Introduction
Metabolic diseases (MDs), including obesity,
elevated triglyceride (TG) levels, reduced levels of high-density
lipoprotein cholesterol, elevated blood pressure and impaired
glucose metabolism, greatly increase the risk of atherosclerotic
cardiovascular disease, hyperlipidemia, hypertension and type 2
diabetes mellitus (1,2). Obesity due to adipocyte hypertrophy
leads to alterations in adipocytokine profiles involved in the
development of insulin resistance, and in the production of
signaling molecules, including peroxisome proliferator-activated
receptor (PPAR)γ, adipocyte protein 2 and leptin. Specifically,
PPARs, ligand-activated nuclear transcription factors, regulate
gene expression associated with adipocyte differentiation,
lipogenesis and glucose metabolism. Furthermore, PPARs are involved
in an array of MDs, including type 2 diabetes mellitus, obesity,
hyperlipidemia, atherosclerosis and cardiovascular disease
(3–5).
Blood stasis syndrome (BSS) is an important
pathological concept in Traditional Korean Medicine (TKM). BSS
refers to the pathological stagnation of blood circulation, delayed
blood flow, dysfunction of endothelial cells or metabolic disorder.
It was first recorded in Huangdi's Inner Classic (6). Numerous studies have reported that
BSS is associated with MDs, including atherosclerosis,
hypertension, coronary artery lesions, cardiac function, blood
lipid, diabetes mellitus and insulin resistance (7–9).
The properties of Do In Seung Gi-Tang (DISGT), a
traditional herbal formula used to treat BSS, have been recorded in
the Dongui Bogam (10). It is
primarily used in TKM for treating obesity and hypertension,
diabetes mellitus, inflammation, immunity and gynecological
diseases, including menstrual irregularity, by promoting blood
circulation (11–13).
Although clinical studies have assessed the symptom
relief effects of DISGT treatment, few studies have investigated
the underlying mechanism of DISGT in the pathology or biology of
adipocytes. As a result, this underlying mechanism remains unclear.
Therefore, the present study investigated the potential
anti-adipogenesis effect of DISGT on 3T3-L1 adipocyte
differentiation and regulation of protein expression associated
with lipid metabolism.
Materials and methods
Materials
The 3T3-L1 mouse fibroblast cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), penicillin-streptomycin (P&S), bovine calf serum (NCS)
and Dulbecco's phosphate-buffered saline (DPBS) were obtained from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Dimethyl
sulfoxide (DMSO), formaldehyde, dexamethasone (DEX),
3-isobutyl-1-methylisobutylxanthine (IBMX), insulin, triton X-100
and Oil Red O staining powder were obtained from Sigma-Aldrich;
Merck Millipore (Darmstadt, Germany). Cell Counting kit-8 (CCK-8)
was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). The EnzyChrom™ TG assay kit was purchased from BioAssay
Systems (Hayward, CA, USA) and the Mouse/Rat Leptin Quantakine
ELISA kit (cat. no. MOB00) was purchased from R&D Systems, Inc.
(Minneapolis, MI, USA). A MILLIPLEX® MAP Mouse Adipocyte
Magnetic Bead Panel kit was obtained from EMD Millipore (Billerica,
MA, USA). The P38 inhibitor SB203580 (cat. no. 5633) was purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Mouse
anti-PPARγ (cat. no. sc-7273), rabbit anti-CCAAT/enhancer binding
protein (C/EBP)α (cat. no. sc-61) and mouse anti-β-actin (cat. no.
sc-81178) primary antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Horseradish peroxidase
(HRP)-conjugated secondary antibodies, goat anti-rabbit IgG (cat.
no. 170-6515) and goat anti-mouse IgG (cat. no. 170-6516), were
obtained from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). All
other reagents from commercial sources were of analytical
grade.
Preparation of herbal extracts
DISGT was prepared by extracting the five different
types of herb listed in Table I.
Each herb, including DISGT, was purchased from Omniherb (Daegu,
Korea) in 2012. A voucher specimen (BS-1) was stored at the Korea
Medicine Fundamental Research Division, Korea Institute of Oriental
Medicine (Daejeon, Korea). The extract was crushed using a grinder
and extracted using distilled water. The solution was filtered
through cotton and extracted at 100°C for 3 h using a COSMOS-660
Vacuum Extractor (Kyungseo E&P, Incheon, Korea), following
which it was concentrated using a vacuum evaporator (EYELA N-12;
EYELA CA-1112; Tokyo Rikakikai Co., Ltd., Tokyo, Japan). The
extract was freeze-dried (PVTFD-100; ilShin BioBase Co., Ltd.,
Dongducheon, Korea) to create a powder (extraction yield, 26.63%).
The prepared powder was stored at −70°C until further use.
 | Table I.Composition of Do In Seung
Gi-Tang. |
Table I.
Composition of Do In Seung
Gi-Tang.
| Compound |
|
|---|
|
|
|---|
| Herbal name | Scientific name | Dosage (g) |
|---|
| Rhai Rhizoma | Rheum
officinale | 12.00 |
|
| Baillon |
| Persicae semen | Prunus
persica | 2.00 |
|
| Batsch |
| Cinnammomi |
Cinnamomum | 8.00 |
| Cortex | loureirii
Nees |
| Natrii Sulfas | Conyza
canadensis | 8.00 |
| Glycyrrhizae | Glycyrrhiza
uralensis | 4.00 |
| Radix | Fisch |
|
Cell culture and differentiation
For cell culture and differentiation, 3T3-L1
preadipocytes were grown and seeded into 6-well plates containing
DMEM supplemented with 10% NCS and 1% P&S at 37°C in a
humidified atmosphere of 5% CO2. Once cells had reached
confluency (day 0) they were exposed to differentiation medium:
DMEM supplemented with 10% FBS, 1% P&S, and a mixture of 0.5 mM
IBMX, 1 µM DEX and 1 µg/ml insulin (MDI), and were treated with
62.5, 125, 250 or 500 µg/ml DISGT, and 10 µM of SB203580, which is
a p38 mitogen-activated protein kinase (MAPK) inhibitor and was
used as a positive control to confirm the efficacy of DISGT, for 48
h (from days 0 to 2). Cells were subsequently maintained in
post-differentiation medium: DMEM containing 1 µg/ml insulin in the
absence of IBMX or DEX, and subsequently treated with various
concentrations of DISGT and 10 µM SB203580 for 72 h (from days 2 to
5). Following this, the medium was replaced with
post-differentiation medium DMEM and cells were treated with
various concentrations of DISGT and 10 µM SB203580 for 48 h (from
days 5 to 7).
Cell cytotoxicity
The effect of DISGT on cell viability was determined
by a CCK-8 assay. 3T3-L1 cells were seeded at a density of
1.5×103 cells into 48-well plates. After 2 days, cells
reached confluency and were treated with 0, 10, 20, 50, 100, 200,
500 or 1000 µg/ml DISGT for 7 days. Following incubation with DISGT
for 7 days, cells were treated with CCK-8 and incubated at 37°C for
4 h. Following treatment, absorbance was measured at a wavelength
of 450 nm using a Benchmark Plus microplate reader (Bio-Rad
Laboratories, Inc.). Data were calculated as percentage of cell
viability compared with control cells.
Oil Red O staining
To observe the fat droplets in adipocytes, cells
were stained with Oil Red O on day 7 following differentiation
induction. Cells treated with various concentrations of DISGT were
washed twice with DPBS and fixed with 10% formalin for 30 min.
Following this, cells were stained with 0.3% Oil Red O in 60%
isopropanol for 1 h at room temperature. Cells were subsequently
washed three times with distilled water and imaged using a light
microscope (Olympus Corporation, Tokyo, Japan). Fat droplets
stained with Oil Red O solution were dissolved with 100% DMSO and
quantified by measuring the optical absorbance at a wavelength of
530 nm using a Benchmark Plus microplate reader.
TG and leptin production
TG and leptin contents were measured in
differentiated 3T3-L1 cells on day 7. A TG analysis was performed
on lipid droplets collected from each sample. TGs were quantified
using an EnzyChrom™ TG assay according to the manufacturer's
protocol and a colorimetric detection method (absorbance measured
at a wavelength of 570 nm) (14).
Leptin was quantified using a mouse leptin-specific ELISA kit
according to the manufacturer's protocol. The detection limit of
the assay was typically <22 pg/ml (15).
Adipocyte detection
A MILLIPLEX MAP Mouse Adipocyte Magnetic Bead Panel
kit was used to detect adipocytes in the supernatant. Measurements
of adipocyte concentrations were conducted according to the
manufacturer's protocol. A total of seven standard samples were
set; two quality controls and the standard samples were run in
duplicate. Detection samples were assayed in triplicate. Signal
values were detected on a Bio-Plex® 200 system and
Bio-Plex Pro II Wash Station (xMAP® Technology; Luminex
Corporation, Austin, TX, USA). Briefly, the binding of specific
factors begins in the bead mixture suspended with a detection
sample. Following an overnight incubation at 4°C, a biotinylated
detection antibody was introduced. A subsequent incubation with a
streptavidin-phycoerythrin conjugate was performed to complete the
reaction on the microplates. Finally, each assay was analyzed using
a Bio-Plex 200 system. Concentrations were calculated according to
a standard curve.
Protein preparation and western blot
analysis
Cells were washed and harvested with ice-cold DPBS.
Lysates were prepared using radioimmunoprecipitation cell lysis
buffer containing 0.5 M Tris-HCl (pH 7.4), 1.5 M NaCl, 2.5%
deoxylcholic acid, 10% NP-40 and 10 mM EDTA. Protein concentration
was measured using a Bicinchoninic Acid Protein assay kit (Thermo
Fisher Scientific Inc.). Cell lysates (30 µg) were separated by
4–20% Criterion™ TGX™ precast gel (Bio-Rad Laboratories, Inc.)
electrophoresis and subsequently transferred onto polyvinylidene
difluoride membranes (GE Healthcare Life Sciences, Chalfont, UK).
Membranes were blocked with 5% skimmed milk and incubated overnight
at 4°C with C/EBPα, PPARγ and β-actin primary antibodies at a
dilution of 1:1,000. After 1 h incubation at room temperature with
HRP-conjugated anti-mouse for or anti-rabbit secondary antibodies
(1:3,000), protein bands were detected with an Enhanced
Chemiluminescence assay kit (Thermo Fisher Scientific, Inc.).
Images were captured using a ChemiDoc™ XRS+ image analyzer (Bio-Rad
Laboratories, Inc.). Relative density of C/EBPα and PPARγ were
normalized to β-actin and quantified using Image Lab™ software
version 4.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean + standard error
(n=3) and were compared using one-way analysis of variance followed
by a Bonferroni correction, in SPSS software version 13.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Herbal formula and cell viability
The yield of the DISGT water extract was 26.63%
(w/w) following freeze-drying. CCK-8 analysis was performed to
evaluate the cytotoxicity of DISGT. DISGT did not demonstrate
significant cytotoxicity at 500 µg/ml (data not shown). The present
study used DISGT at concentrations of 62.5, 125, 250 and 500
µg/ml.
Effects of DISGT on intracellular
function in differentiated 3T3-L1 adipocytes
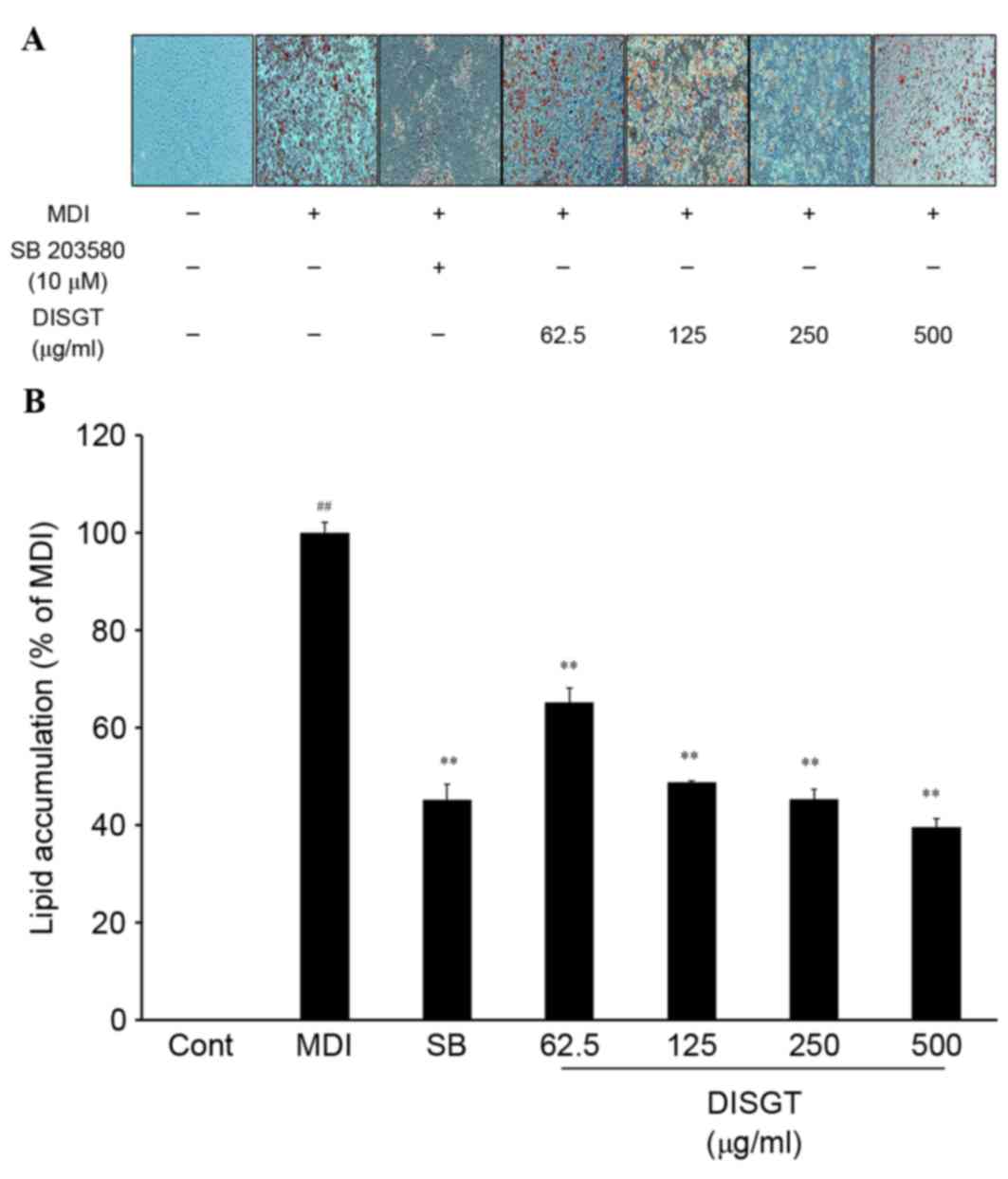
To assess the effect of DISGT on adipogenesis,
intracellular lipid accumulation was analyzed in mature adipocytes.
Following treatment with various concentrations of DISGT for 7
days, lipid accumulation was significantly suppressed in a
dose-dependent manner (Fig.
1).
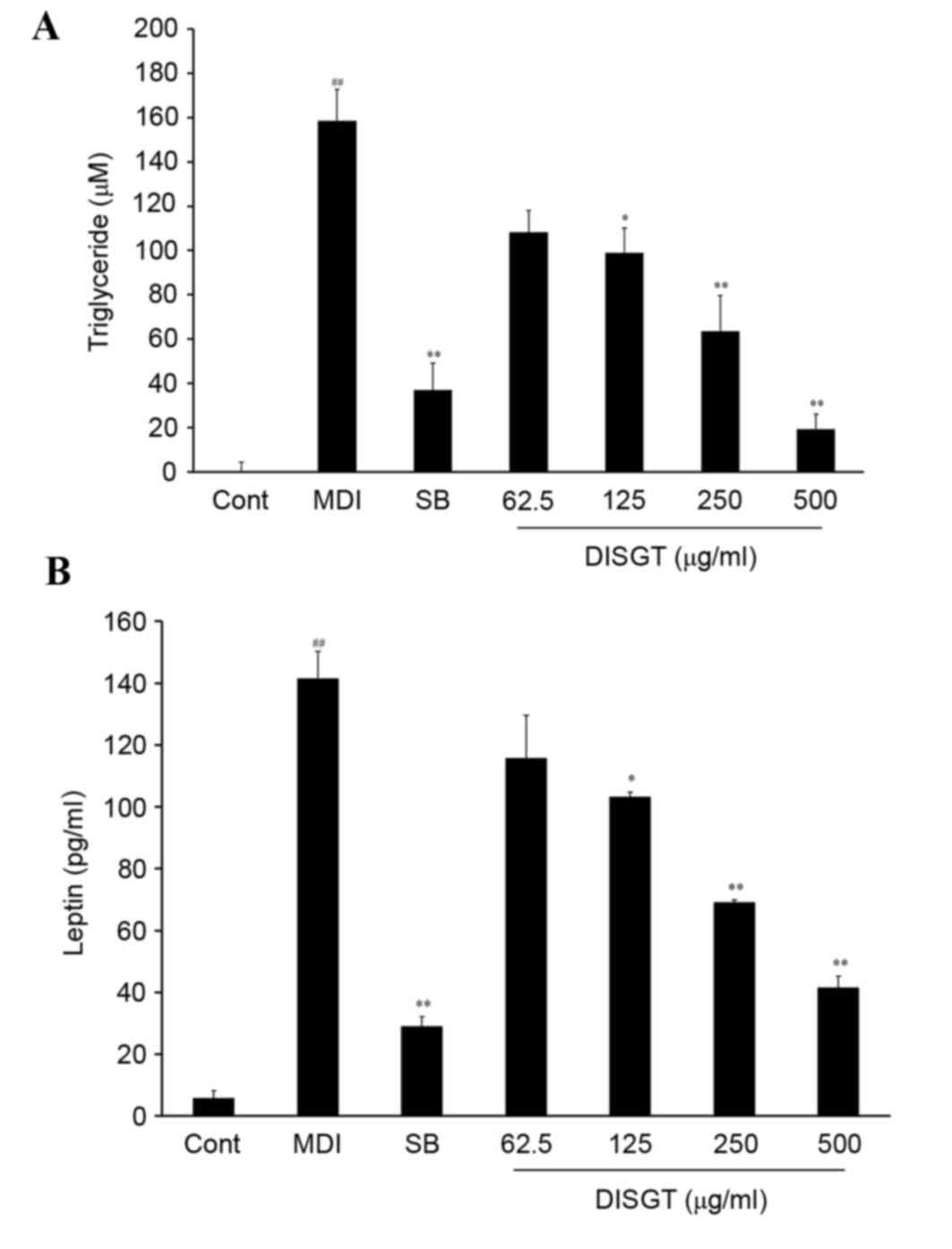
For intracellular lipid regulation, TG levels in the
lysate and leptin levels in the supernatant were measured. The TG
concentration of the control group was 0.0±4.26 µM. Following
treatment with MDI, the TG levels increased to 158.67±14.13 µM.
DISGT treatment significantly suppressed TG release in a
dose-dependent manner (P<0.001). SB203580 is a well-established
p38 MAPK inhibitor. A previous study demonstrated that when p38
MAPK was inhibited, adipocyte differentiation was inhibited by
downregulation of adipogenic-specific transcription factors,
including C/EBPs and PPARγ (16).
SB203580 (10 µM), which was used as a positive control, reduced the
TG concentration to 36.90±12.31 µM (P<0.01 vs. MDI). DISGT (500
µg/ml) treatment was more effective compared with SB203580, and
reduced the TG concentration to 19.32±6.72 µM (P<0.01 vs. MDI;
Fig. 2A). To determine the effect
of leptin inhibition on adipogenesis in adipocytes, 3T3-L1
adipocytes were treated with MDI. As presented in Fig. 2B, MDI treatment of 3T3-L1
adipocytes significantly increased leptin levels compared with the
control (P<0.001). When MDI-differentiated 3T3-L1 cells were
treated with DISGT, leptin release was significantly inhibited in a
dose-dependent manner (P<0.001).
Effects of DISGT on the secretion of
adipokines in the 3T3-L1 adipocytes
To examine the effect of DISGT on MDI-differentiated
3T3-L1 adipocytes, a multiplex assay was performed to detect
adiponectin (Fig. 3A), resistin
(Fig. 3B) and plasminogen
activator inhibitor-1 (PAI-1; Fig.
3C) in the cell supernatants. All factors were increased
following MDI treatment. DISGT significantly and dose-dependently
suppressed the release of adiponectin and resistin (P<0.001).
The positive control SB203580 additionally inhibited release of
adiponectin and PAI-1. DISGT treatment suppressed the release of
adiponectin and resistin to a greater extent compared with SB203580
(10 µM). Furthermore, DISGT treatment inhibited the release of
PAI-1 by MDI-differentiated 3T3-L1 adipocytes. DISGT and SB203580
inhibited PAI-1 levels to a similar extent.
 | Figure 3.Inhibitory effect of DISGT on
adipokine production in 3T3-L1 adipocytes. 3T3-L1 preadipocytes
were differentiated into adipocytes and treated with 0, 62.5, 125,
250 or 500 µg/ml DISGT for 7 days. (A) Adiponectin, (B) resistin
and (C) PAI-1 levels were assessed following DISGT treatment. Data
are presented as the mean + standard error (n=3).
##P<0.01 vs. Cont; *P<0.05 and **P<0.01 vs.
MDI. DISGT, Do In Seung Gi-Tang; PAI-1, plasminogen activator
inhibitor-1; Cont, control; MDI, mixture of
3-isobutyl-1-methylisobutylxanthine, dexamethasone and insulin; SB,
SB203580. |
Effect of DISGT on the expression of
adipocyte-associated proteins during adipogenesis
To determine if DISGT affects adipogenesis during
adipocyte differentiation from 3T3-L1 preadipocytes, protein
expression of C/EBPα and PPARγ were examined by western blotting
following treatment with various concentrations of DISGT for 7
days. At 7 days post-incubation, these proteins were dramatically
upregulated and expressed in the differentiated 3T3-L1 cells.
However, DISGT treatment decreased the expression of C/EBPα and
PPARγ compared with cells cultured with MDI alone. The expression
of C/EBPα and PPARγ were markedly decreased following treatment
with 500 µg/ml DISGT (Fig. 4).
Discussion
BSS, known as Eohyul in TKM, refers to stagnation of
blood circulation, retained blood and vascular obstruction; studies
on its underlying mechanisms and treatment have been performed in
Korea and China (11,12). BSS is one of the most common
illnesses in TKM and is associated with MDs, including obesity,
atherosclerotic cardiovascular disease, hyperlipidemia,
hypertension and type 2 diabetes mellitus (17). Obesity is characterized by an
excess of body fat and adipose tissue and is caused by an imbalance
between calorie intake and usage. It involves MDs and chronic
inflammatory responses (18–21).
The present study detected an anti-adipogenesis
effect of DISGT treatment on MDI-differentiated 3T3-L1 adipocytes.
DISGT, an agent that has been used to treat various symptoms
associated with BSS in TKM, consists of five types of herbal
ingredients. It is considered to improve blood circulation by
removing blood stasis. Furthermore, it has been investigated for
the treatment of various clinical conditions, including
atherosclerotic disease, gynecological disease, diabetes mellitus
and chronic pyelonephritis, by promoting blood flow (22–24).
Therefore, the present study focused on the anti-adipogenesis
mechanism associated with adipokine levels and
intracellular-associated factors, including TG, leptin,
adiponectin, resistin and PAI-1. The results revealed that 0–1,000
µg/ml DISGT was not cytotoxic to adipocytes. Oil Red O staining was
used to visualize the TG-containing fat droplets in adipocytes;
DISGT treatment significantly reduced the fat droplets in
MDI-differentiated 3T3-L1 adipocytes. DISGT treatment significantly
inhibited the release of certain factors associated with
adipokines, including leptin, TG, adiponectin and resistin in a
dose-dependent manner, however, this was not observed for PAI-1,
and inhibited the PPARγ signaling pathway. These results
demonstrated that DISGT treatment dose-dependently inhibited
differentiation and lipid accumulation in 3T3-L1 adipocytes.
Pathological adipocyte differentiation markedly
increases the level of adipocytokines, or adipokines, including
tumor necrosis factor-α, leptin, adiponectin and resistin, which
affects systemic energy balance (25). Therefore, inhibiting adipocyte
differentiation may be important as a therapeutic approach for the
treatment and prevention of MDs related to obesity.
Together with high blood pressure and reduced
high-density lipoprotein levels, elevated serum TG levels are a
primary marker of obesity (26).
Lipid formation in adipose tissue occurs at a late stage during
adipogenesis and is associated with elevated TG levels and adipose
tissue mass (27,28). In the present study, DISGT
treatment markedly suppressed morphological differentiation and TG
accumulation by inhibiting fat droplet formation during 3T3-L1
adipocyte differentiation.
Leptin concentrations in humans and rodents have
been closely associated with adiposity and body weight alterations.
Furthermore, plasma leptin has been demonstrated to be highly
correlated with body mass index in rodents and obese humans
(29). As a hormone, it links
lipid storage, regulates body weight and is influenced by
environmental factors or other hormones, inluding MDI and DEX.
Blood leptin levels are known to be closely connected with TG
levels and the quantity of adipose tissue in the body (30,31).
The present study demonstrated that DISGT treatment significantly
suppressed TG and leptin concentrations. These results suggested
that DISGT may have a biological role in decreasing lipid formation
and TGs and acting as a negative regulator of adipogenesis.
In the present study, DISGT treatment inhibited
adiponectin, resistin and PAI-1 levels in differentiated 3T3-L1
cells. Reduced circulating adiponectin concentrations are
associated with insulin resistance and MDs (32). Resistin, additionally named
adipocyte secreted factor, is a member of the resistin-like
molecules family of proteins with cysteine-rich structures.
Resistin concentrations are increased in numerous
inflammation-associated disorders, including atherosclerosis,
chronic inflammatory bowel disease, chronic renal disease and
arthritis (33).
C/EBPβ and C/EBPδ are rapidly triggered by hormones
including DEX and methylisobutylxanthine during early adipocyte
differentiation. Expression levels of these proteins are
subsequently reduced, and expression of C/EBPα and PPARγ promptly
increase in the mid and late stages of adipogenesis (34–36).
PPARγ is a critical transcription factor markedly expressed in
adipose tissue that stimulates adipocyte differentiation.
Furthermore, it is a key nuclear receptor that regulates an array
of diverse functions in numerous cell types, including the
regulation of genes associated with growth and differentiation
(37). Its primary function is to
directly regulate the development of adipose tissue, which involves
coordinating the expression of a variety of genes responsible for
the formation of the mature adipocyte phenotype (5). C/EBPα and PPARγ individually or in
combination regulate the activation of adipose-specific genes
critical to the development of the adipose phenotype and adipose
biosynthesis, including fatty acid synthase, acetyl-coenzyme A
synthetase 1 and fatty acid binding protein (38).
The present study investigated alterations in the
protein expression levels of C/EBPα and PPARγ following adipocyte
differentiation for 7 days to evaluate the effects of DISGT
treatment on the activity of these adipogenesis-associated
transcription factors. DISGT treatment significantly suppressed
MDI-induced protein expression levels of C/EBPα and PPARγ, which
are primary markers of adipogenesis. These results demonstrated
that DISGT treatment significantly blocked adipocyte
differentiation and lipid accumulation by suppressing adipogenic
gene expression. Thus, the present study suggested the potential of
DISGT as a therapeutic agent for the treatment of MDs associated
with BSS.
Acknowledgements
The present study was supported by the Korea
Institute of Oriental Medicine (grant no. K16110).
References
|
1
|
Eckel RH, Grundy SM and Zimmet PZ: The
metabolic syndrome. Lancet. 365:1415–1428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaur J: A comprehensive review on
metabolic syndrome. Cardiol Res Pract. 2014:9431622014.PubMed/NCBI
|
|
3
|
Shin SS, Jung YS, Yoon KH, Choi S, Hong Y,
Park D, Lee H, Seo BI, Lee HY and Yoon M: The Korean traditional
medicine gyeongshingangjeehwan inhibits adipocyte hypertrophy and
visceral adipose tissue accumulation by activating PPARalpha
actions in rat white adipose tissues. J Ethnopharmacol. 127:47–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pakala R, Kuchulakanti P, Rha SW, Cheneau
E, Baffour R and Waksman R: Peroxisome proliferator-activated
receptor gamma: Its role in metabolic syndrome. Cardiovasc Radiat
Med. 5:97–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farmer SR: Regulation of PPARgamma
activity during adipogenesis. Int J Obes (Lond). 29 Suppl
1:S13–S16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li SM, Xu H and Chen KJ: The diagnostic
criteria of blood-stasis syndrome: Considerations for
standardization of pattern identification. Chin J Integr Med.
20:483–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou XQ, Liang H, Sun X and Zhou HT:
Correlation between TCM blood stasis pattern of coronary heart
disease and coronary angiography result: A meta-analysis. Chin J
Evid Based Med. 12:1470–1477. 2012.
|
|
8
|
Ji W, Zhou J, Wang S and Ji Z: Logistic
regression analysis of relevance between different blood stasis
syndromes and related factors in angina pectoris patients. Chin
Arch Tradit Chin Med. 7(040)2013.(In Chinese).
|
|
9
|
Yu X, Zhang L and Xu H: Progress in
research on relevant factors affecting TCM syndrome differentiation
of CHD. Chin J Int Med Cardio/Cerebrovasc Dis. 7:581–584. 2009.(In
Chinese).
|
|
10
|
Heo J: Donguibogam. Namsandang Publ.
Corp.; Seoul: pp. 4622014
|
|
11
|
Lee YJ, Kim EK, Kim HY, Yoon JJ, Lee SM,
Lee GM, Kang DG and Lee HS: Therapeutic effect of doinseunggi-tang
on diabetic vascular dysfunction. HFS. 21:119–130. 2013.
|
|
12
|
Kosuge T, Ishida H and Ishii M: Studies on
active substances in the herbs used for oketsu (‘stagnant blood’)
in Chinese medicine. II. On the anticoagulative principle in
persicae semen. Chem Pharm Bull (Tokyo). 33:1496–1498. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge RY, Zhou CH and She YC: Influences of
stigma croci and semen persicae on function of ovary-uterus in
pseudopregnant rats. J Tradit Chin Med. 3:23–26. 1983.PubMed/NCBI
|
|
14
|
Orban T, Palczewska G and Palczewski K:
Retinyl ester storage particles (retinosomes) from the retinal
pigmented epithelium resemble lipid droplets in other tissues. J
Biol Chem. 286:17248–17258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Columba-Cabezas S, Iaffaldano G, Chiarotti
F, Alleva E and Cirulli F: Early handling increases susceptibility
to experimental autoimmune encephalomyelitis (EAE) in C57BL/6 male
mice. J Neuroimmunol. 212:10–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Engelman JA, Lisanti MP and Scherer PE:
Specific inhibitors of p38 mitogen-activated protein kinase block
3T3-L1 adipogenesis. J Biol Chem. 273:32111–32120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen KJ: Blood stasis syndrome and its
treatment with activating blood circulation to remove blood stasis
therapy. Chin J Integr Med. 12:891–896. 2012. View Article : Google Scholar
|
|
18
|
Spiegelman BM and Flier JS: Adipogenesis
and obesity: Rounding out the big picture. Cell. 87:377–389. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sartipy P and Loskutoff DJ: Monocyte
chemoattractant protein 1 in obesity and insulin resistance. Proc
Natl Acad Sci USA. 100:pp. 7265–7270. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wofford MR, Andrew ME, Brown A, King D,
Pickett RA, Stevens J, Wyatt S and Jones DW: Obesity hypertension
in the atherosclerosis risk in communities cohort: Implications of
obesity guidelines. J Clin Hypertens. 1:27–32. 1999.
|
|
21
|
Pi-Sunyer FX: The obesity epidemic:
Pathophysiology and consequences of obesity. Obes Res. 10 Suppl
2:S97–S104. 2002. View Article : Google Scholar
|
|
22
|
Yoon JJ, Lee YJ, Park OJ, Lee SM, Lee YP,
Cho NG, Kang DG and Lee HS: Doinseunggitang ameliorates endothelial
dysfunction in diabetic atherosclerosis. Evid Based Complement
Alternat Med. 2013:7835762013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sung YY, Kim DS, Choi G, Kim SH and Kim
HK: Dohaekseunggi-tang extract inhibits obesity, hyperlipidemia,
and hypertension in high-fat diet-induced obese mice. BMC
Complement Altern Med. 14:3722014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Q: Pharmacology and application of
Chinese herbs. 16th. SMC Publ. Inc.; Taipei: pp. 4511989, (In
Chinese).
|
|
25
|
Fasshauer M and Paschke R: Regulation of
adipocytokines and insulin resistance. Diabetologia. 46:1594–1603.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rosen ED and Spiegelman BM: Adipocytes as
regulators of energy balance and glucose homeostasis. Nature.
444:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shao D, Rangwala SM, Bailey ST, Krakow SL,
Reginato MJ and Lazar MA: Interdomain communication regulating
ligand binding by PPAR-gamma. Nature. 396:377–380. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lefterova MI and Lazar MA: New
developments in adipogenesis. Trends Endocrinol Metab. 20:107–114.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Havel PJ: Role of adipose tissue in
body-weight regulation: Mechanisms regulating leptin production and
energy balance. Proc Nutr Soc. 59:pp. 359–371. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Auwerx J and Staels B: Leptin. Lancet.
351:737–742. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Isaia GC, D'Amelio P, Di Bella S and
Tamone C: Is leptin the link between fat and bone mass? J
Endocrinol Invest. 28(Suppl 10): S61–S65. 2005.
|
|
32
|
Liu M and Liu F: Regulation of adiponectin
multimerization, signaling and function. Best Pract Res Clin
Endocrinol Metab. 88:25–31. 2014. View Article : Google Scholar
|
|
33
|
Axelsson J, Bergsten A, Qureshi AR,
Heimbürger O, Bárány P, Lönnqvist F, Lindholm B, Nordfors L,
Alvestrand A and Stenvinkel P: Elevated resistin levels in chronic
kidney disease are associated with decreased glomerular filtration
rate and inflammation, but not with insulin resistance. Kidney Int.
69:596–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lefterova MI, Zhang Y, Steger DJ, Schupp
M, Schug J, Cristancho A, Feng D, Zhuo D, CJ Jr, Liu XS Stoeckert
and Lazar MA: PPARgamma and C/EBP factors orchestrate adipocyte
biology via adjacent binding on a genome-wide scale. Genes Dev.
22:2941–2952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang QQ, Zhang JW and Lane M Daniel:
Sequential gene promoter interactions of C/EBPbeta, C/EBPalpha, and
PPARgamma during adipogenesis. Biochem Biophys Res Commun.
319:235–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman
MW, Gonzalez FJ and Spiegelman BM: C/EBPalpha, induces adipogenesis
through PPARgamma: A unified pathway. Genes Dev. 16:22–26. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schoonjans K, Staels B and Auwerx J: The
peroxisome proliferator activated receptors (PPARS) and their
effects on lipid metabolism and adipocyte differentiation. Biochim
Biophys Acta. 1302:93–109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Otto TC and Lane MD: Adipose development:
From stem cell to adipocyte. Crit Rev Biochem Mol Biol. 40:229–242.
2005. View Article : Google Scholar : PubMed/NCBI
|