Introduction
Cancer is a leading cause of human mortality
worldwide, accounting for 12% of global mortality each year
(1). An increasing body of
evidence has demonstrated that the mitochondria may be a promising
target for cancer therapy (2). A
number of anticancer strategies associated with
mitochondria-targeted imaging have been reported to be efficient,
however, they have limited clinical application due to their low
specificity for tumor targeting, lack of a sensitive modality to
visualize therapeutic responses in real time and safety concern
regarding their potential toxicity (3–5).
Therefore, anticancer agents with tumor mitochondria-targeting and
imaging are capabilities are in demand.
The mitochondrial membrane typically has a potential
of −180 mV, which means positively charged molecules, including
rhodamine and cyanine (Cy) dyes, easily traverse the plasma and
mitochondrial membranes via the proton gradient (6) and accumulate in the mitochondria
(7). Therefore, these molecules
may represent an attractive agent for cancer mitochondria-targeted
imaging. The near infrared (NIR) heptamethine Cy with anticancer
activity has previously been verified for tumor targeting and
imaging (8–11). Kim et al (12) conjugated the anticancer drug
paclitaxel with NIR cyanine dye Cy5.5 labeled tumor-homing
nanoparticles and demonstrated that these novel nanoparticles could
be simultaneously applied in cancer diagnosis and treatment. In
addition, IR-780 dye was revealed to specifically accumulate in the
mitochondria of tumor cells and could be applied in tumor imaging
with deep tissue penetration, high sensitivity and signal-to-noise
ratio (13). However, its clinical
application is still limited due to low anticancer activity and
poor solubility.
The present study synthesized and screened a series
of IR-780 analogs. In addition, the present study investigated the
biochemical and biophysical characterization of the selected
compound heptamethine Cy-triphenylphosphonium (Cy-TPP), and
evaluated its optical properties, subcellular localization and
anticancer activity.
Materials and methods
Chemicals and materials
Organic solvents were obtained from Chengdu KeLong
Chemical Co., Ltd. (Chengdu, China) and were used directly without
further purification unless specified. Anhydrous dichlormethane was
further purified by distillation and was dried over molecular
sieves prior to use. Aqueous solutions were all prepared using
phosphate-buffered saline (PBS; pH 7.4). Cyclohexanone,
2,3,3-trimethylindolenine, iodoethane, oxalyl chloride,
trimethylamine, oxalyl chloride and (3-carboxypropyl)
triphenylphosphonium bromide were purchased from Shanghai Aladdin
Biochemical Technology Co., Ltd. (Shanghai, China), and
2-(4,5-dimethyl-2-thiazolyl)-3,5-diphenyl-bromide (MTT) was
obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The
green fluorescent mitochondria tracker (MitoTracker®
Green) and nucleic acid stain (Hoechst 33342) were purchased from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA), and
used according to the manufacturer's protocols. B16 melanoma cells
were obtained from the American Type Culture Collection (Manassas,
VA, USA).
Synthesis of Cy-OH
Cy7.Cl was synthesized by our laboratory according
to the previously described method (14). The chemical structure was further
identified by proton nuclear magnetic resonance spectroscopy
(1HNMR; Bruker-400 MHz NMR; Bruker Corporation,
Billerica, MA, USA) and Time of Flight Mass Spectrometer (TOF-MS;
Agilent Technologies, Inc., Santa Clara, CA, USA). The chemical
shifts of Cy7.Cl reported in ppm (DMSO-d6; TMS as internal
standard) were as follows: 1HNMR (400 MHz,
DMSO-d6) δ=8.27 (d, J=14.0 Hz, 2H), 7.65 (d, J=7.4 Hz,
2H), 7.53–7.38 (m, 4H), 7.30 (t, J=6.6 Hz, 2H), 6.33 (d, J=14.2 Hz,
2H), 4.26 (d, J=6.9 Hz, 4H), 2.73 (s, 4H), 1.89 (d, J=20.2 Hz, 2H),
1.67 (s, 12H), 1.31(t, J=7.1 Hz, 6H). TOF-MS: calculated for
C34H40ClN2+,
[M+H]+=512.1,575, found 512.2,881.
Cy7.Cl (639.0 mg, 1.0 mmol) and AcONa (820.0 mg,
10.0 mmol) were dissolved in 10 ml anhydrous N,N-dimethylformamide
(DMF) under 100% nitrogen atmosphere for 3 h at 80°C. Then the
mixture was washed three times with saturated KI solution, and
extracted using CH2Cl2 (50 ml) for 3 times.
All organic mixtures were dried using anhydrous sodium sulfate,
concentrated on a rotary evaporator and the residues were purified
by silica chromatography (500–800 mesh) eluted with petroleum
ether: Ethyl acetate=1:1 (v/v) to give a dark red solid 209.0 mg.
The yield of Cy-OH was 42.5% The H-shifts of Cy-OH were as follows:
1H NMR (400 MHz, DMSO-d6) δ 7.93 (d, J=13.3
Hz, 2H), 7.33 (d, J=7.2 Hz, 2H), 7.19 (t, J=8.0 Hz, 2H), 6.90 (dd,
J=7.3, 4.4 Hz, 2H), 5.48 (d, J=13.4 Hz, 2H), 3.80 (dd, J=14.0, 7.0
Hz, 4H), 2.58–2.53 (t, 4H), 1.79–1.69 (m, 4H), 1.56 (s, 12H),
1.16(t, J=7.0 Hz, 6H). TOF-MS: Calculated for
C34H40N2O,
[M+H]+=492.7,070, found 492.7,128.
Synthesis of Cy-TPP
(3-Carboxypropyl) triphenylphosphonium Bromide
(443.0 mg, 1.0 mmol) and 2 drops of DMF were dissolved in 10 ml
anhydrous CH2Cl2, prior to the addition of
chloroglyoxylate (1 ml). The mixture was stirred at 0°C for 4 h.
Then the solvent was evaporated for further use without additional
purification.
Under N2 conditions, Cy-OH (246.0 mg, 0.5
mmol) and triethylamine (50 µl) were dissolved in 5 ml anhydrous
CH2Cl2. TPP+ (462.0 mg, 1.0 mmol)
was dissolved in 5 ml anhydrous CH2Cl2 and
added into the reaction mixture dropwise at 0°C for 30 min; the
mixture was maintained at 25°C for 12 h. The mixture was washed 3
times with saturated KI solution, and extracted using
CH2Cl2 (50 ml) for 3 times. The organic
mixture solvent was dried using anhydrous sodium sulfate,
evaporated on a rotary evaporator and the residues were purified by
silica chromatography (500–800 mesh) eluted with
dichloromethane:methanol (5:1, v/v) to give 164.5 mg of green
solid. The yield of Cy-TPP was 35.0%. The H-shift of Cy-TPP were as
follows: 1H NMR (400 MHz, DMSO-d6) δ 8.32 (d,
J=13.3 Hz, 2H), 7.33–7.38 (m, 17H), 7.53–7.38 (t, J=8.0 Hz, 4H),
7.32 (d, J=7.3, 4.4 Hz, 2H), 6.34 (d, J=13.4 Hz, 2H), 4.27 (d,
J=14.0, 7.0 Hz, 4H), 2.58–2.53 (t, 8H), 1.79–1.69 (m, 4H), 1.56 (s,
12H), 1.16 (t, J=7.0 Hz, 6H). TOF-MS: calculated for
C56H61N2O2P2+,
[M+H]+=825.0887, found 825.0596.
Optical properties of Cy-TPP
The UV-Vis absorption and fluorescence emission (B)
spectra of Cy-TPP (2 µM) were conducted in MeOH, PBS and 100% FBS.
Fluorescence spectra was obtained by fluorescence spectrometer
(F7000 Fluorescence Spectrophotometer; Hitachi High-Technologies
Corporation, Tokyo, Japan) with a Xenon lamp and 1.0-cm quartz
cells at the slits of 5.0/5.0 nm. Absorption spectra was recorded
by UV-Vis spectrophotometer (DU800; Beckman Coulter. Inc., Brea,
CA, USA). The responses of Cy-TPP (5 µM) with different analytes
(50 mM for K+, Na+, 10 mM for Arg, Lys, Glu,
1 mg/ml for BSA, 25 mM for Ca2+, Mg2+,
Al3+, Cu2+, Fe3+, Cr3+,
Co3+, Mn2+,
S2O32−,
H2O2) were conducted by incubating them for
30 min at 37°C and measured by fluorescence spectrometer. The
fluorescence responses of Cy-TPP to various concentrations of BSA
(0.1–1 mg/ml) were also measured by fluorescence spectrometer.
Cytotoxicity assay
In order to evaluate the cellular toxicity of
different doses of Cy-TPP, the compound treated cells were
illuminated using series of concentrations. Typically, B16 cells
(5×104 cells/well) were cultured in RPMI-1640 medium
(Hyclone; GE Healthcare Life Sciences, Logan, Utah, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences) in an incubator with 5% CO2 at
37°C for 24 h. Prior to any experiments, the cell medium was
removed and an increasing concentration (0.05, 1, 2, 5 and 10 µM)
of probe Cy-TPP was added. The cells were then incubated for a
further 24 h, and MTT assays were performed according to the
manufacturer's instructions. Briefly, the medium was replaced with
fresh medium containing 10% MTT (v/v). Following 3 h, the medium
was replaced with 100 µl DMSO to solubilize the formed formazan.
Absorption was measured at 490 nm for each well using a microplate
reader. An untreated assay with RPMI-1640 was also conducted under
the same conditions, which was recorded as the relative cell
viability.
Confocal imaging
Fluorescent images were acquired on a confocal
laser-scanning microscope (Nikon Eclipse Ti; Nikon Corporation,
Tokyo, Japan) with an oil lens (magnification, ×60). B16 cells
(5×104 cells/well) were plated in cell culture
Petri-dishes (F=20 mm) in RPMI-1640 supplemented with 10% FBS in an
incubator with 5% CO2 at 37°C. Following 24 h, the
medium was removed and the cells were washed three times with PBS
buffer. The cells were then incubated with the probe Cy-TPP (2.0
µM) in serum-free medium. Following 2 h, MitoTracker®
Green (200 nM) was added, followed by Hoechst 33342 (1 µM).
Following incubation for 15 min, the medium was removed. Cell
imaging was carried out following cell washing with PBS three times
to remove the excess compounds. Hoechst fluorescence was analyzed
with an excitation at 405 nm and a scan range of 440 to 500 nm;
MitoTracker Green fluorescence was analyzed with an excitation at
488 nm and a scan range of 520 to 570 nm; probe Cy-TPP was analyzed
with an excitation at 770 nm and a scan range of 790 to 810 nm.
Images were processed using Image Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. The data were analyzed by two-way analysis of variance
followed by Dunnett's tests using GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Synthesis and structure
characterization of Cy-TPP
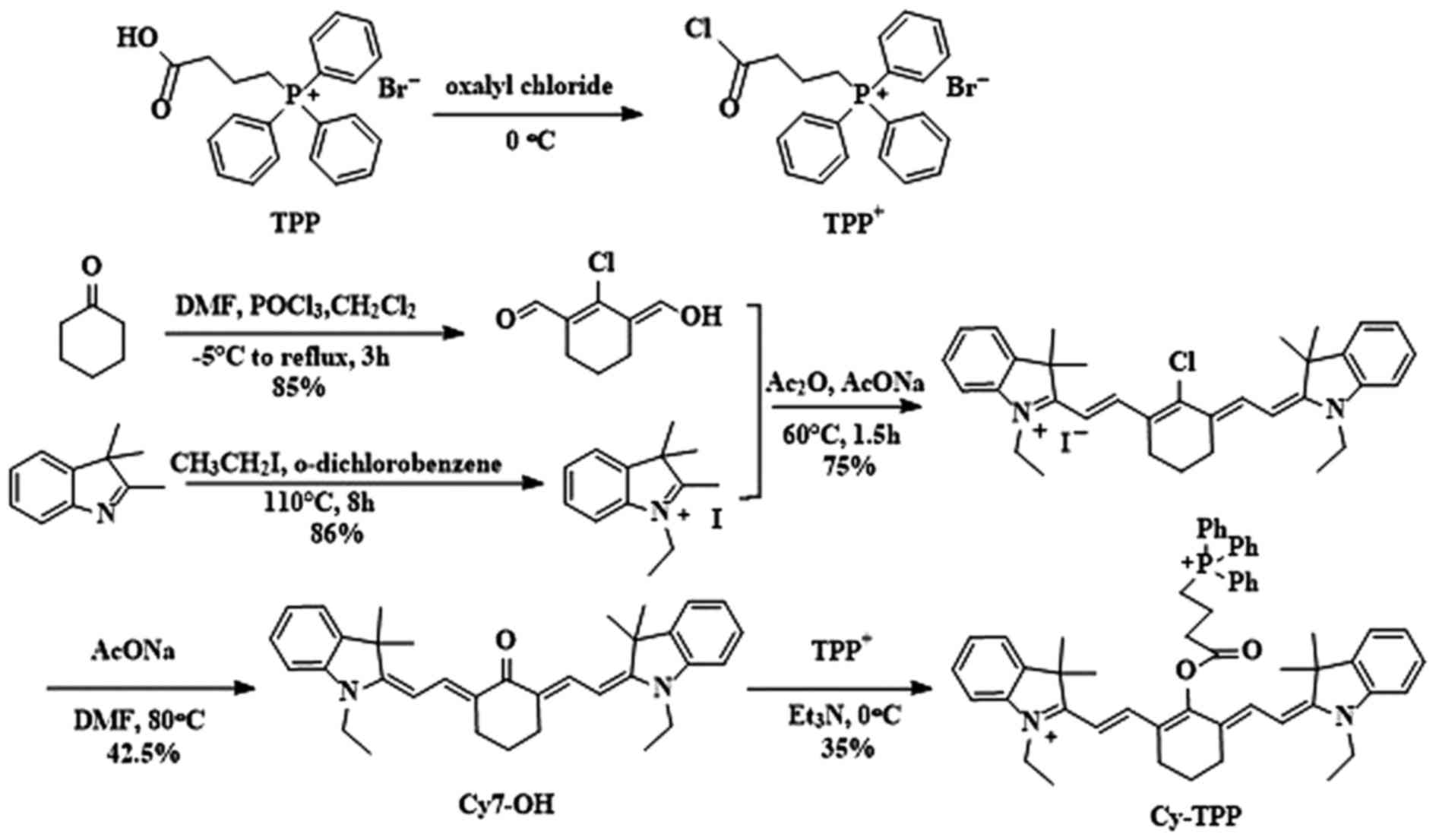
Cy-TPP was designed and synthesized by adding TPP
onto the Cy skeleton. The details of the synthetic routes and
characterizations of Cy-TPP are outlined in Fig. 1. This design was chosen for two
purposes. Firstly, it is well known that many lipophilic cations,
such as TPP and Cy, possess an overall positive charge, which
facilitates their uptake and accumulation in the mitochondria
(15). Thus, heptamethine Cy was
chosen as a signal transducer due to its favorable stability and
NIR absorption and emission profile. Secondly, the TPP moiety has
also been confirmed to be essential for antitumor activity
(16). With an aim to increase the
cytotoxic activity of heptamethine cyanine, TPP was used to enhance
its lipophilic nature and cationic charge, which can facilitate the
mitochondrial membrane potential dependent accumulation of TPP
within the negatively charged mitochondrial matrix (17). The structure of Cy-TPP was
identified by 1HNMR and MS.
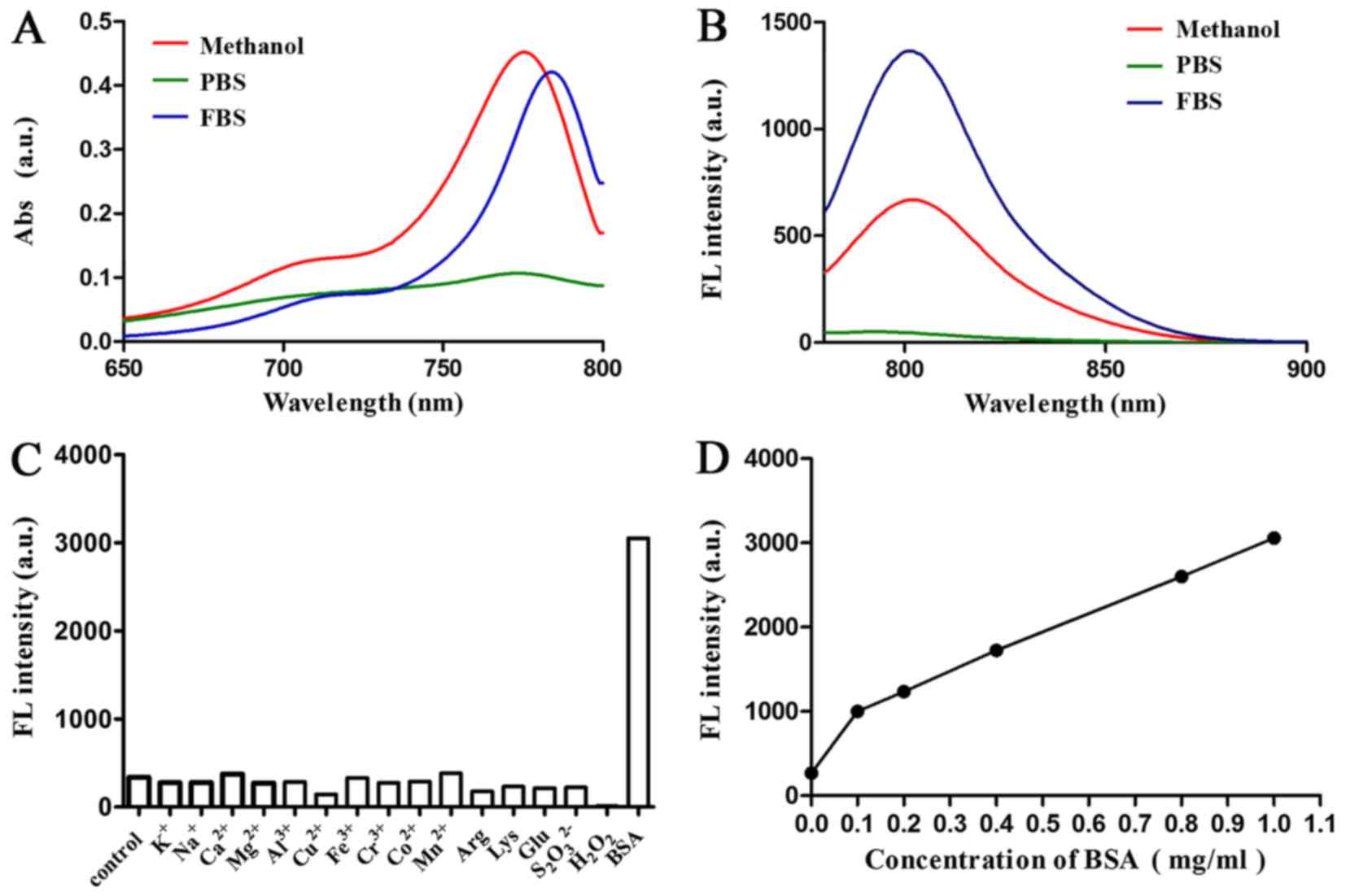
Optical properties of Cy-TPP. The optical properties
of Cy-TPP in methanol, 100% FBS and PBS were then determined
(pH=7.4). The absorption and emission peaks of Cy-TPP were all in
the NIR region (700 to 900 nm), providing 17 to 26 nm Stokes shifts
(Fig. 2A and B). The fluorescence
responses of Cy-TPP (5 mM) to various species, including
K+, Na+, Ca2+, Mg2+,
Al3+, Cu2+, Fe3+, Co2+,
Mn2+, arginine, lysine, glucose and bovine serum albumin
(BSA) was then investigated. The present study demonstrated that
the fluorescence intensity of Cy-TPP markedly increased following
the addition of BSA (Fig. 2C).
Therefore, the effect of different doses of BSA on the fluorescence
intensity of Cy-TPP was further investigated (Fig. 2D). Serum significantly increased
the intensity fluorescence intensity of Cy-TPP, indicating it may
have potential for biomedical application.
Cytotoxicity assay of Cy-TPP
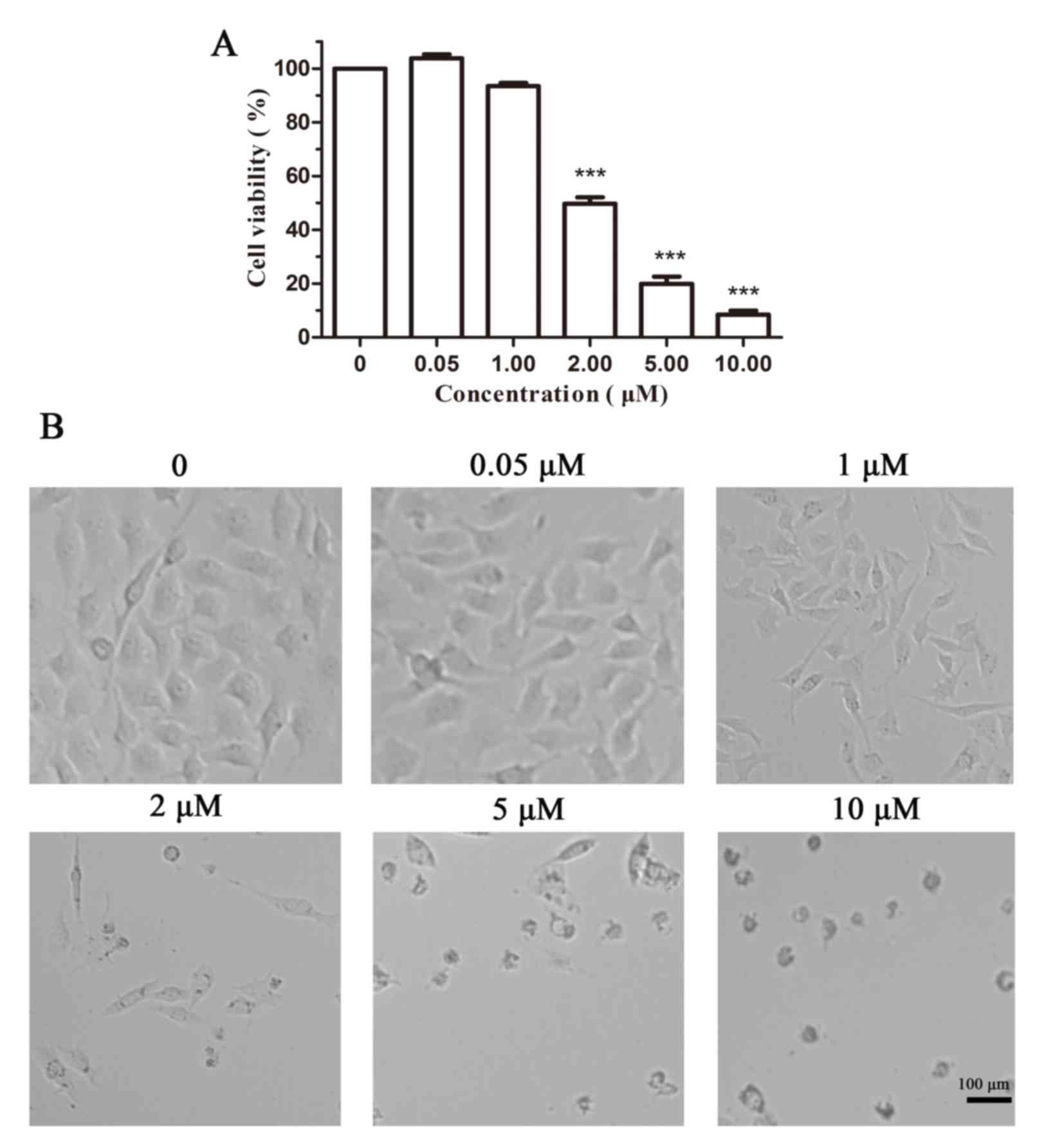
An MTT assay in B16 cells was performed to evaluate
the ability of Cy-TPP to inhibit cell proliferation. As displayed
in Fig. 3A, the inhibition rate of
cell viability in B16 cells incubated with 2.0 µM Cy-TPP was
<50%, which is essential for its further applications in the
anti-proliferation of cancer cells. Fig. 3B reveals cellular morphological
alterations in B16 cells following the addition of Cy-TPP at doses
0–10 µM. An IC50, the half-maximal inhibitory
concentration, value of 3.04 µM also indicated that Cy-TPP
significantly inhibits the proliferation of B16 cells.
Subcellular localization of
Cy-TPP
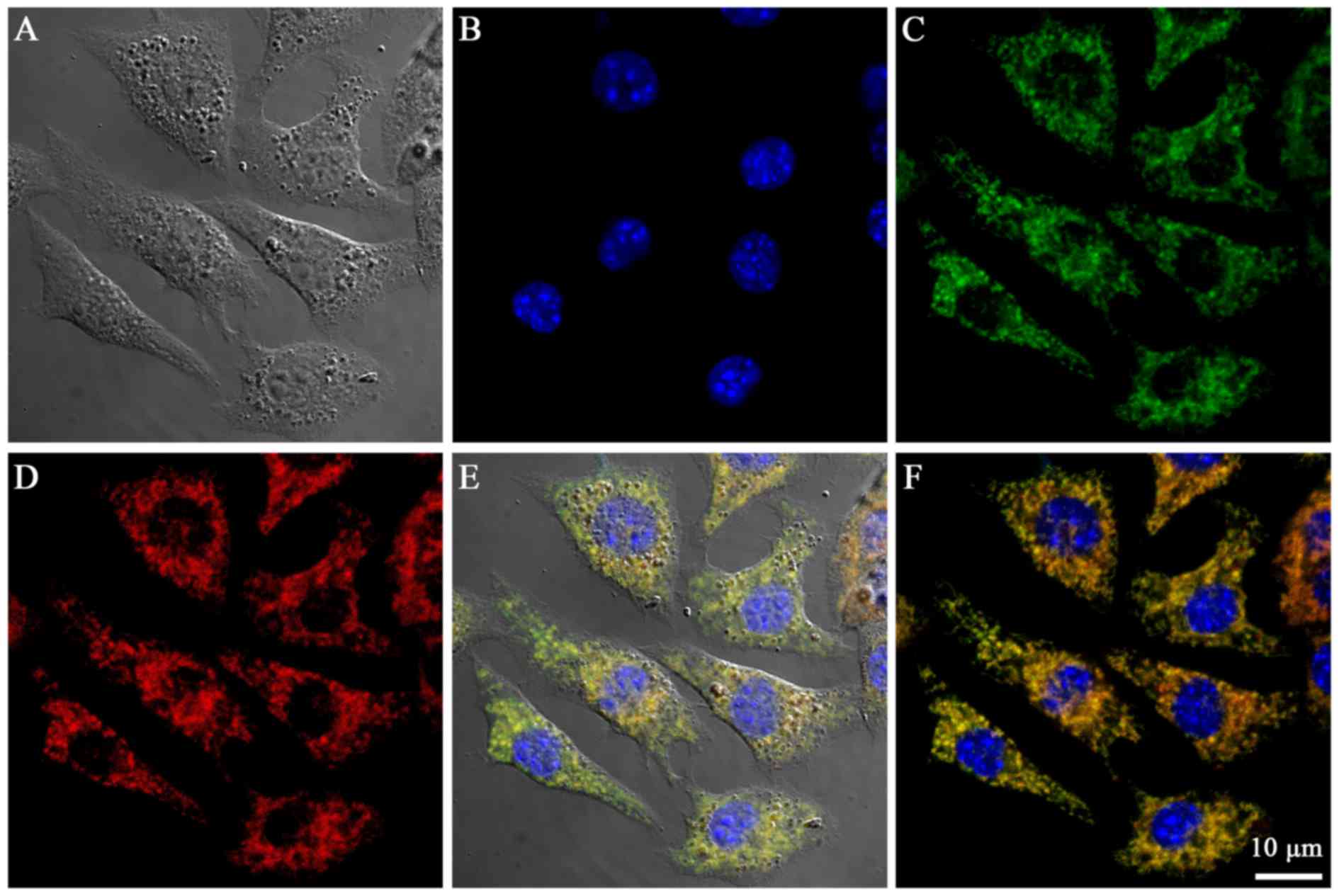
The mitochondria-targeting properties of the Cy-TPP
were determined by incubating the probes with B16 cells (Fig. 4A-D). The confocal laser scanning
microscopy analysis of the cells treated with Cy-TPP revealed
accumulation in the mitochondria (Fig.
4E and F). Quantitative analysis using the Imagine-Pro Plus 6.0
‘colocalization analysis’ tool revealed the significant
colocalization of Cy-TPP with MitoTracker Green in the mitochondria
of the cells (Pearson's correlation coefficient=8.1). The highly
efficient mitochondrial targeting ability of Cy-TPP may be
attributed to their high buffering capacity, which is generated by
lipophilic TPP.
Discussion
The most recent strategy for developing anticancer
agents with cancer mitochondria targeting and NIR fluorescence
imaging is to conjugate a fluorescent probe with functional agents
or materials (18). Heptamethine
Cy dyes, which are associated with NIR absorption and mitochondria
targeting, are promising candidates for cancer therapy and tumor
imaging. Therefore, Cy dyes may be efficient as signal transducers
to visualize therapeutic responses to anticancer drug in real
time.
In our preliminary research, TPP moiety was
demonstrated to be essential for mitochondria targeting and
antitumor activity (16).
Therefore, the aim of the present study was to conjugate the IR-780
analog Cy7-Cl with a TPP moiety to develop a novel
mitochondria-targeted heptamethine Cy derivative. As expected, the
novel compound Cy-TPP exhibited an NIR absorption and emission
profile. Confocal laser scanning microscopy analysis indicated that
Cy-TPP may specifically accumulate in the mitochondria. In
addition, Cy-TPP significantly inhibited the proliferation of B16
cells (IC50=3.04 µM) in a concentration-dependent
manner. The results of the present study demonstrated that Cy-TPP
is a multifunctional agent for mitochondria-targeted imaging and
cancer therapy, and suggest that Cy-TPP may be further developed to
combat other limitations, including multidrug resistance (MDR). It
has been previously demonstrated that MDR is significantly
associated with poor success rates in the majority of anticancer
drugs used in cancer treatments (19). It is widely accepted that
lysosome-mediated clearance of anticancer drugs, such as
doxorubicin, induces the development of MDR (20). However, mitochondria-targeted
anticancer drugs directly exert their effect on the mitochondria,
thereby evading clearance by lysosomes. Thus, the
mitochondria-targeted heptamethine Cy derivative Cy-TPP may have
the potential to reverse MDR.
In conclusion, the present study successfully
designed and synthesized a mitochondria-targeted NIR visualized
anticancer agent, Cy-TPP, with absorption and emission profiles
located in the NIR region (600–900 nm). Subcellular localization
and cell viability analysis demonstrated its mitochondria-targeted
NIR imaging capability and efficient anti-proliferation effects
(IC50=3.04 µM). In this regard, Cy-TPP may be a novel
anticancer agent with mitochondria-targeting ability and can be
visualized by NIR fluorescence imaging.
Acknowledgements
The present study was partly supported by the China
National ‘12.5’ Foundation (grant no. 2011BAJ07B04) and the
National Natural Science Foundation of China (grant no.
20972105).
References
|
1
|
Kumar R, Chaudhary K, Gupta S, Singh H,
Kumar S, Gautam A, Kapoor P and Raghava GP: CancerDR: Cancer drug
resistance database. Sci Rep. 3:14452013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strohecker AM and White E: Targeting
mitochondrial metabolism by inhibiting autophagy in BRAF-driven
cancers. Cancer Discov. 4:766–772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Humphrey RW, Brockway-Lunardi LM, Bonk DT,
Dohoney KM, Doroshow JH, Meech SJ, Ratain MJ, Topalian SL and
Pardoll DM: Opportunities and challenges in the development of
experimental drug combinations for cancer. J Natl Cancer Inst.
103:1222–1226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richards MA: The size of the prize for
earlier diagnosis of cancer in England. Brit J Cancer. 101 Suppl
2:S125–S129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dickinson BC, Srikun D and Chang CJ:
Mitochondrial-targeted fluorescent probes for reactive oxygen
species. Curr Opin Chem Biol. 14:50–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Z and Xu L: Fluorescent probes for the
selective detection of chemical species inside mitochondria. Chem
Commun (Camb). 52:1094–1119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Liu T, Su Y, Luo S, Zhu Y, Tan X,
Fan S, Zhang L, Zhou Y, Cheng T and Shi C: A near-infrared
fluorescent heptamethine indocyanine dye with preferential tumor
accumulation for in vivo imaging. Biomaterials. 31:6612–6617. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang X, Shi C, Tong R, Qian W, Zhau HE,
Wang R, Zhu G, Cheng J, Yang VW, Cheng T, et al: Near IR
heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res.
16:2833–2844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo S, Zhang E, Su Y, Cheng T and Shi C: A
review of NIR dyes in cancer targeting and imaging. Biomaterials.
32:7127–7138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan X, Luo S, Wang D, Su Y, Cheng T and
Shi C: A NIR heptamethine dye with intrinsic cancer targeting,
imaging and photosensitizing properties. Biomaterials.
33:2230–2239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim K, Kim JH, Park H, Kim YS, Park K, Nam
H, Lee S, Park JH, Park RW, Kim IS, et al: Tumor-homing
multifunctional nanoparticles for cancer theragnosis: Simultaneous
diagnosis, drug delivery, and therapeutic monitoring. J Control
Release. 146:219–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Majumdar D, Peng XH and Shin DM: The
medicinal chemistry of theragnostics, multimodality imaging and
applications of nanotechnology in cancer. Curr Top Med Chem.
10:1211–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Sun J, Zhang W, Ma X, Lv J and
Tang B: A near-infrared ratiometric fluorescent probe for rapid and
highly sensitive imaging of endogenous hydrogen sulfide in living
cells. Chem Sci. 4:2551–2556. 2013. View Article : Google Scholar
|
|
15
|
Xu W, Teoh CL, Peng J, Su D, Yuan L and
Chang YT: A mitochondria-targeted ratiometric fluorescent probe to
monitor endogenously generated sulfur dioxide derivatives in living
cells. Biomaterials. 56:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pathania D, Millard M and Neamati N:
Opportunities in discovery and delivery of anticancer drugs
targeting mitochondria and cancer cell metabolism. Adv Drug Deliv
Rev. 61:1250–1275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ross MF, Kelso GF, Blaikie FH, James AM,
Cochemé HM, Filipovska A, Da Ros T, Hurd TR, Smith RA and Murphy
MP: Lipophilic triphenylphosphonium cations as tools in
mitochondrial bioenergetics and free radical biology. Biochemistry
(Mosc). 70:222–230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan X, Luo S, Wang D, Su Y, Cheng T and
Shi C: A NIR heptamethine dye with intrinsic cancer targeting,
imaging and photosensitizing properties. Biomaterials.
33:2230–2239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saw PE, Park J, Jon S and Farokhzad OC: A
drug-delivery strategy for overcoming drug resistance in breast
cancer through targeting of oncofetal fibronectin. Nanomedicine.
13:713–722. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhitomirsky B and Assaraf YG: Lysosomes as
mediators of drug resistance in cancer. Drug Resist Updat.
24:23–33. 2016. View Article : Google Scholar : PubMed/NCBI
|