Introduction
The inability to restore the damaged myocardium
causes the major pathologies of cardiovascular disease. Therefore,
induction of cardiomyocyte proliferation is a promising approach to
reverse myocardial attrition, which may be applied to heart failure
therapy (1,2). Previous studies have confirmed that
there is limited proliferative potential in the adult mammalian
heart (3,4). In the adult mouse heart, ~10% of
cardiomyocytes are mononuclear, of which ~0.3% were induced to
restart cytokinesis by the Neuregulin 1 signaling pathway (5). Previous studies have also provided
certain information about cardiomyocyte renewal in humans, which
indicated a rate of 1% at the age of 25 and 0.45% at the age of 75
(6,7). Although the information gained about
cardiomyocyte renewal in the adult mammal heart provided promise
for cardiomyocyte proliferation, information regarding the detailed
mechanisms of the modulation of cardiomyocyte proliferation is
limited.
A previous study has confirmed the capacity for
restoration in neonatal mouse hearts (8) and advanced research demonstrated that
hypoxia promoted this regenerative process (9). The research suggested that hypoxia
reduces oxidative stress, which relieves the DNA damage response,
and the transition between oxygen levels in the embryonic and
postnatal circulation was considered to be an important reason for
cell-cycle arrest of mammalian cardiomyocytes soon after birth
(9). This explanation is in
accordance with the phenomenon of cardiac regeneration that occurs
in urodele amphibians and zebrafish, which have an internal
environment that is sustained at a lower oxygen state compared with
mammals (10,11). However, the phenomenon of
hypoxia-induced cardiac proliferation remains unconfirmed in human
hearts and the regulation of hypoxia-induced cardiac proliferation
is not fully understood.
Macrophages are versatile immune cells that have a
critical role in angiogenesis, organ development and injured tissue
repair (12). Multiple reports
have indicated an association between the immune system and
regeneration in lower vertebrates, and supported a hypothesis that
the mammalian immune system may influence the proliferative
capacity. Specifically, macrophage infiltration was essential for
limb regeneration in newts (13),
indicating that alterations in macrophages represents a potential
mechanism for the induction of tissue proliferation. Notably, a
recent study confirmed that macrophages were essential for neonatal
cardiomyocyte proliferation through promotion of myocardial
angiogenesis (14). Further
research indicated that distinct macrophage lineages had opposite
effects on the restoration of the injured myocardium and the
macrophages that promoted cardiac restoration are primarily
composed of cardiac resident macrophages (15,16).
Although there are numerous existing associations between
macrophages and cardiac restoration, whether macrophages are
involved in hypoxia-induced cardiomyocyte proliferation has not
previously been determined. The present study confirmed that
cardiomyocyte proliferation occurred in human infant hearts, which
was more significant in infants that suffered from chronic hypoxia.
Furthermore, it was demonstrated that hypoxia increased the
proportion of cardiac resident macrophages and this further
contributed to postnatal cardiac proliferation.
Materials and methods
Patient samples
The present study was performed according to the
principles outlined in the Declaration of Helsinki, informed
consent was obtained from all subjects according to the procedures.
The study was approved by the Human Ethics Committee of Xinqiao
Hospital (Chongqing, China). The biopsy was taken from the stenotic
right ventricular outflow tract and stored at −80°C immediately. A
total of 22 acyanotic and 29 cyanotic patients who had undergone
cardiac surgery in Xinqiao Hospital (Chongqing, China) from June
2014-October 2015 were included. Patients were divided into 3
subgroups based on their age when surgery was performed, clinical
characteristics are presented in Table
I.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
|
| Infant | Adolescent | Adult |
|---|
|
|
|
|
|
|---|
|
| Acyanotic | Cyanotic | Acyanotic | Cyanotic | Acyanotic | Cyanotic |
|---|
| No. | 13 | 18 | 6 | 7 | 3 | 4 |
| Age,
years | 1.6 (0.3–4.0) | 1.4 (0.5–3.5) | 8.5 (6.0–14.0) | 10.0 (6.0–15.0) | 25.0 (21.0–30.0) | 31.0 (28.0–36.0) |
| Gender,
male/female | 8/5 | 12/6 | 4/2 | 2/5 | 1/2 | 1/3 |
| Oxygen
saturation, % n | 98.0 | 75.2 | 99.0 | 76.0 | 99.0 | 82.0 |
|
| (97–100) |
(65.0–80.0)a | (98.0–100) |
(70.0–80.0)a | (97.0–100) |
(80.0–86.0)a |
| Pathology |
|
|
|
|
|
|
| TOF |
| 15 |
| 7 |
| 4 |
| VSD and
PA |
| 3 |
|
|
|
|
| VSD | 6 |
|
|
|
|
|
| RVOS |
|
| 2 |
|
|
|
| VSD and
RVOS | 7 |
| 4 |
| 3 |
|
Animal breeding
C57BL/6J mice were bred in the animal facility of
Xinqiao hospital. The investigation conformed to the Guide for the
Care and Use of Laboratory Animals (17). All experiments were approved by the
Animal Welfare Committee of Xinqiao Hospital. A total of 110
1-day-old mice were divided randomly into a normoxia group (n=30),
hypoxia group (n=30), normoxia vehicle group (n=25) and normoxia
CCR2 inhibitor group (n=25). The mice were fed by the mother at
room temperature with free access to food and water and kept the
rhythm of a 12 h light/dark cycle. The age- and sex-matched mice
were sacrificed at differing times from 1 to 14 days following
birth as described below. 5 mice per group were used for each
experiment; n=5.
Hypoxia treatment
Hypoxia treatment was performed using a hypoxia
workstation (Baker Ruskinn InvivO2 1000; Ruskinn
Technology, Ltd., Bridgend, UK) to maintain the environment at 15%
O2 content and 23°C. The control group was treated in
the same system but with 21% O2 content.
Drug injection
A C-C chemokine receptor type 2 (CCR2) inhibitor
(RS504393; Abcam, Cambridge, UK) was reconstituted in dimethyl
sulfoxide to concentrations of 2.7 mg/ml for storage and diluted in
PBS prior to use. Mice were weighed daily and 4 mg/kg CCR2
inhibitor was subcutaneously injected daily from days 0–10.
Immunostaining
Frozen human and mouse tissues were embedded in
optimal cutting temperature (OCT) compound (tissue-tek 4583; Sakura
Finetek USA Inc., USA) and 10 µm cryosections were cut. Following
fixation with 4% paraformaldehyde for 30 min at room temperature,
sections were blocked with 10% goat serum (AR0009; Boster Systems,
Inc., Pleasanton, CA, USA) for 1 h at 37°C and incubated with
primary antibodies overnight at 4°C. Subsequently, sections were
washed with PBS and incubated with secondary antibodies for 1 h at
room temperature. The following primary antibodies were used:
Anti-cardiac troponin T (TnT; cat. no. ab8295; 1:400; Abcam);
anti-phospho histone H3 Ser10 (pH3; cat. no. 06-570; 1:200; EMD
Millipore, Billerica, MA, USA); anti-Aurora B (cat. no. ab2254;
1:200; Abcam); and anti-wheat germ agglutinin (WGA) conjugated to
Alexa Fluor 488 (cat. no. W11261; 1:200; Invitrogen; Thermo Fisher
Scientific, Inc.). The following secondary antibodies were used:
Alexa Fluor 488-labeled Goat Anti-Rabbit IgG (cat. no. A0423;
1:400; Beyotime Institute of Biotechnology, Haimen, China) and
Alexa Fluor 647-labeled Goat Anti-Mouse IgG (cat. no. A0473; 1:400;
Beyotime Institute of Biotechnology, Haimen, China).
Immunofluorescence was visualized on a Leica confocal microscopy
system (Leica Microsystems, Inc., Buffalo Grove, IL, USA) with the
function of z-tack which revealed the vertical plane images of
tissue sections. DAPI was used to stain nuclei. Results were
quantified by examining ≥3 similarly oriented sections from 3
independent samples in a blinded manner using ImageJ 2.1 software
(National Institutes of Health, Bethesda, MD, USA).
Cell isolation
Mice were anesthetized with isoflurane and
decapitated. Hearts were perfused with cold PBS, finely minced and
digested with 1 mg/ml collagenase II (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 0.15 mg/ml DNase I (cat. no. 10104159001;
Roche Diagnostics GmbH, Mannheim, Germany) for 30 min at 37°C
during constant agitation. The digested material was filtered
through 40 mm filters and centrifuged at 4°C in 400 × g for
5 min in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 2% fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Red
blood cells were lysed in ammonium-chloride-potassium lysis buffer
(Tiangen Biotech Co., Ltd., Beijing, China) and resuspended in
fluorescence-activated cell sorting buffer (FACS; PBS containing 2%
FCS and 2 mM EDTA), as previously described (13).
Flow cytometry
Cell suspensions (1×107 cells in 100 µl)
were incubated with Fc Block (cat. no. 101319; 1:100; BioLegend,
Inc., San Diego, CA, USA) at 4°C for 5 min and labeled with the
following fluorescently conjugated antibodies: Anti-CD45 APC (cat.
no. 103111; 1:100) anti-Ly-6 G PerCP/Cy5.5 (cat. no. 127165;
1:100); anti-F4/80 PE/Cy7 (cat. no. 123113; 1:100); anti-MHC-II
FITC (cat. no. 116405; 1:100) all obtained from BioLegend, Inc. and
anti-CCR2 PE (cat. no. FAB5538P; R&D systems, Minneapolis, MN,
USA) for 30 min at 4°C. Cells were washed twice in FACS buffer.
Flow cytometry analysis was performed on a flow cytometer (BD
FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA) and data
analysis was performed using the FlowJo 10.0 software (Tree Star,
Inc., Ashland, OR, USA).
Statistical analysis
Data were analyzed using GraphPad Prism 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA) and are presented as
the mean ± standard error of the mean. Comparisons between groups
were performed using an unpaired two-tailed Student's t-test. Each
experiment was repeated 3 times. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics of
patients
A total of 22 acyanotic and 29 cyanotic patients
were included in this study, patients were divided into the
following subgroups based on their age at the time of the
operation: Infant group; adolescent group; and adult group.
Clinical data are presented in Table
I. Patients in the cyanotic group primarily underwent
operations for Tetralogy of Fallot, while patients that suffered
from ventricular septal defect combined with right ventricular
outflow tract stenosis functioned as controls. The 2 groups were
matched for age. Oxygen saturation of arterial blood was the
primary difference between the 2 groups.
Cardiomyocyte proliferation in
cyanotic and acyanotic patients
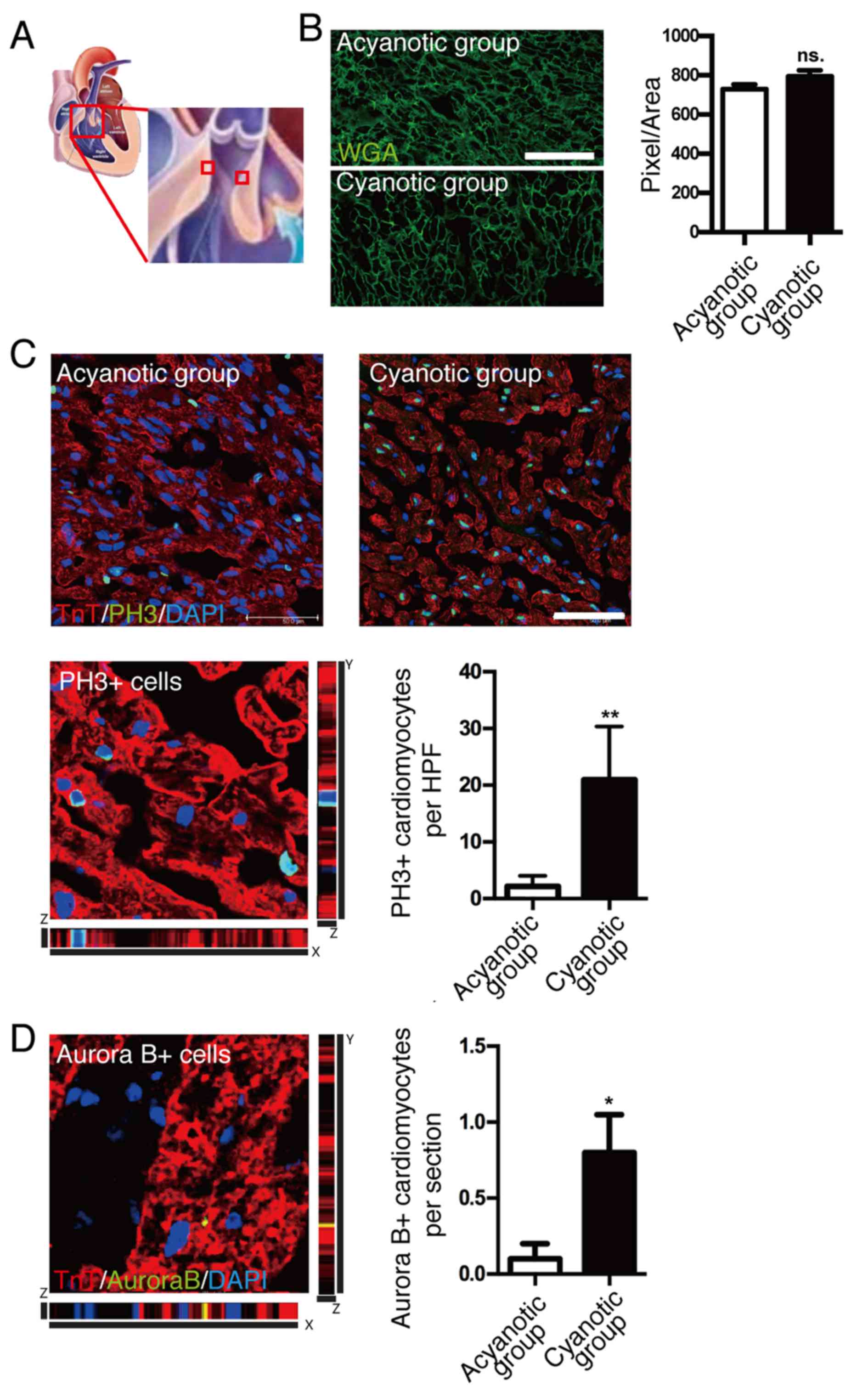
To exclude the potential effects of pressure
overload on cardiomyocyte proliferation, cardiac samples were taken
from the highest point of right ventricular outflow tract
obstruction (Fig. 1A) (18). Cell size quantification
demonstrated no significant difference between cardiomyocyte cell
size of infants with and without cyanosis (Fig. 1B). To investigate cardiomyocyte
proliferation in infants with and without cyanosis, cardiomyocyte
mitosis was investigated by immunostaining using anti-pH3, a
specific marker of G2-M progression, while cardiomyocytes were
marked by anti-cardiac TnT antibody. High-resolution confocal
z-stacking microscopy, a gold standard method for identifying
colocalizations, was used to confirm the colocalization of pH3
signal and cardiomyocyte nucleus (Fig.
1C, bottom left). Quantification of the cardiomyocytes with
nuclear pH3 signal (Fig. 1C,
bottom right) demonstrated that pH3-positive cardiomyocytes were
~10-fold higher in the cyanotic infant group compared with the
acyanotic infant group, which indicated that infant cardiomyocytes
had mitotic potential and hypoxia enhanced this capacity.
A previous report indicated that the loss of
regenerative capacity of adult cardiomyocytes was due to an
inability to undergo cytokinesis, which may explain why the
majority of adult cardiomyocytes were reported to exhibit
polyploidy (19). Therefore, the
present study investigated the cytokinesis of cardiomyocytes to
further investigate the proliferation of cardiomyocytes in
vivo. The presence of the cytokinesis marker, Aurora B kinase,
was quantified and a significant increase in Aurora B-positive
cardiomyocytes was observed in cyanotic samples compared with
acyanotic samples (P<0.05; Fig.
1D). Strict z-stack imaging, without the use of 2-dimensional
imaging, was also performed as Aurora B may localize in the
cleavage furrow between 2 myocytes that are not necessarily in the
same horizontal plane. This technique increases the sensitivity and
specificity of identifying true cardiomyocyte cytokinesis. These
results indicated that sustaining hypoxia in the human heart
following birth may enhance cardiomyocyte proliferation.
Effect of hypoxia on cardiomyocyte
proliferation
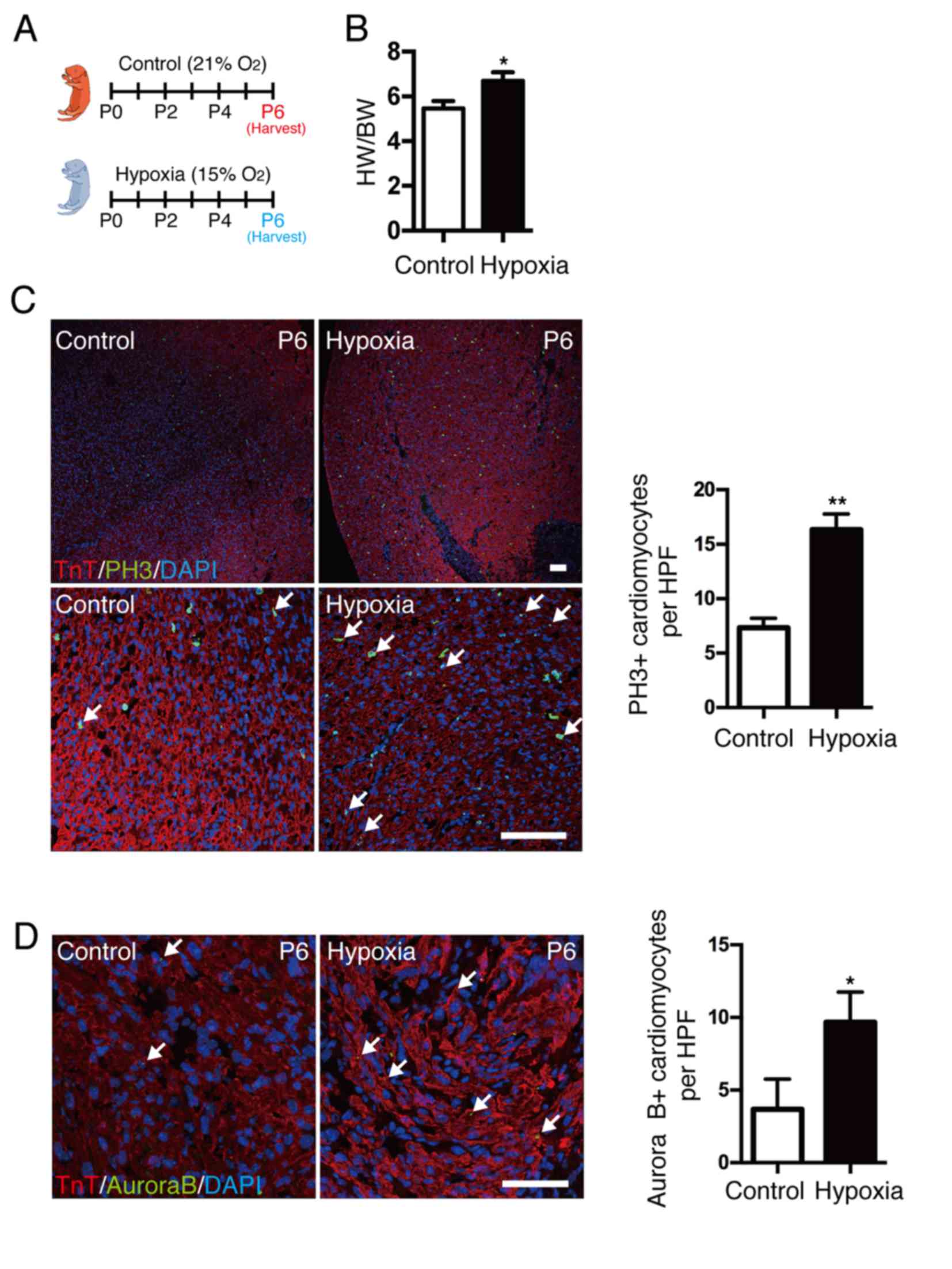
To demonstrate the association between hypoxia and
cardiomyocyte proliferation, neonatal mice were exposed to a mild
hypoxic (15% O2 content) or normoxic (21% O2
content) environment at the time of birth for several days
(Fig. 2A). Hypoxia was confirmed
by oxygen saturation, as the mean value was ~85% in the hypoxic
group. The heart weight to body weight (HW/BW) ratio was
significantly increased in neonates under hypoxia compared with
control mice (P<0.05; Fig. 2B).
Cardiomyocyte mitosis was measured by coimmunostaining with
anti-pH3, anti-TnT antibody and DAPI (Fig. 2C), and results demonstrated that
cardiomyocyte mitosis was significantly increased in the hypoxic
group compared with control mice (P<0.01). In addition,
localization of Aurora B kinase at the cleavage furrow (Fig. 2D) was significantly increased in
the hypoxic group compared with control mice (P<0.05). The
results indicate that a postnatal hypoxic state may directly
enhance cardiomyocyte proliferation. To understand the regulative
mechanism of cardiomyocyte proliferation in hypoxia, neonatal mouse
cardiomyocytes were isolated and cultured in a hypoxic environment
(1% O2). The proliferation of cardiomyocytes at
different time point was subsequently investigated. Unfortunately,
after testing pH3 or Aurora B kinase-positive cardiomyocytes by
immunostaining, no significant difference was observed between
normoxic and hypoxic groups, which indicated that hypoxia alone may
not promote the proliferation of isolated cardiomyocytes, and the
microenvironment of cardiomyocytes may have a critical role in
cardiomyocyte proliferation.
Effect of hypoxia on distinct cardiac
macrophage subsets
A previous study indicated that macrophages were
indispensable for neonatal heart regeneration (12). As distinct subsets of cardiac
macrophages had the opposite effects (13), it was crucial to identify the
subsets of macrophages that had a critical role in cardiomyocyte
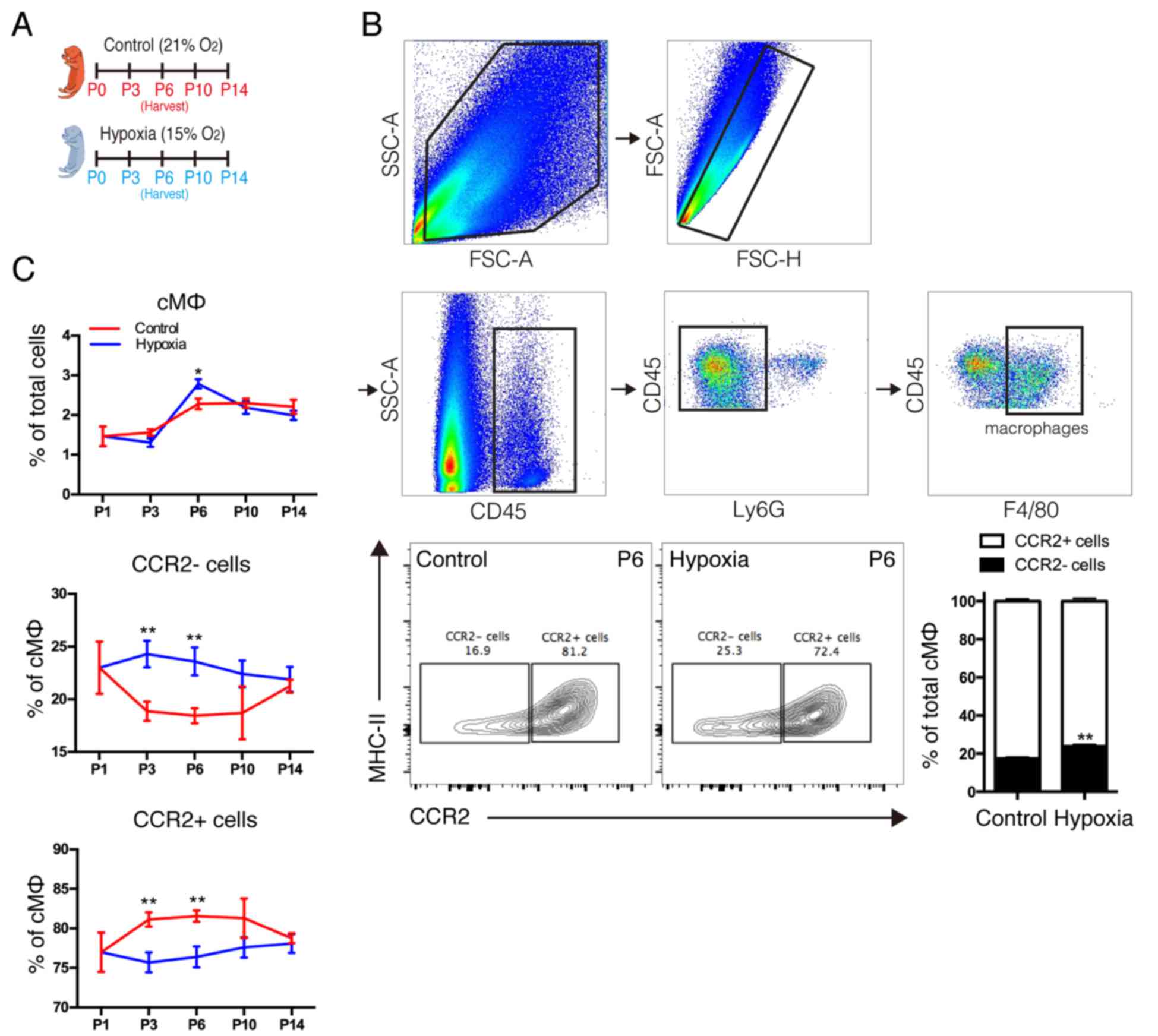
proliferation. Therefore, the present study examined the dynamics
of cardiac macrophage subsets under sustained hypoxia following
birth (Fig. 3A) and analyzed the
macrophage subsets using the gating strategy as presented in
Fig. 3B. The results demonstrated
that the neonatal heart primarily contains 2 macrophage subsets; 1
resident macrophage (MHC-IIlowCCR2-) and 1
monocyte-derived immature macrophage (MHC-IIlowCCR2+).
In response to hypoxia, the number of MHC-IIlowCCR2+
monocyte-derived macrophages in the neonatal heart was reduced and
the number of MHC-IIlowCCR2-resident macrophages was
increased (Fig. 3B). In addition,
it was observed that hypoxia decreased monocyte-derived macrophages
and increased resident macrophages in postnatal hearts along with
the growth in a short time following birth, however, significant
differences were not observed at postnatal days 10 or 14 (Fig. 3C). Notably, it was observed that
the dynamic of distinct subsets is in accordance with the
physiological rule of neonatal cardiac proliferation (8,9). The
results indicated that distinct subsets of cardiac macrophages may
be responsible for the distinct effects on hypoxia-induced
cardiomyocyte proliferation, however, the role of distinct
macrophage subsets remains unclear.
Effect of cardiac resident macrophages
on cardiomyocyte proliferation
To investigate whether the dynamics of distinct
cardiac macrophage subsets contribute to cardiomyocyte
proliferation in postnatal hearts, the recruitment of mononuclear
phagocytes was inhibited by a selective CCR2 inhibitor (Fig. 4A). Although the absolute number of
F4/80+ mononuclear phagocytes was only marginally affected, CCR2
inhibition resulted in significant alterations in resident
macrophage subsets (Fig. 4B). CCR2
inhibition significantly reduced the recruitment of
monocyte-derived macrophages into the neonatal heart and expanded
the resident subsets (Fig. 4C).
Colocalization of nuclear pH3 signal and cardiomyocytes indicated
that cardiomyocyte mitosis was significantly increased in the CCR2
inhibition group compared with the vehicle group (P<0.01;
Fig. 4D). Cardiomyocyte
cytokinesis was measured by localization of Aurora B kinase at the
cleavage furrow, and the results demonstrated that Aurora B
kinase-positive cardiomyocytes were significantly increased in the
CCR2 inhibition group compared with the vehicle group (P<0.05;
Fig. 4E). These results
demonstrated that the dynamics of distinct cardiac macrophage
subsets influenced the proliferation of cardiomyocytes in postnatal
hearts and resident macrophages served an essential role in the
promotion of postnatal cardiomyocyte proliferation.
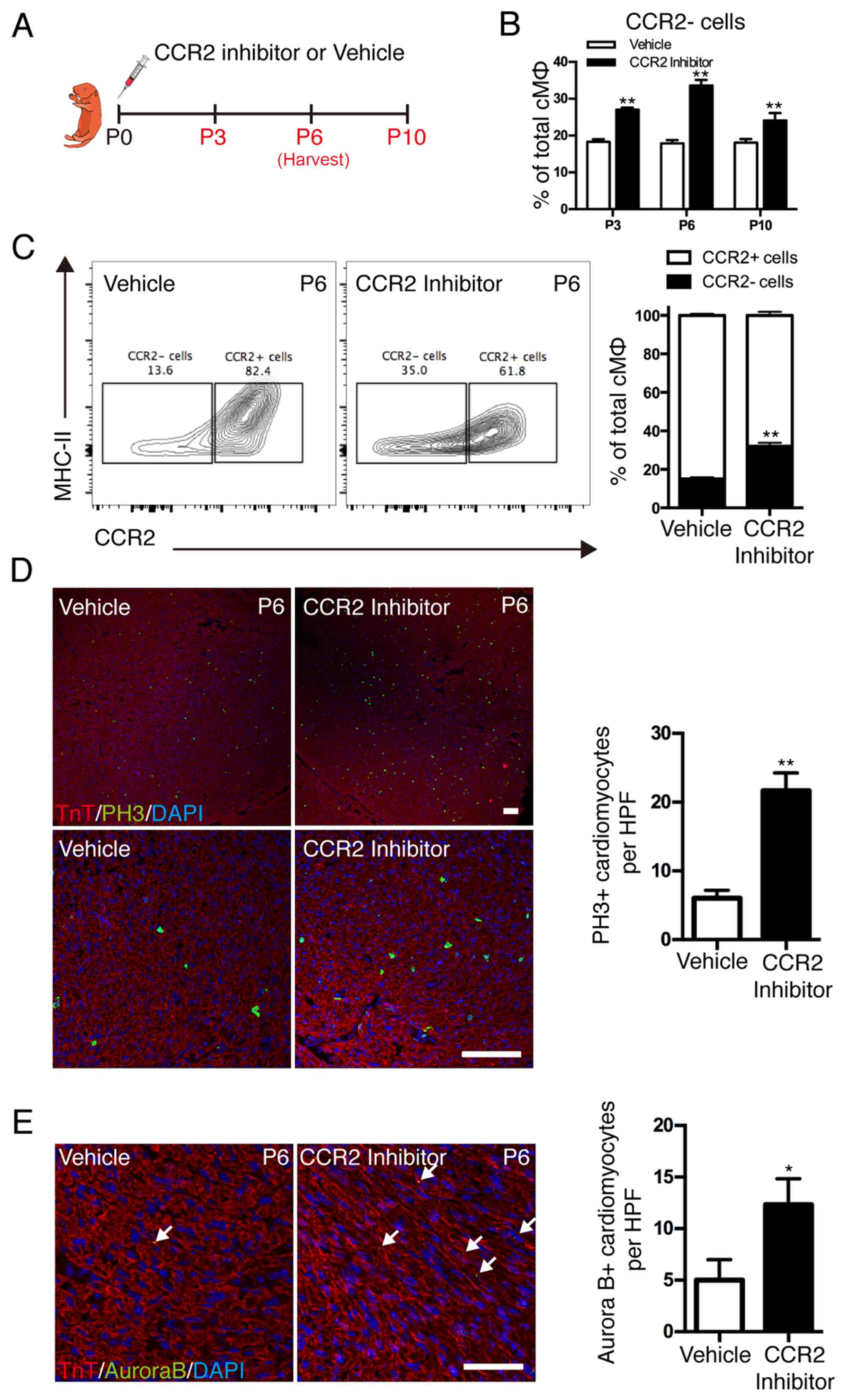 | Figure 4.Effect of cardiac resident macrophages
on postnatal cardiomyocyte proliferation. (A) CCR2 inhibitor (3
mg/kg, daily) was injected subcutaneously after birth for 2 weeks
and hearts were harvested at different time points. (B)
Quantification of cardiac macrophage populations. (C)
Representative image of fluorescence activated cell sorting
analysis of cardiac macrophage populations on the 6th day. (D)
Co-immunostaining with anti-pH3 and anti-TnT antibodies
demonstrated a significant increase in cardiomyocyte mitosis in the
CCR2 inhibitor-treated subjects; scale bar, 100 µm. (E)
Coimmunostaining with anti-Aurora B and anti-TnT antibodies
demonstrated increased cytokinesis in the hearts of CCR2
inhibitor-treated subjects; scale bar, 50 µm. Data are presented at
the mean ± standard error of the mean. *P<0.05 and **P<0.01
vs. vehicle. CCR2, C-C chemokine receptor type 2; PH3, phospho
histone H3 Ser10; TnT, troponin T; P, days after birth; MHC II,
histocompatibility-2 MHC; cMØ, cardiac macrophages; HPF, high power
field. |
Discussion
The association between oxygen state and the
proliferation of mammalian cardiomyocytes following birth has been
previously demonstrated in animal models (9,11),
however, similar findings have not been reported in humans due to
the difficulty of collecting appropriate samples. The present study
collected myocardial samples from cyanotic congenital heart disease
(CHD) patients, whose internal environment has a sustained hypoxic
state since birth, which were compared with samples from acyanotic
CHD patients. This is an appropriate study to investigate whether a
sustained hypoxic state has an effect on the promotion of
cardiomyocyte proliferation following birth in humans. In addition,
the present study also performed analysis on adolescent and adult
heart tissues; however, no significant differences between the
cyanotic and acyanotic groups were observed and results were not
presented. Combined with the results demonstrated by the hypoxic
mouse model, the current study demonstrated that hypoxia promoted
cardiomyocyte proliferation following birth. The present study, to
the best of our knowledge, was the first to demonstrate that
hypoxia affected the composition of macrophages in postnatal
hearts, which may further contribute to cardiomyocyte
proliferation. Several previous studies have demonstrated that
macrophages were associated with the angiogenesis that promoted the
repair of the infarct region of the myocardium (1,12–15).
However, there were no previous reports that indicated that
macrophages may be involved in hypoxia-induced cardiomyocyte
proliferation. We hypothesize that macrophage-associated
angiogenesis may be important for the effects observed on
cardiomyocyte proliferation. The results have revealed a novel
mechanism for the association among hypoxia, macrophages and
cardiomyocyte proliferation; however, the details of this
phenomenon require further investigation.
The ability of cardiac embryonic-derived
macrophages, also known as cardiac resident macrophages, to renew
the population reduces with increasing age and they are gradually
replaced by monocyte-derived macrophages (16). The present study demonstrated that
increasing cardiac resident macrophages, or reducing
monocyte-derived macrophages, promoted the proliferation of
cardiomyocytes. Furthermore, with increasing age, the decrease in
the number of resident macrophages and their replacement with
monocyte-derived macrophages may be partially explain why
cardiomyocytes exit the cell cycle soon after birth. However, to
determine the reason for this in detail, the differences in the
excreted factors between cardiac resident macrophages and
monocyte-derived macrophages requires investigation.
Despite the importance of the results presented, the
present study does not provide a clear understanding of the
mechanism of cardiomyocyte proliferation caused by increased
resident macrophages and decreased monocyte-derived macrophages.
Other limitations also include the small sample size in humans.
More importantly, although there is clear evidence of
hypoxia-induced cardiomyocyte proliferation via altered composition
of cardiac macrophages, it is unclear whether it leads to an
increased contractile function in the long term.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81370004, 81270228
and 81471408).
References
|
1
|
Batty JA, Lima JA Jr and Kunadian V:
Direct cellular reprogramming for cardiac repair and regeneration.
Eur J Heart Fail. 18:145–156. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinagra G and Fabris E: Direct cellular
reprogramming: The hopes and the hurdles. Eur J Heart Fail.
18:157–159. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kühn B, del Monte F, Hajjar RJ, Chang YS,
Lebeche D, Arab S and Keating MT: Periostin induces proliferation
of differentiated cardiomyocytes and promotes cardiac repair. Nat
Med. 13:962–969. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Canseco DC, Kimura W, Garg S, Mukherjee S,
Bhattacharya S, Abdisalaam S, Das S, Asaithamby A, Mammen PP and
Sadek HA: Human ventricular unloading induces cardiomyocyte
proliferation. J Am Coll Cardiol. 65:892–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bersell K, Arab S, Haring B and Kühn B:
Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and
repair of heart injury. Cell. 138:257–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergmann O, Bhardwaj RD, Bernard S, Zdunek
S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA,
Druid H, et al: Evidence for cardiomyocyte renewal in humans.
Science. 324:98–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mollova M, Bersell K, Walsh S, Savla J,
Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S
and Kühn B: Cardiomyocyte proliferation contributes to heart growth
in young humans. Proc Natl Acad Sci USA. 110:pp. 1446–1451. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Porrello ER, Mahmoud AI, Simpson E, Hill
JA, Richardson JA, Olson EN and Sadek HA: Transient regenerative
potential of the neonatal mouse heart. Science. 331:1078–1080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puente BN, Kimura W, Muralidhar SA, Moon
J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R,
Garcia JA, et al: The oxygen-rich postnatal environment induces
cardiomyocyte cell-cycle arrest through DNA damage response. Cell.
157:565–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poss KD, Wilson LG and Keating MT: Heart
regeneration in zebrafish. Science. 298:2188–2190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jopling C, Suñé G, Faucherre A, Fabregat C
and Belmonte JC Izpisua: Hypoxia induces myocardial regeneration in
zebrafish. Circulation. 126:3017–3027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Godwin JW, Pinto AR and Rosenthal NA:
Macrophages are required for adult salamander limb regeneration.
Proc Natl Acad Sci USA. 110:pp. 9415–9420. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aurora AB, Porrello ER, Tan W, Mahmoud AI,
Hill JA, Bassel-Duby R, Sadek HA and Olson EN: Macrophages are
required for neonatal heart regeneration. J Clin Invest.
124:1382–1392. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lavine KJ, Epelman S, Uchida K, Weber KJ,
Nichols CG, Schilling JD, Ornitz DM, Randolph GJ and Mann DL:
Distinct macrophage lineages contribute to disparate patterns of
cardiac recovery and remodeling in the neonatal and adult heart.
Proc Natl Acad Sci USA. 111:pp. 16029–16034. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Molawi K, Wolf Y, Kandalla PK, Favret J,
Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B,
et al: Progressive replacement of embryo-derived cardiac
macrophages with age. J Exp Med. 211:2151–2158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
U.S. Department of Heatth and Human
Services, Public Health Service, National Institutes of Health
(NIH), . Guide for the care and use of laboratory animals. NIH;
Bethesda, MD: 1985
|
|
18
|
Brickner ME, Hillis LD and Lange RA:
Congenital heart disease in adults. N Engl J Med. 342:988–a. 2000.
View Article : Google Scholar
|
|
19
|
Senyo SE, Steinhauser ML, Pizzimenti CL,
Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP and Lee
RT: Mammalian heart renewal by pre-existing cardiomyocytes. Nature.
493:433–436. 2013. View Article : Google Scholar : PubMed/NCBI
|