Introduction
Mechanical ventilation may induce and exacerbate
lung injury, in contrast to its lifesaving effects. It has
previously been demonstrated that biotrauma is an underlying
mechanism of lung injury, important factors of which include
inflammation and the innate immune response (1). Biological markers of
ventilator-associated lung injury (VALI) are important for early
diagnosis and prognosis, and provide novel insights into the
mechanisms of VALI. Currently identified biomarkers of VALI are key
elements of the inflammatory and immune response pathways; however,
the precise function of each mediator remains to be elucidated. To
the best of our knowledge, no studies have validated the
sensitivity or specificity of any biological markers that may aid
early diagnosis or treatment of VALI (2,3).
Further studies are required in order to identify novel, sensitive
and specific biological markers.
Neutrophils (polymorphonuclear leukocytes, PMNs)
have been demonstrated to be important in the development and
progression of VALI and may lie at the center of a positive
feedback loop that results in lung injury (4). Therefore, PMNs or key mediators of
the PMN regulatory signaling pathway may be molecular candidates
for the occurrence of VALI (5).
Neutrophil gelatinase-associated lipocalin (NGAL), a 25-kDa protein
of the lipocalin family, was originally isolated from specific
neutrophil granules (6,7). In addition to excretion by activated
neutrophils, NGAL may be released in small quantities by epithelial
and kidney tubular cells, and during inflammation or injury.
Elevated NGAL is an early blood-based marker of acute kidney injury
and is detectable within 2–12 h following an ischemic/toxic insult
in patients with cardiovascular diseases (8,9).
The function of NGAL in the regulation of alveolar
epithelial cells and in VALI remains to be elucidated. The
expression level of NGAL, which is extensively involved in
inflammation and the immune response, is an indicator of PMN
activation. As an acute and secretory phase protein, NGAL is easily
detected in serum and bronchoalveolar lavage (BAL) fluid (10,11).
The aim of the present study was to demonstrate the use of NGAL as
a novel diagnostic marker for VALI. The results indicated that NGAL
levels increase in lung tissue, BAL fluid and serum during VALI in
mice. Therefore, NGAL may represent a novel diagnostic marker for
the early prediction of patients at high risk of VALI.
Materials and methods
Animals and experimental protocol
Male C57BL/6 mice, (20±2 g, 6–8 weeks and free of
murine specific pathogens), were obtained from the Experimental
Animal Center of Guangdong Province (Guangzhou, China). The mice
were housed in a specific pathogen free animal house at a
temperature of 22±2°C and a relative humidity of 55±5% with a 12:12
h light/dark cycle. Throughout the experimental process, the mice
were housed in a laminar flow cabinet and maintained on standard
laboratory food ad libitum. The study was approved by the
ethics committee of Guangzhou University of Traditional Chinese
Medicine.
Experiments were conducted on 42 wild-type mice,
which were divided into 7 groups. The protocol for the control
group was spontaneous breathing for 2 h. The protocol for the high
peak inflation pressure (high-PIP) group was breathing under the
condition of 50 cm H2O of PIP and 2.5 cm H2O
positive end-expiratory pressure (PEEP) at 17 breaths/min for 2 h
with a tidal volume of 1 ml. The protocol for low peak inflation
pressure (low-PIP) group was breathing under the condition of 15 cm
H2O of PIP and 0 cm H2O PEEP for 2 h at 120
breaths/min with a tidal volume of 0.29 ml. The protocol for
high-volume for 2 h (HV-2) group was breathing under the condition
of 30 ml/kg, 0 cm H2O PEEP for 2 h at 65 breaths/min.
The protocol for low-volume for 2 h (LV-2) group was breathing
under the condition of 6 ml/kg, 5 cm H2O PEEP for 2 h at
135 breaths/min. The protocol for high-volume for 4 h (HV-4) group
was breathing under the condition of 30 ml/kg, 0 cm H2O
PEEP for 4 h at 65 breaths/min. The protocol for low-volume for 4 h
(LV-4) group was breathing under the condition of 6 ml/kg, 5 cm
H2O PEEP for 4 h at 135 breaths/min. The experimental
protocols for each group are detailed in Table I.
 | Table I.Experimental protocol for each
group. |
Table I.
Experimental protocol for each
group.
| Main group | Subgroup | Protocols |
|---|
| Control | Control | Spontaneous breathing
for 2 h |
| Ventilation for 2 h
group | High-PIP (n=6) | 50 cm H2O
of PIP and 2.5 cm H2O PEEP at 17 breaths/min for 2 h
with tidal volume of 1 ml |
|
| Low-PIP (n=6) | 15 cm H2O
of PIP and 0 cm H2O PEEP for 2 h at 120 breaths/min with
a tidal volume of 0.29 ml |
|
| HV2 (n=6) | 30 ml/kg, 0 cm
H2O PEEP for 2 h at 65 breaths/min |
|
| LV2 (n=6) | 6 ml/kg, 5 cm
H2O PEEP for 2 h at 135 breaths/min |
| Ventilation for 4 h
group | HV4 (n=6) | 30 ml/kg, 0 cm
H2O PEEP for 4 h at 65 breaths/min |
|
| LV4 (n=6) | 6 ml/kg, 5 cm
H2O PEEP for 4 h at 135 breaths/min |
Collection of BAL fluid, serum or lung
tissue
Following treatment, animals were sacrificed via
administration of 50 mg/kg sodium pentobarbital (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Peripheral blood was extracted by
removing the eyeball. Following overnight storage at 4°C, serum was
collected via centrifugation at 1,000 × g for 30 min at 4°C.
Immediately post-mortem, the heart-lung block was dissected and BAL
fluid was obtained using the method previously described (12,13).
BAL fluid was centrifuged at 1,000 × g for 10 min at 4°C and the
supernatant was collected for western blotting. For reverse
transcription-polymerase chain reaction (RT-qPCR) and western
blotting of lung samples, the right lung was cut into samples (~1×1
cm) and flash-frozen in liquid nitrogen. For immunohistochemical
and hematoxylin and eosin (H&E) staining, the left lung was
fixed as previously described (14). The vertical axis of each left lung
was identified and the lung was cut perpendicular to this axis into
4-mm thick slices.
RT-qPCR
Total RNA was extracted from frozen lung samples
using TRIzol reagent (Ambion; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocols. RNA
was quantified by measuring absorption at wavelengths of 260 and
280 nm using the NanoDrop system (NanoDrop; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). and stored at −70°C until
required. A total of 1 µg total RNA was reverse-transcribed to cDNA
using the AffinityScript QPCR cDNA Synthesis kit (Agilent
Technologies, Inc., Santa Clara, CA, USA), and RT-qPCR was
performed using the Brilliant II SYBR-Green QPCR Master Mix kit
(Agilent Technologies, Inc.) under the conditions as follows:
preheating at 95°C for 10 min, followed by 40 cycles of 95°C for 10
sec, 60°C for 20 sec and 72°C for 10 sec. The primers were
purchased from Sangon Biotech Co., Ltd. (Shanghai, China) and the
sequences were as follows: Forward, 5′-CCCTGAACTGAAGGAACG-3′ and
reverse, 5′-TTGGTATGGTGGCTGGTG-3′ for NGAL, and forward,
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and reverse,
5′-ATGGAGCCACCGATCCACA-3′ for β-actin. PCR reactions were performed
on an iQ5 Multicolor Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Gene expression was
measured in triplicate, quantified using the 2−ΔΔCq
method (15) and normalized to the
β-actin internal control.
Western blotting
Lung tissue protein was extracted using ice-cold
RIPA buffer (Beyotime Institute of Biotechnology, Nantong, China)
according to the manufacturer's protocols. Protein concentration
was determined using the Bicinchoninic Acid Protein Assay kit
(Thermo Fisher Scientific, Inc.). Total proteins (30 µg) were
separated by 8–12% SDS-PAGE at 120 V, and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA) at 250 mA for 90 min via wet transfer. Following blocking
non-specific binding sites with 5% non-fat milk in Tris-buffered
saline (TBS) with Tween-20 (20 mM/l TBS pH 7.5, 500 mM/l NaCl and
0.1% Tween-20) for 1 h, the blots were probed with mouse monoclonal
anti-β-actin (sc-47778; 1:500; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and anti-NGAL (MAB1857; 1:1,000; R&D Systems,
Inc., Minneapolis, MN, USA) primary antibodies at 37°C for 1 h.
Bands were detected using an anti-mouse horseradish
peroxidase-conjugated secondary antibody (7076; 1:2,000; Cell
Signaling Technology, Inc.) at 37°C for 40 min followed by
treatment with SuperSignal® West Pico Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc.) and X-ray film exposure
(Kodak, Rochester, NY, USA). The film was scanned, and
densitometric analysis was performed using QuantityOne software
version 4.6.2 (Bio-Rad Laboratories, Inc.). Squares of equal size
were drawn around each band to measure the density, and the value
was adjusted based on the density of the background near that band.
Densitometric analysis was repeated three times. Results were
expressed as a ratio of the target protein compared with the
reference protein. The ratio of the target protein from the control
group was arbitrarily set to 1.
H&E staining
Lung samples were fixed in 10% formalin solution at
4°C for 24 h, and dehydrated. The samples were wax embedded and cut
into 4–6 µm sections on a microtome. The sections were flattened,
mounted and heated on blank glass slides. Histological evaluations
were performed by H&E staining and pathological examination
using a CTR 6500 microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Immunohistochemical staining of
NGAL
Following deparaffinization and dehydration, the
sections were immersed in 10 mM sodium citrate buffer (pH 6.0) with
0.05% Tween-20, for 5 min at 58°C, and subsequently treated with
0.25% Triton X-100 for 5 min. Following blocking with 5% bovine
serum albumin (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) at room temperature for 20 min, the slides were
incubated at 4°C with a rat monoclonal anti-NGAL antibody (MAB1857;
1:100; R&D Systems, Inc.) overnight. Finally, slides were
incubated with a rabbit anti-rat biotin-conjugated IgG H&L
secondary antibody (ab6733; 1:1,000; Abcam, Cambridge, MA, USA) at
room temperature for 1 h, and detected using an avidin-biotin
complex system (Vectastain ABC-kit; Vector Laboratories, Inc.,
Burlingame, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). Data are
expressed as the mean ± standard deviation. Statistical comparisons
were performed using one-way analysis of variance, followed by
Scheffe's test. Statistical differences between two groups were
determined using the unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Different modalities of mechanical
ventilation affect the extent of lung injury
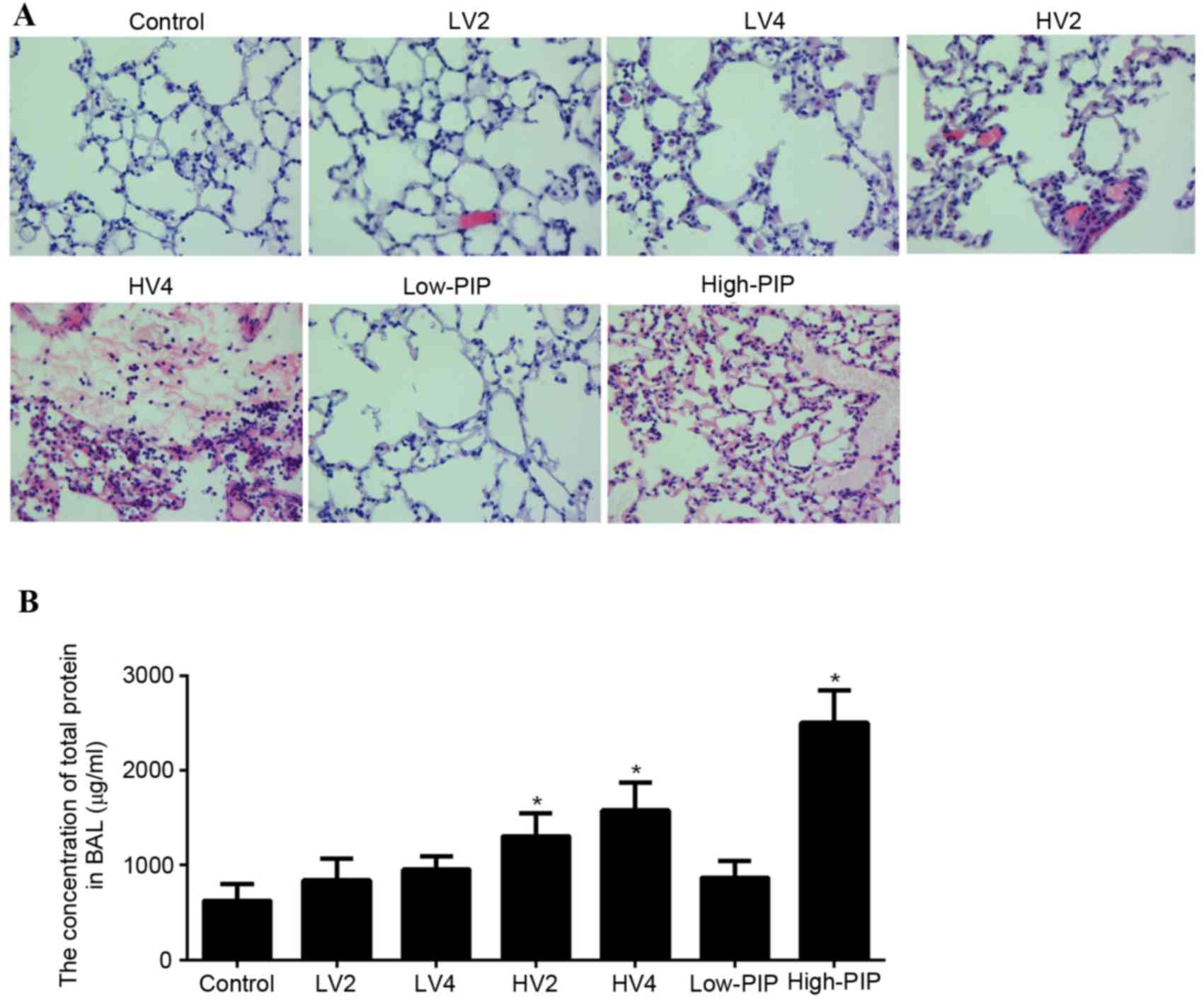
Following the application of different modalities of
mechanical ventilation, lung tissue was collected and evaluated via
H&E staining, as presented in Fig.
1A. The control, LV2, LV4 and low-PIP groups exhibited a normal
histology. HV2, HV4 and high-PIP groups presented an abnormal
histology compared with the control group. These abnormalities
included alveolar septal thickening indicative of edema formation,
mononuclear cell infiltration of the alveolar walls, hemorrhage,
fibrin exudation and intra-alveolar erythrocyte infiltration.
To validate the VALI model in mice, total protein
from BAL fluid was detected. As presented in Fig. 1B, total protein concentration in
BAL fluid of LV2, LV4 and low-PIP groups did not differ from the
control group. Total protein concentration in BAL fluid of HV2, HV4
and high-PIP groups was greater compared with the control group.
Therefore, these observations suggested that LV2, LV4 and low-PIP
modalities of mechanical ventilation are safe, whereas HV2, HV4 and
high-PIP may result in lung injury.
NGAL expression increases upon
mechanical ventilation
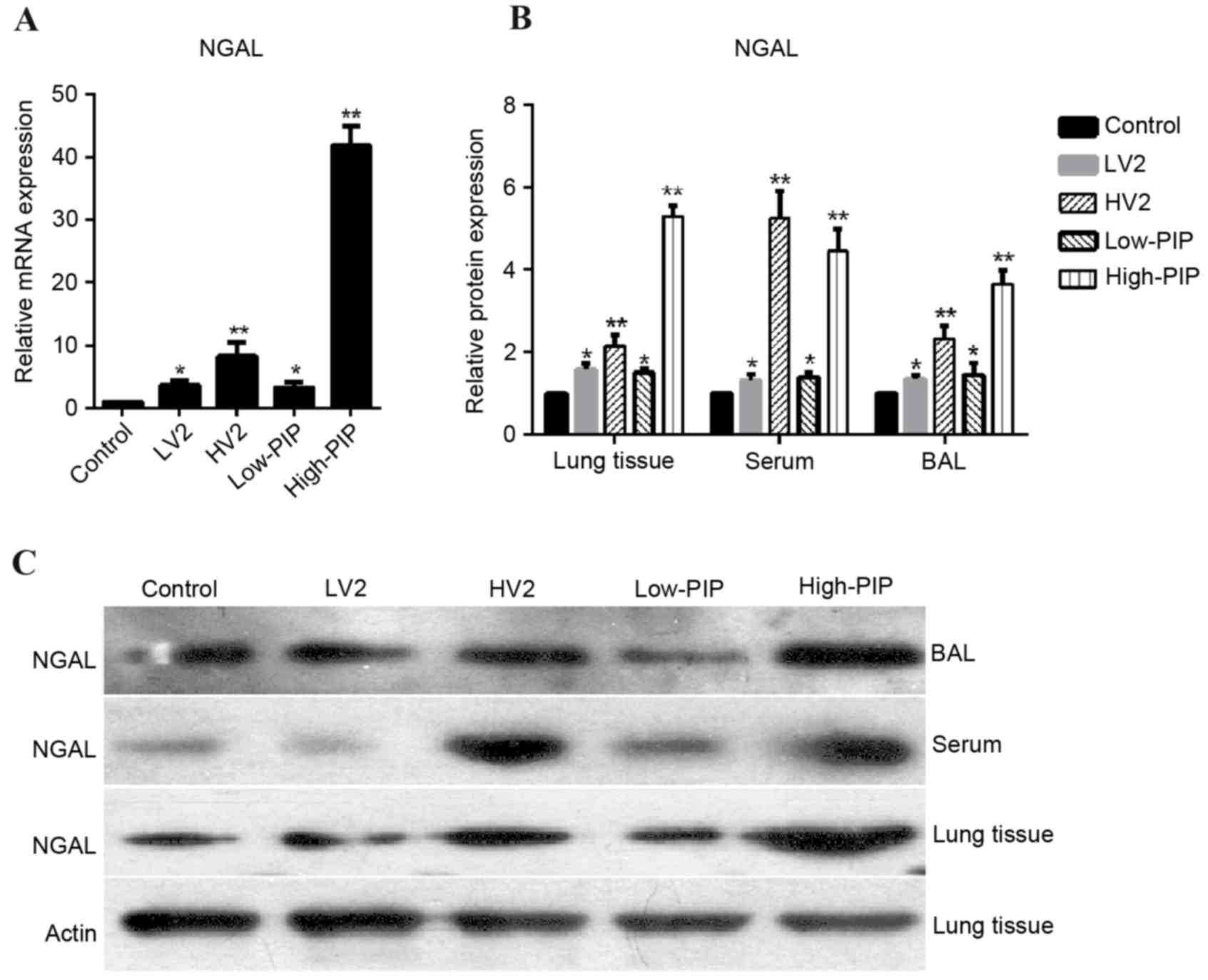
To investigate NGAL expression in a mouse model of
VALI, lung tissues, BAL fluid and serum were collected from
control, LV2, HV2 low-PIP and high-PIP groups. RT-qPCR demonstrated
that NGAL mRNA expression in lung tissue was increased in all
groups compared with the control group, particularly the HV2 and
high-PIP groups (Fig. 2A). As
presented in Fig. 2B and C, NGAL
protein expression levels were increased in all groups compared
with the control group, particularly in the HV2 and high-PIP
groups.
NGAL expression increase is
time-dependent under high-volume mechanical ventilation
treatment
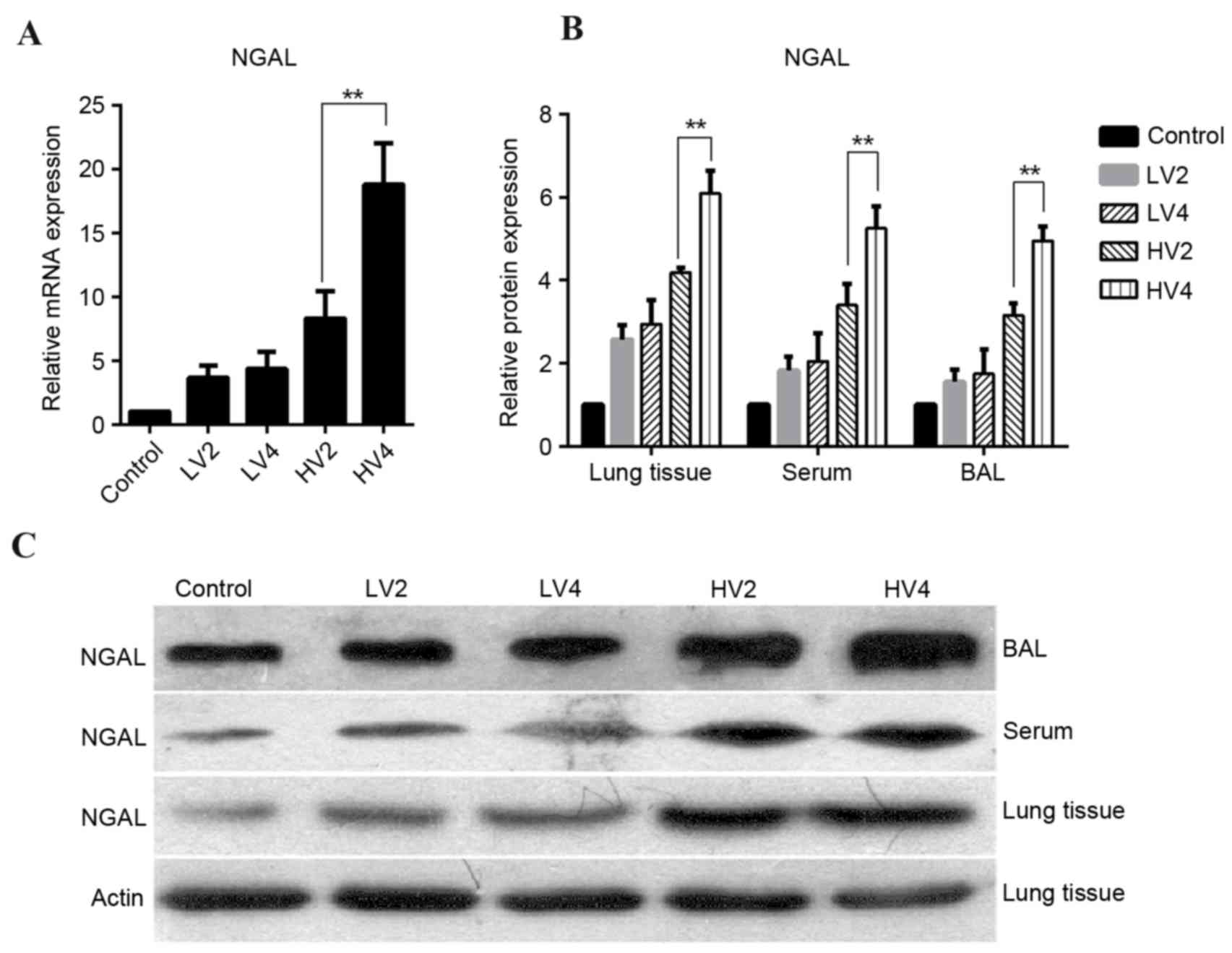
To investigate the expression of NGAL in a mouse
model of VALI, mice were subjected to low- or high-volume
mechanical ventilation for 2 h or 4 h. As presented in Fig. 3A, NGAL mRNA expression levels in
lung tissue demonstrated no difference between LV2 and LV4
exposure. However, a significant increase was present in HV4
compared with the HV2 group. Similarly, NGAL protein expression
levels in lung tissue did not differ between the LV2 and LV4
groups; however, they were markedly greater in the HV4 group
compared with the HV2 group (Fig. 3B
and C). Protein expression profiles of NGAL in BAL fluid and
serum were similar to those in lung tissue (Fig. 3B and C).
Localization of NGAL expression in
murine lungs
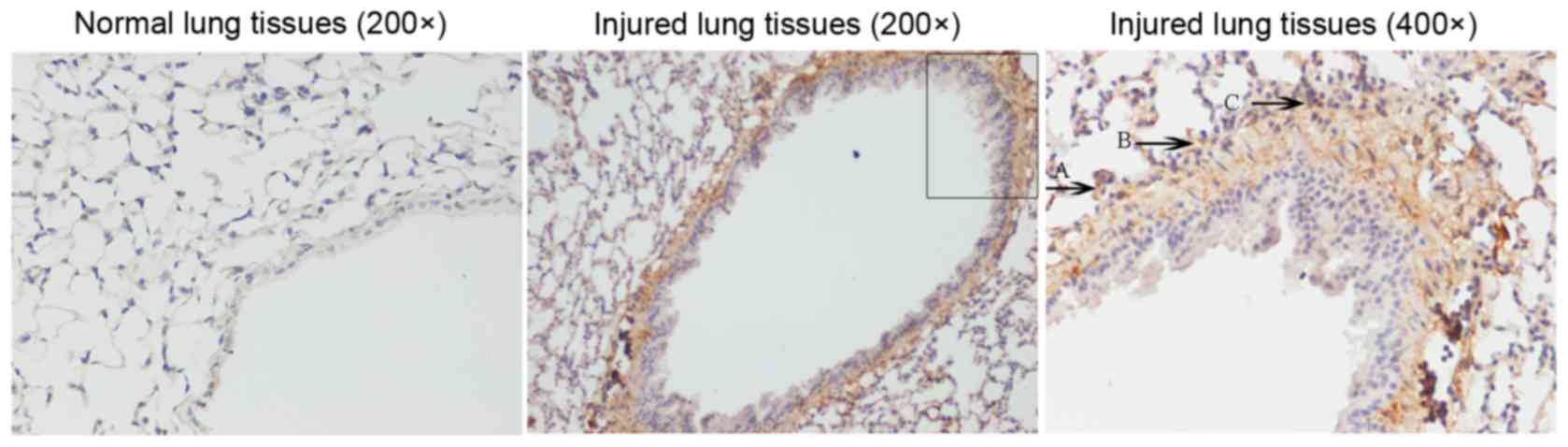
To evaluate the spatial localization of NGAL
expression, immunohistochemical staining of murine lung tissue
samples from injured and control mice was performed. As presented
in Fig. 4, strong murine NGAL
expression was detected in the lung tissue from injured mice. In
addition, NGAL expression was increased in lung vascular
endothelial cells and infiltrating neutrophils of the injured
lung.
Discussion
VALI has recently been of primary concern for
clinicians and researchers. The reduction in mortality associated
with low tidal volume ventilation in patients with acute lung
injury, and acute respiratory distress syndrome has resulted in an
increased research interest toward the underlying mechanism of VALI
(16). The roles of the innate
immune response and inflammation in the pathogenesis of VALI have
been extensively studied in recent years. It has previously been
suggested that inflammation may not be integral to the initiation
of VALI; however, prevalent data in the field suggests a major
pathogenetic role of inflammation and lung neutrophil recruitment.
Therefore, investigators have searched for biological markers of
VALI and therapeutic targets that may facilitate treatment.
NGAL of the lipocalin superfamily, was originally
isolated from specific neutrophil granules (6,7).
NGAL protein levels are typically very low in various biological
fluids (17). Previous studies
have demonstrated that NGAL expression is associated with acute
injury, particularly acute kidney injury. However, an association
between NGAL and acute lung injury has not yet been reported. The
present study characterized the expression profile of NGAL in mice
subjected to different mechanical ventilation protocols. NGAL mRNA
and protein expression levels in lung tissue increased under all
mechanical ventilation treatments; however, a significant increase
was observed following high-volume or high-PIP mechanical
ventilation. A similar NGAL increase was further detected in BAL
fluid and serum, and was notably increased following high-volume or
high-PIP mechanical ventilation. NGAL expression was time-dependent
under high-volume mechanical ventilation treatment.
Immunohistochemical localization studies revealed increased NGAL
expression in lung endothelium, alveolar epithelial cells, and
infiltrating neutrophils. NGAL is easily detected in serum and BAL
fluid (10,11). The results of the present study
verify the role of NGAL as a novel and potential biomarker for
VALI. A previous study revealed that plasma concentrations of NGAL
were increased in oleic acid-induced acute lung injury and a
conventional mechanical ventilation model in piglets (18). Serum NGAL levels were additionally
significantly increased in lung transplant patients (19).
In conclusion, the present study used an animal
model to identify NGAL as a potential novel biomarker for
mechanical-induced lung injury. Further studies are required to
define the functional role of NGAL and the pathophysiology of
altered NGAL expression in VALI.
References
|
1
|
Slutsky AS: Ventilator-induced lung
injury: From barotrauma to biotrauma. Respir Care. 50:646–659.
2005.PubMed/NCBI
|
|
2
|
Parsons PE, Eisner MD, Thompson BT,
Matthay MA, Ancukiewicz M, Bernard GR and Wheeler AP: NHLBI Acute
Respiratory Distress Syndrome Clinical Trials Network: Lower tidal
volume ventilation and plasma cytokine markers of inflammation in
patients with acute lung injury. Crit Care Med. 33:1–6. 230–232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frank JA, Parsons PE and Matthay MA:
Pathogenetic significance of biological markers of
ventilator-associated lung injury in experimental and clinical
studies. Chest. 130:1906–1914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee WL and Downey GP: Neutrophil
activation and acute lung injury. Curr Opin Crit Care. 7:1–7. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choudhury S, Wilson MR, Goddard ME, O'Dea
KP and Takata M: Mechanisms of early pulmonary neutrophil
sequestration in ventilator-induced lung injury in mice. Am J
Physiol Lung Cell Mol Physiol. 287:L902–L910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu SY, Carlson M, Engström A, Garcia R,
Peterson CG and Venge P: Purification and characterization of a
human neutrophil lipocalin (HNL) from the secondary granules of
human neutrophils. Scand J Clin Lab Invest. 54:365–376. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bolignano D, Donato V, Lacquaniti A, Fazio
MR, Bono C, Coppolino G and Buemi M: Neutrophil
gelatinase-associated lipocalin (NGAL) in human neoplasias: A new
protein enters the scene. Cancer Lett. 288:10–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Helanova K, Spinar J and Parenica J:
Diagnostic and prognostic utility of neutrophil
gelatinase-associated lipocalin (NGAL) in patients with
cardiovascular diseases-review. Kidney Blood Press Res. 39:623–629.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clerico A, Galli C, Fortunato A and Ronco
C: Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker
of acute kidney injury: A review of the laboratory characteristics
and clinical evidences. Clin Chem Lab Med. 50:1505–1517. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reghunathan R, Jayapal M, Hsu LY, Chng HH,
Tai D, Leung BP and Melendez AJ: Expression profile of immune
response genes in patients with severe acute respiratory syndrome.
BMC Immunol. 6:22005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gwira JA, Wei F, Ishibe S, Ueland JM,
Barasch J and Cantley LG: Expression of neutrophil
gelatinase-associated lipocalin regulates epithelial morphogenesis
in vitro. J BiolChem. 280:7875–7882. 2005.
|
|
12
|
O'Croinin DF, Nichol AD, Hopkins N, Boylan
J, O'Brien S, O'Connor C, Laffey JG and McLoughlin P: Sustained
hypercapnic acidosis during pulmonary infection increases bacterial
load and worsens lung injury. Crit Care Med. 36:2128–2135. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Croinin DF, Hopkins NO, Moore MM, Boylan
JF, McLoughlin P and Laffey JG: Hypercapnic acidosis does not
modulate the severity of bacterial pneumonia-induced lung injury.
Crit Care Med. 33:2606–2612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayes M, Curley GF, Masterson C, Devaney
J, O'Toole D and Laffey JG: Mesenchymal stromal cells are more
effective than the MSC secretome in diminishing injury and
enhancing recovery following ventilator-induced lung injury.
Intensive Care Med Exp. 3:292015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altemeier WA, Matute-Bello G, Gharib SA,
Glenny RW, Martin TR and Liles WC: Modulation of
lipopolysaccharide-induced gene transcription and promotion of lung
injury by mechanical ventilation. J Immunol. 175:3369–3376. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuwabara T, Mori K, Mukoyama M, Kasahara
M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, et
al: Urinary neutrophil gelatinase-associated lipocalin levels
reflect damage to glomeruli, proximal tubules, and distal nephrons.
Kidney Int. 75:285–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu AJ, Ling F, Li ZQ, Li XF, Liu YL, Du J
and Han L: Effect of oleic acid-induced acute lung injury and
conventional mechanical ventilation on renal function in piglets.
Chin Med J. 126:2530–2535. 2013.PubMed/NCBI
|
|
19
|
Szewczyk M, Wielkoszyński T, Zakliczyński
M and Zembala M: Plasma neutrophil gelatinase-associated lipocalin
(NGAL) correlations with cystatin c, serum creatinine, and
glomerular filtration rate in patients after heart and lung
transplantation. Transplant Proc. 41:3242–3243. 2009. View Article : Google Scholar : PubMed/NCBI
|