Introduction
Dyschromatosis symmetrica hereditaria (DSH; OMIM no.
127400) is a rare type of autosomal dominant genodermatosis that is
characterized by hyper- and hypo-pigmented macules on the dorsal
aspects of the extremities (1).
The causative gene for DSH was identified as adenosine deaminase
acting on RNA 1 (ADAR1 or DSRAD) (2). Disease onset typically occurs during
infancy or childhood, and 73% of patients develop skin lesions
<6 years of age (3). Case
reports of patients presenting with skin lesions at birth are
rarely reported; only one case was reported by Hemmati and Lam in
2009 (4). Moreover, there have
been limited significant non-cutaneous associations previously
reported with DSH. Notably, although there have been numerous
reports of associated dystonia, patients with comorbid congenital
heart disease (CHD) and DSH have not been reported before.
The present study examined two sporadic cases of
DSH, with confirmation of the diagnosis by identification of two
novel mutations in the ADAR1 gene. Notably, one of the
affected patients additionally presented with CHD and succumbed to
heart failure at the age of 2 years.
Materials and methods
Ethical approval
The study protocol was approved by the Shanghai Skin
Disease Hospital (Shanghai, China). All patients and 120 ethnically
matched control individuals were informed about the purpose of the
study and written consent was obtained prior to recruitment and
sampling. Informed written consent of minors was obtained from
their guardians.
Clinical report
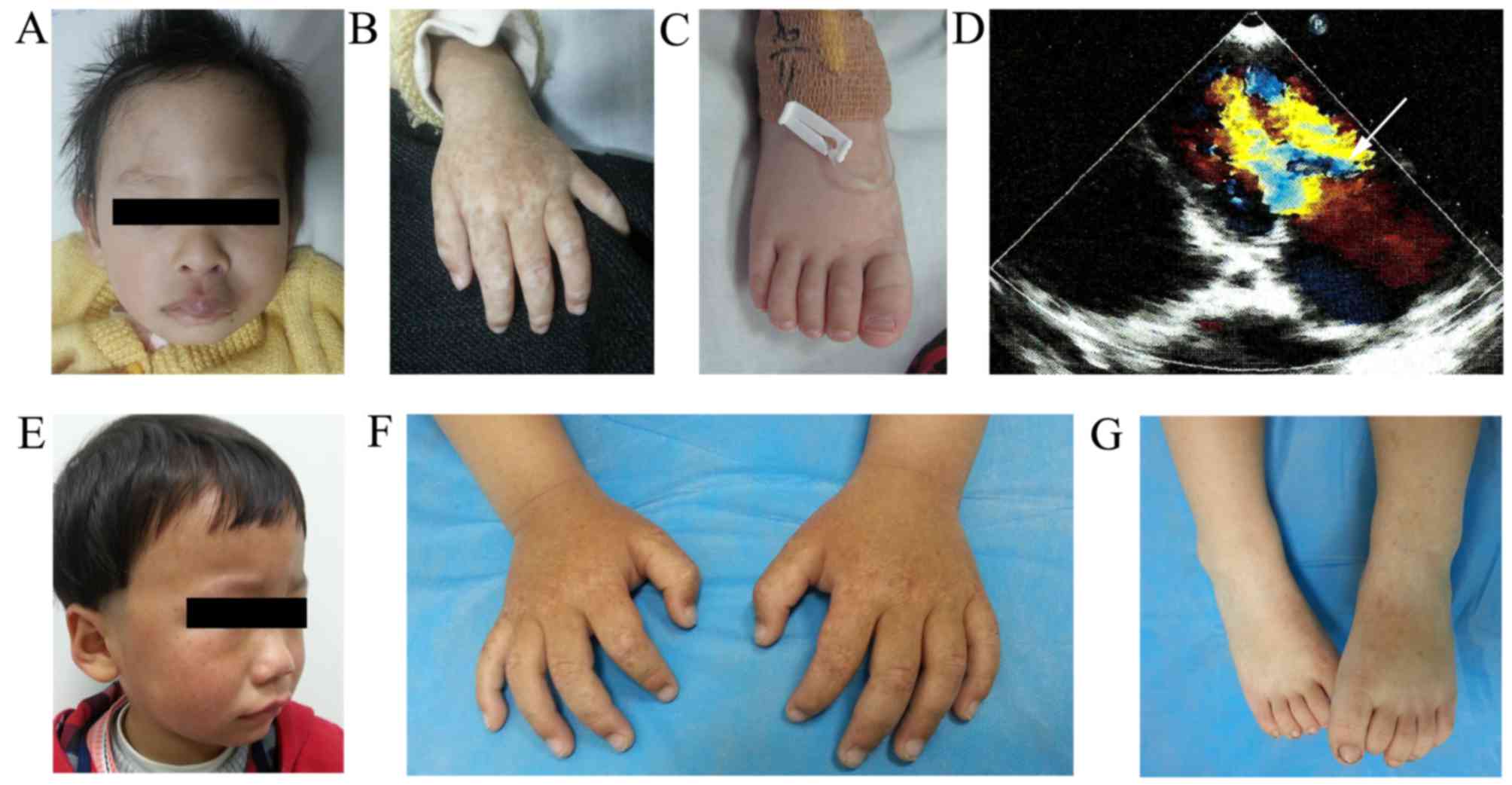
The first patient, an 8-month-old girl, was
delivered at 34 weeks by cesarean section due to fetal distress
with a birth weight of 1.750 kg. She presented with CHD and hyper-
and hypo-pigmented macules on the dorsal aspects of her extremities
from birth. On examination at the age of 3 months, an ultrasound
revealed a hemangioma and patent foramen ovale. A physical
examination revealed a hyperplastic plaque with a healthy skin
color (diameter, 2–3 cm) on the central segment of the upper lip
(Fig. 1A), and hyper- and
hypo-pigmented macules were distributed on the back of her hands
(Fig. 1B) and feet (Fig. 1C). Cardiac auscultation revealed a
diastolic murmur at the apex, and a diastolic thrill. Levels of
creatine kinase isoenzyme-MB were typical; however, the N-terminal
pro-brain natriuretic peptide level was 5,140 ng/ml. An
echocardiogram disclosed severe mitral valve stenosis and
regurgitation (Fig. 1D), mild to
moderate aortic regurgitation and left atrium and left ventricle
dilation. An electrocardiogram revealed sinus tachycardia and a
right bundle branch block. A chest computed tomography scan
demonstrated an enlarged cardiac shadow and bilateral large pleural
effusions. Ultrasonography revealed a small pleural effusion and
fluid in the peritoneal cavity. Based on the clinical features and
investigation findings, the diagnoses of CHD, DSH and hemangioma
were confirmed.
At the age of 6 months, mitral valve surgery was
undertaken. An echocardiogram following mitral valve surgery
revealed moderate to severe mitral valve stenosis and regurgitation
with anterior mitral leaflet prolapse, mild to moderate aortic
valve regurgitation and left ventricular and left atrium dilation.
At 4 days afterwards, similar results were observed in a second
echocardiogram, with the exception of rupture of the chordae
tendinae. These findings placed the patient at high risk of acute
heart failure, which was the cause of death at age 2.
The second patient was a 2-year-old Chinese boy,
born following a full-term pregnancy by healthy delivery. He
presented with asymptomatic hyper- and hypo-pigmented macules over
the dorsum of the hands and feet from birth, and developed
freckle-like macules on his face at 1-year-old. Gradually, these
lesions progressed. Physical examination revealed that the patient
had small freckle-like pigmented macules on his face (Fig. 1E), and hypo- and hyper-pigmented
macules on the back of the knees (Fig.
1F) and extremities (Fig. 1G)
of irregular shape and size. The rest of the physical examination
presented no abnormalities.
ADAR1 mutation analysis
Following informed consent, genomic DNA was
extracted from the patients' peripheral blood lymphocytes according
to the standard protocol. The present study designed primers
flanking all 15 coding exons and intron-exon boundaries of the
ADAR1 gene using the web-based version of the Primer 3.0
program (www.genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi).
A polymerase chain reaction (PCR) was performed in a 15 µl reaction
volume containing 20 ng genomic DNA, 0.3 mM deoxunucleotides, 0.3
µM each primer, 3.0 mM MgCl2 and 0.1 U Taq DNA
polymerase (Roche Diagnostics, Basel, Switzerland). The PCR
conditions were as follows: Taq activation at 95°C for 15 min,
followed by 40 cycles of denaturation at 94°C for 40 sec, annealing
at 58°C for 60 sec and extension at 72°C for 55 sec, except that in
the first 10 cycles when annealing temperature decreased from 63 to
58°C by 0.5°C per cycle. The final extension was 72°C for 10 min.
Following amplification, the products were purified using a
QIAquick PCR Purification kit (Qiagen GmbH, Hilden, Germany).
ADAR1 was sequenced using an ABI PRISM® 3730
automated sequencer (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Sequence comparisons and analysis were
performed using Phred-Phrap-Consed software version 12.0
(http://www.phrap.org/phredphrapconsed.html). In
addition, samples from 120 unrelated population-matched controls
were sequenced to exclude the possibility that any discovered
mutations were polymorphisms in ADAR1. Mutations were
identified by comparing these results with the reported cDNA
reference sequence (GenBank accession no. NM_001111).
Results
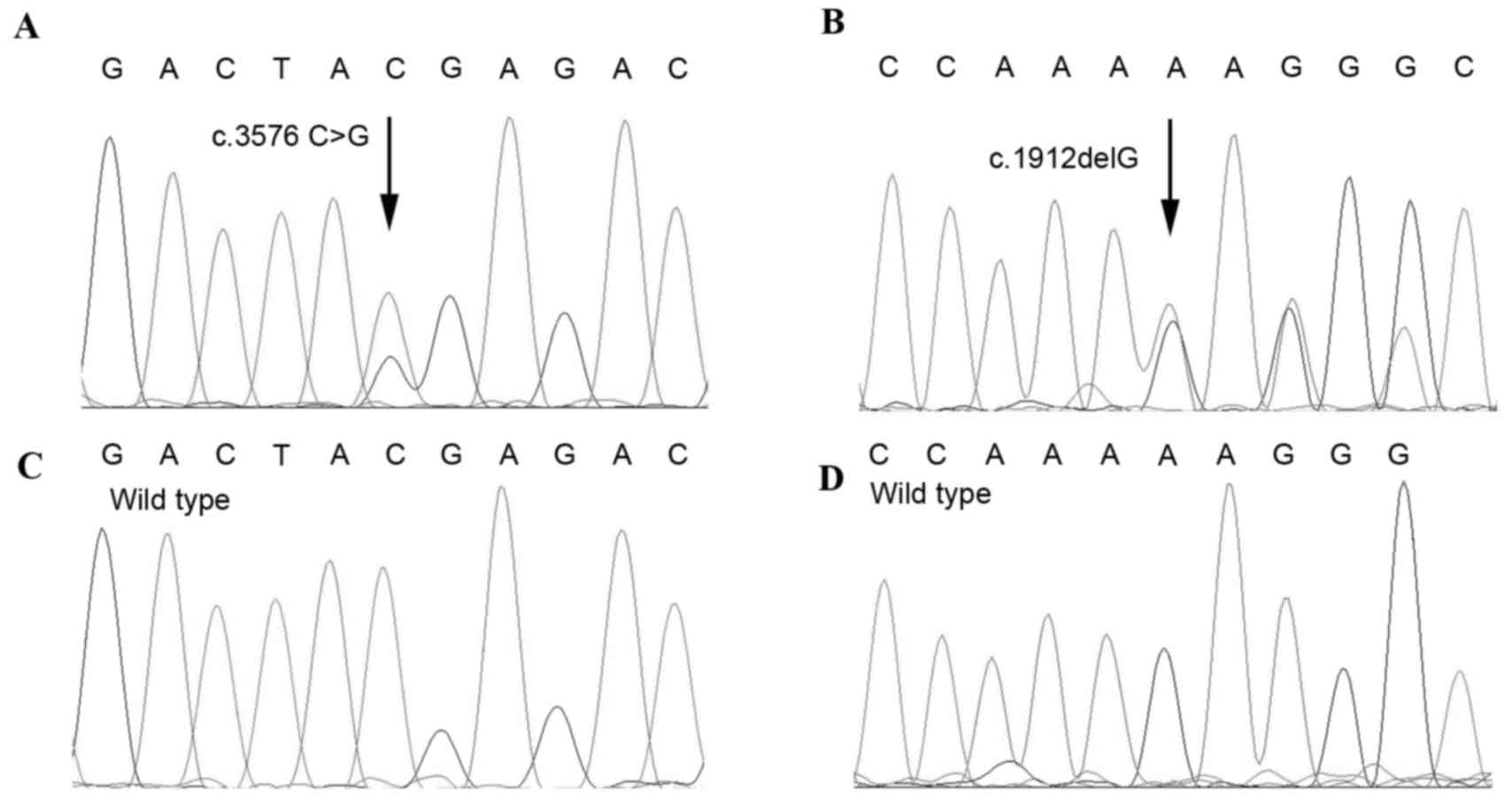
Sequencing analysis of the ADAR1 gene from
the two DSH patients was performed. A nonsense mutation at c.3576
C>G (p.Y1192X) in exon 15 of the ADAR1 gene was
identified in Patient 1 (Fig. 2A).
The resulting truncated protein was predicted to lack 105 amino
acids. A single-base frameshift deletion, c.1912delG, was
identified within exon 4 in Patient 2, which generated a
pre-terminating codon 15 codons downstream of the deletion site
(Fig. 2B). These two mutations,
which have not been described in previous studies, were not
detected in the 120-unrelated healthy Chinese individuals (Fig. 2C and D), suggesting that they are
not common polymorphisms.
Discussion
DSH is an autosomal-dominant skin disorder,
characterized by the presence of hyper- and hypo-pigmented macules
primarily on the dorsal aspects of the extremities that appear in
infancy or early childhood (5,6).
Mutations in the ADAR1 gene have been identified as the
molecular basis of DSH (2). To
date, >120 mutations, distributed primarily in the deaminase
domain of ADAR1 (~62.3%), have been reported (7–9).
Although the lesions in DSH are common in infancy and early
childhood, and typically stop spreading prior adolescence and then
remain unaltered for the rest of life, only one patient with DSH
since birth has been reported previously (4). In 2009, Hemmati and Lam (4) first reported a 4-year-old Chinese
girl who presented at the authors' pediatric dermatology clinic
with reticulate hyper- and hypo-pigmentation of the dorsum of the
hands and feet that were present since birth; however, gene
analysis was not performed. The two patients examined in the
present study developed DSH at birth. Sequencing of the overall
coding sequence of ADAR1 revealed a nonsense mutation at
c.3576C>G (p.Y1192X) in Patient 1 and a single-base deletion
c.1912delG (p.Glu673ValfsX652) in Patient 2. To the best of the
authors' knowledge, these are novel mutations in the ADAR1
gene. The present study raises the question of whether these two
novel ADAR1 mutations are associated with the earlier
emergence of DSH. Further investigations of ADAR1 mutations
are necessary to confirm this possibility.
Although the comorbidity of DSH and other diseases
is uncommon, various complications, including dystonia (10–13),
acral hypertrophy (14), psoriasis
(15,16) and depression (15) have been reported in patients with
DSH. However, a patient with DSH complicated by CHD has not
previously been reported. The etiology of CHD is complex and is
associated with environmental and genetic causes. Studies in human
genetics have led to the identification of >50 human genes
(17) involved in isolated CHD or
in genetic syndromes where CHD is part of the phenotype. However,
none of these genes were primarily associated with malfunctions of
the mitral valve; therefore, the present study did not perform
mutation analyses on them. Patient 1, who had concurrent CHD,
succumbed to congestive heart failure and was identified as having
a p.Y1192X mutation in ADAR1. CHD and DSH are hypothesized
to be different entities. There is no conclusive evidence that the
mutation in ADAR1 causes CHD. Therefore, the coexistence of
these two diseases may be a simple coincidence rather than an
intrinsic underlying mechanism.
In conclusion, the present study performed a
mutation analysis of the ADAR1 gene in two sporadic patients
with typical DSH from birth, and identified two novel mutations. To
the best of the authors' knowledge, only one case of the existence
of DSH since birth has previously been reported. Additionally, the
coexistence of DSH and CHD diseases was first reported, and the
affected patient succumbed to heart failure. The identification of
additional ADAR1 mutations may facilitate understanding of
the association between genotype and phenotype in DSH.
Acknowledgements
The authors would like to thank all the subjects for
their ongoing participation in the present study. The present study
was supported by grants from National Natural Science Foundation of
China (grant nos. 81201222 and 81272990).
References
|
1
|
Zhang G, Shao M, Li Z, Gu Y, Du X, Wang X
and Li M: Genetic spectrum of dyschromatosis symmetrica hereditaria
in Chinese patients including a novel nonstop mutation in ADAR1
gene. BMC Med Genet. 17:142016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyamura Y, Suzuki T, Kono M, Inagaki K,
Ito S, Suzuki N and Tomita Y: Mutations of the RNA-specific
adenosine deaminase gene (DSRAD) are involved in dyschromatosis
symmetrica hereditaria. Am J Hum Genet. 73:693–699. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomita Y and Suzuki T: Genetics of
pigmentary disorders. Am J Med Genet C Semin Med Genet. 131C:75–81.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hemmati I and Lam J: Hyper-and
hypopigmented macules over palms and soles since birth-a case of
dyschromatosis symmetrica hereditaria. Dermatol Online J.
15:52009.PubMed/NCBI
|
|
5
|
Toyama I: Dyschromatosis symmetrica
hereditaria. Jpn J Dermatol. 29:95–96. 1929.
|
|
6
|
Ostlere LS, Ratnavel RC, Lawlor F, Black
MM and Griffiths WA: Reticulate acropigmentation of Dohi. Clin Exp
Dermatol. 20:477–479. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Yang L, Li C, Jin C, Lai M, Zhang G,
Hu Y, Ji J and Yao Z: Mutational spectrum of the ADAR1 gene in
dyschromatosis symmetrica hereditaria. Arch Dermatol Res.
302:469–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stenson PD, Mort M, Ball EV, Howells K,
Phillips AD, Thomas NS and Cooper DN: The human gene mutation
database: 2008 update. Genome Med. 1:132009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai ML, Yang LJ, Zhu XH and Li M: A novel
mutation of the DSRAD gene in a Chinese family with dyschromatosis
symmetrica hereditaria. Genet Mol Res. 11:1731–1737. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo T, Suzuki T, Ito S, Kono M, Negoro T
and Tomita Y: Dyschromatosis symmetrica hereditaria associated with
neurological disorders. J Dermatol. 35:662–666. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tojo K, Sekijima Y, Suzuki T, Suzuki N,
Tomita Y, Yoshida K, Hashimoto T and Ikeda S: Dystonia, mental
deterioration, and dyschromatosis symmetrica hereditaria in a
family with ADAR1 mutation. Mov Disord. 21:1510–1513. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patrizi A, Manneschi V, Pini A, Baioni E
and Ghetti P: Dyschromatosis symmetrica hereditaria associated with
idiopathic torsion dystonia. A case report. Acta Derm Venereol.
74:135–137. 1994.PubMed/NCBI
|
|
13
|
Kaliyadan F, Vinayan KP, Fernandes B and
Jayasree MG: Acral dyschromatosis with developmental regression and
dystonia in a seven-year-old child: Dyschromatosis symmetrica
hereditaria variant or a new syndrome? Indian J Dermatol Venereol
Leprol. 75:412–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murata T, Yagi Y, Tanioka M, Suzuki T,
Miyachi Y, Morita K and Utani A: Dyschromatosis symmetrica
hereditaria with acral hypertrophy. Eur J Dermatol. 21:649–650.
2011.PubMed/NCBI
|
|
15
|
Luo S, Zheng Y, Ni H, Liu Y, Liu Y, Li X
and Liu Q: Novel clinical and molecular findings in Chinese
families with dyschromatosis symmetrica hereditaria. J Dermatol.
39:556–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi BJ, Xue M, Liu Y, Jiang Y and Diao QC:
First report of the coexistence of dyschromatosis symmetrica
hereditaria and psoriasis: One novel TCT to A mutation in the
double-RNA-specific adenosine deaminase gene. J Eur Acad Dermatol
Venereol. 26:657–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andersen TA, Kde L Troelsen and Larsen LA:
Of mice and men: Molecular genetics of congenital heart disease.
Cell Mol Life Sci. 71:1327–1352. 2014. View Article : Google Scholar : PubMed/NCBI
|