Introduction
Breast cancer has one of the highest mortality rates
among malignant tumors in women worldwide, and since it is also
characterized by high incidence and morbidity rates, it poses a
major public health concern (1,2).
Tumor metastasis is one of the main causes underlying
cancer-associated mortality. Although only 5–10% of newly diagnosed
breast cancer patients exhibit metastasis to distant organs, the
risk of metastasis in patients with localized primary disease,
following successful primary tumor resection and adjuvant therapy,
remains high (3,4). Several diagnostic biomarkers and
therapeutic targets, such as the estrogen receptor (ER) and
progesterone receptor (PR), and the human epidermal growth factor
receptor (HER) 2 are already being used in clinical practice;
however, variations among individual patients hinder the diagnosis
and effective treatment of breast cancer (5). Therefore, the need to identify
reliable biomarkers for the diagnosis, prognosis and treatment of
patients with breast cancer is urgent.
Epithelial cell adhesion molecule (EpCAM) is a
glycosylated, type I transmembrane protein, which is overexpressed
in several neoplasms, such as breast cancer, hepatocellular
carcinoma (6), glioma (7) and colorectal cancer (8). Since EpCAM has been associated with
cancer progression and prognosis, it is used as a diagnostic and
prognostic marker for various types of disease (9).
Our previous studies have indicated that EpCAM may
serve a regulatory role during epithelial-mesenchymal transition
(EMT) in breast cancer cells (10), whereas knockdown of EpCAM inhibited
breast cancer cell growth and metastasis via inhibition of the
Ras/Raf/extracellular signal-regulated kinase signaling pathway and
matrix metalloproteinase (MMP)-9 (11). These results suggested that EpCAM
may serve a role in the regulation of cancer cell growth and may
hold potential as a prognostic marker in breast cancer.
Cyclooxygenase (COX)-2 is the key enzyme regulating
prostaglandin synthesis and is involved in inflammatory processes.
COX-2 is expressed in several tissues, and its expression is
induced and regulated by tumor promoters, cytokines, endotoxins,
growth factors and prostaglandins (12). High levels of prostaglandins,
resulting from the overexpression of COX-2, have been implicated in
the pathogenesis of numerous malignancies, including colon, breast,
and lung cancer, and have been associated with carcinogenesis,
particularly neoangiogenesis and tumor progression (13–18).
However, the relationship between EpCAM and COX-2 in breast cancer
has yet to be elucidated.
In the present study, an immunohistochemical
approach was used to evaluate the expression of EpCAM and COX-2 in
tissue samples derived from patients with breast cancer, and to
determine whether a correlation can be established between them.
The results revealed that the expression of EpCAM exhibited a
statistically significant, positive correlation with COX-2
expression, thus suggesting a combined prognostic value for EpCAM
and COX-2 in breast cancer.
Materials and methods
Tissue microarray (TMA) and
immunohistochemistry (IHC)
TMAs (cat. no. 140317A; AlenaBio Biotechnology Ltd.,
Xi'an, China) with samples from healthy and breast cancer tissue,
with stage and grade information, were purchased from US Biomax,
Inc. (Rockville, MD, USA). For IHC analysis, TMA sections were
deparaffinized in 100% xylene and rehydrated in graded ethanol
solutions. The sections were then boiled under pressure in citrate
buffer (pH 6.0) for 5 min for antigen retrieval. TMA sections were
incubated at 37°C for 1 h with EpCAM (1:200 dilution; cat no.
21050-1-AP; Wuhan Sanying Biotechnology, Wuhan, China,) and COX-2
(1:100 dilution; cat. no. 12375-1-AP; Wuhan Sanying Biotechnology)
antibodies in TBS containing 1% bovine serum albumin (Sangong
Pharmaceutical Co., Ltd., Shanghai, China). After washing with PBS,
the sections were incubated with anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibody (1:500 dilution; cat. no.
SA00001-2; Wuhan Sanying Biotechnology). Signal development was
performed by adding 250 µl 3,3′diaminobenzidine (Sangong
Pharmaceutical Co., Ltd.) substrate solution to each slide and
incubating for 3 min in the dark. Finally, slides were washed 3
times in water and drained. Images were captured using an Aperio
ScanScope® CS system (Nikon Instruments Inc., Vista, CA,
USA). EpCAM or COX-2 positive staining on TMA sections was
semi-quantitatively analyzed by two independent investigators using
the following criteria: 0, background staining; 1, weakly positive;
2, moderately positive; 3, strongly positive staining.
Cell culture and transfection
The MCF-7 and MDA-MB-231 human breast adenocarcinoma
cell lines were purchased from American Type Culture Collection
(Manassas, VA, USA). MCF-7 cells were maintained in DMEM-F12
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% calf serum (Gibco; Thermo Fisher Scientific,
Inc.), 1% penicillin/streptomycin, 1 mM sodium pyruvate, 1.5 g/l
sodium bicarbonate, and 10 mM HEPES. MDA-MB-231 cells were cultured
in Leibovitz's L-15 medium (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% calf serum, 1% penicillin/streptomycin and 10
mM HEPES. All cells were incubated in a 5% CO2
humidified atmosphere at 37°C.
Cells that were in the logarithmic growth phase were
transfected with complementary DNAs encoding human EpCAM and
control empty plasmid (cat. no. BC014785; Wuhan Sanying
Biotechnology), or small interfering (si)RNA targeting EpCAM
(si-EpCAM) and control scrambled siRNA (sequences
5′-UGCUCUGAGCGAGUGAGAATT-3′ and 5′-UUCUCACUCGCUCAGAGCATT-3′,
respectively; GenePharma Co., Ltd., Shanghai, China), using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) as the delivery agent, according to the
manufacturer's protocol. Subsequent experiments were performed 48 h
post-transfection.
Western blot analysis
To prepare whole cell extracts, cells at 90%
confluence were washed in PBS prior to incubation with lysis buffer
containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris (pH 7.4), 1 mM
EDTA, 1 mM EGTA (pH 8.0), 0.2 mM Na3VO4, 0.2
mM phenylmethylsulfonyl fluoride and 0.5% Nonidet P-40 on ice for
10 min. Debris was removed from the lysates by centrifugation at
9,000 × g for 10 min at 4°C and the supernatants were
collected. Protein concentration was determined with the Coomassie
Protein Assay Reagent using bovine serum albumin as a standard.
Equal amounts of protein (50 µg) were separated by 10% SDS-PAGE and
transferred onto nitrocellulose membranes, which were blocked with
TBS containing 0.5% Tween-20 and 5% fat-free dry milk for 2 h at
37°C. The membranes were then incubated for 3 h at 37°C with EpCAM
(1:1,000 dilution; cat. no. 21020-1-AP; Wuhan Sanying
Biotechnology), COX-2 (1:800 dilution; cat. no. 12375-1-AP; Wuhan
Sanying Biotechnology) and GAPDH (1:2,000 dilution; cat. no.
10494-1-AP; Wuhan Sanying Biotechnology) primary antibodies.
Following incubation with a HRP-conjugated anti-goat secondary
antibody (1:1,000 dilution; cat. no. SA00001-2; Wuhan Sanying
Biotechnology) for 40 min at 37°C, the protein bands were
visualized using an enhanced chemiluminescence detection system (GE
Healthcare Life Sciences, Chalfont, UK). Western blots presented
are representative of at least 3 independent experiments. Protein
expression was quantified using densitometry analysis with Labworks
software version 4.6 (Labworks LLC, Lehi, UT, USA). Band intensity
is expressed as the mean ± standard error of 3 experiments for each
group. GAPDH was used as the loading control.
Statistical analysis
Immunohistochemical scores for EpCAM and COX-2 were
tabulated, and the χ2 test for trend analysis was
performed to investigate the relationship between EpCAM and COX-2
expression and pathological diagnostic criteria for breast cancer.
Spearman's correlation coefficient analysis was performed to test
for positive or negative correlations between EpCAM and COX-2
expression across breast cancer subtypes and diagnostic parameters.
Statistical significance was analyzed using GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Correlation between EpCAM and COX-2
expression and clinicopathological parameters in breast cancer
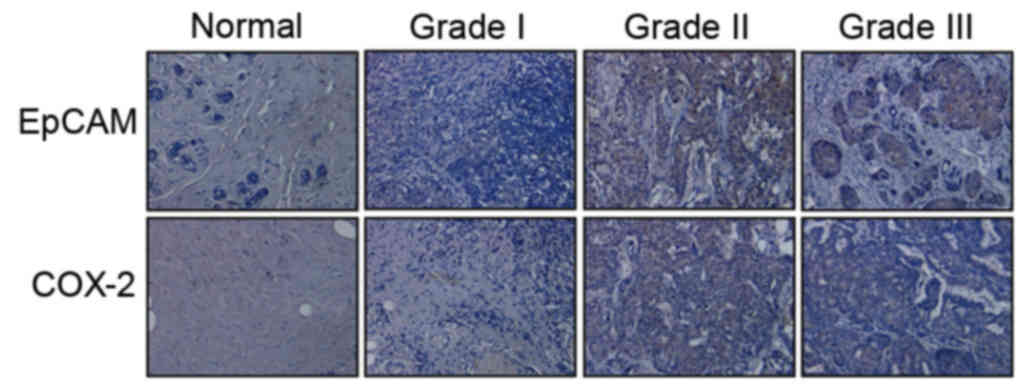
To investigate the expression of EpCAM and COX-2 in
breast cancer tissue, immunohistochemistry was performed on a
series of 134 human breast cancer samples within TMAs.
Representative EpCAM and COX-2 staining is presented in Fig. 1. A total of 92 (68.66%) samples
highly expressed EpCAM and COX-2, whereas 19 (4.17%) samples
exhibited low EpCAM and COX-2 expression; a total of 18 (13.43%)
samples exhibited high EpCAM and low COX-2 expression, whereas 5
(3.73%) samples exhibited low EpCAM and high COX-2 expression. A
positive correlation was revealed between EpCAM and COX-2
expression in breast cancer (r=0.63, P=0.009; Table I).
 | Table I.Correlation between EpCAM and COX-2
expression in breast cancer. |
Table I.
Correlation between EpCAM and COX-2
expression in breast cancer.
|
| EpCAM |
|
|
|---|
|
|
|
|
|
|---|
|
| Low | High | r | P-value |
|---|
| COX-2 |
| Low | 19 | 18 | 0.63 | 0.009 |
| High | 5 | 92 |
|
|
To examine the relationship between EpCAM and COX-2
expression and clinicopathological characteristics of the disease,
the correlation between EpCAM and COX-2 expression and the
following parameters was investigated: Age at the time of
diagnosis, tumor differentiation, lymph node metastasis, as well as
the expression of ER, PR, HER2, p53 and the proliferation marker
Ki-67 (Table II). The results
suggested that EpCAM and COX-2 expression were significantly
correlated with the histological grade of the tumor (P<0.05).
High expression of EpCAM and COX-2 was more frequently observed in
higher grade (poorly differentiated) tumors compared with in lower
grade tumors. Furthermore, a significant correlation was revealed
between EpCAM and COX-2 expression and the expression of ER, PR and
Ki-67 (P<0.05); however, no correlation was apparent with lymph
node metastasis and p53 expression. Notably, the expression of
EpCAM was positively correlated with the expression of HER2
(P<0.05), whereas no correlation was revealed between COX-2 and
HER2 expression (Table II).
 | Table II.Relationship between the expression of
EpCAM and COX-2 and clinicopathological parameters of breast
cancer. |
Table II.
Relationship between the expression of
EpCAM and COX-2 and clinicopathological parameters of breast
cancer.
|
| EpCAM |
| COX-2 |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Low | High | P-value | Low | High | P-value |
|---|
| Age (years) |
|
|
|
|
| 0.278 |
| ≤50 | 14 | 76 | 0.324 | 21 | 62 |
|
|
>50 | 10 | 34 |
| 16 | 35 |
|
| Histological
grade |
|
|
|
|
| 0.014 |
| G1 | 13 | 13 | 0.024 | 18 | 6 |
|
| G2 | 8 | 33 |
| 12 | 32 |
|
| G3 | 3 | 64 |
| 7 | 59 |
|
| Lymph node
status |
|
|
|
|
| 0.428 |
|
Negative | 14 | 42 | 0.358 | 20 | 38 |
|
|
Positive | 10 | 68 |
| 17 | 59 |
|
| AJCC stage |
|
|
|
|
| 0.019 |
| I | 11 | 19 | 0.015 | 5 | 25 |
|
| II | 9 | 63 |
| 12 | 60 |
|
| III | 4 | 28 |
| 7 | 25 |
|
| ER |
|
|
|
|
| 0.001 |
|
Negative | 18 | 24 | 0.008 | 19 | 15 |
|
|
Positive | 6 | 86 |
| 18 | 82 |
|
| PR |
|
|
|
|
| 0.015 |
|
Negative | 17 | 42 | 0.025 | 23 | 23 |
|
|
Positive | 7 | 68 |
| 14 | 74 |
|
| HER2 |
|
|
|
|
| 0.591 |
|
Negative | 14 | 35 | 0.035 | 17 | 49 |
|
|
Positive | 10 | 75 |
| 20 | 48 |
|
| p53 |
|
|
|
|
| 0.628 |
|
Negative | 12 | 62 | 0.349 | 20 | 45 |
|
|
Positive | 12 | 48 |
| 17 | 52 |
|
| Ki-67 |
|
|
|
|
| 0.024 |
|
Negative | 18 | 28 | 0.019 | 26 | 15 |
|
|
Positive | 6 | 82 |
| 11 | 82 |
|
| Total | 24 | 110 |
| 37 | 97 |
|
Correlation between EpCAM and COX-2
expression in breast cancer cell lines
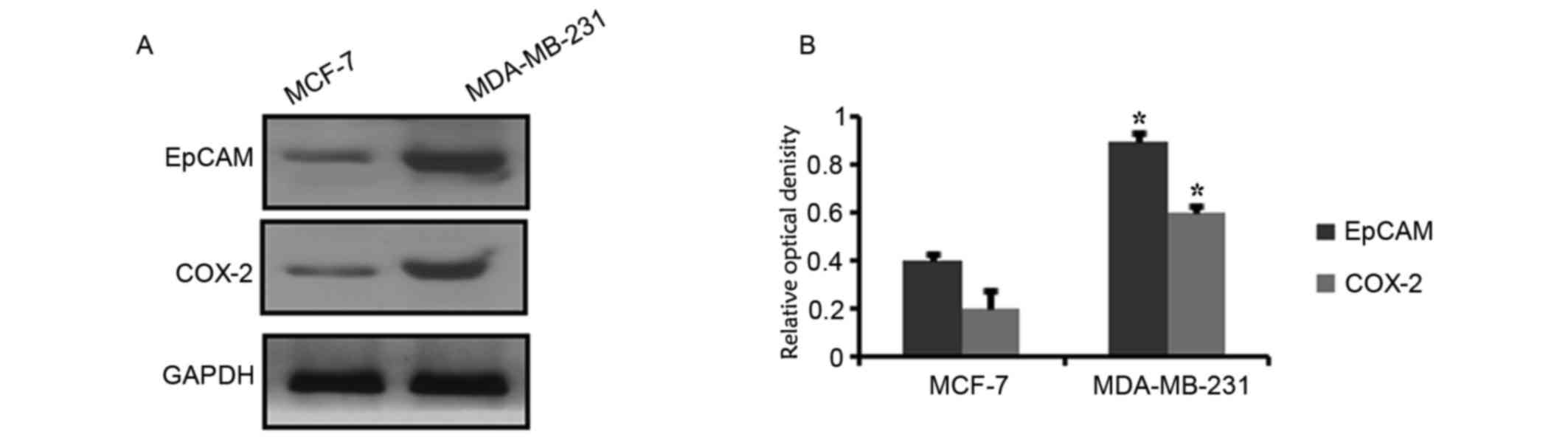
The aforementioned results demonstrated that EpCAM
and COX-2 expression were positively correlated in breast cancer
tissue samples. The expression levels of EpCAM and COX-2 were also
detected in two breast cancer cell lines. Western blot analysis
demonstrated that EpCAM and COX-2 protein expression levels varied
between these two cell lines. The expression of EpCAM and COX-2
appeared higher in MDA-MB-231 cells compared with MCF-7 cells
(Fig. 2).
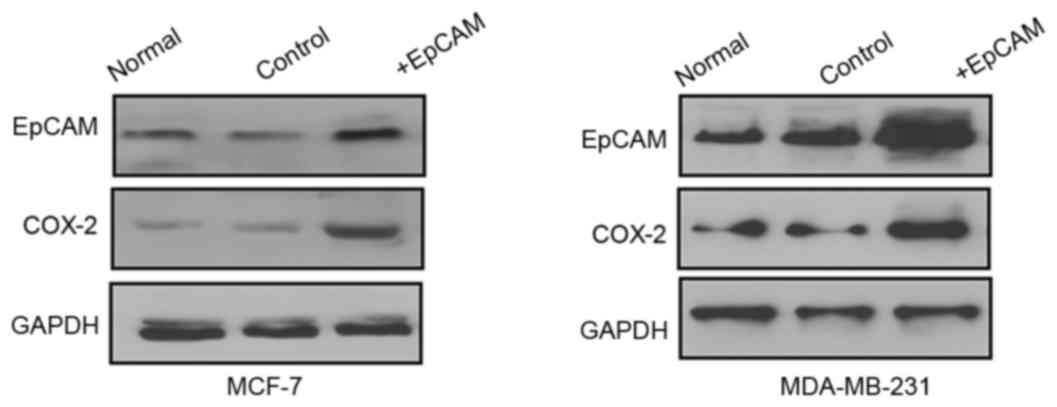
Regulation of COX-2 by EpCAM
To investigate whether EpCAM was involved in the
regulation of COX-2 in breast cancer, MCF-7 and MDA-MB-231 breast
cancer cells were transfected with EpCAM overexpression plasmid or
control. The results demonstrated that overexpression of EpCAM
promoted the expression of COX-2 (Fig.
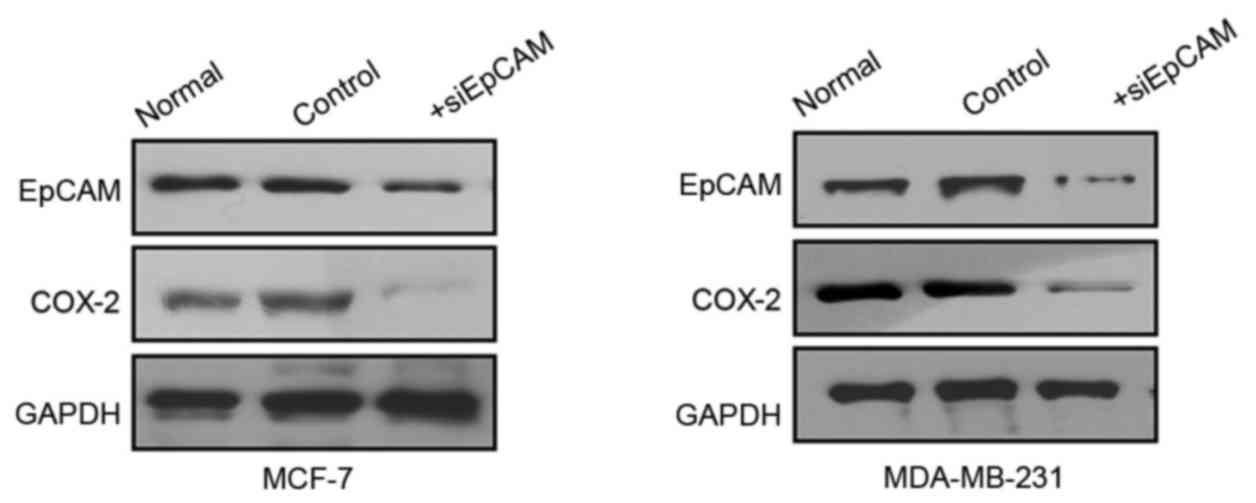
3). Furthermore, MCF-7 and MDA-MB-231 cells were transfected
with siEpCAM or control in order to silence the expression of
EpCAM. Western blot analysis revealed that EpCAM silencing
decreased the expression of COX-2 in both cell lines (Fig. 4). These results suggested that
EpCAM may be involved in the regulation of COX-2 expression.
Discussion
EpCAM expression is frequently increased in breast
cancer. It has previously been demonstrated that EpCAM may be
involved in breast cancer cell growth and metastasis (11). The present study revealed a
positive correlation between the expression of EpCAM and COX-2 in
breast cancer tissue samples. High EpCAM and high COX-2 expression
were more commonly detected in poorly differentiated tumors
compared with well and moderately differentiated tumors. The
correlation between EpCAM and COX-2 expression was also observed in
breast cancer cell lines. These results suggested that EpCAM and
COX-2 may have underlying biological connections in breast
cancer.
The results of the present study, combined with
results from our previous preclinical studies (10,19),
suggested a potential for EpCAM as a therapeutic target in breast
cancer. The regulatory role of EpCAM in gene expression has
previously been reported, including MMP-9 expression (20), thus suggesting that inhibition of
the regulatory functions of EpCAM may suppress various tumor cell
processes that drive carcinogenesis. In addition, EpCAM has
previously been suggested as a potential protein marker for cells
undergoing enhanced EMT or for cancer cells with aggressive
phenotypes (11,21), and the transcription factor
activator protein 1 has been reported to be involved in the
transcriptional activation of the EpCAM gene (22). Furthermore, important roles for
EpCAM have been suggested in the promotion of tumorigenic or
metastatic behavior of breast cancer cells. Specifically, EpCAM was
demonstrated to serve a role in mediating the effects of epidermal
growth factor in human ovarian cancer cell migration (23) and was associated with prostate
cancer metastasis via the phosphatidylinositol-4,5-bisphosphate
3-kinase/Akt/mechanistic target of rapamycin signaling pathway
(24). High EpCAM expression was
also associated with gastric cancer cell proliferation and disease
progression (25).
COX-2 has previously been used as a prognostic
factor for malignancy and has been associated with carcinogenesis.
The COX-2 pathway has been implicated in various processes
associated with tumor progression, such as angiogenesis,
proliferation and invasion (26).
Therefore, it may be hypothesized that COX-2 has potential as a
prognostic biomarker for breast cancer. Previous studies have
reported that COX-2 was upregulated and associated with tumor
invasiveness and clinical outcome in numerous types of human cancer
(27–30). In the present study, a correlation
was revealed between high COX-2 expression and poor differentiation
status (P<0.05). COX-2 expression was also correlated with
factors of poor prognosis, such as high Ki-67 proliferative rate
and poor differentiation. In relation with the aforementioned
findings regarding the involvement of EpCAM in the regulation of
COX-2 expression in breast cancer cells, these results suggested
that EpCAM expression may modulate COX-2 expression in human breast
cancer, and that various subtypes of COX-2-positive carcinomas may
respond to therapeutic strategies that target EpCAM.
In conclusion, the present study identified a
positive correlation between EpCAM and COX-2 expression in breast
cancer cell lines and tissue specimens. EpCAM and COX-2 were
associated with the prognosis of breast cancer patients, with a
high EpCAM/COX-2 ratio being indicative of poor prognosis. In
addition, EpCAM was reported to potentially regulate COX-2
expression in breast cancer cells. These results demonstrated that
EpCAM may serve an important role in COX-2 regulation, and
suggested that the inhibition of these proteins may hold potential
as a multi-target therapeutic approach for the treatment of
patients with breast cancer.
Acknowledgements
The present study was supported by the Major State
Basic Research Development Program of China (grant no.
2012CB822103), the National Natural Science Foundation of China
(grant nos. 30800195 and 31270866) and the National Natural Science
Foundation of Liaoning Province (grant no. 2013023046).
References
|
1
|
Jin X and Mu P: Targeting breast cancer
metastasis. Breast Cancer (Auckl). 9 Suppl 1:S23–S34. 2015.
|
|
2
|
Kimbung S, Loman N and Hedenfalk I:
Clinical and molecular complexity of breast cancer metastases.
Semin Cancer Biol. 35:85–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bill R and Christofori G: The relevance of
EMT in breast cancer metastasis: Correlation or causality? FEBS
Lett. 589:1577–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kodack DP, Askoxylakis V, Ferraro GB,
Fukumura D and Jain RK: Emerging strategies for treating brain
metastases from breast cancer. Cancer Cell. 27:163–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milroy MJ: Breast cancer screening. S D
Med: Spec No. 69–73. 2015.
|
|
6
|
Xu M, Qian G, Xie F, Shi C, Yan L, Yu L,
Zheng T, Wei L and Yang J: Expression of epithelial cell adhesion
molecule associated with elevated ductular reactions in
hepatocellar carcinoma. Clin Res Hepatol Gastroenterol. 38:699–705.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Ma WY, Xu SC, Liang Y, Fu YB, Pang
B, Xin T, Fan HT, Zhang R, Luo JG, et al: The overexpression of
epithelial cell adhesion molecule (EpCAM) in glioma. J Neurooncol.
119:39–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goossens-Beumer IJ, Zeestraten EC, Benard
A, Christen T, Reimers MS, Keijzer R, Sier CF, Liefers GJ, Morreau
H, Putter H, et al: Clinical prognostic value of combined analysis
of Aldh1, Survivin, and EpCAM expression in colorectal cancer. Br J
Cancer. 110:2935–2944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van der Gun BT, Melchers LJ, Ruiters MH,
de Leij LF, McLaughlin PM and Rots MG: EpCAM in carcinogenesis: The
good, the bad or the ugly. Carcinogenesis. 31:1913–1921. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao J, Yan Q, Wang J, Liu S and Yang X:
Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated
by AP1-dependent EpCAM expression in MCF-7 cells. J Cell Physiol.
230:775–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao J, Liu X, Yang F, Liu T, Yan Q and
Yang X: By inhibiting Ras/Raf/ERK and MMP-9, knockdown of EpCAM
inhibits breast cancer cell growth and metastasis. Oncotarget.
6:27187–27198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao Y, Sun K, Xu W, Li XL, Shen H and Sun
WH: Helicobacter pylori infection, gastrin and cyclooxygenase-2 in
gastric carcinogenesis. World J Gastroenterol. 20:12860–12873.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoellen F, Kelling K, Dittmer C, Diedrich
K, Friedrich M and Thill M: Impact of cyclooxygenase-2 in breast
cancer. Anticancer Res. 31:4359–4367. 2011.PubMed/NCBI
|
|
14
|
Harris RE, Casto BC and Harris ZM:
Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J
Clin Oncol. 5:677–692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thill M, Terjung A and Friedrich M: Breast
cancer-new aspects of tumor biology: Are calcitriol and
cyclooxygenase-2 possible targets for breast cancer? Eur J Gynaecol
Oncol. 35:341–358. 2014.PubMed/NCBI
|
|
16
|
Cheng J and Fan XM: Role of
cyclooxygenase-2 in gastric cancer development and progression.
World J Gastroenterol. 19:7361–7368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Misra S and Sharma K: COX-2 signaling and
cancer: New players in old arena. Curr Drug Targets. 15:347–359.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiel A, Mrena J and Ristimäki A:
Cyclooxygenase-2 and gastric cancer. Cancer Metastasis Rev.
30:387–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Yan Q, Liu S and Yang X: Knockdown
of EpCAM enhances the chemosensitivity of breast cancer cells to
5-fluorouracil by downregulating the antiapoptotic factor Bcl-2.
PLoS One. 9:e1025902014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eberlein C, Rooney C, Ross SJ, Farren M,
Weir HM and Barry ST: E-Cadherin and EpCAM expression by NSCLC
tumour cells associate with normal fibroblast activation through a
pathway initiated by integrin alphavbeta6 and maintained through
TGFβ signalling. Oncogene. 34:704–716. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Philip R, Heiler S, Mu W, Büchler MW,
Zöller M and Thuma F: Claudin-7 promotes the epithelial-mesenchymal
transition in human colorectal cancer. Oncotarget. 6:2046–2063.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sankpal NV, Mayfield JD, Willman MW,
Fleming TP and Gillanders WE: Activator protein 1 (AP-1)
contributes to EpCAM-dependent breast cancer invasion. Breast
Cancer Res. 13:R1242011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan Q, Cheng JC, Qiu X, Chang HM and Leung
PC: EpCAM is up-regulated by EGF via ERK1/2 signaling and
suppresses human epithelial ovarian cancer cell migration. Biochem
Biophys Res Commun. 457:256–261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ni J, Cozzi P, Hao J, Beretov J, Chang L,
Duan W, Shigdar S, Delprado W, Graham P, Bucci J, et al: Epithelial
cell adhesion molecule (EpCAM) is associated with prostate cancer
metastasis and chemo/radioresistance via the PI3K/Akt/mTOR
signaling pathway. Int J Biochem Cell Biol. 45:2736–2748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kroepil F, Dulian A, Vallböhmer D, Geddert
H, Krieg A, Vay C, Topp SA, Am Esch JS, Baldus SE, Gires O, et al:
High EpCAM expression is linked to proliferation and lauren
classification in gastric cancer. BMC Res Notes. 6:2532013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang D and Dubois RN: Eicosanoids and
cancer. Nat Rev Cancer. 10:181–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deshpande R, Mansara P and Kaul-Ghanekar
R: Alpha-linolenic acid regulates Cox2/VEGF/MAP kinase pathway and
decreases the expression of HPV oncoproteins E6/E7 through
restoration of p53 and Rb expression in human cervical cancer cell
lines. Tumour Biol. 37:3295–3305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kosaka T, Davydova J, Ono HA, Akiyama H,
Hirai S, Ohno S, Takeshita F, Aoki K, Ochiya T, Yamamoto M, et al:
Imaging and antitumoral effect of a Cyclo-oxygenase 2-specific
replicative adenovirus for small metastatic gastric cancer lesions.
Anticancer Res. 35:5201–5210. 2015.PubMed/NCBI
|
|
29
|
Yu S, Hou Q, Sun H, Liu J and Li J:
Upregulation of C-C chemokine receptor type 7 expression by
membrane-associated prostaglandin E synthase-1/prostaglandin E2
requires glycogen synthase kinase 3β-mediated signal transduction
in colon cancer cells. Mol Med Rep. 12:7169–7175. 2015.PubMed/NCBI
|
|
30
|
Perez AA, Balabram D, Rocha RM, da Silva
Souza Á and Gobbi H: Co-Expression of p16, Ki67 and COX-2 Is
associated with basal phenotype in high-grade ductal carcinoma in
situ of the breast. J Histochem Cytochem. 63:408–416. 2015.
View Article : Google Scholar : PubMed/NCBI
|