Introduction
Keratoconus is a progressive disease characterized
by thinning and protrusion of the cornea, resulting in an
irregular, conical shape (1). The
characteristics of keratoconus have been known for at least 200
years, but causes of keratoconus have not been clearly established.
Case reports showed associations between keratoconus and atopy
(2), allergy (3), family history (4), other systemic genetic disorders
(4), contact lens wear (5), exposure to ultraviolet radiation
(6), eye-rubbing (7), disorders of enzyme function (6), and epithelial trauma and the
production of inflammatory mediators (8). However, in a multivariate analysis of
keratoconus risk factors, eye-rubbing was the most significant
predictor for the development and/or progression of this disease
(9).
Two large studies of keratoconus indicated that
approximately 50% of subjects reported frequent, chronic, vigorous
or abnormal rubbing of at least one eye (10,11).
Vigorous eye-rubbing in keratoconus has been shown to be up to 10
times more forceful than normal eye-rubbing (12,13).
Previous studies have found that the values of corneal
biomechanical measurements are significantly lower in keratoconic
eyes than in normal eyes (14,15).
A study of the application of experimental digital forces to human
eyes revealed that, for an open eye with normal intraocular
pressure (IOP) of 15 mm Hg, light and firm digital forces increased
IOP by 100 and 300%, respectively (16). The IOP in keratoconus responses may
be greater when more force is used and rubbing episodes are longer.
In addition, some studies have reported that the human cornea is a
viscoelastic tissue (17) and
depending on the nature of mechanical forces to which the human
cornea is subjected, there may be an (almost) instantaneous
deformation, which is the elastic part of the viscoelasticity
(18,19). We therefore hypothesized that human
keratoconus fibroblasts (HKCFBs) within the intraocular environment
may be lengthened in response to the dynamic forces applied to the
cornea caused by constant changes in the levels of IOP.
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent enzymes that are responsible for the degradation of
extracellular matrix (ECM) and connective tissue protein (20). Elevated levels of MMPs are found in
keratoconus corneas, and considerable degradation of the
extracellular matrix occurs, indicating that MMPs may be involved
in the pathogenesis of keratoconus (21–25).
Tissue inhibitors of metalloproteinases (TIMPs) inhibit the
activity of MMPs by binding to them (20). The balance between the MMPs and
TIMPs determines the extent of proteolysis linked with tissue
remodeling or degradation of ECM components including collagen and
elastin (20).
Several studies have suggested that many diseases
have been associated with an imbalance of ECM synthesis and
degradation and mechanical factors have been found involved in the
pathogenesis of these diseases (26,27).
Mechanical stimulation is involved in the regulation of MMPs in
some ocular tissues, such as sclera (28), trabecular meshwork (29) and lamina cribrosa cells (30). The cornea is a dynamic tissue,
capable of altering its ECM composition and biomechanical
properties in response to changes in the visual environment
(31,32). In addition, corneal fibroblasts
have been demonstrated to respond actively to local tension changes
in the ECM (33), and fibroblasts
are a major type of mechanoresponsive cell (34). However, to date, there have been no
reports regarding the effect of mechanical stretch on expression of
MMP and TIMP in HKCFBs.
In our experiment, HKCFBs were subjected to cyclical
mechanical stretch in vitro and the expression of MMP-1,
MMP-3, TIMP-1, and TIMP-2 were evaluated. The present study appears
to be the first experimental evidence to show significantly induced
levels of MMP and reduced levels of TIMP in HKCFBs after mechanical
stretch, thereby providing support for the possible association
between the thinning and ectasia of a keratoconus cornea and
mechanical stretch. In addition, we used IL-6 antibody (IL-6 Ab) to
examine the role of IL-6 on stretch mediated regulation of MMP in
HKCFBs. Results indicated that IL-6 may be a potential mediator of
stretch-induced effects on expression of MMP and IL-6 Ab might
provide a new modality to prevent further progression of some forms
of keratoconus.
Materials and methods
Isolation and cell culture
Keratoconus cornea was collected from patient with
keratoconus (a 16 year old donor) who was undergoing corneal
transplantation within 6–12 h following surgery from Shanxi Eye
Hospital (Tai yuan, Shanxi, China). The study was undertaken with
the approval of the local ethics committee and after obtaining
written informed consent from patient. The epithelium and
endothelium of cornea were mechanically removed. The corneal stroma
was cut into several pieces with diameter of 1.0 mm. Subsequently,
the small tissue pieces were digested into single cells with
DMEM/F12 medium containing 2 mg/ml of type II collagenase. Cells
were cultured in DMEM/F12 containing fetal bovine serum and in an
air −5% CO2 incubator at 37°C. Experiments were
performed using HKCFBs from passages 3–5.
Mechanical stretch application
For experiment, HKCFBs were seeded at 6-well
Bioflex® plates (Flexcell Int. Corp., Hillsborough, NC,
USA) with an initial density of 5×105/well. After the
cells reached subconfluency, the cells were serum starved using
DMEM/F12 with 0.1% FBS for 24 h. After 24 h, the media was replaced
with FBS-free media (DMEM/F12). HKCFBs were then subjected to
cyclical stretch (1 Hz) at 10% maximum elongation for 6 h using a
Flexcell® Tension Plus™ FX-4000™ system (Flexcell Int.
Corp., Hillsborough, NC, USA) at 37°C in a humidified incubator
with an atmosphere containing 5% CO2. Cells plated on
Bioflex® plates but not subjected to stretch served as
controls. At the end of the experiment, the cells and supernatants
from cell cultures were collected for gene or protein detection,
respectively.
Treatment with IL-6
The relationship between IL-6 and expression of MMP
and TIMP was investigated. HKCFBs were seeded at 6-well
Bioflex® plates (Flexcell Int. Corp., Hillsborough, NC,
USA) with an initial density of 5×105/well. When the
cultured cells became confluent, the cells were serum starved using
DMEM/F12 with 0.1% FBS for 24 h. After 24 h, the media was replaced
with FBS-free media (DMEM/F12) and treated with IL-6 (R&D
Systems, Minneapolis, MN, USA) at a final concentration of 0, 12.5,
25 or 50 ng/ml. The cells were cultured for 6 h. No significant
cytotoxic effect on cell proliferation (data not shown). At the end
of the experiment, the cells and supernatants from cell cultures
were collected for gene or protein detection, respectively.
Effect of IL-6 Ab
IL-6 Ab (Peprotech, Rocky Hill, NJ, USA) was used to
determine if mechanical stretch-induced expression of MMP by HKCFBs
was mediated by IL-6. The stretch experiments were repeated in the
presence and absence of a IL-6 Ab (15, 30 or 60 ng/ml). No
significant cytotoxic effect on cell proliferation (data not
shown). At the end of the experiment, the supernatants from cell
cultures were collected for protein detection.
Real-time polymerase chain reaction
(RT-PCR)
The procedure used for RT-PCR was similar to that
described elsewhere. Briefly, total RNA was extracted by TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to
manufacturer-recommended procedures. 1 µg of RNA was converted to
cDNA with the PrimeScript™ RT reagent Kit (TaKaRa Biotechnology
CO., Dalian, China) by gradient PCR device (Eppendorf, Germany).
Reverse transcription was performed for 2 min at 42°C, 15 min at
37°C, and 5 sec at 85°C, followed by cooling to 4°C. Then, 2 µl of
30-fold-diluted cDNA products were amplified with SYBR®
Premix Ex Taq™ ӀӀ (TaKaRa Biotechnology CO) by the StepOnePlus™
RT-PCR System (Applied Biosystems, USA) using the gene specific
primers (Table I) designed by
Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China). For
quantification, all target mRNA expression were normalized to the
expressed housekeeping gene glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). RT-PCR conditions were as follows: 95°C for
30 sec and then 40 cycles at 95°C for 5 sec, 60°C for 30 sec, and
72°C for 1 min, followed by 72°C for 10 min. The relative quantity
of mRNA was calculated using the 2−ΔΔCт method, in which
Cт is the threshold cycle. The resulting data were expressed as a
ratio to the control value denoted as one.
 | Table I.Primer sequences for RT-PCR. |
Table I.
Primer sequences for RT-PCR.
| Gene | 5′-3′ sequences
(forward; reverse) |
|---|
| MMP-1 | For:
GGGAGATCATCGGGACAACTC |
|
| Rev:
GGGCCTGGTTGAAAAGCAT |
| MMP-3 | For:
TGGCATTCAGTCCCTCTATGG |
|
| Rev:
AGGACAAAGCAGGATCACAGTT |
| TIMP-1 | For:
TTGTTGCTGGGCTGATAGC |
|
| Rev:
CAGGATTCAGGCTATCTGGG |
| TIMP-2 | For:
GCACATCACCCTCTGTGACTT |
|
| Rev: AGCGCGTGAT CTT
GCACT |
| IL-6 | For:
CCTGAACCTTCCAAAGATGGC |
|
| Rev:
CTTGGGGTTCTTGCTGATGT |
| GAPDH | For:
AAGGTCGGAGTGAACGGATTTG |
|
| Rev:
TTCACCAGGCAAGTCTCCTCA |
Enzyme-linked immunosorbent assay
(ELISA)
Human MMP-1, MMP-3, TIMP-1, TIMP-2, and IL-6 ELISA
kits (BioSource International, Camarillo, CA, USA) were used to
measure levels of MMP-1, MMP-3, TIMP-1, TIMP-2, and IL-6 in the
cell supernatants according to the manufacturer's recommendations.
The optical density was measured at 450 nm in a microplate reader
(Multiskan Go, Thermo Scientific, USA).
Statistical analysis
All results were presented as the mean ± standard
deviation of three independent experiments and statistically
performed by using SPSS v.19.0 software and one-way analysis of
variance (ANOVA) analysis. P<0.05 was considered to denote a
statistically significant difference.
Results
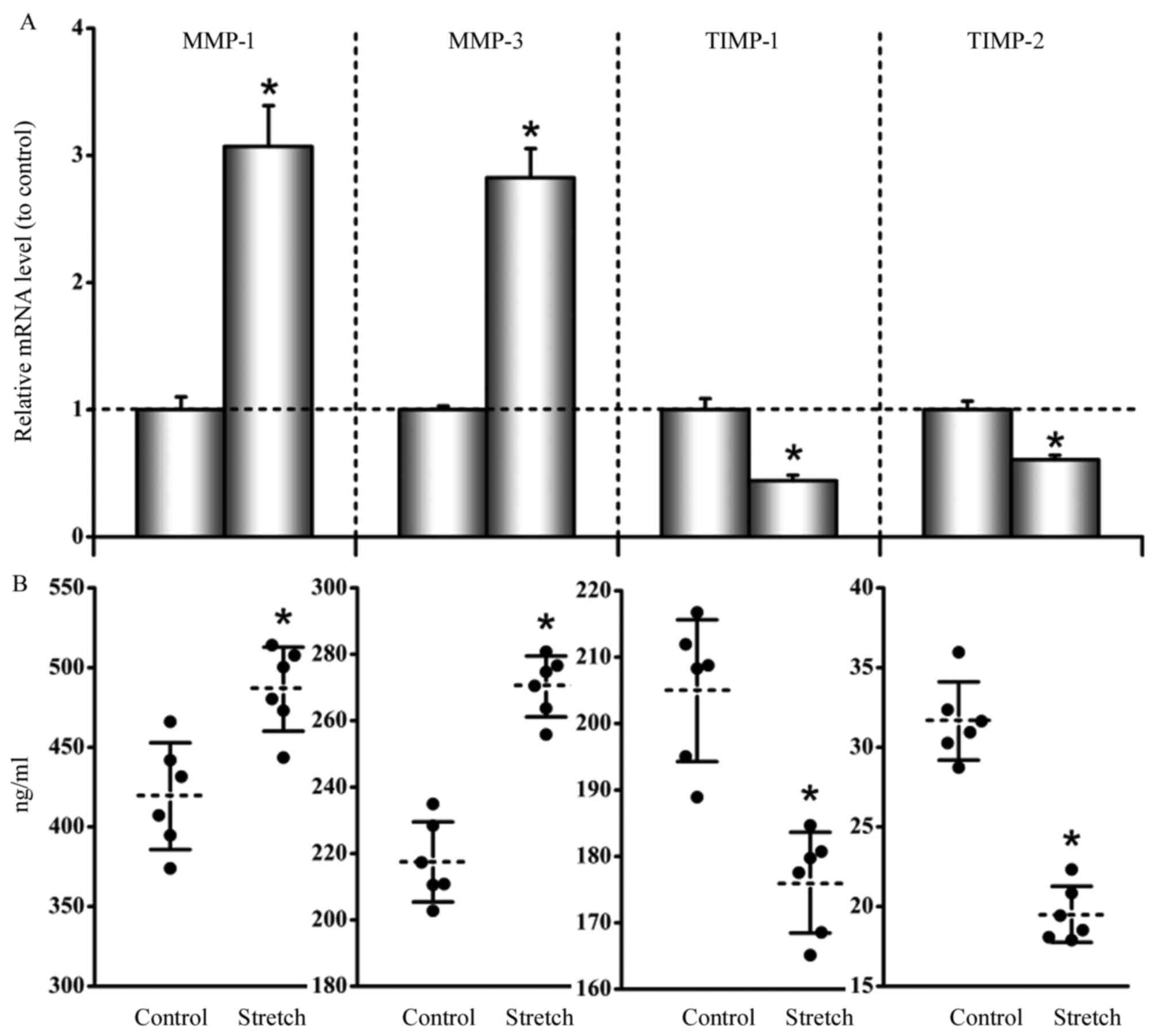
Cyclical stretch up-regulates MMP-1
and −3 and down-regulates TIMP-1 and −2
The results indicated that the mRNA expression of
MMP-1 and −3 were up-regulated by the cyclic stretch, while in
contrast the mRNA expression of TIMP-1 and −2 were down-regulated
by the cyclic stretch. The ratios of stretch to nonstretched
control values of MMP-1, MMP-3, TIMP-1, and TIMP-2 were 3.07±0.31,
2.83±0.22, 0.44±0.04, and 0.61±0.04, respectively (P<0.05;
Fig. 1A).
The concentrations of MMP-1 and −3 in cell culture
supernatants showed an increase in stretched cells relative to
unstretched cells, while in contrast the concentrations of TIMP-1
and −2 showed a decrease in stretched cells compared to unstretched
cells: MMP-1 in unstretched cells, 419.27±33.58 ng/ml, and
stretched cells, 486.48±26.32 ng/ml (P<0.05); MMP-3 in
unstretched cells, 217.44±12.08 ng/ml, and stretched cells,
270.34±10.15 ng/ml (P<0.05); TIMP-1 in unstretched cells,
204.94±10.67 ng/ml, and stretched cells, 176.06±8.58 ng/ml
(P<0.05); and TIMP-2 in unstretched cells, 31.65±2.45 ng/ml, and
stretched cells, 19.51±1.55 ng/ml (P<0.05; Fig. 1B).
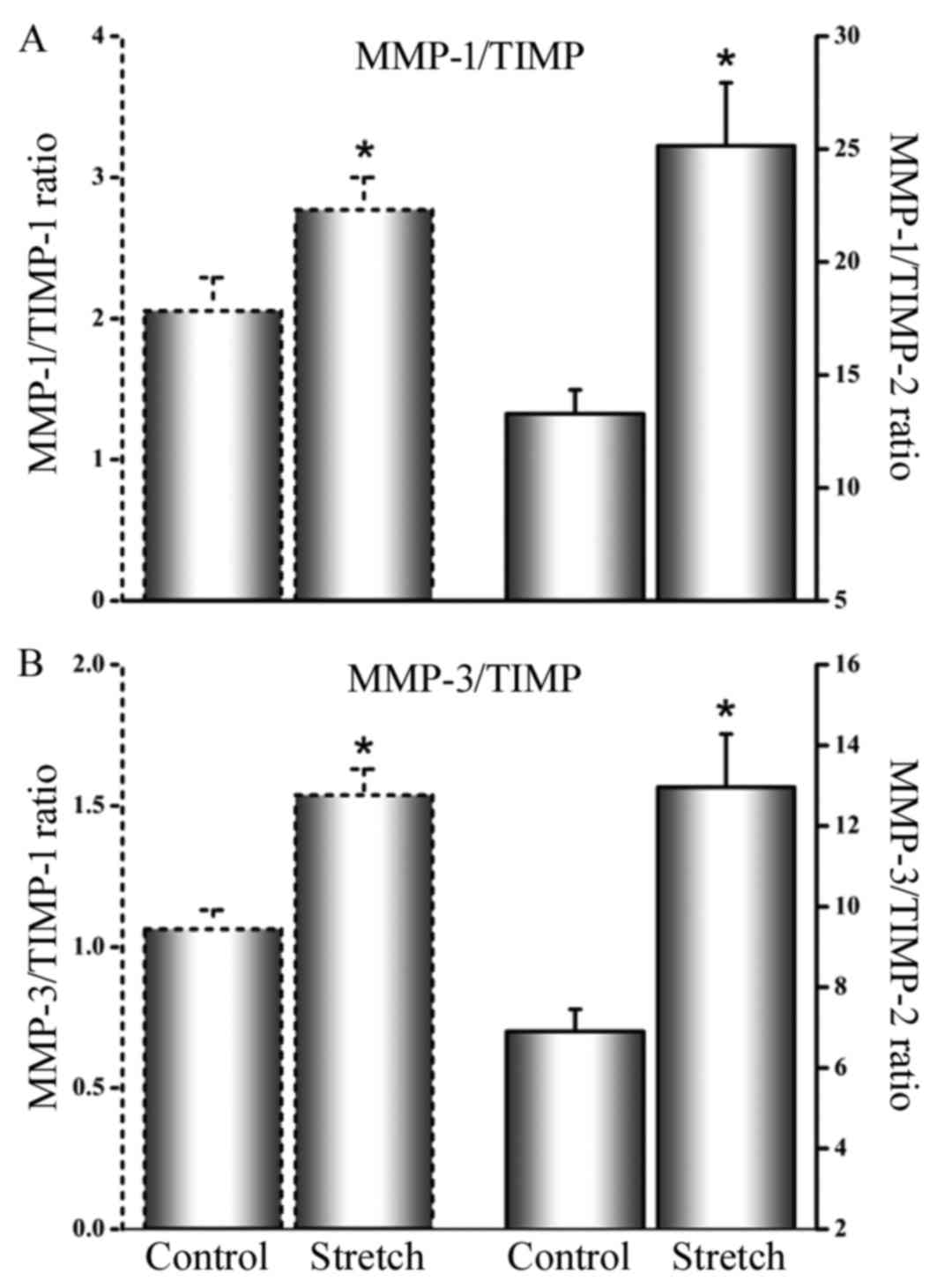
Cyclical stretch increases
concentration ratios of MMP/TIMP
The MMP/TIMP ratios in cell culture supernatants
were increased in stretched cells relative to unstretched cells:
MMP-1/TIMP-1 ratio in unstretched cells was 2.05±0.24 and 2.77±0.23
in stretched cells (P<0.05; Fig.
2A); MMP-1/TIMP-2 ratio in unstretched cells was 13.28±1.05
compared to 25.14±2.13 in stretched cells (P<0.05; Fig. 2A); MMP-3/TIMP-1 ratio in
unstretched cells was 1.06±0.07 as opposed to 1.55±0.09 in
stretched cells (P<0.05; Fig.
2B); MMP-3/TIMP-2 ratio in unstretched cells was 6.89±0.56
compared with 12.96±1.32 in stretched cells (P<0.05; Fig. 2B).
Cyclical stretch causes secretion of
IL-6
Cyclical stretch augmented IL-6 mRNA expression and
protein synthesis. The ratio of stretch to nonstretched control
mRNA value of IL-6 was 3.21±0.24 (P<0.05; Fig. 3A). It was found that the mean
concentration of IL-6 in cell culture supernatants rose from
7.12±0.58 ng/ml before stretch to 9.77±0.85 ng/ml after stretch
(P<0.05; Fig. 3B).
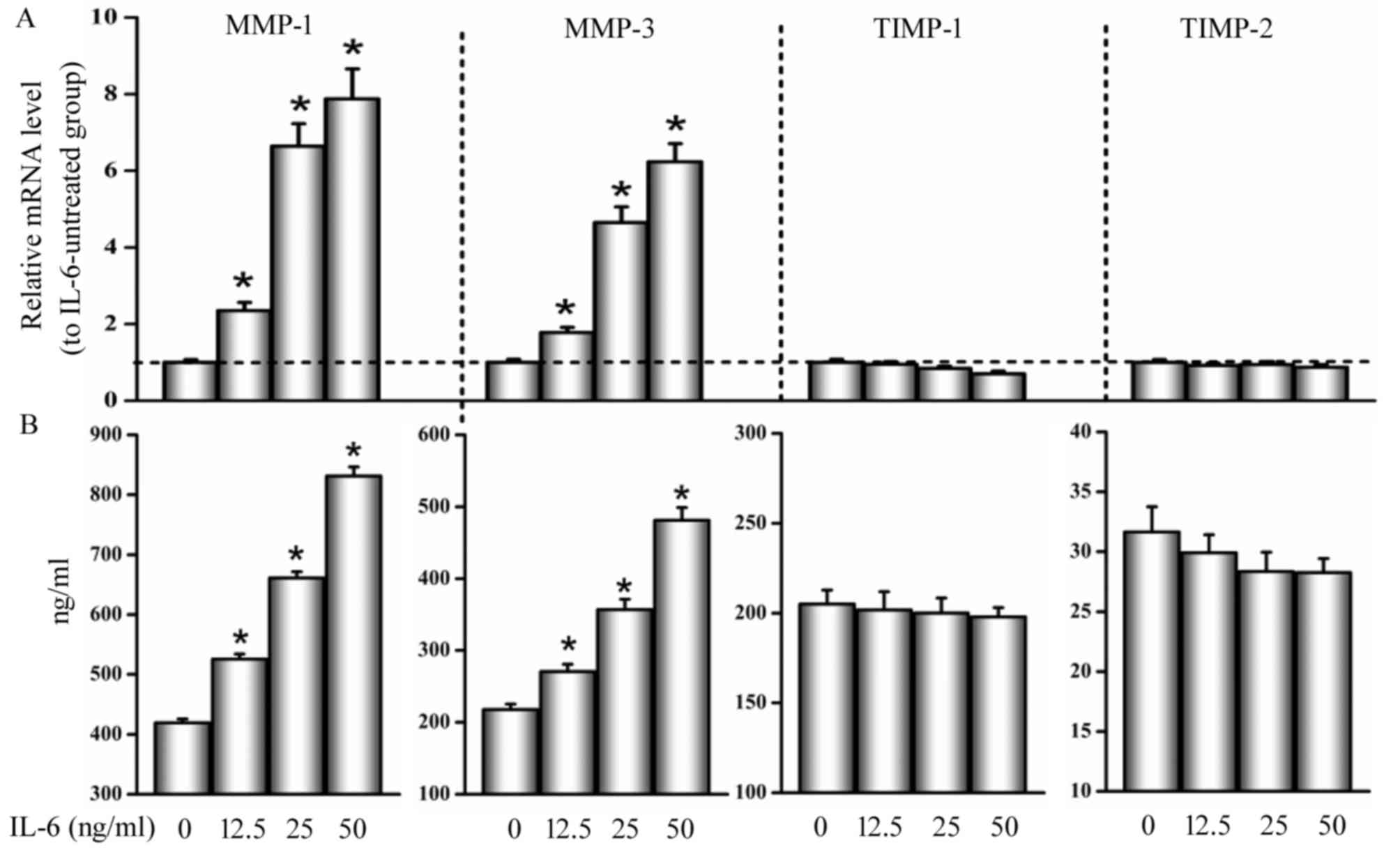
IL-6 induces MMP-1 and −3
expression
Fig. 4A showed that
MMP-1 and −3 mRNA expression were enhanced in the presence of IL-6
with a dose-dependent manner. The ratios of mRNA for MMP-1 and −3
to IL-6-untreated control were 2.35±0.21 and 1.78±0.13 at a
concentration of 12.5 ng/ml, 6.64±0.58 and 4.65±0.41 at 25 ng/ml,
and 7.87±0.74 and 6.23±0.49 at 50 ng/ml, respectively
(P<0.05).
As shown in Fig.
4B, IL-6 induced MMP-1 and −3 protein synthesis in a
dose-dependent manner. Similarly, the ratios of protein for MMP-1
and −3 to IL-6-untreated control were 1.27±0.09 and 1.25±0.08 at a
concentration of 12.5 ng/ml, 1.58±0.11 and 1.66±0.12 at 25 ng/ml,
and 1.98±0.13 and 2.22±0.17 at 50 ng/ml, respectively
(P<0.05).
In addition, no significant differences or changes
were observed in mRNA and protein levels of TIMP-1 and −2 between
cells treated with and without IL-6 (P>0.05; Fig. 4).
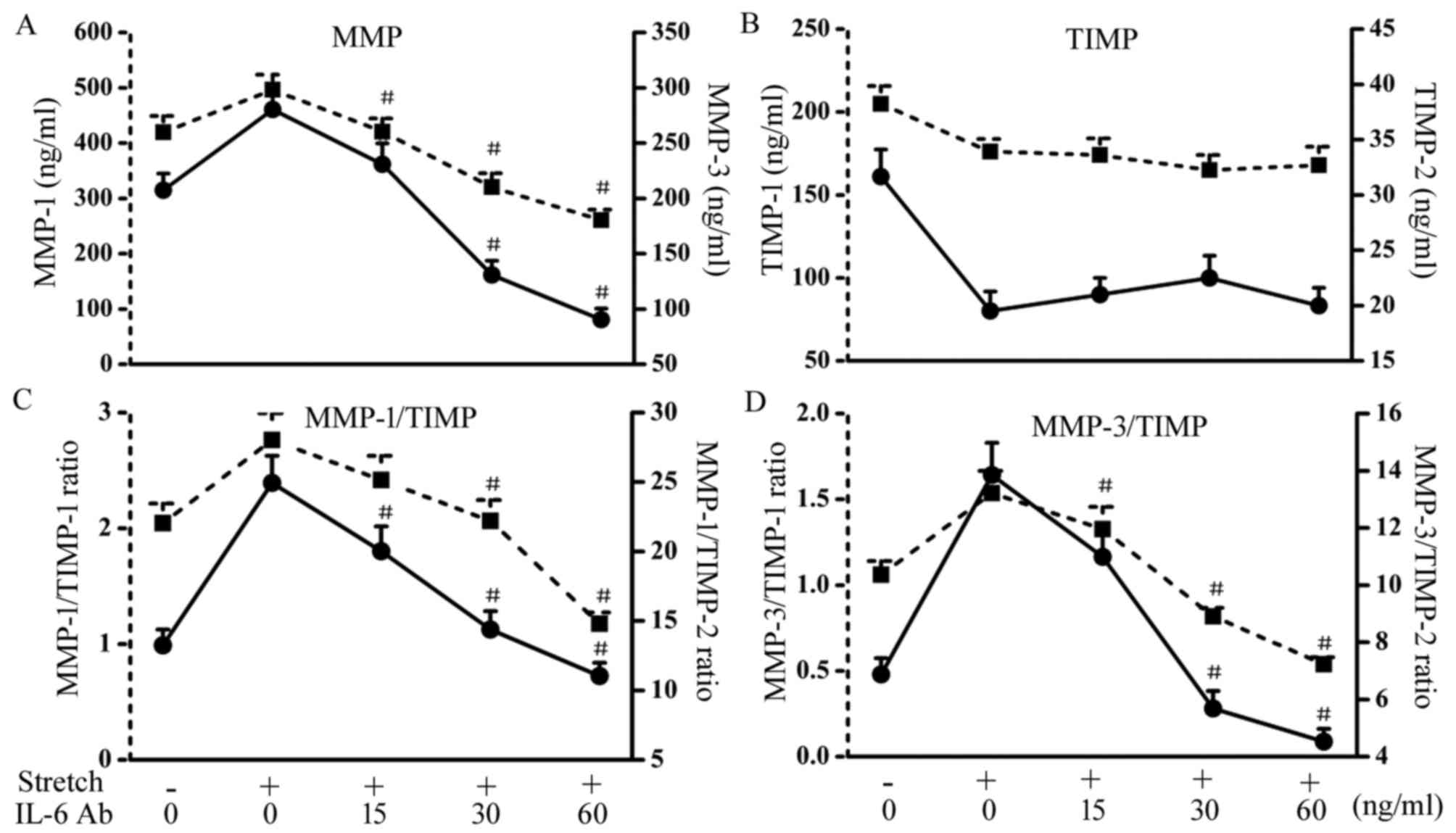
IL-6 Ab inhibits stretch-induced MMP-1
and −3 expression
As depicted in Fig.
5A, the protein expression of MMP-1 and −3 induced by
mechanical stretch were significantly reduced in a dose-dependent
manner by the specific IL-6 Ab treatment. IL-6 Ab markedly
decreased mechanical stretch-induced MMP-1 and −3 protein
production by 16±1.2 and 18±1.4% at a concentration of 15 ng/ml,
35±2.9 and 54±4.9% at 30 ng/ml, and 51±4.5 and 68±6.1% at 60 ng/ml,
respectively (P<0.05). Additionally, TIMP-1 and −2
concentrations reduced by mechanical stretch were not affected by
the addition of IL-6 Ab (P>0.05; Fig. 5B).
IL-6 Ab down-regulates concentration
ratios of MMP/TIMP
The fact that decreases in mechanical
stretch-induced MMP-1 and −3 secretion after IL-6 Ab treatment were
not accompanied by changes in the amount of secreted TIMP-1 and −2,
resulted in shifting the MMP/TIMP ratios. For example,
concentration ratios of MMP-1/TIMP-1 and MMP-1/TIMP-2 showed
decrease in cells stretched in the presence of IL-6 Ab relative to
the IL-6 Ab-untreated stretched cells by 13±0.9 and 17±1.3% at a
concentration of 15 ng/ml, 27±2.2 and 42±3.6% at 30 ng/ml, and
61±5.5 and 56±4.3% at 60 ng/ml, respectively (P<0.05; Fig. 5C). Similarly, a significant
reduction was demonstrated for the MMP-3/TIMP-1 and MMP-3/TIMP-2
ratios in stretched cells treated with IL-6 Ab, compared with the
IL-6 Ab-untreated stretched cells by 14±1.0 and 21±1.6% at a
concentration of 15 ng/ml, 47±4.1 and 59±5.5% at 30 ng/ml, and
67±5.8 and 69±6.2% at 60 ng/ml, respectively (P<0.05; Fig. 5D).
Discussion
It might be thought that the eye-rubbing is
coincidental and not causal, but the large proportion of
keratoconus giving a history of chronic habits of abnormal
eye-rubbing appeared leaves little doubt that chronic and abnormal
eye-rubbing may cause the cornea to give way and is also possibly
responsible for the progression of some forms of keratoconus
(7). Based on the above
hypothesis, we speculate that corneal stromal fibroblasts may be
exposed to mechanical stress caused by abnormal chronic eye-rubbing
in some patients with keratoconus. In vitro cell stretch
systems have rapidly become standard models for studying the
effects of mechanical forces on a variety of cell types, including
scleral fibroblasts (28),
trabecular meshwork cells (29)
and lamina cribrosa cells (30).
These studies have attempted to model intraocular forces by
introducing mechanical distortion (4–15% amplitude strain) to
ocular cells for static or dynamic (0.3 Hz or 1 Hz) loading for 30
min −72 h (28–30). Because the forces exerted at the
cornea represent transient changes in force caused by constant
changes in the levels of IOP, we selected a stretch protocol for
HKCFBs in vitro employing a regimen of cyclical dynamic
stretch. In our study, flexible-bottom culture dishes were
subjected to distension, using the Flexcell® Tension
Plus™ FX-4000™ system to deliver a highly controlled regimen of
sinusoidal stretch with a strain amplitude of 10% at a frequency of
1 Hz (60 cycles per min) for 6 h.
In the human eye, studies of MMPs and TIMPs have
been performed in the aqueous humor (35), vitreous (36), retina (37), trabecular meshwork (38), keratoconus corneas (39), and corneas during wound healing
(40). Large studies have revealed
a relationship between mechanical stretch and MMPs and TIMPs in
many kinds of eye cells. For example, Shelton et al
(28) reported that mechanical
strain increased levels of MMP-2 and reduced levels of TIMP-2 by
human scleral fibroblasts. Kirwan et al (30) reported that cyclical stretch
induced MMP-2 expression in human lamina cribrosa cells.
Mechanically stretched trabecular meshwork cells have reported
increases in MMP-2 protein and gene expression (29,41),
increased MMP-14 protein levels (29), and either no change or a decrease
in TIMP-2 (29,42). In these cultured cells, mechanical
stretch shifted the MMP-TIMP balance and these results suggest
mechanical stretch may be involved in the pathogenesis of these
diseases (29,30).
MMP-1, −2, −3, −7, −9 and −13 have been found to be
elevated in the tear film of patients with keratoconus (22–25,43,44)
and decreased levels of TIMP-1 have been found in keratoconus
corneas and their cell cultures (6,45),
indicating that MMPs and TIMPs may be involved in the pathogenesis
of keratoconus. Our studies have focused primarily on MMP-1, MMP-3,
TIMP-1, and TIMP-2. To the best of our knowledge, this was the
first investigation to determine the effect of cyclical mechanical
stretch on MMP-1, MMP-3, TIMP-1 and TIMP-2 mRNA expression and
protein synthesis in HKCFBs in vitro.
Our data showed that mechanical stretch increased
MMP-1 and −3 production and decreased TIMP-1 and −2 production,
resulting in an increased MMP-1/TIMP-1 ratio, MMP-1/TIMP-2 ratio,
MMP-3/TIMP-1 ratio, and MMP-3/TIMP-2 ratio. MMP-1, an interstitial
collagenase, can degrade native fibrillar collagen types I, II,
III, IX, and XI (20). MMP-3, or
stromelysin-1, has a broad substrate specificity that includes
casein, proteoglycans, fibronectin, elastin, and laminin, as well
as collagen types III, IV, V, IX, and IX (20). Type I and type III collagen
accounts for about 85% of corneal ECM (46). Keratoconus corneal stroma has
decreased levels of type I, III, V, XII and VI collagens (47). Therefore, it is conceivable that
the increase in the ratios of MMP/TIMP by stretched HKCFBs may
facilitate the degradation of the corneal ECM. A balance between
ECM synthesis and degradation is a prerequisite for maintaining the
structural and functional integrity of the cornea (27). Moreover, previous studies have
revealed that the thinning and ectasia of a keratoconus cornea has
been mainly attributed to the increased degradation of ECM
(21,25,48).
Since keratoconus is characterized by the thinning of the corneal
stroma, taken together, the up-regulation of MMP/TIMP ratios caused
by mechanical stretch which may represent one of the main causes of
the damage of corneal tissue, thereby contribute to the development
and/or progression of some forms of keratoconus.
Keratoconus is defined as a non-inflammatory disease
of the cornea, however, in recent years, several groups have
demonstrated that the tear film in keratoconus showed increased
levels of inflammatory molecules (IL-4, −5, −6, −8, TNF-α, -β)
compared with normal controls (22,23,25,49).
The extent of the increase was found to be associated with the
severity of keratoconus (22,23,49).
This suggested that the pathogenesis of keratoconus may involve
chronic inflammatory events.
IL-6 is a pleiotropic cytokine with a wide range of
biological activities in immune regulation, hematopoiesis,
inflammation and oncogenesis and IL-6 could play an important role
in the generation of inflammation within the cornea (50). A recent study has indicated that
IL-6 may rank as important factors in the pathogenesis of
keratoconus (25). Previous
studies have documented the effect of mechanical stretch on IL-6
synthesis in a variety of cell types (51). Additionally, IL-6 can modulate the
expression of MMPs and TIMPs by human corneal epithelial cells
(52). However, to date, there
have been no reports regarding the correlation between mechanical
stretch and IL-6 expression and the effect of IL-6 on the
production of MMPs and TIMPs in HKCFBs.
Our study showed that cyclical stretch increased
IL-6 levels, and we also found IL-6 induced MMP-1 and −3 expression
in a dose-dependent manner, but did not modify TIMP-1 and TIMP-2
levels, indicating that IL-6 induces a increase of the MMP/TIMP
ratios in HKCFBs. The synthesis of larger amounts of IL-6 by
epithelial cells could influence a number of immunity and
inflammation activities which could lead to corneal damage within
the eye (53). We postulate that
IL-6 may play a role in the degradation of ECM in keratoconus
corneas by up-regulating the MMP/TIMP ratios. Balasubramanian et
al (54) used specific ELISA
to measure the amount of inflammatory markers 60 sec after
eye-rubbing in normal subjects. They found an increased expression
in tear MMP-13, IL-6 and TNF-α in normal subjects. These
inflammatory changes after eye-rubbing are thought contribute to
the pathogenesis. Put together, our results provide further support
for that the pathogenesis of keratoconus may involve chronic
inflammatory events and matrix degradation. Moreover, the
mechanical stress caused by eye-rubbing may be a cause of
development of inflammatory events and matrix degradation in some
types of keratoconus.
Wang et al (55) reported that TNF-α mediates the
stretch-induced MMP genes expression in human umbilical vein
endothelial cells. TNF-α is also widely considered an important
cytokine mediator of inflammation and immune regulation that can
cause a range of local and systemic biological effects in various
cell types (56). Similarly, we
speculate that the stretch-induced MMP expression in HKCFBs is
dependent on IL-6. In our experiment, IL-6 Ab was used to determine
whether mechanical stretched-induced MMP by HKCFBs was mediated by
IL-6. Our data showed that the stretch-induced expression of MMP-1
and −3 were markedly inhibited in the presence of IL-6 Ab, whereas
no significant changes in TIMP-1 and −2 protein levels were
detected in stretched cells with the presence or absence of the
IL-6 Ab. Moreover, the IL-6 Ab inhibited significantly the
stretch-induced increase in MMP-1 and −3 in culture supernatants in
a dose-dependent manner.
Previous work reported that heat shock can increase
MMP-1 and −3 and IL-6 at both the mRNA and protein levels in
cultured skin fibroblasts and the expression of MMP-1 and −3
increased by heat shock significantly were blocked after treatment
with a monoclonal antibody against IL-6, indicating that IL-6
mediates the heat shock-induced MMP-1 and −3 expression (57). Similarly, our study demonstrates
that mechanical stretch up-regulated MMP-1 and −3 expression via
IL-6 in HKCFBs.
A recent study has found that subsequent treatment
of keratoconus patients with cyclosporine A (CyA) reduced tear
MMP-9 levels significantly and led to local reduction in corneal
curvatures, indicating that CyA might be a novel treatment strategy
in keratoconus (58). In addition,
it is worth mentioning that several IL-6 blocking agents such as a
humanized anti-IL-6 receptor antibody has been developed and
successfully applied in clinical trials for the treatment of
several diseases (59,60).
Our data showed that MMP/TIMP ratios up-regulated by
cyclical stretch were attenuated by IL-6 Ab in a dose-dependent
manner. A significant reduction in the MMP/TIMP ratios by IL-6 Ab
might provide a new modality to treat patients with
keratoconus.
Further studies are required to determine which
signaling pathway being involved in the process: Stretch → IL-6 →
MMP in keratoconus. It is possible that inhibiting IL-6 may help to
dampen degradation of the corneal ECM mediated by mechanical
stretch thereby reducing damage to the cornea. This may be realized
by inhibiting IL-6 receptors or inhibition of targets downstream of
the IL-6 signaling pathway or other pathways targeting IL-6 for
treating keratoconus.
In conclusion, our findings indicate that cyclically
mechanical stretch induces levels of MMP-1 and −3 and IL-6 and
reduces levels of TIMP-1 and −2 in HKCFBs; IL-6 mediates the
stretch-induced MMP expression in keratoconus.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (grant no. 11402161, 11402162,
11572213, 31300770, 31271005).
References
|
1
|
Rabinowitz YS: Keratoconus. Surv
Ophthalmol. 42:297–319. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harrison RJ, Klouda PT, Easty DL, Manku M,
Charles J and Stewart CM: Association between keratoconus and
atopy. Br J Ophthalmol. 73:816–822. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McMonniesc CW and Boneham GC: Keratoconus,
allergy, itch, eye-rubbing and hand-dominance. Clin Exp Optom.
86:376–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szczotka-Flynn L, Slaughter M, Mcmahon T,
Barr J, Edrington T, Fink B, Lass J, Belin M and Iyengar SK; CLEK
Study Group, : Disease severity and family history in keratoconus.
Br J Ophthalmol. 92:1108–1111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McMonnies CW: The biomechanics of
keratoconus and rigid contact lenses. Eye Contact Lens. 31:80–92.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cristina Kenney M and Brown DJ: The
cascade hypothesis of keratoconus. Cont Lens Anterior Eye.
26:139–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ridley F: Eye-rubbing and contact lenses.
Br J Ophthalmol. 45:6311961. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson SE, He YG, Weng J, Li Q, McDowall
AW, Vital M and Chwang EL: Epithelial injury induces keratocyte
apoptosis: Hypothesized role for the interleukin-1 system in the
modulation of corneal tissue organization and wound healing. Exp
Eye Res. 62:325–327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bawazeer AM, Hodge WG and Lorimer B: Atopy
and keratoconus: A multivariate analysis. Br J Ophthalmol.
84:834–836. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zadnik K, Barr JT, Edrington TB, Everett
DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H and
Gordon MO: Baseline findings in the collaborative longitudinal
evaluation of keratoconus (CLEK) study. Invest Ophthalmol Vis Sci.
39:2537–2546. 1998.PubMed/NCBI
|
|
11
|
Weed KH, MacEwen CJ, Giles T, Low J and
McGhee CN: The Dundee university Scottish keratoconus study:
Demographics, corneal signs, associated diseases, and eye rubbing.
Eye (Lond). 22:534–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korb D, Leahy C and Greiner J: Prevalence
and characteristics of eye-rubbing for keratoconic and
non-keratoconic subjects. Invest Ophthalmol Vis Sci.
32:10571991.
|
|
13
|
McMonnies CW: Abnormal rubbing and
keratectasia. Eye Contact Lens. 33:265–271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ortiz D, Piñero D, Shabayek MH,
Arnalich-Montiel F and Alió JL: Corneal biomechanical properties in
normal, post-laser in situ keratomileusis, and keratoconic eyes. J
Cataract Refract Surg. 33:1371–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shah S, Laiquzzaman M, Bhojwani R, Mantry
S and Cunliffe I: Assessment of the biomechanical properties of the
cornea with the ocular response analyzer in normal and keratoconic
eyes. Invest Ophthalmol Vis Sci. 48:3026–3031. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McMonnies CW and Boneham GC: Corneal
curvature stability with increased intraocular pressure. Eye
Contact Lens. 33:130–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwartz NJ, Mackay RS and Sackman JL: A
theoretical and experimental study of the mechanical behavior of
the cornea with application to the measurement of intraocular
pressure. Bull Math Biophys. 28:5851966. View Article : Google Scholar
|
|
18
|
Humphrey JD and Delange SL: An
introduction to biomechanics: Solids and fluids, analysis and
design. Springer; New York, NY: pp. 1293. 2007
|
|
19
|
Liu WC, Lee SM, Graham AD and Lin MC:
Effects of eye rubbing and breath holding on corneal biomechanical
properties and intraocular pressure. Cornea. 30:855–860. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collier SA: Is the corneal degradation in
keratoconus caused by matrix-metalloproteinases? Clin Exp
Ophthalmol. 29:340–344. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lema I and Durán JA: Inflammatory
molecules in the tears of patients with keratoconus. Ophthalmology.
112:654–659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lema I, Sobrino T, Durán JA, Brea D and
Díez-Feijoo E: Subclinical keratoconus and inflammatory molecules
from tears. Br J Ophthalmol. 93:820–824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balasubramanian SA, Pye DC and Willcox MD:
Are proteinases the reason for keratoconus? Curr Eye Res.
35:185–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balasubramanian SA, Mohan S, Pye DC and
Willcox MD: Proteases, proteolysis and inflammatory molecules in
the tears of people with keratoconus. Acta Ophthalmol.
90:e303–e309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun HB: Mechanical loading, cartilage
degradation, and arthritis. Ann N Y Acad Sci. 1211:37–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lenz O, Elliot SJ and Stetler-Stevenson
WG: Matrix metalloproteinases in renal development and disease. J
Am Soc Nephrol. 11:574–581. 2000.PubMed/NCBI
|
|
28
|
Shelton L and Rada JS: Effects of cyclic
mechanical stretch on extracellular matrix synthesis by human
scleral fibroblasts. Exp Eye Res. 84:314–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradley JM, Kelley MJ, Zhu X, Anderssohn
AM, Alexander JP and Acott TS: Effects of mechanical stretching on
trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci.
42:1505–1513. 2001.PubMed/NCBI
|
|
30
|
Kirwan RP, Fenerty CH, Crean J, Wordinger
RJ, Clark AF and O'Brien CJ: Influence of cyclical mechanical
strain on extracellular matrix gene expression in human lamina
cribrosa cells in vitro. Mol Vis. 11:798–810. 2005.PubMed/NCBI
|
|
31
|
Petroll WM, Cavanagh HD and Jester JV:
Dynamic three-dimensional visualization of collagen matrix
remodeling and cytoskeletal organization in living corneal
fibroblasts. Scanning. 26:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grabner G, Eilmsteiner R, Steindl C,
Ruckhofer J, Mattioli R and Husinsky W: Dynamic corneal imaging. J
Cataract Refract Surg. 31:163–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Petroll WM, Vishwanath M and Ma L: Corneal
fibroblasts respond rapidly to changes in local mechanical stress.
Invest Ophthalmol Vis Sci. 45:3466–3474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Derderian CA, Bastidas N, Lerman OZ, Bhatt
KA, Lin SE, Voss J, Holmes JW, Levine JP and Gurtner GC: Mechanical
strain alters gene expression in an in vitro model of hypertrophic
scarring. Ann Plast Surg. 55:69–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Määttä M, Tervahartiala T, Harju M,
Airaksinen J, Autio-Harmainen H and Sorsa T: Matrix
metalloproteinases and their tissue inhibitors in aqueous humor of
patients with primary open-angle glaucoma, exfoliation syndrome,
and exfoliation glaucoma. J Glaucoma. 14:64–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takano A, Hirata A, Inomata Y, Kawaji T,
Nakagawa K, Nagata S and Tanihara H: Intravitreal plasmin injection
activates endogenous matrix metalloproteinase-2 in rabbit and human
vitreous. Am J Ophthalmol. 140:654–660. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Flaxel C, Bradle J, Acott T and Samples
JR: Retinal pigment epithelium produces matrix metalloproteinases
after laser treatment. Retina. 27:629–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pang IH, Hellberg PE, Fleenor DL, Jacobson
N and Clark AF: Expression of matrix metalloproteinases and their
inhibitors in human trabecular meshwork cells. Invest Ophthalmol
Vis Sci. 44:3485–3493. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Predović J, Balog T, Marotti T, Gabrić N,
Bohac M, Romac I and Dekaris I: The expression of human corneal
MMP-2, MMP-9, proMMP-13 and TIMP-1 in bullous keratopathy and
keratoconus. Coll Antropol. 32:15–19. 2008.
|
|
40
|
Daniels JT, Geerling G, Alexander RA,
Murphy G, Khaw PT and Saarialho-Kere U: Temporal and spatial
expression of matrix metalloproteinases during wound healing of
human corneal tissue. Exp Eye Res. 77:653–664. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vittal V, Rose A, Gregory KE, Kelley MJ
and Acott TS: Changes in gene expression by trabecular meshwork
cells in response to mechanical stretching. Invest Ophthalmol Vis
Sci. 46:2857–2868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lubis AM and Lubis VK: Matrix
metalloproteinase, tissue inhibitor of metalloproteinase and
transforming growth factor-beta 1 in frozen shoulder, and their
changes as response to intensive stretching and supervised neglect
exercise. J Orthop Sci. 18:519–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mackiewicz Z, Määttä M, Stenman M,
Konttinen L, Tervo T and Konttinen YT: Collagenolytic proteinases
in keratoconus. Cornea. 25:603–610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pannebaker C, Chandler HL and Nichols JJ:
Tear proteomics in keratoconus. Mol Vis. 16:1949–1957.
2010.PubMed/NCBI
|
|
45
|
Kenney MC, Chwa M, Atilano SR, Tran A,
Carballo M, Saghizadeh M, Vasiliou V, Adachi W and Brown DJ:
Increased levels of catalase and cathepsin V/L2 but decreased
TIMP-1 in keratoconus corneas: Evidence that oxidative stress plays
a role in this disorder. Invest Ophthalmol Vis Sci. 46:823–832.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Karamichos D, Funderburgh ML, Hutcheon AE,
Zieske JD, Du Y, Wu J and Funderburgh JL: A role for topographic
cues in the organization of collagenous matrix by corneal
fibroblasts and stem cells. PLoS One. 9:e862602014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chaerkady R, Shao H, Scott SG, Pandey A,
Jun AS and Chakravarti S: The keratoconus corneal proteome: Loss of
epithelial integrity and stromal degeneration. J Proteomics.
87:122–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matthews FJ, Cook SD, Majid MA, Dick AD
and Smith VA: Changes in the balance of the tissue inhibitor of
matrix metalloproteinases (TIMPs)-1 and −3 may promote keratocyte
apoptosis in keratoconus. Exp Eye Res. 84:1125–1134. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jun AS, Cope L, Speck C, Feng X, Lee S,
Meng H, Hamad A and Chakravarti S: Subnormal cytokine profile in
the tear fluid of keratoconus patients. PLoS One. 6:e164372011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Field G, Conn CA, McClanahan TB, Nao BS,
Kluger MJ and Gallagher KP: Tumor necrosis factor and interleukin-6
are not elevated in venous blood from ischemic canine myocardium.
Proc Soc Exp Biol Med. 206:pp. 384–391. 1994; View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kanwar YS, Wada J, Sun L, Xie P, Wallner
EI, Chen S, Chugh S and Danesh FR: Diabetic nephropathy: Mechanisms
of renal disease progression. Exp Biol Med (Maywood). 233:4–11.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tang CH, Chen CF, Chen WM and Fong YC:
IL-6 increases MMP-13 expression and motility in human
chondrosarcoma cells. J Biol Chem. 286:11056–11066. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cubitt CL, Lausch RN and Oakes JE:
Differences in interleukin-6 gene expression between cultured human
corneal epithelial cells and keratocytes. Invest Ophthalmol Vis
Sci. 36:330–336. 1995.PubMed/NCBI
|
|
54
|
Balasubramanian SA, Pye DC and Willcox MD:
Effects of eye rubbing on the levels of protease, protease activity
and cytokines in tears: Relevance in keratoconus. Clin Exp Optom.
96:214–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang BW, Chang H, Lin S, Kuan P and Shyu
KG: Induction of matrix metalloproteinases-14 and −2 by cyclical
mechanical stretch is mediated by tumor necrosis factor-alpha in
cultured human umbilical vein endothelial cells. Cardiovasc Res.
59:460–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tracey KJ and Cerami A: Tumor necrosis
factor and regulation of metabolism in infection: Role of systemic
versus tissue levels. Proc Soc Exp Biol Med. 200:pp. 233–239. 1992;
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim
KH, Eun HC and Chung JH: Heat shock-induced matrix
metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and
JNK activation and via an autocrine interleukin-6 loop. J Invest
Dermatol. 123:1012–1019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shetty R, Ghosh A, Lim RR, Subramani M,
Mihir K, Reshma AR, Ranganath A, Nagarai S, Nuijts RM, Beuerman R,
et al: Elevated expression of matrix metalloproteinase-9 and
inflammatory cytokines in keratoconus patients is inhibited by
cyclosporine A. Invest Ophthalmol Vis Sci. 56:738–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jones SA: Directing transition from innate
to acquired immunity: Defining a role for IL-6. J Immunol.
175:3463–3468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nishimoto N and Kishimoto T: Interleukin
6: From bench to bedside. Nat Clin Pract Rheumatol. 2:691. 2006.
View Article : Google Scholar
|