Introduction
Gastric cancer (GC) is one of the most common types
of cancer worldwide and its mortality rate is the second highest
among all malignancies (1).
Recurrence following treatment is the primary cause of
GC-associated mortality (2).
Therefore, it is important to elucidate the mechanism of drug
resistance and identify strategies to prevent the recurrence of GC
following treatment. Cancer stem cells (CSCs) are the origin of
uncontrolled cancer cell growth. The elimination of CSCs is
considered the only way to fully eradicate tumors (3). Thus, identification, isolation and
validation of gastric CSCs (GCSCs) may provide novel clues for GC
treatment. However, data currently available regarding the
isolation, characterization and functional investigations of CSCs
are inconsistent and controversial, particularly in GCSCs. For
example, Fukuda et al (4)
obtained GCSCs from MKN-45 cells via side population (SP) cell
sorting, whereas Zhang et al (5), found that the SP cell sorting method
did not apply to all types of GC cell. Takaishi et al
(6) isolated GCSCs from MKN-45,
MKN-74 and N-87 GC cell lines when CD44+ was used as a
marker, however, no significant differences in tumor formation were
found between the SP cells and non-SP cells. Others have reported
that CD44+ cells show no correlation with the malignancy
of GC cells (7). Thus, it is
important to isolate pure GCSCs by applying the appropriate methods
and markers.
The CSC theory holds that the development of tumors
derives from CSCs with unlimited self-renewal ability to generate
cancer progenitor cells (CPCs), which have limited self-renewal
ability and differentiate into large quantities of regular cancer
cells. However, the majority of studies on CSCs do not distinguish
between CSCs and CPCs in cell populations with stemness, as CPCs
also have self-renewal ability and stemness (8). As CSCs and CPCs may have
significantly different biological characteristics, it is important
to distinguish between CSCs and CPCs in stem-like cells.
Aldehyde dehydrogenase (ALDH) is a marker, which can
be used to distinguish between the high degree of stemness of CSCs
and the low degree of stemness of CPCs from stem-like cell
populations. ALDH is an enzyme, which detoxifies and is important
in stem cell proliferation. Its activity reflects the degree of
cell stemness (9–13). Accordingly, several studies have
acquired CSCs from ALDH+ tumor cells by assessing ALDH
activity (14–19). Although these studies did not
distinguish between CSCs and CPCs in acquired stem-like cells, this
method can detect the levels expression of ALDH in ALDH+
cell populations. Consequently, the present study hypothesized that
pure CSCs are ALDH+ cells with high ALDH activity and
CPCs are ALDH+ cells with low ALDH activity. In our
previous study, ALDH high (ALDHhigh), low
(ALDHlow) and negative (ALDHneg) subgroups we
successfully sorted in H22 mouse hepatic cancer cells, and it was
found that the characteristics of these cells were similar to those
of CSCs, CPCs and regular cancer cells, respectively (20). These results suggested that sorting
of ALDHhigh and ALDHlow populations may
assist in isolating and characterizing GCSCs and gastric CPCs
(GCPCs).
In order to elucidate the causes of the conflicting
results in previous studies of gastric cancer stem cells, in the
present study ALDHhigh, ALDHlow and
ALDHneg were successfully sorted from 615 murine GC
(MFC) cells using an ALDH assay. It was found that
ALDHhigh and ALDHlow cells exhibited
characteristics of GCSCs and GCPCs, respectively. These findings
suggested that the MFC stem-like cells had two cell subpopulations
with distinct characteristics and that CPCs require exclusion for
the investigation of CSCs.
Materials and methods
Cell lines and cell culture
MFC cells were purchased from the Chinese Academy of
Sciences Typical Culture Preservation Committee Cell Bank
(Shanghai, China). The cells were cultured in humidified air at
37°C with 5% CO2 in RPMI-1640 (Sigma-Aldrich; Merck
Millipore; Darmstadt, Germany) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.).
ALDH assay and cell sorting
ALDH activity was determined using an ALDEFLUOR™
assay (Stemcell Technologies, Inc., Vancouver, BC, Canada)
according to the manufacturer's protocol. Briefly, the cells were
suspended in ALDEFLUOR™ assay buffer (2×106 cells/ml).
The ALDH reaction substrate, BODIPY-aminoacetaldehyde, was added to
the experimental groups, whereas ALDH substrate and the inhibitor,
diethylaminobenzaldehyde, were added to the control groups,
followed by incubation at 37°C for 40 min in the dark. An Accuri C6
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) was used
to detect ALDH activity, and analyze the proportion of
ALDH+ and ALDH− cells. Cell sorting was
performed as previously described (20). Briefly, the 1% of viable cells with
the highest fluorescence intensity among the ALDH+ cell
population were selected as ALDHhigh cells, the 1% with
the lowest fluorescence intensity among the ALDH+ cell
population were selected as the ALDHlow cell population
and the 1% with the lowest fluorescence intensity among the
ALDH− cell population were selected as
ALDHneg cells. Flow cytometry was used to select these
cells on a FACS Aria II flow cytometer (BD Biosciences).
Flow cytometry
The cells were suspended in phosphate-buffered
saline (PBS; 2×106 cells/ml). Rat
anti-CD133-phycoerythrin antibody (clone 13A4; 1:50; cat. no.
12-1331-82; eBioscience, San Diego, CA, USA) and rat
anti-CD44-allophycocyanin antibody (clone IM7; 1:50; cat. no.
559250; BD Biosciences) were added to the experimental groups, and
the same quantity of normal isotype IgG was added to the control
groups. Following incubation at 4°C for 30 min in the dark, the
cells were washed with PBS and subjected to flow cytometric
analysis (Accuri C6).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
RNA was extracted from cells using an RNeasy Total
RNA system (Qiagen, Inc, Valencia, CA, USA) according to the
manufacturer's protocol. The quantity and purity of the RNA were
assessed by measuring the absorbance at 260 and 280 nm. The cDNA
was synthesized from total RNA (2 µg) with oligo (dT) primers using
an M-MLV reverse transcriptase first strand kit (Invitrogen; Thermo
Fisher Scientific, Inc.). A 25 µl PCR reaction contained 4 µl cDNA,
2.5 µl buffer, 1 µl forward primer, 1 µl reverse primer, 1 µl dNTP,
1 µl Taq DNA polymerase and 14.5 µl DEPC water. The primers
used were as follows: Octamer-binding transcription factor-4
(OCT-4), forward 5′-TGGGCTAGAGAAGGATGTGG-3′ and reverse
5′-CTGGGAAAGGTGTCCCTGTA-3′; sex determining region Y-box 2 (SOX-2),
forward 5′-GAACGCCTTCATGGTATGGT-3′ and reverse
5′-TCTCGGTCTCGGACAAAAGT-3′; GAPDH, forward
5′-GGTTGTCTCCTGCGACTTCA-3′ and reverse 5′-TGGTCCAGGGTTTCTTACTCC-3′.
The PCR reaction conditions were as follows: 94°C for 5 min,
followed by 30 cycles of 94°C for 40 sec, 61°C for 30 sec and 72°C
for 10 min. The PCR products were analyzed on a 2% agarose gel with
ethidium bromide. The gel images were analyzed using Image-Pro plus
6.0 software (Media Cybernetics, Inc. Rockville, MD, USA).
Spheroid colony formation assay
The cells were inoculated at a density of 5,000
cells/well in ultra-low attachment 6-well plates (Corning
Incorporated, Corning, NY, USA). Stem cell culture medium (2 ml;
Academy of Military Medical Sciences, Beijing, China) was added to
each well. The plate was placed in humidified air at 37°C with 5%
CO2. Every 2 days, 1 ml stem cell medium was added. The
sphere formation of the cells was observed at 7 days under a
fluoresence microscope (TE2000-U; Nikon Corporation, Tokyo,
Japan).
In vivo tumorigenicity
The ALDHhigh, ALDHlow or
ALDHneg cells were suspended in PBS following sorting,
adjusted to 500 or 5,000 cells per 50 µl, and then mixed with 50 µl
Matrigel (BD biosciences). The cells were injected subcutaneously
into 6-week-old female 615 mice (Experimental Animal Center of PLA
General Hospital, Beijing, China). These mice were maintained under
barrier conditions on a 12 h light/dark cycle in a temperature room
at 20–24°C with free access to food and water, and the growth of
tumors was observed every week for 16 weeks. The tumor mass was
monitored using a caliper and the mice were sacrificed by cervical
dislocation at 16 weeks.
Statistical analysis
All data were analyzed using SPSS 19.0 statistical
software (IBM SPSS, Armonk, NY, USA). Data are expressed as the
mean ± standard deviation from three independent experiments.
Comparisons were made using an independent samples Student's
t test between two groups and by one-way analysis among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
ALDH assay and cell sorting in MFC
cells
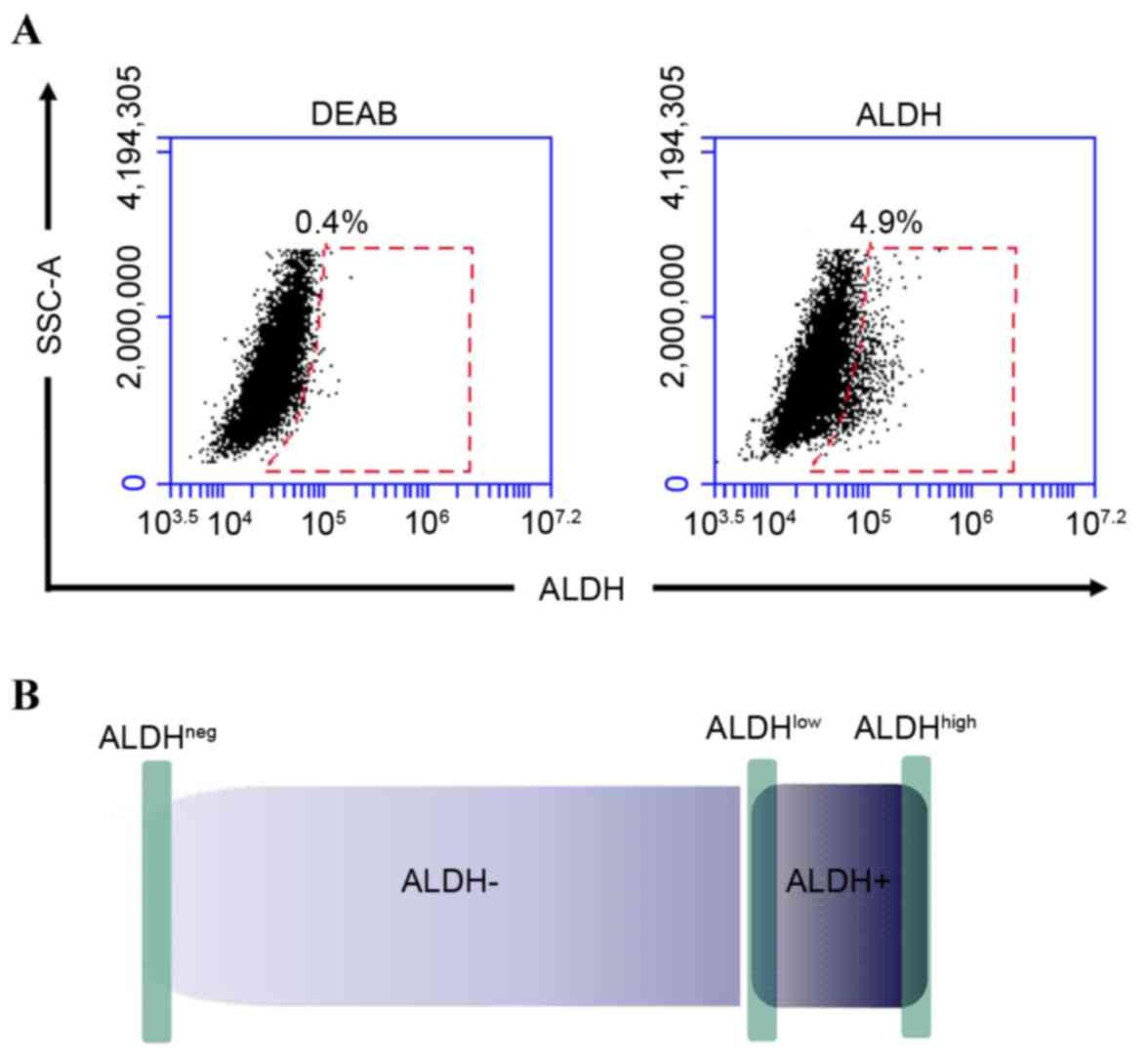
The results of the flow cytometric analyses showed
that the proportion of ALDH+ MFC cells was 5.12±0.91%
(Fig. 1A), which was in accordance
with the relatively low percentages of CSCs in tumors (21). In order to obtain cancer cells with
different differentiation levels, the 1% of the cells with the
highest fluorescence in the ALDH+ population
(ALDHhigh) were selected and considered to be GCSCs. The
1% of the cells with the lowest fluorescence (ALDHlow)
were considered to be GCPCs and the 1% with the lowest fluorescence
in the ALDH− population (ALDHneg) were
considered to be regular cancer cells (Fig. 1B).
Expression of stem cell surface
markers in cells with different ALDH activities
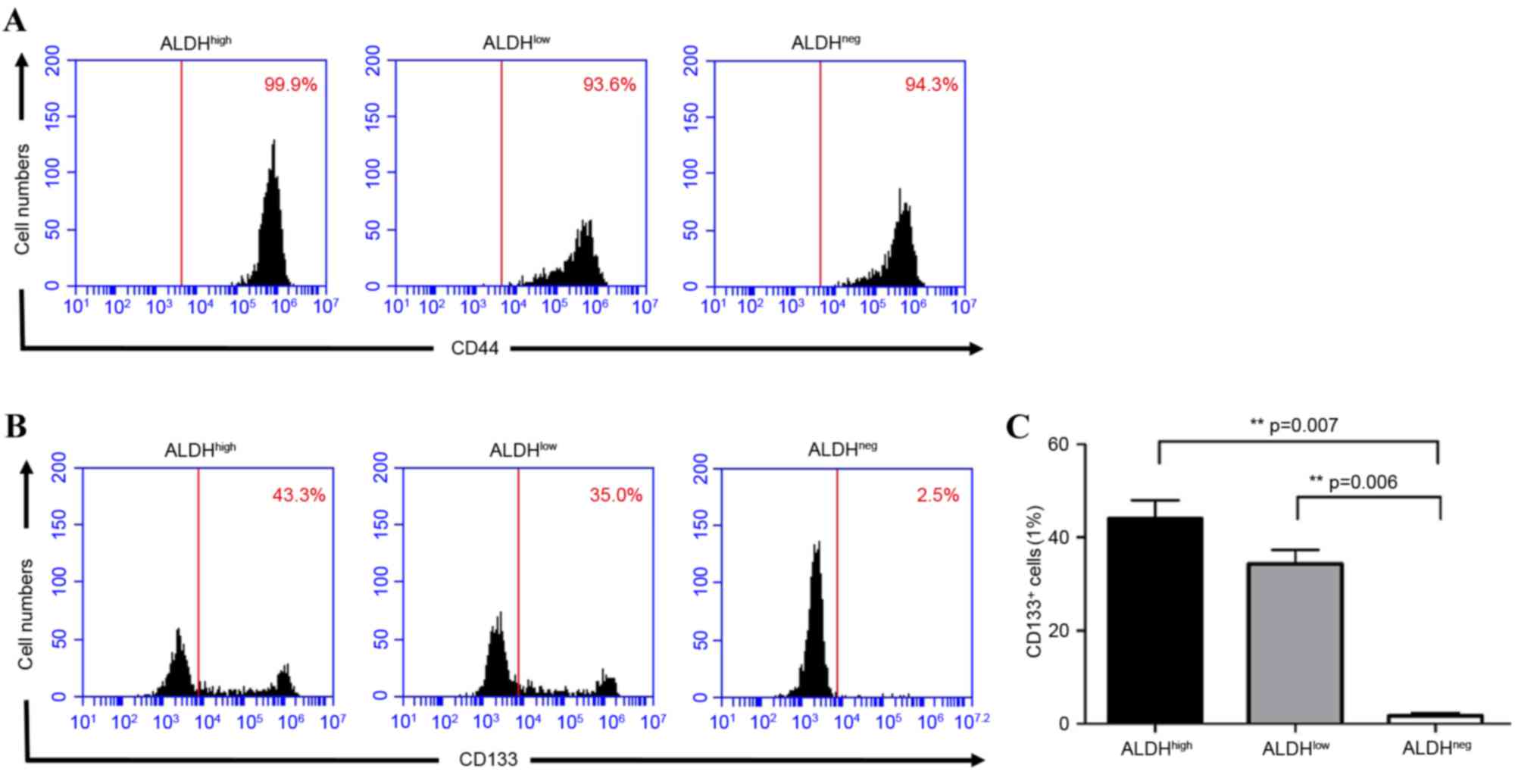
In order to confirm the populations, the stem cell
surface markers, CD44+ and CD133+ (22) were detected in the different cell
populations using flow cytometry. The results showed that
CD44+ was expressed in >90% of the total cell
subpopulation, indicating that CD44+ may not be suitable
as a stem cell marker in MFC cells (Fig. 2A). CD133+ was
significantly higher in the ALDHhigh (44.07±3.97%) and
ALDHlow (34.33±3.06%) cells, compared with the
ALDHneg (1.60±0.66%) cells (high. vs. neg, P=0.007; low,
vs. neg, P=0.006). The expression of CD133+ was higher
in the ALDHhigh cells, compared with the
ALDHlow cells, but this was not significantly different
(P=0.09; Fig. 2B and C).
Gene expression in cells with
different ALDH activity
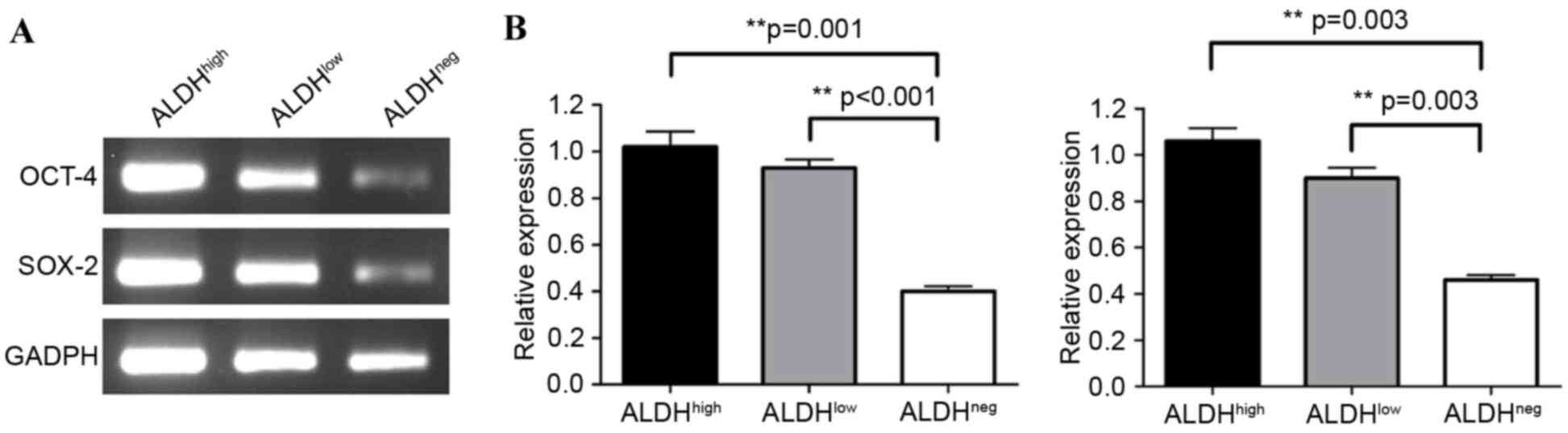
In order to investigate the degree of
differentiation of the cell subgroups, the relative expression of
the stemness-associated genes OCT-4 (23) and SOX-2 (24) were examined using RT-PCR analysis.
The results showed that the relative expression levels of OCT-4
were significantly higher in the ALDHhigh (1.02±0.07)
and ALDHlow cells (0.93±0.04), compared with the
ALDHneg (0.40±0.02) cells; ALDHhigh, vs.
ALDHneg: P=0.001; ALDHlow, vs.
ALDHneg: P<0.001; Fig.
3A and B). The expression of OCT-4 in the ALDHhigh
cells was higher, compared with that in the ALDHlow
cells, although this was not significant (P=0.331). A similar trend
was observed for the expression of SOX-2. The ALDHhigh
(1.06±0.06) and ALDHlow (0.90±0.05) cells had markedly
higher expression of SOX-2, compared with the ALDHneg
(0.46±0.02) cells (ALDHhigh, vs.
ALDHneg: P=0.003; ALDHlow, vs.
ALDHneg; P=0.003), as shown in Fig. 3B. The expression of SOX-2 in the
ALDHhigh cells was higher, compared with that in the
ALDHlow cells, although this was not significant
(P=0.053).
Sphere formation in cells with
differing ALDH activity
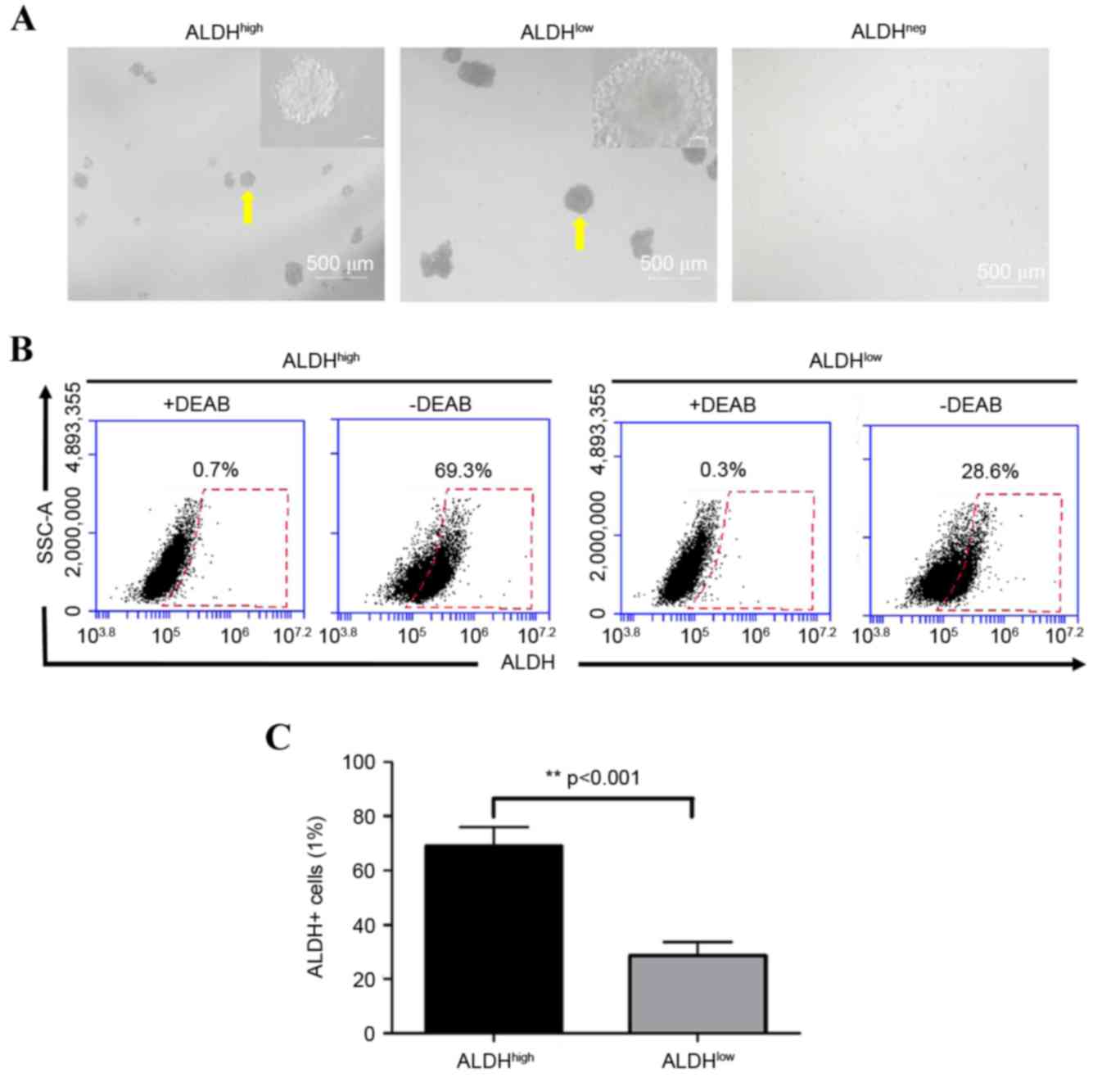
To assess the sphere-forming ability of cells with
differing ALDH activity, ALDHhigh, ALDHlow or
ALDHneg cells were cultured under ultra-low adhesion
conditions without serum. The results showed that
ALDHhigh and ALDHlow cells formed tumor
spheres following 7 days in culture, however, ALDHneg
cells did not form spheres. The tumor sphere volumes of the
ALDHhigh cells were significantly lower, compared with
those of the ALDHlow cells (Fig. 4A). The ALDH activity assays
demonstrated that the percentage of ALDH+ cells in the
spheres formed by ALDHlow cells was significantly lower,
compared with the percentage in the ALDHhigh cell
spheres (30.5±5.7, vs. 70.1±7.1%, respectively; P<0.001;
Fig. 4B and C).
Tumor formation in cells with
differing ALDH activity in mice
In order to assess the tumor-forming ability of
cells with differing ALDH activity, the sorted cells were
subcutaneously injected in mice at different concentrations (500
and 5,000 cells per 50 µl PBS) to observe tumor formation. The
results are shown in Table I.
Tumors appeared 4 weeks following the injection of 5,000 cells and
tumor formation was observed in all mice at 8 weeks in the
ALDHlow group. Of the six transplanted mice, four
developed tumors 16 weeks following the injection of 5,000 cells in
the ALDHhigh group, and only one mouse developed tumors
16 weeks following injection of 5,000 cells in the
ALDHneg group. However, when the injected number of
cells was decreased to 500, tumors first appeared in the
ALDHlow group 8 weeks following injection. The rate of
tumor formation in the ALDHhigh group was higher,
compared with that in the ALDHlow group with extended
duration, and no tumors formed in the ALDHneg group.
 | Table I.Tumor formation in mice injected with
cells of differing ALDH activity. |
Table I.
Tumor formation in mice injected with
cells of differing ALDH activity.
|
|
| Mice with tumors
(n) |
|---|
|
|
|
|
|---|
| Cell type | Cells (n) | 4 weeks | 8 weeks | 12 weeks | 16 weeks |
|---|
|
ALDHhigh | 5,000 | 0 | 1 | 2 | 4 |
|
ALDHlow | 5,000 | 3 | 6 | 6 | 6 |
|
ALDHneg | 5,000 | 0 | 1 | 1 | 1 |
|
ALDHhigh |
500 | 0 | 0 | 1 | 3 |
|
ALDHlow |
500 | 0 | 1 | 1 | 1 |
|
ALDHneg |
500 | 0 | 0 | 0 | 0 |
Discussion
In the present study, it was shown that the
expression of the stemness-associated markers, CD133+,
OCT-4 and SOX-2, decreased with a decrease in ALDH activity in MFC
cells. The ALDHhigh and ALDHlow cells formed
tumor spheres, however the ALDHlow cells formed larger
tumor spheres. In mice transplanted with 5,000 cells, the rate of
tumor formation in the ALDHlow group was significantly
higher, compared with that in the ALDHhigh group. On
injection of the mice with 500 cells, tumor development was
delayed, however, more mice developed tumors in the
ALDHhigh group, compared with the ALDHlow
group. These results demonstrated that ALDHhigh cells
had the characteristics of GCSCs and ALDHlow cells had
the characteristics of GCPCs. These findings suggested that the
separation of GCPCs from GCSCs may be important to elucidate the
biology of GCSCs and for developing strategies to eliminate GCSCs
for the treatment of patients with GC.
The theory of CSCs suggests that tumor cells have a
differentiation level similar to that of stem cells (8). Undifferentiated CSCs initially
generate CPCs and then further differentiate into regular cancer
cells. Although CPCs are important in tumor cells, few studies have
systematically investigated CPCs, and there are no reports
distinguishing CSCs from CPCs in GC. To the best of our knowledge,
only the study by Beier et al (25) and our previous study have sorted
CPCs. Our previous study sorted CPCs in H22 cells using an ALDH
activity assay (20). Beier et
al (25) reported the
successful isolation of CD133− cerebral glioma CPCs from
CD133+ cerebral glioma CSCs under conditions of stem
cell cultivation. However, they did not show the percentage of CSCs
in the CD133+ cells, therefore, it is possible that
CD133− cerebral glioma CPCs also contain regular cancer
cells. In our previous study stemness-like cells were distinguished
according to CSC sorting methods including, stemness-associated
marker sorting, sphere enrichment (21), SP sorting (4) and ALDH activity sorting (26). Although stemness-assocated marker
sorting is the most common way to isolate CSCs, it is not able to
further distinguish CPCs from the selected CSCs. Sphere enrichment
is a useful method by itself, however, the cells in the formed
sphere may contain regular cancer cells (27). The SP method is another way to sort
CSCs, however it requires the chemical, Hoechst 33342, which is
cytotoxic and may affect the reliability of the data. ALDH is
important in stem cell differentiation and proliferation. ALDH
activity reflects the degree of stemness of stem cells and has been
used as a functional stem cell marker in sorting various types of
CSC (14–19). Consistent with the findings of the
present study, Katsuno et al (28) found that the tumor formation of
ALDH+ GC cells is more marked, compared with that of
ALDH− GC cells in GC tissues and cell lines. In
addition, Zhi et al (29)
successfully acquired a GC stem-like cell population via ALDH
activity detection. These findings indicate that ALDH may be a
reliable marker for the acquisition of GCSCs.
The results of the present study showed that ~5% of
the MFC cells were ALDH+ in the total cell population,
which is in accordance with the low percentage of CSCs in solid
tumors (21). Consequently,
ALDH+ cells were defined as stem-like cells and
ALDH− cells were defined as non-stemess cells. In order
to isolate GCSCs and GCPCs from the stem-like cell population, our
previously reported ALDH activity assay was used (20). The 1% of the ALDH+ cells
with the highest activity were selected and considered to be GCSCs.
Although a subset of GCSCs in the ALDH+ cells may be
missed in the low proportion selection method, cells acquired from
the selection are more accurate and the data are more reliable. In
addition, the 1% of ALDH+ cells with the lowest activity
were selected and considered to be GCPCs, as its degree of stemness
was the weakest of the ALDH+ cells and close to
non-stemness cells (ALDH−) at the differentiated stage
of CPCs (30). Finally, the 1% of
the ALDH− cells with the lowest activity were selected
and defined as regular cancer cells.
Further analyses of the stemness-associated markers
revealed that CD44+ was expressed at high levels in all
three cell subpopulations, suggesting that CD44+ was not
a suitable marker for MFC cells; however, the levels of
CD133+, OCT-4 and SOX-2 decreased with a decrease in
ALDH activity, and were lowest in the ALDHneg cells.
These results suggested that the activity of ALDH was positively
correlated with the degree of stemness in the MFC cells, with the
ALDHhigh and ALDHlow cells being stem-like
cells, and ALDHneg cells being regular cancer cells.
A sphere formation assay is one of the classic
methods for detecting CSCs (6).
The present study found that ALDHhigh and
ALDHlow cells formed tumor spheres, which suggested that
the two types of cells have a certain degree of self-renewal
ability. However, the volumes of the spheres were considerably
lower for the ALDHhigh cells, compared with the
ALDHlow cells. In addition, the percentage of
ALDH+ cells in the spheres formed by the
ALDHlow cells was significantly lower, compared with the
spheres formed by the ALDHhigh cells. These results
suggested that ALDHlow cells formred larger tumor
spheres with a large number of ALDH− cells, consistent
with the lower self-renewal but rapid differentiation abilities of
CPCs. By contrast, the ALDHhigh sphere cells were
comprised predominantly of ALDH+ cells, suggesting that
tumor spheres are generated by self-renewal. In addition, the
volumes of the spheres were relatively low, indicating stable and
slow proliferation of ALDHhigh cells, a typical
characteristic of CSCs (21).
However, ALDHhigh and ALDHlow cells formed
spheres under certain conditions, providing evidence that the
purity of CSCs is low by sphere enrichment.
Various studies have suggested that CSCs have higher
tumor-forming abilities, compared with other cell subpopulations
(4,6,21).
However, other studies have reported opposite results. Read et
al (31) found that
tumor-forming cells express markers of neural progenitor cells
rather than stem cell markers in a mouse model of medulloblastoma.
Ucar et al (17) found that
the time for in vivo tumor formation of H522 cells with high
ALDH activity is significantly longer, compared with that of cells
with low ALDH activity.
Combined with the results of the present study,
several novel perspectives have been suggested. The proportions of
CSCs and CPCs are low in solid tumors, and CSCs in a resting state
do not exhibit tumor formation ability in a short duration
(21). However, tumors with
pathological significance require numerous regular cancer cells to
obtain a certain volume. Consequently, if the observational period
is not long enough, it is possibly to falsely conclude that CSCs do
not have stemness-associated properties. Although CPCs have limited
self-renewal ability, they proliferate rapidly (27). When certain numbers of CPCs (5,000
ALDHlow cells) were injected into mice, they showed high
tumor-forming ability, as CPCs generate large numbers of regular
cancer cells. When fewer tumor cells (500 ALDHhigh
cells) were injected into mice, CSCs demonstrated a high
tumor-forming ability when the observation period was long enough.
However, mice injected with CPCs showed weaker tumor formation
ability, compared with the CSC group, although tumors formed at an
early stage in the CPC group, possibly due to the limited
self-renewal ability of CPCs. The characteristics of
ALDHneg cells were in accordance with those of regular
cancer cells, with no self-renewal ability and limited
proliferative ability (27).
Therefore, tumor formation ability was significantly lower,
compared with that of ALDHhigh and ALDHlow
cells. Thus, it was hypothesized that CSCs, CPCs and regular cancer
cells are cell subpopulations with differing differentiation stages
and different proliferative abilities in tumor tissues.
In conclusion, the results of the present study
suggested that GCSCs and GCPCs are two stem-like subgroups with
different characteristics, and these two subgroups exist in the
stem-like cells of MFC cells. Excluding GCPCs from stem-like cells
to achieve a higher purity of GCSCs may benefit future
investigations of GCSCs and CSCs.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81,172,891).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hohenberger P and Gretschel S: Gastric
cancer. Lancet. 362:305–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polyak K and Hahn WC: Roots and stems:
Stem cells in cancer. Nat Med. 12:296–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukuda K, Saikawa Y, Ohashi M, Kumagai K,
Kitajima M, Okano H, Matsuzaki Y and Kitagawa Y: Tumor initiating
potential of side population cells in human gastric cancer. Int J
Oncol. 34:1201–1207. 2009.PubMed/NCBI
|
|
5
|
Zhang H, Xi H, Cai A, Xia Q, Wang XX, Lu
C, Zhang Y, Song Z, Wang H, Li Q, et al: Not all side population
cells contain cancer stem-like cells in human gastric cancer cell
lines. Dig Dis Sci. 58:132–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rocco A, Liguori E, Pirozzi G, Tirino V,
Compare D, Franco R, Tatangelo F, Palaia R, D'Armiento FP,
Pollastrone G, et al: CD133 and CD44 cell surface markers do not
identify cancer stem cells in primary human gastric tumors. J Cell
Physiol. 227:2686–2693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chute JP, Muramoto GG, Whitesides J,
Colvin M, Safi R, Chao NJ and McDonnell DP: Inhibition of aldehyde
dehydrogenase and retinoid signaling induces the expansion of human
hematopoietic stem cells. Proc Natl Acad Sci USA. 103:pp.
11707–11712. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collins SJ: Retinoic acid receptors,
hematopoiesis and leukemogenesis. Curr Opin Hematol. 15:346–351.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Estes BT, Wu AW, Storms RW and Guilak F:
Extended passaging, but not aldehyde dehydrogenase activity,
increases the chondrogenic potential of human adipose-derived adult
stem cells. J Cell Physiol. 209:987–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muramoto GG, Russell JL, Safi R, Salter
AB, Himburg HA, Daher P, Meadows SK, Doan P, Storms RW, Chao NJ, et
al: Inhibition of aldehyde dehydrogenase expands hematopoietic stem
cells with radioprotective capacity. Stem Cells. 28:523–534.
2010.PubMed/NCBI
|
|
13
|
Sladek NE: Human aldehyde dehydrogenases:
Potential pathological, pharmacological, and toxicological impact.
J Biochem Mol Toxicol. 17:7–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burger PE, Gupta R, Xiong X, Ontiveros CS,
Salm SN, Moscatelli D and Wilson EL: High aldehyde dehydrogenase
activity: A novel functional marker of murine prostate
stem/progenitor cells. Stem Cells. 27:2220–2228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clay MR, Tabor M, Owen JH, Carey TE,
Bradford CR, Wolf GT, Wicha MS and Prince ME: Single-marker
identification of head and neck squamous cell carcinoma cancer stem
cells with aldehyde dehydrogenase. Head Neck. 32:1195–1201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lingala S, Cui YY, Chen X, Ruebner BH,
Qian XF, Zern MA and Wu J: Immunohistochemical staining of cancer
stem cell markers in hepatocellular carcinoma. Exp Mol Pathol.
89:27–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ucar D, Cogle CR, Zucali JR, Ostmark B,
Scott EW, Zori R, Gray BA and Moreb JS: Aldehyde dehydrogenase
activity as a functional marker for lung cancer. Chem Biol
Interact. 178:48–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van den Hoogen C, van der Horst G, Cheung
H, Buijs JT, Lippitt JM, Guzmán-Ramírez N, Hamdy FC, Eaton CL,
Thalmann GN, Cecchini MG, et al: High aldehyde dehydrogenase
activity identifies tumor-initiating and metastasis-initiating
cells in human prostate cancer. Cancer Res. 70:5163–5173. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng S, Yang X, Lassus H, Liang S, Kaur S,
Ye Q, Li C, Wang LP, Roby KF, Orsulic S, et al: Distinct expression
levels and patterns of stem cell marker, aldehyde dehydrogenase
isoform 1 (ALDH1), in human epithelial cancers. PLoS One.
5:e102772010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding Y, Liang W and Hu X: Analysis of
heterogenous distribution of different stem characteristic subset
in H22 cells. Armed Police Med. 23:415–418. 2012.
|
|
21
|
Boman BM and Wicha MS: Cancer stem cells:
A step toward the cure. J Clin Oncol. 26:2795–2799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quante M and Wang TC: Stem cells in
gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol.
6:724–737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koo BS, Lee SH, Kim JM, Huang S, Kim SH,
Rho YS, Bae WJ, Kang HJ, Kim YS, Moon JH and Lim YC: Oct4 is a
critical regulator of stemness in head and neck squamous carcinoma
cells. Oncogene. 34:2317–2324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tam WL and Ng HH: Sox2: Masterminding the
root of cancer. Cancer Cell. 26:3–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beier F, Beier CP, Aschenbrenner I,
Hildebrandt GC, Brümmendorf TH and Beier D: Identification of
CD133(−)/telomerase(low) progenitor cells in glioblastoma-derived
cancer stem cell lines. Cell Mol Neurobiol. 31:337–343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marcato P, Dean CA, Giacomantonio CA and
Lee PW: Aldehyde dehydrogenase: Its role as a cancer stem cell
marker comes down to the specific isoform. Cell Cycle.
10:1378–1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beier D, Hau P, Proescholdt M, Lohmeier A,
Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U and Beier
CP: CD133(+) and CD133(−) glioblastoma-derived cancer stem cells
show differential growth characteristics and molecular profiles.
Cancer Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katsuno Y, Ehata S, Yashiro M, Yanagihara
K, Hirakawa K and Miyazono K: Coordinated expression of REG4 and
aldehyde dehydrogenase 1 regulating tumourigenic capacity of
diffuse-type gastric carcinoma-initiating cells is inhibited by
TGF-β. J Pathol. 228:391–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhi QM, Chen XH, Ji J, Zhang JN, Li JF,
Cai Q, Liu BY, Gu QL, Zhu ZG and Yu YY: Salinomycin can effectively
kill ALDH(high) stem-like cells on gastric cancer. Biomed
Pharmacother. 65:509–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bosch X: Bone-marrow-derive cells
implicated in gastric cancer. Lancet Oncol. 6:82005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Read TA, Fogarty MP, Markant SL, McLendon
RE, Wei Z, Ellison DW, Febbo PG and Wechsler-Reya RJ:
Identification of CD15 as a marker for tumor-propagating cells in a
mouse model of medulloblastoma. Cancer Cell. 15:135–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|