF or WE with or without growth factors,
samples from the culture media were subjected to luciferase and
SEAP assays. Oncostatin M supplementation in WE media was observed
to stimulate AFP promoter activity most strongly and significantly
(Fig. 1A), while no growth factor
tested significantly affected the promoter activity of the albumin
gene (Fig. 1B).
 | Figure 2.Effect of growth factors on AFP and
albumin gene promoter activities. 201B7 cells were transfected with
luciferase reporter plasmids driven by either the promoter of (A)
AFP or (B) albumin. A pSEAP2-control vector was transfected in
parallel to monitor transfection efficiency. Following
transfection, cells were cultured in FF medium or in WE medium with
or without (−) growth factors for 2 days, and analyzed by
luciferase and alkaline phosphatase assays. Gene promoter activity
is reported as the relative ratio of luciferase vs. SEAP
measurement for each gene. *P<0.05 vs. FF. AFP; α-fetoprotein;
SEAP, secreted alkaline phosphatase; FF, ReproFF; WE, William's E;
FGF, fibroblast growth factor; BMP4, bone morphogenetic protein 4;
OncoM, oncostatin M; EGF, epidermal growth factor; NGF, nerve
growth factor; RA, retinoic acid; TGF-β, transforming growth factor
β; HGF, hepatocyte growth factor; Dex, dexamethasone; ITS,
insulin-transferrin-selenium. |
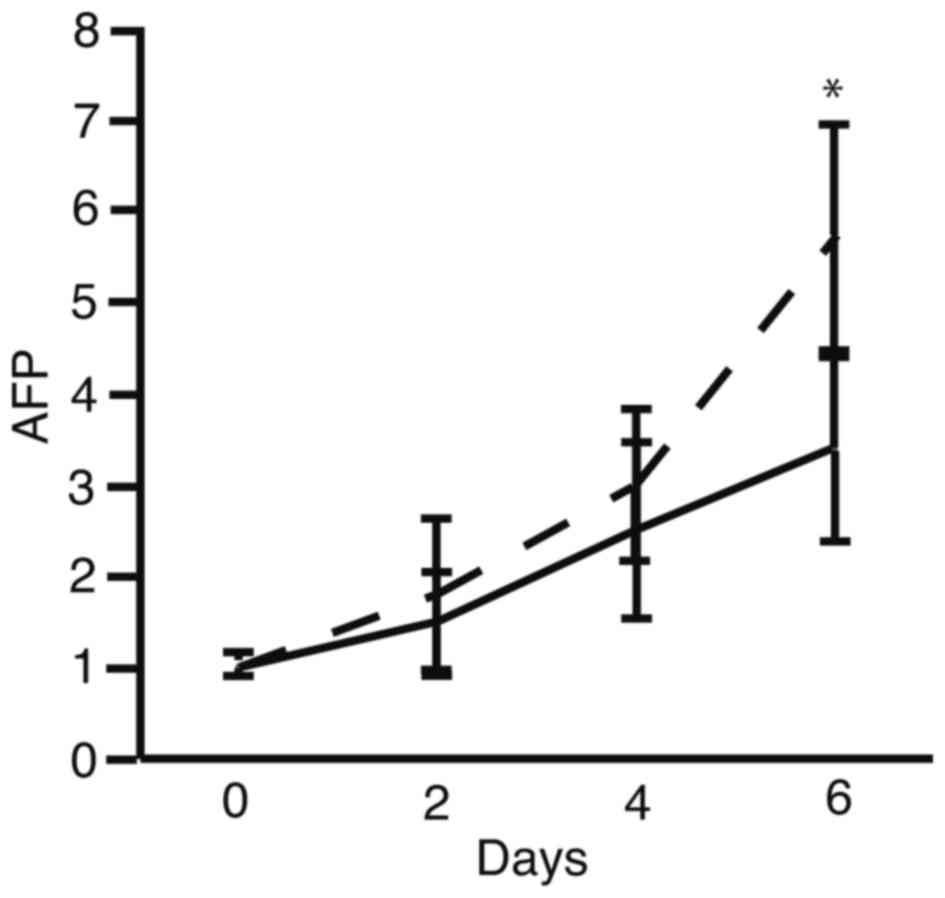
The present results suggested that oncostatin M may
be suitable for the initiation of hepatocyte differentiation in iPS
cells. Time course analysis of the AFP mRNA expression pattern was
performed to identify the timing of the stimulation of 201B7 cells
towards the initiation of hepatocyte lineage differentiation
(Fig. 3). The mRNA expression
levels of AFP increased on the sixth day following the initiation
of culture in the oncostatin M-supplemented WE medium, compared
with cells cultured in WE medium alone (Fig. 3).
Discussion
Oncostatin M belongs to the interleukin-6 subfamily
(16) and it is secreted by
hematopoietic cells during endoderm formation (17). Oncostatin M is important in the
maturation of immature hepatocytes (18) and in the long-term culture of human
primary hepatocytes (19). In the
present study, it was demonstrated that oncostatin M initiated the
differentiation of iPS cells to hepatocyte lineage. The mechanism
of the initiation of hepatocyte differentiation of iPS cells
remains unclear. The present data is consistent with previous
studies from our own group (5,20)
and a study from another group that has demonstrated that
oncostatin M promotes differentiation of iPS cells to hepatocytes
(21). Therefore, oncostatin M is
suggested to be suitable for the initiation of hepatocyte
differentiation from iPS cells.
There is the potential that some of the immature iPS
cells cultured in WE medium supplemented with oncostatin M remain
after differentiation initiation. To counter this, hepatocyte
selection medium (HSM) could be used to eliminate undifferentiated
iPS cells from the culture. Cells do not survive in media without
glucose or arginine, because these two ingredients are essential
for their survival. However, the gluconeogenesis pathway and the
urea cycle occur in hepatocytes, thereby allowing them to produce
glucose from galactose and arginine from ornithine, respectively
(18,19). Hence, HSM is routinely prepared
without glucose or arginine, but supplemented with galactose and
ornithine (22), and culturing in
HSM results is elimination of undifferentiated iPS cells, however
survival of hepatocytes (23).
Since iPS cells cannot survive in media without glucose, it is
hypothesized that the potentially remaining iPS cells in the
differentiation conditions tested in the present study may be
eliminated by subsequent culture in HSM.
A limitation of the present study is that the
expression levels of AFP and albumin were analyzed only at the mRNA
level, and not at the protein level. In addition, the expression
levels of liver-specific genes, including cytochrome P450 3A4,
aldehyde dehydrogenase 2, and glucose-6-phosphatase were not
analyzed. Finally, Fig. 3
demonstrated that AFP expression increased on the sixth day of
culture post-stimulation with oncostatin M in WE medium compared
with WE medium alone, however, the time course analysis was only
performed up to 6 days and therefore, it remains unclear whether
longer culture would increase AFP expression further or whether
this was the maximum. Further studies are required to culture iPS
cells in WE supplemented with oncostatin M for longer than 6 days,
and to analyze the protein expression of AFP and albumin, in
addition to the expression of liver-specific gene markers.
Acknowledgements
The present study was supported by a Grant-in-Aid
for Scientific Research from the Japan Society for the Promotion of
Science (grant no. 15K09032).
References
|
1
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takayama K, Inamura M, Kawabata K,
Sugawara M, Kikuchi K, Higuchi M, Nagamoto Y, Watanabe H, Tashiro
K, Sakurai F, et al: Generation of metabolically functioning
hepatocytes from human pluripotent stem cells by FOXA2 and HNF1α
transduction. J Hepatol. 57:628–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takayama K, Inamura M, Kawabata K,
Katayama K, Higuchi M, Tashiro K, Nonaka A, Sakurai F, Hayakawa T,
Furue Kusuda M and Mizuguchi H: Efficient generation of functional
hepatocytes from human embryonic stem cells and induced pluripotent
stem cells by HNF4α transduction. Mol Ther. 20:127–137. 2012.
View Article : Google Scholar
|
|
4
|
Basma H, Soto-Gutiérrez A, Yannam GR, Liu
L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, et al:
Differentiation and transplantation of human embryonic stem
cell-derived hepatocytes. Gastroenterology. 136:990–999. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Single-step protocol for the
differentiation of human-induced pluripotent stem cells into
hepatic progenitor-like cells. Biomed Rep. 1:18–22. 2013.PubMed/NCBI
|
|
6
|
Inamura M, Kawabata K, Takayama K, Tashiro
K, Sakurai F, Katayama K, Toyoda M, Akutsu H, Miyagawa Y, Okita H,
et al: Efficient generation of hepatoblasts from human ES cells and
iPS cells by transient overexpression of homeobox gene HEX. Mol
Ther. 19:400–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka A, Woltjen K, Miyake K, Hotta A,
Ikeya M, Yamamoto T, Nishino T, Shoji E, Sehara-Fujisawa A, Manabe
Y, et al: Efficient and reproducible myogenic differentiation from
human iPS cells: Prospects for modeling Miyoshi Myopathy in vitro.
PLoS One. 8:e615402013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Sueishi M: Dual gene expression in
embryoid bodies derived from human induced pluripotent stem cells
using episomal vectors. Tissue Eng Part A. 20:3154–3162. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Improved survival and
initiation of differentiation of human induced pluripotent stem
cells to hepatocyte-like cells upon culture in William's E medium
followed by hepatocyte differentiation inducer treatment. PLoS One.
11:e01534352016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu D, Ramin SA and Cederbaum AI: Effect of
pyridine on the expression of cytochrome P450 isozymes in primary
rat hepatocyte culture. Mol Cell Biochem. 173:103–111. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takeba Y, Matsumoto N, Takenoshita-Nakaya
S, Harimoto Y, Kumai T, Kinoshita Y, Nakano H, Ohtsubo T and
Kobayashi S: Comparative study of culture conditions for
maintaining CYP3A4 and ATP-binding cassette transporters activity
in primary cultured human hepatocytes. J Pharmacol Sci.
115:516–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamiya A, Kojima N, Kinoshita T, Sakai Y
and Miyaijma A: Maturation of fetal hepatocytes in vitro by
extracellular matrices and oncostatin M: induction of tryptophan
oxygenase. Hepatology. 35:1351–1359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamiya A, Kinoshita T, Ito Y, Matsui T,
Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, Kishimoto T
and Miyajima A: Fetal liver development requires a paracrine action
of oncostatin M through the gp130 signal transducer. EMBO J.
18:2127–2136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tam S, Clavijo A, Engelhard EK and
Thurmond MC: Fluorescence-based multiplex real-time RT-PCR arrays
for the detection and serotype determination of foot-and-mouth
disease virus. J Virol Methods. 161:183–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urbańska-Ryś H, Wiersbowska A, Stepień H
and Robak T: Relationship between circulating interleukin-10
(IL-10) with interleukin-6 (IL-6) type cytokines (IL-6,
interleukin-11 (IL-11), oncostatin M (OSM)) and soluble
interleukin-6 (IL-6) receptor (sIL-6R) in patients with multiple
myeloma. Eur Cytokine Netw. 11:443–451. 2000.PubMed/NCBI
|
|
17
|
Kinoshita T, Sekiguchi T, Xu MJ, Ito Y,
Kamiya A, Tsuji K, Nakahata T and Miyajima A: Hepatic
differentiation induced by oncostatin M attenuates fetal liver
hematopoiesis. Proc Natl Acad Sci USA. 96:7265–7270. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye JS, Su XS, Stoltz JF, de Isla N and
Zhang L: Signalling pathways involved in the process of mesenchymal
stem cells differentiating into hepatocytes. Cell Prolif.
48:157–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levy G, Bomze D, Heinz S, Ramachandran SD,
Noerenberg A, Cohen M, Shibolet O, Sklan E, Braspenning J and
Nahmias Y: Long-term culture and expansion of primary human
hepatocytes. Nat Biotechnol. 33:1264–1271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: An optimal medium
supplementation regimen for initiation of hepatocyte
differentiation in human induced pluripotent stem cells. J Cell
Biochem. 116:1479–1489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo Y, Iwao T, Nakamura K, Sasaki T,
Takahashi S, Kamada N, Matsubara T, Gonzalez FJ, Akutsu H, Miyagawa
Y, et al: An efficient method for differentiation of human induced
pluripotent stem cells into hepatocyte-like cells retaining drug
metabolizing activity. Drug Metab Pharmacokinet. 29:237–243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomizawa M, Toyama Y, Ito C, Toshimori K,
Iwase K, Takiguchi M, Saisho H and Yokosuka O: Hepatoblast-like
cells enriched from mouse embryonic stem cells in medium without
glucose, pyruvate, arginine and tyrosine. Cell Tissue Res.
333:17–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Survival of primary human
hepatocytes and death of induced pluripotent stem cells in media
lacking glucose and arginine. PLoS One. 8:e718972013. View Article : Google Scholar : PubMed/NCBI
|