Introduction
Gastric cancer (GC) is associated with high
mortality worldwide, but its occurrence is particularly high in
China and other Asian countries (1,2).
Many patients are diagnosed in the late stages of GC, and thus
cannot undergo surgery and are subjected to chemotherapy instead.
However, the efficacy of 5-fluorouracil- and cisplatin-based
regimens during the late stages of GC is limited (3,4).
Currently, the prognosis for GC patients is very poor; thus, novel
therapeutic targets and alternative treatment strategies are
urgently required.
Tumour protein 53 (p53) was first described in 1979
(5), as a protein that bound to
the simian virus large T antigen. Inactivation of p53 is evident in
more than half of all human cancers (6), and it is caused by mutations or
deletions in the TP53 gene itself (7,8) or
by changes in alternative splicing (9–11).
Different isoforms of p53 are expressed in different tissues,
including various types of normal, precancerous and malignant
tissues (12–14). Previous results have linked several
factors, including the presence of Helicobacter pylori
infection, chronic gastritis, and p53 isoforms, with the occurrence
of GC (15,16).
Tumour necrosis factor-α (TNF-α) is a potential
anticancer agent, effective against various malignant tumours. The
therapeutic benefit from TNF-α can be ascribed to its
anti-proliferative effects, and its ability to increase the
penetration of chemotherapeutic agents into tumour tissues
(17). An increasing body of
evidence suggests that recombinant mutated human TNF (rmhTNF) acts
synergistically with traditional chemotherapeutic drugs to exert
enhanced antitumor effects (18).
Recently, rmhTNF has been administered to patients with non-small
cell lung cancer, non-Hodgkin lymphoma, or malignant pleura and
ascites, when other therapies failed (19). In the present study, the effects of
combination treatment of the commonly used cytotoxic agent
cisplatin and the novel agent rmhTNF were examined on two GC cell
lines expressing either wild type or mutated p53 isoform β
(p53β).
Materials and methods
Cells
The SGC-7901 (expressing mutant p53) and the MKN45
(expressing wild-type p53) GC cell lines were obtained from the
Central Laboratory of Weifang Medical College (Weifang, China).
Cell lines were passaged four times prior to harvesting for RNA
isolation. All human cell lines were cultured in RPMI-1640 medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 5% foetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) at 37°C/5%
CO2.
Inhibition of cell growth
MKN-45 and SGC-7901 cells were seeded in 96-well
plates at a density of 5×104 cells/ml and incubated at
37°C/5% CO2 for 24 h, in order to achieve the
exponential phase of cell growth. The supernatant was then
discarded, and fresh culture medium was added to the wells.
Treatments were performed by adding to the culture medium the
following: i) 4 µg/ml cisplatin (Deyao Pharmaceutical Co., Ltd.,
Dezhou, China); ii) 50 IU/ml rmhTNF (Shanghai Weike
Biopharmaceutical Co., Ltd., Shanghai, China); iii) 100 IU/ml
rmhTNF; iv) 200 IU/ml rmhTNF; v) 4 µg/ml cisplatin and 50 IU/ml
rmhTNF; and vi) 4 µg/ml cisplatin and 100 IU/ml rmhTNF. Control
wells contained medium alone (untreated cells). Following culture
for 24 h at 37°C/5% CO2, the medium was replaced with
110 µl10% Cell Counting Kit-8 medium (Yesen Biotechnology
Scientific Inc., Shanghai, China), and cells were incubated for
another 2 h at 37°C/5% CO2. The absorbance at 450 nm
(A450) was determined using a Bio-Tek PowerWave XS
microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
The mean absorbance values were determined from four wells for each
treatment group, and the growth inhibition rate was calculated
using the following formula:
IC=(AExp–AC)(AC–AEmp)x100
IC denotes the growth inhibition rate (%),
AExp the mean absorbance for the treatment group,
AC the mean absorbance for the control group, and
AEmp the mean absorbance for wells without cells or
reagents added.
Reverse transcription-polymerase chain
reaction (RT-PCR)
4×104 MKN45 and SGC7901 cells in the
exponential phase of growth were seeded in per well in 6-well
plates and cultured for 24 h in medium supplemented with 4 µg/ml
cisplatin, 50 U/ml rmhTNF, 100 U/ml rmhTNF, 200 U/ml rmhTNF, 4
µg/ml cisplatin and 50 U/ml rmhTNF, or 4 µg/ml cisplatin and 100
U/ml rmhTNF. As a control, normal growth medium without supplements
was used (untreated cells). TRIzol® RNA isolation,
M-MuLV first-chain synthesis and PCR amplification kits were all
bought from Shanghai Sangon Biotech Corporation Ltd. (Shanghai,
China). The concentration of total RNA in each sample was
determined with a 2000c UV-Vis spectrophotometer (Thermo Fisher
Scientific, Inc.). Each 20 µl reverse transcription reaction
contained 4 µl 25 mM MgCl2, 2 µl 10xPCR Buffer II, 1 µl
double-distilled water, 8 µl premixed deoxyribonucleoside
triphosphates, 1 µl RNA inhibitor solution (20 U/µl), 1 µl random
hexamers, 1 µl MuLV reverse transcriptase, and 2 µl total RNA (≤1
µg), as per the kit's protocols (Shanghai Sangon Biotech
Corporation Ltd.). Total RNA was added just prior to the start of
the reaction. Reactions were incubated at 25°C for 10 min, then at
42°C for 30 min, followed by 95°C for 5 min and then cooled at 5°C
for 5 min. Samples were then diluted 1:4 to obtain a final
concentration of 10 ng/µl cDNA. The PCR step was carried out on a
PE-5700 MyCycler (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and involved 35 amplification cycles at 94°C for 1 min, 58°C
for 50 sec and 72°C for 1 min. Specific oligonucleotide primers
were used as listed in Table I.
Amplicons were subjected to 1% (w/v) agarose gel electrophoresis,
and gels were analysed with a BioSpectrum AC Gel Imaging System
(Alpha Innotech Corp., San Leandro, CA, USA). The relative
expression levels of target genes were calculated using the
following formula:
 | Table I.Primers used for polymerase chain
reaction. |
Table I.
Primers used for polymerase chain
reaction.
| Gene | Primer sequence
(5′-3′) | Product (bp) |
|---|
| β-actin | F:
GTGGGGCGCCCCAGGCACCA |
539 |
|
| R:
CTCCTTAATGTCACGCACGATTTC |
|
| bcl-2 | F:
CGCGACTCCTGATTCATT |
316 |
|
| R:
TGCATTCTTGGACGAGGG |
|
| p53β |
| 1,050 |
|
Outer | F:
GTCACTGCCATGGAGGAGCCGCA |
|
|
primers | R:
GACGCACACCTATTGCAAGCAAG GGTTC |
|
|
Inner | F:
ATGGAGGAGCCGCAGTCAGAT |
|
|
primers | R:
TTGAAAGCTGGTCTGGTCCTGA |
|
Relativegeneexpression=AbsorbanceintegralareaoftargetgeneAbsorbanceintegralareaofβ–actin
Statistical analysis
SPSS 20.0 (IBM SPSS, Armonk, NY, USA) was used in
order to analyse experimental data, with values presented as the
mean ± standard deviation. The difference among groups was assessed
by one-way analysis of variance. Comparisons between two groups
were conducted using the Student-Newman-Keuls method or Student's
t-test. The relationship between two variables was analysed using
Pearson's correlation coefficient. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of cisplatin and rmhTNF on GC
cell growth
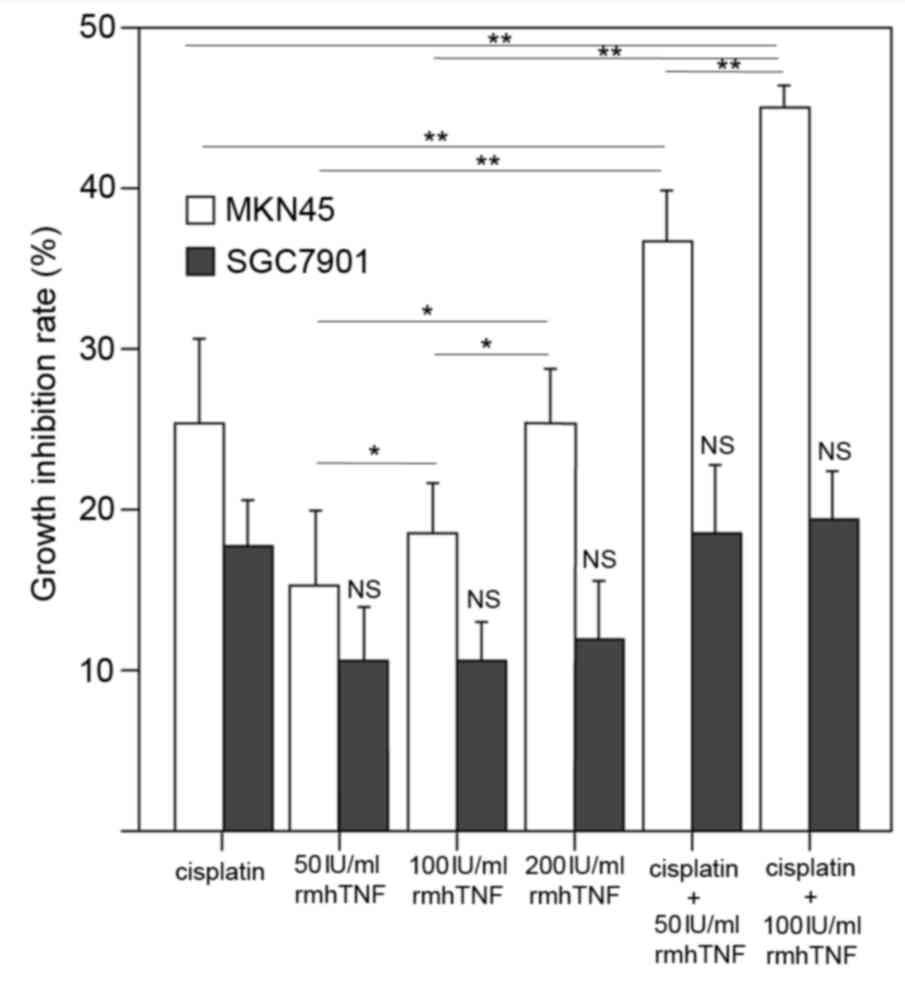
Following treatment with rmhTNF for 24 h, a
significant inhibition of cell growth was observed in MKN45 cells
in a dose-dependent manner (P<0.05; Fig. 1). However, no significant effect
was observed on the cell growth of the mutant p53-expressing
SGC7901 cells with rmhTNF treatment (Fig. 1). Combination treatment of
cisplatin and rmhTNF acted synergistically to further enhance the
inhibitory effect on the growth of MKN45 cells, compared with
either cisplatin or rmhTNF treatments alone (P<0.01; Fig. 1). A synergistic effect of cisplatin
and rmhTNF was not observed in the mutant p53-expressing SGC7901
cells (Fig. 1). The combination
treatment of cisplatin was performed with two different doses of
rmhTNF (50 and 100 IU/ml) and the growth inhibition observed was
dose-dependent in MKN45 cells (P<0.01; Fig. 1).
Expression levels of p53β and bcl-2
apoptosis regulator (bcl-2) mRNA in GC cells
The mRNA expression of bcl-2 and p53β in MKN45 cells
(Fig. 2A) and bcl-2 in SGC7901
cells (Fig. 2B) were determined.
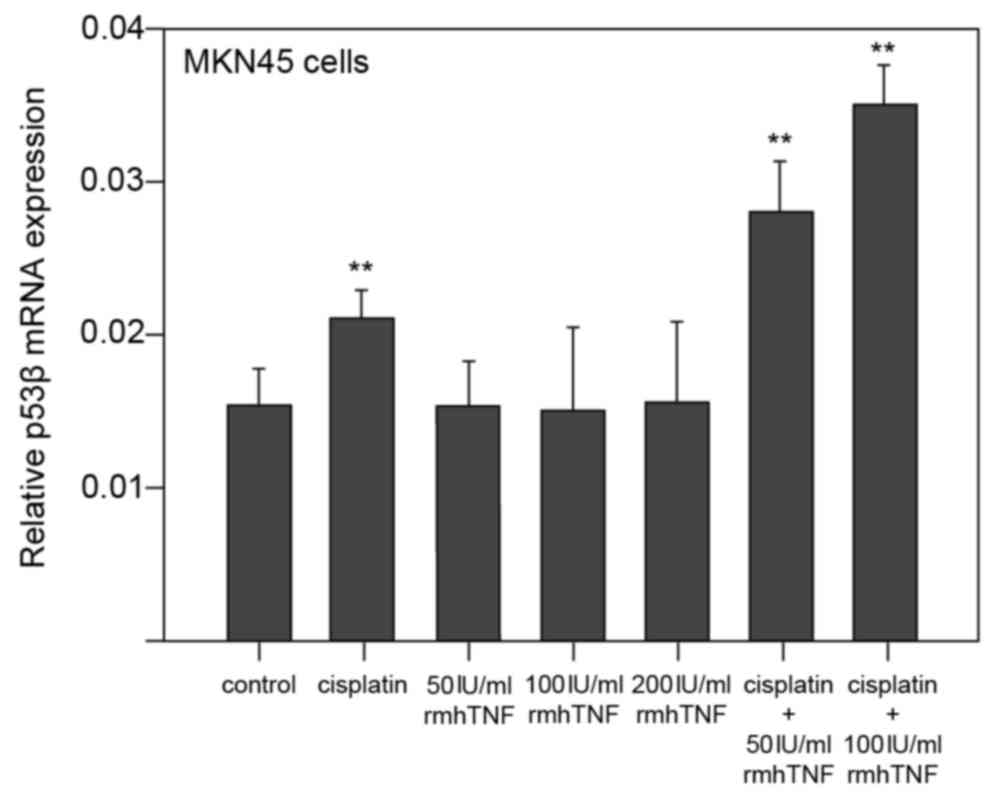
In MKN45 cells, p53β mRNA expression levels were significantly
increased by cisplatin alone compared with untreated cells
(P<0.01; Figs. 2A and 3). Treatment of MKN45 cells with rmhTNF
alone had no effect on p53β mRNA expression compared with untreated
cells (Figs. 2A and 3). However, when cisplatin was used in
combination with rmhTNF, p53β mRNA expression levels were further
increased compared with cells treated with cisplatin alone
(P<0.01; Figs. 2A and 3), suggesting again that rmhTNF and
cisplatin act synergistically in MKN45 GC cells.
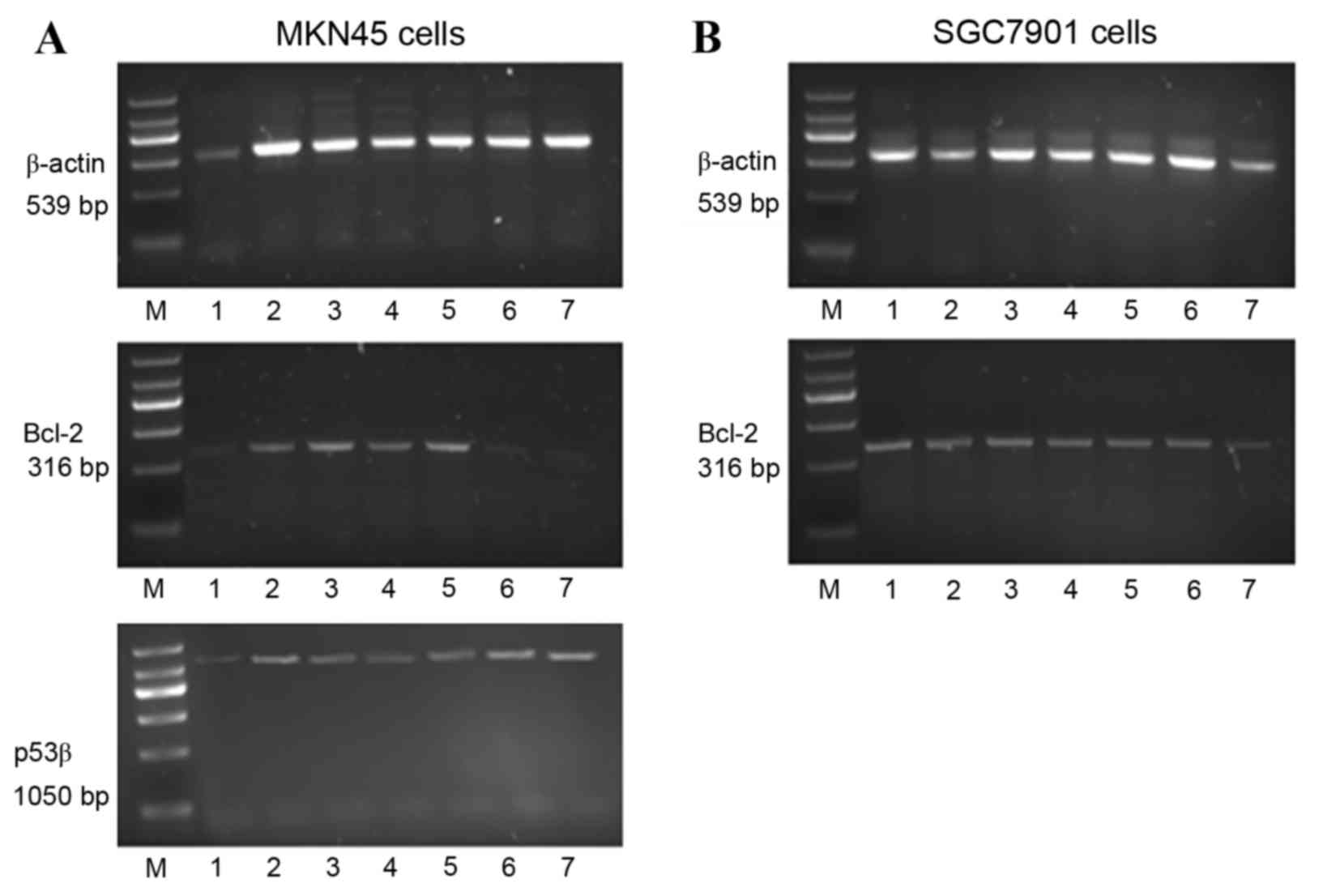 | Figure 2.Analysis of β-actin, bcl-2 and p53β
mRNA expression in (A) MKN45 and (B) SGC7901 cells. Cells were
either untreated (control) or treated with cisplatin and/or rmhTNF
for 24 h. Reverse transcription was performed on extracted RNA with
resulting cDNA amplified by polymerase chain reaction. The
resulting amplicons were visualised by agarose gel electrophoresis.
Lane M, DNA marker; lane 1, control; lane 2, cisplatin 4 µg/ml;
lane 3, rmhTNF 50 IU/ml; lane 4, rmhTNF 100 IU/ml; lane 5, rmhTNF
200 IU/ml; lane 6, rmhTNF 50 IU/ml plus cisplatin 4 µg/ml; lane 7,
rmhTNF 100 IU/ml plus cisplatin 4 µg/ml; Bcl-2, bcl-2 apoptosis
regulator; p53β, tumour protein 53 isoform β; rmhTNF, recombinant
mutated human tumour necrosis factor. |
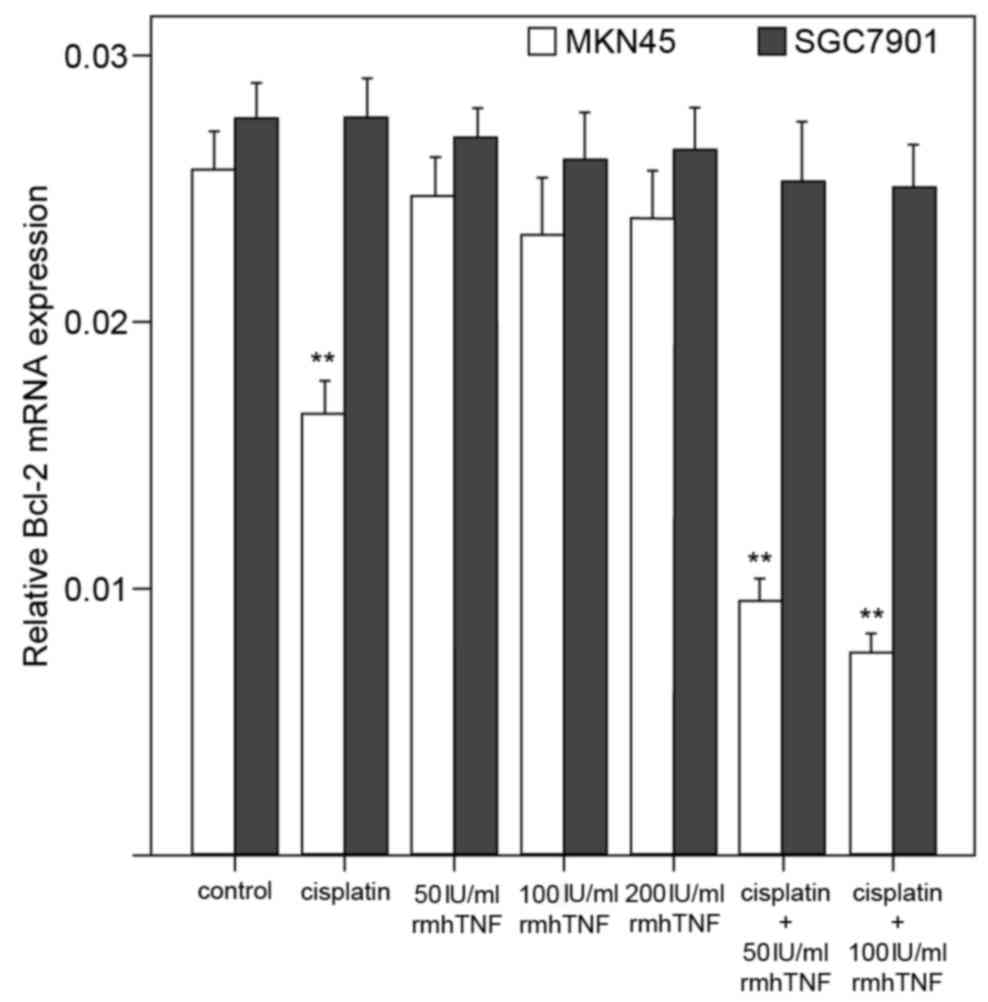
In MKN45 cells, bcl-2 mRNA expression levels were
significantly downregulated by cisplatin alone, or by combined
cisplatin and rmhTNF treatment, compared with untreated cells
(P<0.01; Figs. 2A and 4). However, no significant effect on
bcl-2 mRNA expression levels was detected with rmhTNF treatment
alone in MKN45 cells, compared with untreated cells (P>0.05;
Figs. 2A and 4). In the mutant p53-expressing SGC7901
cells, bcl-2 mRNA levels were not significantly altered in either
of the various treatment groups (P>0.05; Figs. 2B and 4).
Correlation between p53β and bcl-2
mRNA expression levels in MKN45 cells
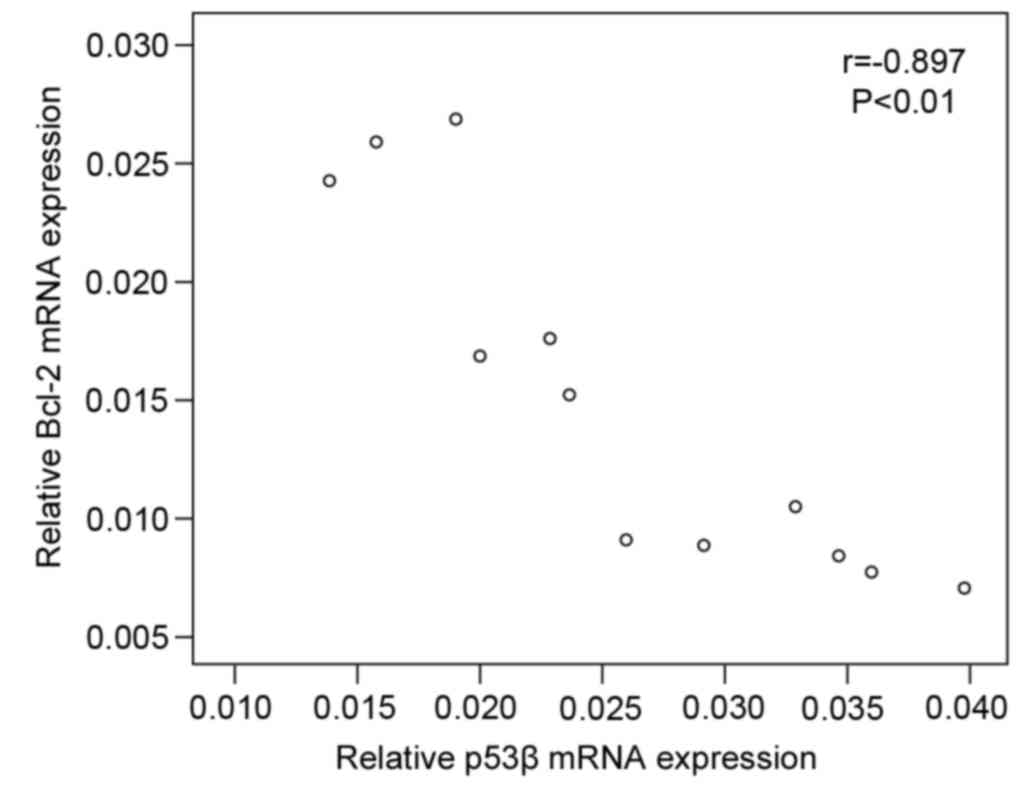
Correlation analysis was performed to assess whether
bcl-2 and p53β mRNA expression are associated with each other and
with the growth inhibition phenotype. The results indicated that
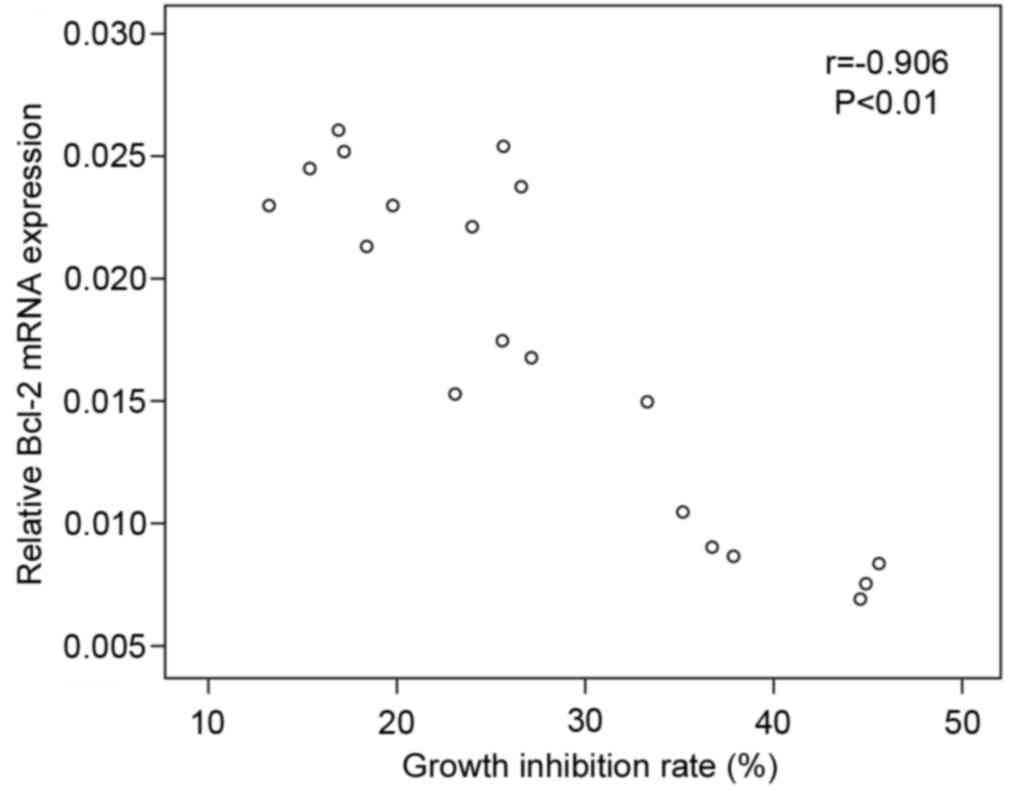
bcl-2 mRNA expression in MKN45 cells was negatively correlated with
mRNA expression of p53β (r=−0.897; P<0.01; Fig. 5), and negatively correlated with
the cell growth inhibition rate (r=−0.906; P<0.01; Fig. 6).
Discussion
Novel therapeutic targets and alternative therapies
are urgently required to improve the prognosis of invasive GC. For
more effective therapies, a prerequisite may be that the target of
choice is commonly involved in GC. The p53 protein has suppressive
effects in tumours, and p53 gene alterations are widespread in
cancer (7,8,20–22).
Although mutated forms of p53 are common in GC tissues, their
pharmacological significance remains unclear. Previous results
indicated that p53 isoforms and infection with H. pylori
have a strong association with the development of GC (13). In a previous small-scale study
(23), p53β downregulation and
Δ133p53 upregulation was associated with superficial gastritis,
atrophic gastritis, paracancerous areas, and eventually invasive
GC. These findings indicated that p53 isoforms are involved in
gastric carcinogenesis and could be potential targets for GC
therapies.
The different p53 isoforms exhibit varying levels of
prognostic significance in various cancer types, including breast
(24,25) and ovarian (26–28)
cancer. It remains unknown whether any of these isoforms could be
used as actual targets for therapeutic development. The present
study was designed to investigate the role of p53β in two GC cell
lines with differing p53 status, that were treated with cisplatin
and/or rmhTNF. The MKN45 GC cell line expresses wild-type p53
(29), while the SGC7901 cell line
expresses a mutated form of p53 (GAG→GCG in exon six, corresponding
to Glu→Ala in codon 204; (30).
The growth of MKN45 cells was inhibited by rmhTNF alone, while the
growth of SGC7901 cells was unaffected by rmhTNF. The inhibitory
effect of cisplatin on the growth of MKN45 cells was enhanced by
rmhTNF; however, this was not observed in SGC7901 cells. RT-PCR
analysis revealed that cisplatin alone, but not rmhTNF, resulted in
significant upregulation of p53β and downregulation of bcl-2 mRNA
expression in MKN45 cells. The effect of cisplatin on p53β and
bcl-2 mRNA expression was significantly enhanced by rmhTNF.
Taken together, the present study indicated that
p53β serves a role in the inhibitory effects exerted by cisplatin
on MKN45 GC cells, suggesting that p53β is a key target of
cisplatin. Members of the bcl-2 family, which are downstream of p53
signalling, exhibit pro- or anti-apoptotic activity. The final
action of bcl-2 family members is determined by the ratio of these
mutually antagonistic members (31,32).
It is currently unclear how p53β regulates expression of bcl-2 and
other downstream targets of p53. One possibility is that tetramers
with wild-type p53 are formed (33,34).
In wild-type p53-negative H1299 cells, p53β is important in
sensitizing these cells to chemotherapy (35). The precise function of p53β
requires further clarification; however, the therapeutic
significance of this p53 isoform is evident by its negative
correlation to breast tumour size, and its positive association
with disease-free survival periods (36,37).
In the present study, bcl-2 expression was negatively correlated
with p53β expression in MKN45 cells, which contain wild-type p53.
This was not observed in SGC7901 cells, which contain a mutated
form of p53, thereby indicating a p53-dependent mechanism for
cisplatin.
The present study indicated that rmhTNF is not able
to exert its effects on the p53β-bcl-2 pathway directly, but only
when in combination with cisplatin. rmhTNF may function as an
enhancer, improving the effects of cisplatin on the inhibition of
cellular growth, upregulation of p53β, and downregulation of bcl-2,
through mechanisms that are yet to be elucidated. Previous findings
revealed that Δ133p53 is involved in the progression from chronic
gastric inflammation to carcinoma (15,38–40).
An association between Δ133p53, rmhTNF, and treatment of gastric
carcinoma is evident, but further investigations are required to
clarify this. In addition, further work is required to screen for
targets of rmhTNF and to determine whether Δ133p53 is a target of
rmhTNF. Determining the genotype or phenotype that is most closely
associated with rmhTNF may improve drug regimens against GC.
In summary, the present study indicated that p53β is
involved in the cisplatin-mediated growth inhibition of MKN45 GC
cells. The effects of cisplatin on these cells were enhanced when
combined with rmhTNF. The mechanism of action for the synergistic
effect of cisplatin and rmhTNF remains unknown; however, the
present study indicated that p53β may be a critical target in the
pharmacology of GC.
Acknowledgements
The present study was supported by Shandong
Provincial Award Foundation for Youth and Middle-aged Scientist
(grant no. BS2010SW034).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schirren R, Reim D and Novotny AR:
Adjuvant and/or neoadjuvant therapy for gastric cancer cancer? A
perspective review. Ther Adv Med Oncol. 7:39–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito S, Oki E, Nakashima Y, Ando K, Hiyoshi
Y, Ohgaki K, Saeki H, Morita M, Sakaguchi Y and Maehara Y: Clinical
significance of adjuvant surgery following chemotherapy for
patients with initially unresectable stage IV gastric cancer.
Anticancer Res. 35:401–406. 2015.PubMed/NCBI
|
|
5
|
Lane DP and Crawford LV: T antigen is
bound to a host protein in SV40-transformed cells. Nature.
278:261–263. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stegh AH: Targeting the p53 signaling
pathway in cancer therapy-the promises, challenges and perils.
Expert Opin Ther Targets. 16:67–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Busuttil RA, Zapparoli GV, Haupt S,
Fennell C, Wong SQ, Pang JM, Takeno EA, Mitchell C, Di Costanzo N,
Fox S, et al: Role of p53 in the progression of gastric cancer.
Oncotarget. 5:12016–12026. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshimura A, Sugihara H, Ling ZQ, Peng DF,
Mukaisho K, Fujiyama Y and Hattori T: How wild-type TP53 is
inactivated in undifferentiated-type gastric carcinomas: Analyses
of intratumoral heterogeneity in deletion and mutation of TP53.
Pathobiology. 73:40–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan WM and Poon RY: The p53 Isoform
Deltap53 lacks intrinsic transcriptional activity and reveals the
critical role of nuclear import in dominant-negative activity.
Cancer Res. 67:1959–1969. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Courtois S, Verhaegh G, North S, Luciani
MG, Lassus P, Hibner U, Oren M and Hainaut P: DeltaN-p53, a natural
isoform of p53 lacking the first transactivation domain,
counteracts growth suppression by wild-type p53. Oncogene.
21:6722–6728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marcel V, Perrier S, Aoubala M, Ageorges
S, Groves MJ, Diot A, Fernandes K, Tauro S and Bourdon JC: Δ160p53
is a novel N-terminal p53 isoform encoded by Δ133p53 transcript.
FEBS Lett. 584:4463–4468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chambers SK and Martinez JD: The
significance of p53 isoform expression in serous ovarian cancer.
Future Oncol. 8:683–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Philipova T, Baryawno N, Hartmann W,
Pietsch T, Druid H, Johnsen JI and Ekström TJ: Differential forms
of p53 in medulloblastoma primary tumors, cell lines and
xenografts. Int J Oncol. 38:843–849. 2011.PubMed/NCBI
|
|
14
|
Goldschneider D, Horvilleur E, Plassa LF,
Guillaud-Bataille M, Million K, Wittmer-Dupret E, Danglot G, de Thé
H, Bénard J, May E and Douc-Rasy S: Expression of C-terminal
deleted p53 isoforms in neuroblastoma. Nucleic Acids Res.
34:5603–5612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei J, Noto J, Zaika E, Romero-Gallo J,
Correa P, El-Rifai W, Peek RM and Zaika A: Pathogenic bacterium
Helicobacter pylori alters the expression profile of p53 protein
isoformsand p53 response to cellular stresses. Proc Natl Acad Sci
USA. 109:pp. E2543–E2550. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei J, O'Brien D, Vilgelm A, Piazuelo MB,
Correa P, Washington MK, El-Rifai W, Peek RM and Zaika A:
Interaction of Helicobacter pylori with gastric epithelial cells is
mediated by the p53 protein family. Gastroenterology.
134:1412–1423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roberts NJ, Zhou S, Diaz LA Jr and
Holdhoff M: Systemic use of tumor necrosis factor alpha as an
anticancer agent. Oncotarget. 2:739–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang C, Niu J, Li M, Teng Y, Wang H and
Zhang Y: Tumor vasculature-targeted recombinant mutated human TNF-α
enhanced the antitumor activity of doxorubicin by increasing tumor
vessel permeability in mouse xenograft models. PLoS One.
9:e870362014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Xu T, Zhang Z, Xue X, Zhang C, Qin
X, Li W, Hao Q, Zhang W and Zhang Y: Phase II multicenter,
randomized, double-blind study of recombinant mutated human tumor
necrosis factor-α in combination with chemotherapies in cancer
patients. Cancer Sci. 103:288–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kafshdooz T, Tabrizi AD, Ardabili SM
Mohaddes, Kafshdooz L, Ghojazadeh M, Gharesouran J, Abdii A and
Alizadeh H: Polymorphism of p53 gene codon 72 in endometrial
cancer: Correlation with tumor grade and histological type. Asian
Pac J Cancer Prev. 15:9603–9606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J, Dong H, Shen W, He S, Li H, Lu Y, Wu
ZS and Jia L: New molecular beacon for p53 gene point mutation and
significant potential in serving as the polymerization primer.
Biosens Bioelectron. 66:504–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nason KS: Predicting response to
neoadjuvant therapy in esophageal cancer with p53 genotyping: A
fortune-teller's crystal ball or a viable prognostic tool? J Thorac
Cardiovasc Surg. 148:2286–2287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji W, Zhang N, Zhang H, Ma J, Zhong H,
Jiao J and Gao Z: Expression of p53β and Δ133p53 isoforms in
different gastric tissues. Int J Clin Exp Pathol. 8:10468–10474.
2015.PubMed/NCBI
|
|
24
|
Marcel V, Fernandes K, Terrier O, Lane DP
and Bourdon JC: Modulation of p53β and p53γ expression by
regulating the alternative splicing of TP53 gene modifies cellular
response. Cell Death Differ. 21:1377–1387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Avery-Kiejda KA, Morten B, Wong-Brown MW,
Mathe A and Scott RJ: The relative mRNA expression of p53 isoforms
in breast cancer is associated with clinical features and outcome.
Carcinogenesis. 35:586–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hofstetter G, Berger A, Fiegl H, Slade N,
Zorić A, Holzer B, Schuster E, Mobus VJ, Reimer D, Daxenbichler G,
et al: Alternative splicing of p53 and p73: The novel p53 splice
variant p53delta is an independent prognostic marker in ovarian
cancer. Oncogene. 29:1997–2004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hofstetter G, Berger A, Berger R, Zorić A,
Braicu EI, Reimer D, Fiegl H, Marth C, Zeimet AG, Ulmer H, et al:
The N-terminally truncated p53 isoform Δ40p53 influences prognosis
in mucinous ovarian cancer. Int J Gynecol Cancer. 22:372–379. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hofstetter G, Berger A, Schuster E, Wolf
A, Hager G, Vergote I, Cadron I, Sehouli J, Braicu EI, Mahner S, et
al: Δ133p53 is an independent prognostic marker in p53 mutant
advanced serous ovarian cancer. Br J Cancer. 105:1593–1599. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berglind H, Pawitan Y, Kato S, Ishioka C
and Soussi T: Analysis of p53 mutation status in human cancer cell
lines: A paradigm for cell line cross-contamination. Cancer Biol
Ther. 7:699–708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji W, Ma J, Zhang H, Zhong H, Li L, Ding
N, Jiao J and Gao Z: Role of p53β in the inhibition of
proliferation of gastric cancer cells expressing wild-type or
mutated p53. Mol Med Rep. 12:691–695. 2015.PubMed/NCBI
|
|
31
|
Zheng S, Zhao M, Ren Y, Wu Y and Yang J:
Sesamin suppresses STZ induced INS-1 cell apoptosis through
inhibition of NF-κB activation and regulation of Bcl-2 family
protein expression. Eur J Pharmacol. 750:52–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cekanova M, Fernando RI, Siriwardhana N,
Sukhthankar M, De la Parra C, Woraratphoka J, Malone C, Ström A,
Baek SJ, Wade PA, et al: BCL-2 family protein, BAD is
down-regulated in breast cancer and inhibits cell invasion. Exp
Cell Res. 331:1–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bourdon JC, Fernandes K, Murray-Zmijewski
F, Liu G, Diot A, Xirodimas DP, Saville MK and Lane DP: p53
isoforms can regulate p53 transcriptional activity. Genes Dev.
19:2122–2137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khoury MP and Bourdon JC: p53 isoforms: An
intracellular microprocessor? Genes Cancer. 2:453–465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Silden E, Hjelle SM, Wergeland L, Sulen A,
Andresen V, Bourdon JC, Micklem DR, McCormack E and Gjertsen BT:
Expression of TP53 isoforms p53β or p53γ enhances chemosensitivity
in TP53 (null) cell lines. PLoS One. 8:e562762013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okumura N, Yoshida H, Kitagishi Y,
Nishimura Y and Matsuda S: Alternative splicings on p53, BRCA1 and
PTEN genes involved in breast cancer. Biochem Biophys Res Commun.
413:395–399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marcel V, Dichtel-Danjoy ML, Sagne C,
Hafsi H, Ma D, Ortiz-Cuaran S, Olivier M, Hall J, Mollereau B,
Hainaut P and Bourdon JC: Biological functions of p53 isoforms
through evolution: Lessons from animal and cellular models. Cell
Death Differ. 18:1815–1824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng P, Shi RH, Zhang HJ, Yu LZ, Zhang GX
and Hao B: Effects of tumor necrosis factor-alpha inducing
protein-alpha secreted by Helicobacter pylori on human gastric
epithelial cells. Zhonghua Yi Xue Za Zhi. 88:1528–1532. 2008.(In
Chinese). PubMed/NCBI
|
|
39
|
Fukui T, Matsui K, Kato H, Takao H,
Sugiyama Y, Kunieda K and Saji S: Significance of apoptosis induced
by tumor necrosis factor-alpha and/or interferon-gamma against
human gastric cancer cell lines and the role of the p53 gene. Surg
Today. 33:847–853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimoyama S, Mochizuki Y, Kusada O and
Kaminishi M: Supra-additive antitumor activity of 5FU with tumor
necrosis factor-related apoptosis-inducing ligand on gastric and
colon cancers in vitro. Int J Oncol. 21:643–648. 2002.PubMed/NCBI
|