Introduction
Bladder cancer is the second most common urologic
malignancy amongst males (1). In
2015 in the United States, it was estimated that there were 74,000
new cases of bladder cancer and 16,000 deaths (2). Transitional cell carcinoma (TCC) or
urothelial carcinoma of the bladder (UCB) account for 90% of all
bladder cancer cases, and are divided in 2 variants: non-muscle
invasive or muscle invasive. The non-muscle invasive form has a
high rate of recurrence (50%), and it progresses to muscle invasive
form in ~11% of the cases (3).
Despite advances in multi-treatment approaches, including surgery,
radiotherapy and chemotherapy, the clinical outcomes of bladder
cancer patients remain unsatisfactory (4,5).
This is due to considerable tumor variability and heterogeneity,
even within the same pathological stage. Predicting a prognosis by
examining the clinicopathological characteristics remains
difficult. Therefore, identification of novel prognostic factors
with high sensitivity and specificity is important to evaluate the
prognosis of UCB and individualize treatment strategies.
CD147 is a member of the immunoglobulin superfamily.
It is widely expressed in human tumors and plays a central role in
the progression of many cancers by stimulating the secretion of
matrix metalloproteinases (MMPs) and cytokines (6). CD147 regulates cell proliferation,
apoptosis, metastasis, differentiation and chemical drug resistance
(6). In addition, recent studies
have demonstrated that the level of CD147 is associated with the
prognosis of patients in many cancers (6). Furthermore, CD147 is recognized as an
effective therapeutic target for hepatocellular carcinoma (HCC)
(7,8) and additional cancers (9,10),
and exciting clinical progress has been made in HCC treatment with
CD147-directed monoclonal antibodies (11).
The activator protein-1 transcription factor, AP-1,
is principally composed of homodimers of the Jun family (c-Jun,
JunB and JunD), heterodimers of the members of Fos (c-Fos, FosB,
Fra-1 and Fra-2) and Jun, or cAMP response element-binding
protein/activating transcription factor family members (12). Homo- or hetero- forms of the AP-1
complex can activate target gene expression by binding to
12-O-Tetradecanoylphorbol-13-acetate-(or TPA)-responsive elements,
which are also known as AP-1 sites (consensus sequence,
5-TGAG/CTCA-3), in promoter or enhancer regions (12). Evidence has demonstrated that AP-1
serves an important role in inflammation, cellular migration,
metastasis and cell proliferation, apoptosis and transformation
(13). More importantly, several
studies have revealed that the AP-1 complex is involved in
carcinogenesis of various tumor types, such as ovarian cancer,
breast cancer and skin cancer. For example, c-Fos has been
identified as independent predictor of decreased survival in breast
cancer (14). High levels of c-Fos
expression are associated with high-grade lesions and poor
prognosis in osteosarcoma and endometrial carcinoma (15,16).
High levels of c-Jun protein have been identified in 31% of primary
and metastatic lung tumors (17).
In breast cancer, activated c-Jun is expressed predominantly at the
invasive front of breast cancer and is associated with
proliferation and angiogenesis (18). However, there has been little
research into the levels of c-Jun and c-Fos in patients with
urothelial carcinoma of bladder tissues.
In the present study, the prognostic significance of
c-Fos, c-Jun and CD147 expression was examined by performing
immunohistochemistry with a tissue microarray of samples from 41
patients with UCB. In addition, the correlation between c-Fos,
c-Jun and CD147 expression in UCB was investigated to supply more
reliable judgments of the prognosis of UCB patients.
Materials and methods
Patients and tissues
A total of 41 bladder cancer tissues with
histologically confirmed UCB were obtained from the Shanghai Outdo
Biotech Co., Ltd. (Shanghai, China) from May 2007 to November 2011,
and 34 para-cancer normal tissues, which were 5 cm far away from
the edge of the tumor, were randomly selected as controls. The
study was approved by the Ethics Committee of the Fourth Military
Medical University (Xi'an, China). Following transurethral
resection and partial or radical cystectomy therapy, all patients
were available for follow-up data and provided written and informed
consent. Clinical pathological information was obtained from
patients' operative and pathological reports, and included: Sex,
age, recurrence, metastasis, invasion tumor size and growth
pattern, WHO grade, TNM stage, AJCC stage and survival time.
The study population comprised 35 men (85.4%) and 6
women (14.6%), with a median age at diagnosis of 71 years (range,
44–85 years). The tumor growth type was papillary in 11 patients
(26.8%) and nonpapillary in 30 patients (73.2%); low-grade tumors
were observed in 3 patients (7.3%) and high-grade tumors were
observed in 38 patients (92.7%). Furthermore, 6 patients (14.6%)
had low-stage (Tis) tumors and 35 patients (85.4%) had high-stage
tumors (T1, 6; T2, 10; T3, 15; T4a, 4; Table I). There was no recurrence or
distant metastasis recorded in all cases. Among these 41 patients,
18 (43.9%) did not survive. The median follow-up period was 35
months (data not shown).
 | Table I.CD147, c-Fos, and c-Jun expression in
41 patients with urothelial carcinoma of the bladder. |
Table I.
CD147, c-Fos, and c-Jun expression in
41 patients with urothelial carcinoma of the bladder.
|
| CD147 (%) |
|---|
|
|
|
|---|
| Factor | +++(n=3) (%) | ++(n=6) (%) | +(n=13) (%) | -(n=19) (%) | P-value |
|---|
| Age (y) |
| >71
(n=20) | 1 (5.0) | 2 (10.0) | 7 (35.0) | 10 (50.0) | 0.393 |
| ≤71
(n=21) | 2 (9.5) | 4 (19.0) | 6 (28.6) | 9 (42.9) |
| Sex |
|
|
|
|
|
| Male
(n=35) | 3 (8.6) | 6 (17.1) | 11 (31.4) | 15 (42.9) | 0.165 |
| Female
(n=6) | 0 (0) | 0 (0) | 2 (33.3) | 4 (66.7) |
| Growth pattern |
|
Papillary (n=11) | 0 (0) | 2 (18.2) | 5 (45.5) | 4 (36.4) | 0.964 |
|
Nonpapillary (n=30) | 3 (10.0) | 4 (13.3) | 8 (26.7) | 15 (50.0) |
| WHO grade |
|
|
|
|
|
| Low
(n=3) | 0 (0) | 1 (33.3) | 2 (66.7) | 0 (0) | 0.338 |
| High
(n=38) | 3 (7.9) | 5 (13.2) | 11 (28.9) | 19 (50.0) |
| Lymph node
invasion |
|
|
|
|
|
|
Negative (n=37) | 2 (5.4) | 6 (16.2) | 10 (27.0) | 19 (51.4) | 0.136 |
|
Positive (n=4) | 1 (25.0) | 0 (0) | 3 (75.0) | 0 (0) |
| TNM stage |
|
|
|
|
|
| Low
(Tis) (n=6) | 0 (0) | 0 (0) | 4 (66.7) | 2 (33.3) | 0.649 |
| High
(T1-T4a) (n=35) | 3 (8.6) | 6 (17.1) | 9 (25.7) | 17 (48.6) |
| Tumor diameter |
|
|
|
|
|
| >4
cm (n=16) | 0 (0) | 5 (31.3) | 6 (37.5) | 5 (31.3) | 0.355 |
| ≤4 cm
(n=25) | 3 (12.0) | 1 (4.0) | 7 (28.0) | 14 (56.0) |
| AJCC cancer
staging |
|
|
|
|
|
| Tis
(n=12) | 1 (8.3) | 1 (8.3) | 4 (33.3) | 6 (50.0) | 0.554 |
| 1–2
(n=11) | 0 (0) | 2 (18.2) | 4 (36.4) | 5 (45.5) |
| 3–4
(n=18) | 2 (11.1) | 5 (27.8) | 3 (16.7) | 8 (44.4) |
|
|
| c-Fos (%) |
|
|
|
| Factor | +++(n=0) | ++(n=1) (%) | +(n=9) (%) | −(n=31)(%) | P-value |
|
| Age (y) |
|
|
|
|
|
| >71
(n=20) | – | 0 (0) | 6 (30.0) | 14 (70.0) | 0.693 |
| ≤71
(n=21) | – | 1 (4.8) | 3 (14.3) | 17 (81.0) |
| Sex |
|
|
|
|
|
| Male
(n=35) | – | 1 (2.9) | 6 (17.1) | 28 (80.0) | 0.220 |
| Female
(n=6) | – | 0 (0) | 3 (50.0) | 3 (50.0) |
| Growth pattern |
|
|
|
|
|
|
Papillary (n=11) | – | 1 (9.1) | 1 (9.1) | 9 (81.8) | 0.973 |
|
Nonpapillary (n=30) | – | 0 (0) | 8 (26.7) | 22 (73.3) |
| WHO grade |
|
|
|
|
|
| Low
(n=3) | – | 0 (0) | 0 (0) | 3 (100.0) | 0.336 |
| High
(n=38) | – | 1 (2.6) | 9 (23.7) | 28 (73.7) |
| Lymph node
invasion |
|
|
|
|
|
|
Negative (n=37) | – | 1 (2.7) | 7 (18.9) | 29 (78.4) | 0.330 |
|
Positive (n=4) | – | 0 (0) | 2 (50.0) | 2 (50.0) |
| TNM stage |
|
|
|
|
|
| Low
(Tis) (n=6) | – | 1 (16.7) | 0 (0) | 5 (83.3) | 0.731 |
| High
(T1-T4a) (n=35) | – | 0 (0) | 9 (25.7) | 26 (74.3) |
| Tumor diameter |
|
|
|
|
|
| >4
cm (n=16) | – | 1 (6.3) | 3 (18.8) | 12 (75.0) | 0.651 |
| ≤4 cm
(n=25) | – | 0 (0) | 6 (24.0) | 19 (76.0) |
| AJCC cancer
staging |
|
|
|
|
|
| Tis
(n=12) | – | 1 (8.3) | 2 (16.7) | 9 (75.0) | 0.552 |
| 1–2
(n=11) | – | 0 (0) | 3 (27.3) | 8 (72.7) |
| 3–4
(n=18) | – | 0 (0) | 4 (22.2) | 14 (77.8) |
|
|
| c-Jun (%) |
|
|
|
| Factor | +++ (n=0) | ++ (n=0) | + (n=9) (%) | − (n=32) (%) | P-value |
|
| Age (y) |
|
|
|
|
|
| >71
(n=20) | – | – | 2 (10.0) | 18 (90.0) | 0.130 |
| ≤71
(n=21) | – | – | 7 (33.3) | 14 (66.7) |
| Sex |
|
|
|
|
|
| Male
(n=35) | – | – | 8 (22.9) | 27 (77.1) | 1.000 |
| Female
(n=6) | – | – | 1 (16.7) | 5 (83.3) |
| Growth pattern |
|
|
|
|
|
|
Papillary (n=11) | – | – | 2 (18.2) | 9 (81.8) | 1.000 |
|
Nonpapillary (n=30) | – | – | 7 (23.3) | 23 (76.7) |
| WHO grade |
|
|
|
|
|
| Low
(n=3) | – | – | 0 (0) | 3 (100) | 1.000 |
| High
(n=38) | – | – | 9 (23.7) | 29 (76.3) |
| Lymph node
invasion |
|
|
|
|
|
|
Negative (n=37) | – | – | 7 (18.9) | 30 (81.1) | 0.204 |
|
Positive (n=4) | – | – | 2 (50.00) | 2 (50.0) | – |
| TNM stage |
|
|
|
|
|
| Low
(Tis) (n=6) | – | – | 2 (33.3) | 4 (66.7) | 0.597 |
| High
(T1-T4a) (n=35) | – | – | 7 (20.0) | 28 (80.0) | – |
| Tumor diameter |
|
|
|
|
|
| >4
cm (n=16) | – | – | 5 (31.3) | 11 (68.8) | 0.276 |
| ≤4 cm
(n=25) | – | – | 4 (16.0) | 21 (84.0) | – |
| AJCC cancer
staging |
|
|
|
|
|
| Tis
(n=12) | – | – | 1 (8.3) | 11 (91.7) | 0.038 |
| 1–2
(n=11) | – | – | 1 (9.1) | 10 (90.9) | – |
| 3–4
(n=18) | – | – | 7 (38.9) | 11 (61.1) | – |
|
| – | – | – | – | – |
Immunohistochemical (IHC) staining and
evaluation
The expression of c-Jun, c-Fos and CD147 was
detected by IHC staining. Briefly, 5 µm sections were cut from
formalin-fixed, paraffin-embedded tissues. Following
deparaffinization and dehydration, sections were heated in an
autoclave (~120°C, 200 kPa, 2 to 6 min,) in 10 mM citrate buffer
(pH 6.0) for antigen retrieval, and treated with 3%
H2O2 for 20 min to block the peroxidase.
Sections were blocked in 5% goat serum in PBS, followed by
overnight incubation at 4°C with anti-c-Jun antibody (1:100
dilution; cat. no. #9165 Cell Signaling Technology, Inc., Danvers,
MA, USA), anti-c-Fos antibody (1:500 dilution; cat. no. #2251; Cell
Signaling Technology, Inc.) andanti-CD147 antibody (1:167 dilution;
cat. no. #201508; Cell Engineering Research Center, Fourth Military
Medical University, Xi'an, China). The signal was detected with
streptavidin-peroxidase staining kits (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and DAB substrate (ZSGB-BIO., Beijing,
China). All sections were then counterstained with Mayer's
hematoxylin (0.1%) for 5 to 8 min at room temperature (cat. no
#715012; Baso Diagnostics, Inc., Zhuhai, Guangdong, China) and then
mounted.
Images were taken with an inverted microscope
(CKX41; Olympus Corporation, Tokyo, Japan) equipped with a digital
camera under ×100 and ×400 magnification. Two pathologists
evaluated the results independently, with no knowledge of
clinicopathological features.
The expression of c-Jun, c-Fos and CD147 were scored
in a semi-quantitative manner according both the extent and the
intensity of the staining: IHC negative, absent; IHC +, faint or
weak intensity in >10% of cancer cells; IHC ++, moderate
intensity in >10% of cancer cells; and IHC +++, strong intensity
in >10% of cancer cells.
Statistical analysis
All statistical analyses were performed using SPSS
software (version, 17.0; SPSS Inc., Chicago, IL, USA). The
relationship between the level of three proteins (c-Fos, c-Jun and
CD147) and clinicopathological features was analyzed with
chi-squared statistical test (linear-by-linear association or the
Fisher exact test) or Spearman correlation analysis, while overall
survival (OS) analysis was carried out using the Kaplan-Meier
method and compared by the Log-rank test and the Cox proportional
hazards regression model served to identify relevant prognostic
factors. All P-values were two sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
The expression characteristicss of
c-Jun, c-Fos and CD147 in UCB and normal tissues
A total of 9 of 41 (22.0%) UCB tissues and 1 of 34
(8.8%) para-cancer tissues presented positive staining of c-Jun,
respectively. Tumor staining of c-Jun expression was observed in
the nucleus staining pattern (Fig.
1), however, only negative or weak immunoreactivity of c-Jun
was detected in para-cancer tissues, which indicated that positive
c-Jun was more commonly expressed in human UCB than para-cancer
tissues (P=0.017; Fig. 1).
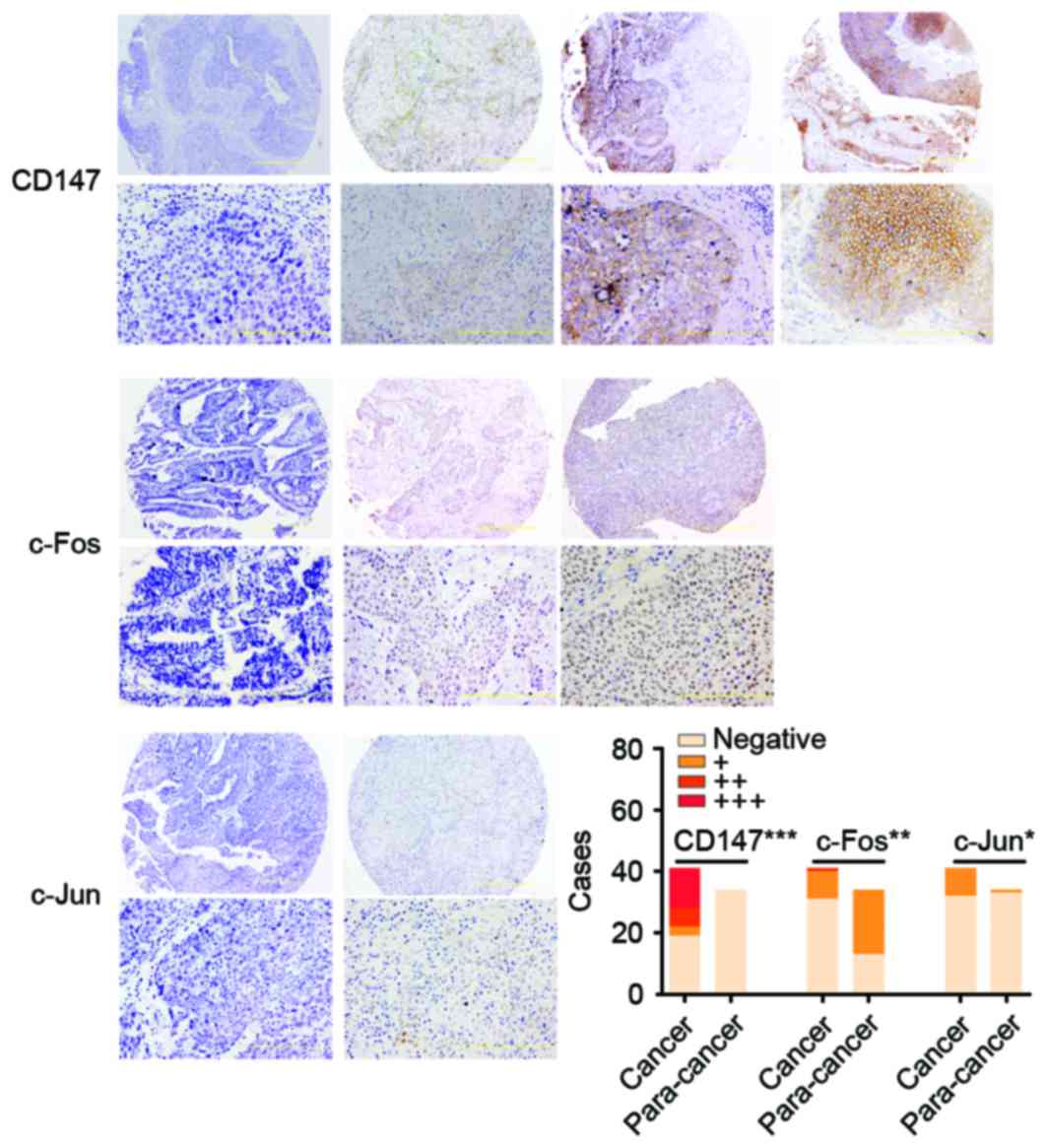 | Figure 1.The immunohistochemical staining of
CD147, c-Fos and c-Jun in UCB. The expression of c-Jun, c-Fos and
CD147 was scored as negative, absence; +, weak; ++, moderate; and
+++, strong. The images were ×100 (upper row) and ×400
magnification (bottom row). The histogram presents the number of
cases with CD147, c-Fos and c-Jun expression in UCB tissues and
para-cancer normal tissues. The distribution of 41 cases of UCB and
34 cases of para-cancer tissues is presented in the bar graph.
CD147 (negative, 19; +, 3; ++, 6; and +++, 13 in UCB. negative, 34
in para-cancer), c-Fos (negative, 31; +, 9; and ++, 1 in UCB.
negative, 13; and +, 21 in para-cancer) and c-Jun (negative, 32; +,
9; in UCB. Negative, 33; and +, 1 in para-cancer), respectively.
***P<0.001, **P<0.01 and *P<0.05 vs. para-cancer. UCB,
urothelial carcinoma of the bladder. |
Following IHC in UCB tissues, 10 of 41 cases (24.4%)
presented c-Fos staining, which was strong in 1 case (2+ or higher,
2.4%), and weak in 9 cases (1+, 22.0%). The other tissues,
including 31 (75.6%) UCB and 13 of 34 (38.2%) normal bladder
tissues, demonstrated no staining. Tumor staining of c-Fos
expression was observed in the nucleus staining pattern (Fig. 1).
CD147 immunoreaction positive (+, ++, +++) was
observed in the 22 cases (53.7%) and most tumor cells with diffuse
plasma membranous staining of CD147, while none of normal
urothelial cells expressed CD147 (Fig.
1), which indicated that the CD147 was more common high
expression in human UCB than that in para-cancer normal tissues
(P<0.001; Fig. 1).
A weak inverse correlation was identified
(rho=−0.310, P=0.049; Table II)
existed in 41 UCB patients between c-Fos expression (negative, +,
++) and CD147 expression (negative, +, ++, +++). However, neither
c-Fos expression (rho =0.233, P=0.142) nor CD147 (rho =0.024,
P=0.881) expression had significant association with c-Jun
expression (negative, +).
 | Table II.CD147, c-Jun and c-Fos expression
correlation in 41 patients with urothelial carcinoma of the
bladder. |
Table II.
CD147, c-Jun and c-Fos expression
correlation in 41 patients with urothelial carcinoma of the
bladder.
|
| CD147 | c-Jun | c-Fos |
|---|
|
|
|
|
|
|---|
| Marker | rho | P-value | rho | P-value | rho | P-value |
| CD147 | – | – | 0.024 | 0.881 | −0.310 | 0.049 |
| c-Jun | 0.024 | 0.881 | – | – | 0.233 | 0.142 |
| c-Fos | −0.310 | 0.049 | 0.233 | 0.142 | – | – |
Clinicopathological parameter
relevance and survival analysis
The protein expression of c-Jun was significantly
associated with AJCC cancer staging (P=0.038), while other
clinicopathological factors, including age, sex, growth pattern,
WHO grade, lymph nodes invasion, tumor node metastatis (TNM) stage
and tumor size, had no apparent relationship with c-Jun, c-Fos or
CD147 expression (Table I).
Neither CD147-c-Jun co-expression (5 cases in 41 UCB patients,
12.2%), CD147-c-Fos co-expression (2 in 41 UCB patients, 5.0%), nor
c-Fos-c-Jun co-expression (4 in 41 UCB patients, 9.8%) were
associated with any clinicopathological parameters (Table III).
 | Table III.CD147, c-Fos, and c-Jun
co-expressions in 41 patients with urothelial carcinoma of the
bladder. |
Table III.
CD147, c-Fos, and c-Jun
co-expressions in 41 patients with urothelial carcinoma of the
bladder.
|
| CD147-c-Jun
co-expression |
|---|
|
|
|
|---|
| Factor | Co-expression (n=5)
(%) | Non-co-expression
(n=36) (%) | P-value |
|---|
| Age (y) |
|
|
|
| >71
(n=20) | 2 (10.0) | 18 (90.0) | 1.000 |
| ≤71
(n=21) | 3 (14.3) | 18 (85.7) |
| Sex |
|
|
|
| Male
(n=35) | 5 (14.3) | 30 (85.7) | 1.000 |
| Female
(n=6) | 0 (0) | 6 (100) |
| Growth pattern |
|
|
|
|
Papillary (n=11) | 1 (9.1) | 10 (90.9) | 1.000 |
|
Nonpapillary (n=30) | 4 (13.3) | 26 (86.7) |
| WHO grade |
|
|
|
| Low
(n=3) | 0 (0) | 5 (100) | 1.000 |
| High
(n=38) | 5 (13.2) | 33 (86.8) |
| Lymph nodes
invasion |
|
|
|
|
Negative (n=37) | 5 (13.5) | 32 (86.5) | 1.000 |
|
Positive (n=4) | 0 (0) | 4 (100) |
| TNM stage |
|
|
|
| Low
(Tis) (n=6) | 0 (0) | 6 (100) | 1.000 |
| High
(T1-T4a) (n=35) | 5 (14.3) | 30 (85.7) |
| Tumor diameter |
|
|
|
| >4
cm (n=16) | 0 (0) | 16 (100) | 0.137 |
| ≤4 cm
(n=25) | 5 (20.0) | 20 (80.0) |
| AJCC cancer
staging |
|
|
|
| Tis
(n=12) | 0 (0) | 12 (100) | 0.068 |
| 1–2
(n=11) | 1 (9.1) | 10 (90.9) |
| 3–4
(n=18) | 4 (22.2) | 14 (77.8) |
|
|
| CD147-c-Fos
co-expression |
|
|
|
| Factor | Co-expression (n=2)
(%) | Non-co-expression
(n=39) (%) | P-value |
|
| Age (y) |
|
|
|
| >71
(n=20) | 2 (10.0) | 18 (90.0) | 0.232 |
| ≤71
(n=21) | 0 (0) | 21 (100) |
| Sex |
|
|
|
| Male
(n=35) | 2 (5.7) | 33 (94.3) | 1.000 |
| Female
(n=6) | 0 (0) | 6 (100) |
| Growth pattern |
|
|
|
|
Papillary (n=11) | 2 (18.2) | 9 (81.8) | 0.067 |
|
Nonpapillary (n=30) | 0 (0) | 30 (100) |
| WHO grade |
|
|
|
| Low
(n=3) | 0 (0) | 3 (100) | 1.000 |
| High
(n=38) | 2 (5.3) | 36 (94.7) |
| Lymph nodes
invasion |
|
|
|
|
Negative (n=37) | 2 (5.4) | 35 (94.6) | 1.000 |
|
Positive (n=4) | 0 (0) | 4 (100) |
| TNM stage |
|
|
|
| Low
(Tis) (n=6) | 0 (0) | 6 (100) | 1.000 |
| High
(T1-T4a) (n=35) | 2 (5.7) | 33 (94.3) |
| Tumor diameter |
|
|
|
| >4
cm (n=16) | 1 (6.2) | 15 (93.8) | 1.000 |
| ≤4 cm
(n=25) | 1 (4.0) | 24 (96.0) |
| AJCC cancer
staging |
|
|
|
| Tis
(n=12) | 0 (0) | 12 (100) | 0.548 |
| 1–2
(n=11) | 1 (9.1) | 10 (90.9) |
| 3–4
(n=18) | 1 (5.6) | 17 (94.4) |
|
|
| c-Fos-c-Jun
co-expression |
|
|
|
| Factor | Co-expression (n=4)
(%) | Non-co-expression
(n=37) (%) | P-value |
|
| Age (y) |
|
|
|
| >71
(n=20) | 1 (5.0) | 19 (95.0) | 0.606 |
| ≤71
(n=21) | 3 (14.3) | 18 (85.7) |
| Sex |
|
|
|
| Male
(n=35) | 3 (8.6) | 32 (91.4) | 0.483 |
| Female
(n=6) | 1 (16.7) | 5 (83.3) |
| Growth pattern |
|
|
|
|
Papillary (n=11) | 0 (0) | 11 (100) | 0.559 |
|
Nonpapillary (n=30) | 4 (13.3) | 26 (86.7) |
| WHO grade |
|
|
|
| Low
(n=3) | 0 (0) | 3 (100) | 1.000 |
| High
(n=38) | 4 (10.5) | 34 (89.5) |
| Lymph nodes
invasion |
|
|
|
|
Negative (n=37) | 4 (10.8) | 33 (89.2) | 1.000 |
|
Positive (n=4) | 0 (0) | 4 (100) |
| TNM stage |
|
|
|
| Low
(Tis) (n=6) | 0 (0) | 6 (100) | 1.000 |
| High
(T1-T4a) (n=35) | 4 (11.4) | 31 (88.6) |
| Tumor diameter |
|
|
|
| >4
cm (n=16) | 0 (0) | 16 (100) | 0.143 |
| ≤4 cm
(n=25) | 4 (16.0) | 21 (84.0) |
| AJCC cancer
staging |
|
|
|
| Tis
(n=12) | 1 (8.3) | 11 (91.7) | 0.383 |
| 1–2
(n=11) | 0 (0) | 11 (100) |
| 3–4
(n=18) | 3 (16.7) | 15 (83.3) |
|
Univariate survival analyses revealed that high
CD147 expression (P=0.038), high c-Jun expression (P=0.008) and
high WHO grade (P<0.001; data not shown) were associated with
poor OS, whereas high c-Fos expression did not significantly
correlate with poor OS for UCB (P=0.225; Fig. 2A-C). Further analysis revealed that
either CD147-c-Fos-c-Jun co-expression (P=0.004) or CD147-c-Jun
co-expression (P=0.037) and c-Fos-c-Jun co-expression (P<0.001)
were also associated with poor OS (Fig. 2D-F). Therefore, it was considered
that OS was better in patients who had c-Fos, c-Jun and CD147 all
negative expression, or only one of them positive than in those who
had double positive or triple positive expression (P=0.008;
Fig. 2H).
 | Figure 2.OS analysis of c-Fos, c-Jun and CD147
expression in 41 patients with urothelial carcinoma of the bladder.
(A) Patients with CD147 positive expression (+, ++, +++) had poorer
overall survival rate than those with CD147 negative expression
(log-rank test, P=0.038). (B) Patients with c-Jun positive
expression (+) had poorer overall survival rate than those with
c-Jun negative expression (log-rank test, P=0.008). (C) c-Fos
positive expression (+, ++) did not significantly correlate with
poor OS (log-rank test, P=0.225). (D) Patients with all the three
proteins co-expressed (CD147-c-Fos-c-Jun co-expression) had poorer
overall survival rate than the others (log-rank test, P=0.004). (E)
Patients with CD147-c-Jun co-expression had poorer overall survival
rate than the others (log-rank test, P=0.037). (F) Patients with
c-Fos-c-Jun co-expression had poorer overall survival rate than the
others (log-rank test, P<0.001). (G) CD147-c-Fos co-expression
did not significantly correlate with poor OS (log-rank test,
P=0.768). (H) OS was better in patients who had c-Fos, c-Jun and
CD147 all negative, or single-protein positive expression than in
those who had double-protein positive or triple-protein positive
expression (log-rank test, P=0.008). OS, overall survival. |
Multivariate analyses (Table IV) demonstrated that high CD147
expression (hazard ratio [HR]=6.889; 95% confidence interval [CI]:
1.315–36.084; P=0.022), high c-Fos expression (HR=4.636; 95%
CI:1.128–19.057; P=0.033), high c-Jun (HR=4.589; 95%
CI:1.172–17.968; P=0.029) and even male sex (HR=0.140; 95%
CI:0.027–0.732; P=0.020) were independent predictors for poor
OS.
 | Table IV.Cox multivariate prognostic analysis
for 41 patients with urothelial carcinoma of the bladder. |
Table IV.
Cox multivariate prognostic analysis
for 41 patients with urothelial carcinoma of the bladder.
|
|
|
|
| 95% CI for HR |
|---|
|
|
|
|
|
|
|---|
| Marker | Risk factor | P-value | HR | Lower | Upper |
|---|
| Sex | Male | 0.020 | 0.140 | 0.027 | 0.732 |
| CD147 | Positive | 0.022 | 6.889 | 1.315 | 36.084 |
| c-Fos | Positive | 0.033 | 4.636 | 1.128 | 19.057 |
| c-Jun | Positive | 0.029 | 4.589 | 1.172 | 17.968 |
Discussion
The present study demonstrated that increased
expression of c-Jun or CD147 proteins was predictive of poor OS for
UCB, although c-Fos protein was not significantly associated with
OS. Furthermore, both CD147 and c-Jun positive expression, or all
positive expression of c-Fos, c-Jun and CD147, served as an index
of poor OS for UCB. All above indicated that overexpression of
c-Jun and CD147, perhaps including c-Fos may contribute to tumor
progression of UCB.
Many genetic factors have been identified as being
associated with bladder cancer (19,20).
However, not much is understood regarding the molecular mechanisms
of its tumorigenesis and tumor progression. CD147 expressed by
tumor cells stimulates peritumoral fibroblasts to produce matrix
metalloproteinases, thus contributing to tumor invasion and
metastasis (21). Previous studies
have highlighted the pivotal role of CD147 protein in
carcinogenesis and tumor progression. CD147 expression in breast
carcinomas is associated with risk factors such as poor
histological grade, negative hormone status, the mitotic index and
tumor size (22). High CD147
immunostaining scores in hepatocellular carcinomas correlate
significantly with tumor grading and tumor-node-metastasis stages
(22). It is reported that CD147
is involved in a regulatory loop of miRNA and transcription factors
in breast cancer invasion and metastasis (23). In gastric carcinoma, CD147
expression is positively correlated with tumor size, depth of
invasion and lymphatic invasion, but not with lymph node
metastasis, staging, or differentiation (24). Previous studies of the authors have
demonstrated that CD147 may be involved in the progression of
prostate cancer and renal cell carcinoma, and can be used as an
independent prognostic factor of these cancers (21,25).
However, CD147 protein expression patterns within esophageal
squamous cell carcinoma and dysplastic lesions are not associated
with any of these clinicopathologic factors (26). In the present study, the results
indicated that CD147 was overexpressed in human bladder cancer.
However, intense expression of CD147 in bladder cancer was not
significantly correlated with the TNM stage, WHO grade and tumor
size (Table I), which disaccorded
with the results of Riethdorf et al (27). In addition, Riethdorf's study
identified that the expression levels of CD147 in invasive
transitional cell carcinomas of bladder were higher than those in
noninvasive tumors (27). These
discrepancies suggest that there are different clinical features of
CD147 expression in bladder cancer cells of different patients of
UCB, in consideration of all cases in the current study with no
recurrence and metastasis following transurethral resection and
partial or radical cystectomy therapy. It has already been
demonstrated that CD147 overexpression is associated with poorer
outcome and shorter survival time in patients with solid tumors
(28,29). The present findings supported this
hypothesis, as CD147 expression was associated with poor survival
in univariate analysis (Fig. 2).
Cumulatively, these results indicated that CD147 may be one of the
key molecular markers to identify high risk of progression in
bladder cancer, but other key molecules involved in tumor
progression such as transcription factors must be identified to
develop more effective therapeutic targets and to supply more
reliable judgments of the prognosis of UCB patients. The study of
Hagemann et al (30)
demonstrated that CD147 activates multiple transcription factors,
including AP-1 (c-Fos, c-Jun and FosB) and NF-κB in cardiomyocytes.
The AP-1 complex consists of two elements, c-Fos and c-Jun
(31). The AP-1 complex has been
implicated in the transformation and progression of cancer
(32). Although a few studies have
discovered some alternative activities of c-Jun like anti-cancer
property (33), most research
suggests that c-Jun contributes to tumor initiation and increased
invasiveness (18,34). In breast cancer, high expression
levels of c-Jun in MCF-7 cells can result in an overall increased
invasion by increased cellular motility and increased expression of
MMP-9 (35). The observed
phenotype for MCF-7 cells with c-Jun overexpression is similar to
that observed clinically in advanced breast cancer, which had
become hormone unresponsive (36).
The other most common element of the AP-1 complex, c-Fos, has also
been identified as independent predictor of poor survival in breast
cancer (14). CD147 has been
regarded as a prognostic marker for breast cancer with MMP-9
(37). It seems that there is a
reason to examine the hypothesis that c-Jun and CD147, and even
c-Fos, are involved in the progression of the human urothelial
carcinoma of the bladder, according to the present study results
and other previously-mentioned studies. However, future research
should aim to elucidate the mechanism of c-Jun, c-Fos and CD147 in
bladder cancer progression by enlarging sample size, and studying
in in vitro and in vivo models.
The present study indicated that increased
expression of the c-Jun and CD147 proteins, as well as
co-expression of CD147-c-Jun, c-Jun-c-Fos, or CD147-c-Jun-c-Fos has
prognostic significance for UCB patients. Therefore, high CD147 and
c-Jun expression may serve roles in UCB progression and may be
diagnostic and therapeutic targets in UCB whether alone or in
combination.
Acknowledgements
This work was supported by grants from National
Science and Technology Major Project (grant no. 2013ZX09301301) and
National High Technology Research and Development Program (grant
no. 2015CB553701).
References
|
1
|
Sharma S, Ksheersagar P and Sharma P:
Diagnosis and treatment of bladder cancer. Am Fam Physician.
80:717–723. 2009.PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Tilborg AA, Bangma CH and Zwarthoff
EC: Bladder cancer biomarkers and their role in surveillance and
screening. Int J Urol. 16:23–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giuliani L, Giberti C, Martorana G,
Bonamini A, Natta GD and Rovida S: Results of radical cystectomy
for primary bladder cancer. Retrospective study of more than 200
cases. Urology. 26:243–248. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pagano F, Bassi P, Galetti TP, Meneghini
A, Milani C, Artibani W and Garbeglio A: Results of contemporary
radical cystectomy for invasive bladder cancer: A
clinicopathological study with an emphasis on the inadequacy of the
tumor, nodes and metastases classification. The J Urol. 145:45–50.
1991.PubMed/NCBI
|
|
6
|
Xiong L, Edwards CK III and Zhou L: The
biological function and clinical utilization of CD147 in human
diseases: A review of the current scientific literature. Int J Mol
Sci. 15:17411–17441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu S, Li Y, Zhang Y, Wang X, Gong L, Han
X, Yao L, Lan M and Zhang W: Expression and clinical implications
of HAb18G/CD147 in hepatocellular carcinoma. Hepatol Res.
45:97–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, Xu HY, Zhang Q, Song F, Jiang JL,
Yang XM, Mi L, Wen N, Tian R, Wang L, et al: HAb18G/CD147 functions
in invasion and metastasis of hepatocellular carcinoma. Mol Cancer
Res. 5:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu XY, Lin N, Li YM, Zhi C and Shen H:
Expression of HAb18G/CD147 and its localization correlate with the
progression and poor prognosis of non-small cell lung cancer.
Pathol Res Pract. 209:345–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhagirath D, Abrol N, Khan R, Sharma M,
Seth A and Sharma A: Expression of CD147, BIGH3 and Stathmin and
their potential role as diagnostic marker in patients with
urothelial carcinoma of the bladder. Clin Chim Acta. 413:1641–1646.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bian H, Zheng JS, Nan G, Li R, Chen C, Hu
CX, Zhang Y, Sun B, Wang XL, Cui SC, et al: Randomized trial of
[131I] metuximab in treatment of hepatocellular carcinoma after
percutaneous radiofrequency ablation. J Natl Cancer Inst.
106:2392014. View Article : Google Scholar
|
|
12
|
Hess J, Angel P and Schorpp-Kistner M:
AP-1 subunits: Quarrel and harmony among siblings. J Cell Sci.
117:5965–5973. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye N, Ding Y, Wild C, Shen Q and Zhou J:
Small molecule inhibitors targeting activator protein 1 (AP-1). J
Med Chem. 57:6930–6948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahner S, Baasch C, Schwarz J, Hein S,
Wölber L, Jänicke F and Milde-Langosch K: C-Fos expression is a
molecular predictor of progression and survival in epithelial
ovarian carcinoma. Br J Cancer. 99:1269–1275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bamberger AM, Milde-Langosch K, Rössing E,
Goemann C and Löning T: Expression pattern of the AP-1 family in
endometrial cancer: Correlations with cell cycle regulators. J
Cancer Res Clin Oncology. 127:545–550. 2001. View Article : Google Scholar
|
|
16
|
Gamberi G, Benassi MS, Bohling T,
Ragazzini P, Molendini L, Sollazzo MR, Pompetti F, Merli M,
Magagnoli G, Balladelli A and Picci P: C-myc and c-fos in human
osteosarcoma: Prognostic value of mRNA and protein expression.
Oncology. 55:556–563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szabo E, Riffe ME, Steinberg SM, Birrer MJ
and Linnoila RI: Altered cJUN expression: An early event in human
lung carcinogenesis. Cancer Res. 56:305–315. 1996.PubMed/NCBI
|
|
18
|
Vleugel MM, Greijer AE, Bos R, van der
Wall E and van Diest PJ: c-Jun activation is associated with
proliferation and angiogenesis in invasive breast cancer. Human
Pathol. 37:668–674. 2006. View Article : Google Scholar
|
|
19
|
Gromova I, Gromov P and Celis JE: bc10: A
novel human bladder cancer-associated protein with a conserved
genomic structure downregulated in invasive cancer. Int J Cancer.
98:539–546. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moreira JM, Gromov P and Celis JE:
Expression of the tumor suppressor protein 14-3-3 sigma is
down-regulated in invasive transitional cell carcinomas of the
urinary bladder undergoing epithelial-to-mesenchymal transition.
Mol Cell Proteomics. 3:410–419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han ZD, He HC, Bi XC, Qin WJ, Dai QS, Zou
J, Ye YK, Liang YX, Zeng GH, Zhu G, et al: Expression and clinical
significance of CD147 in genitourinary carcinomas. J Surg Res.
160:260–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong WD, Chen QB, Ye YK, Han ZD, Bi XC,
Dai QS, Liang YX, Zeng GH, Wang YS, Zhu G, et al: Extracellular
matrix metalloproteinase inducer expression has an impact on
survival in human bladder cancer. Cancer Epidemiol. 34:478–482.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong LM, Liao CG, Zhang Y, Xu J, Li Y,
Huang W, Zhang Y, Bian H and Chen ZN: A regulatory loop involving
miR-22, Sp1, and c-Myc modulates CD147 expression in breast cancer
invasion and metastasis. Cancer Res. 74:3764–3778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng HC, Takahashi H, Murai Y, Cui ZG,
Nomoto K, Miwa S, Tsuneyama K and Takano Y: Upregulated
EMMPRIN/CD147 might contribute to growth and angiogenesis of
gastric carcinoma: A good marker for local invasion and prognosis.
Br J Cancer. 95:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang YX, He HC, Han ZD, Bi XC, Dai QS, Ye
YK, Qin WJ, Zeng GH, Zhu G, Xu CL and Zhong WD: CD147 and VEGF
expression in advanced renal cell carcinoma and their prognostic
value. Cancer Invest. 27:788–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishibashi Y, Matsumoto T, Niwa M, Suzuki
Y, Omura N, Hanyu N, Nakada K, Yanaga K, Yamada K, Ohkawa K, et al:
CD147 and matrix metalloproteinase-2 protein expression as
significant prognostic factors in esophageal squamous cell
carcinoma. Cancer. 101:1994–2000. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riethdorf S, Reimers N, Assmann V,
Kornfeld JW, Terracciano L, Sauter G and Pantel K: High incidence
of EMMPRIN expression in human tumors. Int J Cancer. 119:1800–1810.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong WD, Han ZD, He HC, Bi XC, Dai QS,
Zhu G, Ye YK, Liang YX, Qin WJ, Zhang Z, et al: CD147, MMP-1, MMP-2
and MMP-9 protein expression as significant prognostic factors in
human prostate cancer. Oncology. 75:230–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu W, Liu J, Xiong X, Ai Y and Wang H:
Expression of MMP9 and CD147 in invasive squamous cell carcinoma of
the uterine cervix and their implication. Pathol Res Pract.
205:709–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hagemann T, Wilson J, Kulbe H, Li NF,
Leinster DA, Charles K, Klemm F, Pukrop T, Binder C and Balkwill
FR: Macrophages induce invasiveness of epithelial cancer cells via
NF-kappa B and JNK. J Immunol. 175:1197–1205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bossis G, Malnou CE, Farras R,
Andermarcher E, Hipskind R, Rodriguez M, Schmidt D, Muller S,
Jariel-Encontre I and Piechaczyk M: Down-regulation of c-Fos/c-Jun
AP-1 dimer activity by sumoylation. Mol Cell Biol. 25:6964–6979.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramos-Nino ME and Littenberg B: A novel
combination: Ranpirnase and rosiglitazone induce a synergistic
apoptotic effect by down-regulating Fra-1 and Survivin in cancer
cells. Mol Cancer Ther. 7:1871–1879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang CW, Lee YZ, Hsu HY, Wu CM, Chang HY,
Chao YS and Lee SJ: c-Jun-mediated anticancer mechanisms of
tylophorine. Carcinogenesis. 34:1304–1314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eferl R, Ricci R, Kenner L, Zenz R, David
JP, Rath M and Wagner EF: Liver tumor development. c-Jun
antagonizes the proapoptotic activity of p53. Cell. 112:181–192.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Briggs J, Chamboredon S, Castellazzi M,
Kerry JA and Bos TJ: Transcriptional upregulation of SPARC, in
response to c-Jun overexpression, contributes to increased motility
and invasion of MCF7 breast cancer cells. Oncogene. 21:7077–7091.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith LM, Wise SC, Hendricks DT, Sabichi
AL, Bos T, Reddy P, Brown PH and Birrer MJ: cJun overexpression in
MCF-7 breast cancer cells produces a tumorigenic, invasive and
hormone resistant phenotype. Oncogene. 18:6063–6070. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao S, Ma W, Zhang M, Tang D, Shi Q, Xu
S, Zhang X, Liu Y, Song Y, Liu L and Zhang Q: High expression of
CD147 and MMP-9 is correlated with poor prognosis of
triple-negative breast cancer (TNBC) patients. Med Oncol.
30:3352013. View Article : Google Scholar : PubMed/NCBI
|
















