Introduction
Aminoglycosides are renowned antibiotics primarily
used in clinics for the treatment of tuberculosis, and effectively
cure infections caused by aerobic gram-negative bacteria (1–3).
Gentamicin (GM) is a widely-used aminoglycoside antibiotic that has
attracted increasing attention recently due to its potent ototoxic
side effects. Typically, aminoglycoside antibiotics are absorbed
and accumulated in the inner ear lymph (4–6);
hair cells of the inner ear have a high affinity for them. Previous
studies have demonstrated in an ototoxicity model of GM that death
of hair cells is attributed to apoptosis (7), and apoptosis is closely associated
with autophagy (8).
Autophagy is a series of complex process within the
cell where the components or foreign invaders are digested. It is
an important underlying mechanism that maintains the steady and
resistant adversity of the cells, and regulates dynamic alterations
in cell membrane structure following lysosome-mediated degradation
of cellular proteins and organelles (9–12).
Autophagy is considered to be a general response to stress
contributing to cell death; alternatively, it may be a cell
cytoprotective mechanism (13,14).
Autophagy formation is comprised of the following four processes:
Induction and formation of the pre-autophagosome structure,
autophagosome formation, fusion with lysosomes and degradation
(15). Homologous proteins that
serve important roles in autophagy have additionally been
identified in mammalian cells (12,16),
including microtubule-associated protein 1A/B-light chain 3 (LC3),
the mammalian ortholog of yeast autophagy-related protein 8 (Atg8).
The conjugation of LC3 to lipid phosphatidylethanolamine to form
LC3-II, located in the autophagy body membrane, reflects the level
of autophagy (17,18). To date, 34 different types of Atg
protein have been determined in yeast (19), which serve a key role in formation
of the autophagosome.
Depending on the experimental conditions, autophagy
may directly induce cell death or act as an underlying mechanism
for cytoprotection. Despite recent rapid progress in autophagy
research, little is understood about autophagy in the organ of
Corti in GM-induced cochleotoxicity.
Cochleotoxicity induced by GM is a typical condition
of the cochlea that generates stress, leading to inner ear hair
cell death and tissue damage. To assess whether autophagy occurs in
GM-induced cochlear injury and whether it contributes to inner ear
hair cell loss or survival, the present study used a GM ototoxic
experimental model to examine the underlying mechanisms. Apoptosis
has been reported to contribute to cochleotoxicity (7,20,21);
however, the interactions between autophagy and apoptosis to induce
cochlear pathology remain unclear. Autophagy is suppressed by Bcl-2
and related antiapoptotic family members, by binding to the B cell
lymphoma-2 (Bcl-2) homology 3 (BH3) domain of Beclin-1, which is an
essential component of the class III phosphatidyl
inositol-3-kinase/Vps34 complex necessary for autophagy induction.
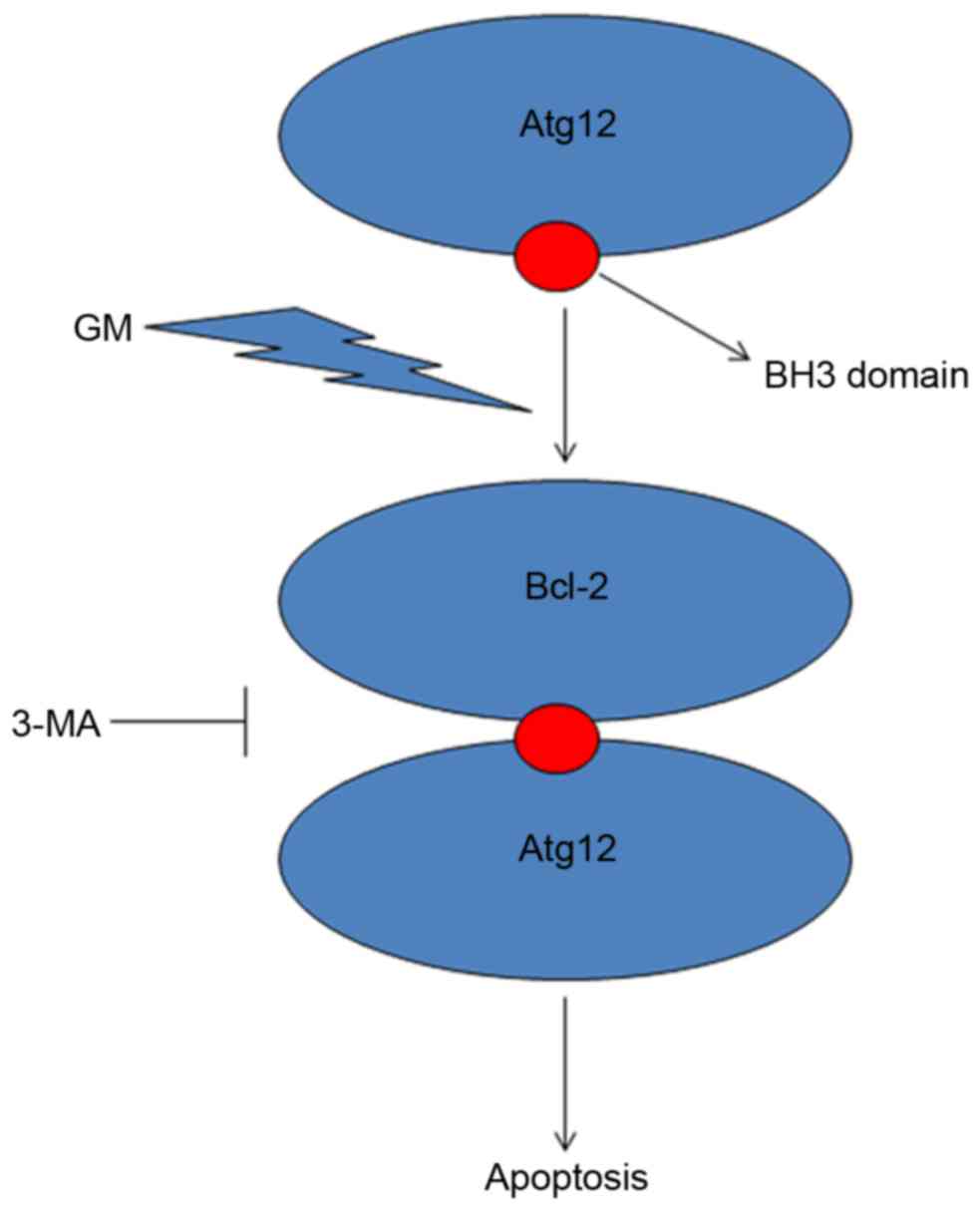
The present study demonstrated that Atg12 interaction with Bcl-2
links autophagy and apoptosis during GM treatment of inner ear hair
cells.
Materials and methods
Materials
GM sulfate (G1272) was purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany); C57BL/6J mice were acquired from
the Model Animal Research Center of Nanjing University (Nanjing,
China); 3-methyladenine (3-MA) was purchased from Sigma-Aldrich;
Merck Millipore (M9281) and Dulbecco's modified Eagle's medium
(DMEM) was obtained from Gibco; Thermo Fisher Scientific, Waltham,
MA, USA. The following primary antibodies were used: Rabbit
anti-Atg12 (2011S) and rabbit anti-Bcl-2 (50E3) from Cell Signaling
Technology, Inc. (Danvers, MA, USA); rabbit anti-LC3 (L7543;
Sigma-Aldrich; Merck Millipore) and mouse anti-Bcl-2 (SC-7382;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The following
secondary antibodies were used: Cyanine (Cy3)-conjugated donkey
anti-rabbit (A31572; Thermo Fisher Scientific, Inc.), horseradish
peroxidase (HRP)-conjugated goat anti-rabbit (111–035-003; Jackson
ImmunoResearch Laboratories, Inc., Westgrove, PA, USA) and IgG
HRP-conjugated goat anti-mouse (SC-2005; Santa Cruz Biotechnology,
Inc.). Fluorescein isothyanate (FITC)-phalloidin (P5282) and DAPI
(D9542) were purchased from Sigma-Aldrich; Merck Millipore, and a
Pierce Classic Immunoprecipitation kit was obtained from Thermo
Fisher Scientific, Inc. (26146).
Dissection and culturing organ of
Corti explants
A total of 160 C57BL/6J mice (age, 4 days;
male:female, 1:1; weight, 3–5 g) were purchased from the Model
Animal Research Center of Nanjing University (Nanjing, China). Mice
were housed under a 12-h light-dark cycle at 22±1°C and with a
humidity of 56±5%, with 5 mice per cage. Mice received an ad
libitum 0.3% sodium diet. The care and use of animals were in
strict accordance with the ‘Guiding Directive for Humane treatment
of Laboratory Animals’ issued by the Chinese National Ministry of
Science and Technology. The Institutional Animal Care and Use
Committee of Nanjing Medical University and Nanjing Drum Tower
Hospital provided ethical approval for all procedures. All efforts
were made to minimize the number of animals used and to prevent
their suffering. Dissection and culturing was performed as
previously described (22).
Following cleaning with 75% ethanol, pups were decapitated and the
cochleae were carefully dissected. Soft tissues and the cartilage
were removed, and the cochlear epithelium was isolated. The
organotypic cultures were maintained in serum-free media consisting
of DMEM supplemented with glucose and 30 U/ml penicillin G
(Sigma-Aldrich; Merck KGaA) at 37°C in a humidified atmosphere
containing 5% CO2. After 24 h, the culture medium was
removed and replaced with fresh media containing 100 µm/l GM or 10
mmol/l 3-MA and incubated at 37°C for 24 h. Cochlear explants were
randomly divided into three groups: Control (culture medium),
GM-treated (culture medium + GM) and GM- and 3-MA-treated (culture
medium + GM + 3-MA).
Immunocytochemical analysis
A total of 48 h after dissection, the organ of Corti
were immediately processed for immunofluorescent staining. Cochlear
explants were fixed with 4% paraformaldehyde for 30 min. Following
fixation, they were rinsed with 0.1 mM phosphate-buffered saline
(PBS) three times and blocked with 5% normal horse serum
(Sigma-Aldrich; Merck KGaA) in PBS (pH 7.4) with 0.1% Triton X-100
for 1 h at room temperature. The tissues were incubated with an
anti-LC3 primary antibody overnight at 4°C. The next day the
tissues were washed three times with 0.1% Tween in PBS, followed by
incubation with the secondary Cy3-conjugated donkey anti-rabbit
antibody for 2 h at room temperature. Following this,
FITC-phalloidin and DAPI were added to label hair cell stereocilia
and the nucleus, respectively. Following immunostaining, samples
were mounted in Southern Biotech Fluoromount-G™ Slide Mounting
medium (Thermo Fisher Scientific, Inc.). Cochleae were dissected
into apical, middle and basal turns, and were imaged using a Nikon
confocal fluorescence microscope (TE2000-U; Nikon Corporation,
Tokyo, Japan).
Cytocochleograms and quantification of
cochlear hair cells
For HC quantification in the GM-treated and GM +
3-MA-treated samples, the entire cochlea was imaged using a
quantified analytic system for cochlea hair cells as previously
described (23,24). Missing inner hair cells (IHCs) and
outer hair cells (OHCs) were counted over 0.24-mm intervals under a
light microscope (magnification, ×400). Cochleograms were generated
demonstrating the percentage hair cell loss as a function of
percentage distance from the apex. Negative controls were included
to determine the amount of background staining. The Cochlea Anatomy
Labshell Program (supplied as a gift from Professor Dalian Ding,
Buffalo University, Buffalo, NY, USA) was used to compute the
percentage of missing hair cells in 10% intervals along the length
of the cochlea, starting from the apex. For each cochlea, the mean
density of HCs was determined (apical, 10–20% distance from the
cochlear apex; middle, 40–50% distance from the cochlear apex;
basal, 70–80% distance from the cochlear apex).
Transmission electron microscopy (TEM)
and scanning electron microscopy (SEM)
Cochlea were collected after 48 h and fixed in 2.5%
glutaraldehyde in 0.1 M PBS at 4°C overnight. The samples were
subsequently post-fixed in 1% osmium tetroxide and further
processed by standard procedures, including dehydration,
infiltration and polymerization in araldite. Ultrathin sections
were post-stained and examined using a H-7650 transmission electron
microscope (Hitachi, Ltd., Tokyo, Japan) (25). For SEM, the fixation procedure was
performed as described above, following which samples were
dehydrated in ethanol, critical point dried with a vacuum and
coated with gold-palladium. The samples were observed by SEM for
morphological description and for potential acoustic trauma
assessment. Samples were imaged under a Hitachi S-3000 N Scanning
Electron Microscope (Hitachi, Ltd.).
Western blot analysis and
co-immunoprecipitation assay
Western blot analysis was performed to assess the
protein expression levels of LC3 and Bcl-2 from cochlear explants
following exposure to the various experimental conditions as
described above. The cochleas were lysed with ice-cold
radioimmunoprecipitation assay buffer (PP109; Protein
Biotechnology, Beijing, China), containing Phosphatase Inhibitor
Cocktail (Roche, Basel, Switzerland), to obtain the tissue
proteins. Protein concentrations were measured using a
bicinchoninic acid Protein Quantification kit (PP202, Protein
Biotechnology) according to the manufacturer's protocol, using
GAPDH (KC-5G4, 1:2,000; KangChen Biotech Inc., Shanghai, China) as
the reference protein. Equivalent amounts of tissue lysate protein
(20 µg) were separated by gel electrophoresis on a 4–20% gradient
SDS-PAGE gel and transferred onto polyvinylidene fluoride
membranes. Blots were blocked with 5% non-fat milk and then
incubated with anti-LC3 (1:1,000) and anti-Bcl-2 (1:1,000) rabbit
polyclonal antibodies at 4°C overnight. Membranes were then washed
with 0.1% Tween20 in PBS and probed with an HRP-conjugated goat
anti-rabbit (1:5,000) secondary antibody for 2 h at room
temperature. The bound antibody complexes were detected using an
ECL Plus kit (Pierce; Thermo Fisher Scientific, Inc.) and a Storm
840 PhosphorImager system (Molecular Devices, LLC, Sunnyvale, CA,
USA).
For endogenous co-immunoprecipitation, a rabbit
monoclonal anti-Atg12 antibody was used. Precleared tissue lysates
were incubated with anti-Atg12 at 4°C overnight, followed by
incubation with protein G agarose beads for 1 h at room
temperature. Beads were washed with lysis buffer and boiled in
sample buffer. Western blot detection of Bcl-2 was performed with
mouse anti-Bcl-2 (1:1,000) at 4°C overnight. Membranes were then
washed with 0.1% Tween-20 in PBS and probed with an HRP-conjugated
goat anti-mouse (1:5,000) secondary antibody for 2 h at room
temperature. The bound antibody complexes were detected using an
ECL Plus kit using a Storm 840 PhosphorImager system.
Statistical analysis
The data were presented as the mean ± standard
deviation. All experiments were repeated at least three times.
Statistical analyses were conducted using Microsoft Excel 2007
(Microsoft Corporation, Redmund, WA, USA) and GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
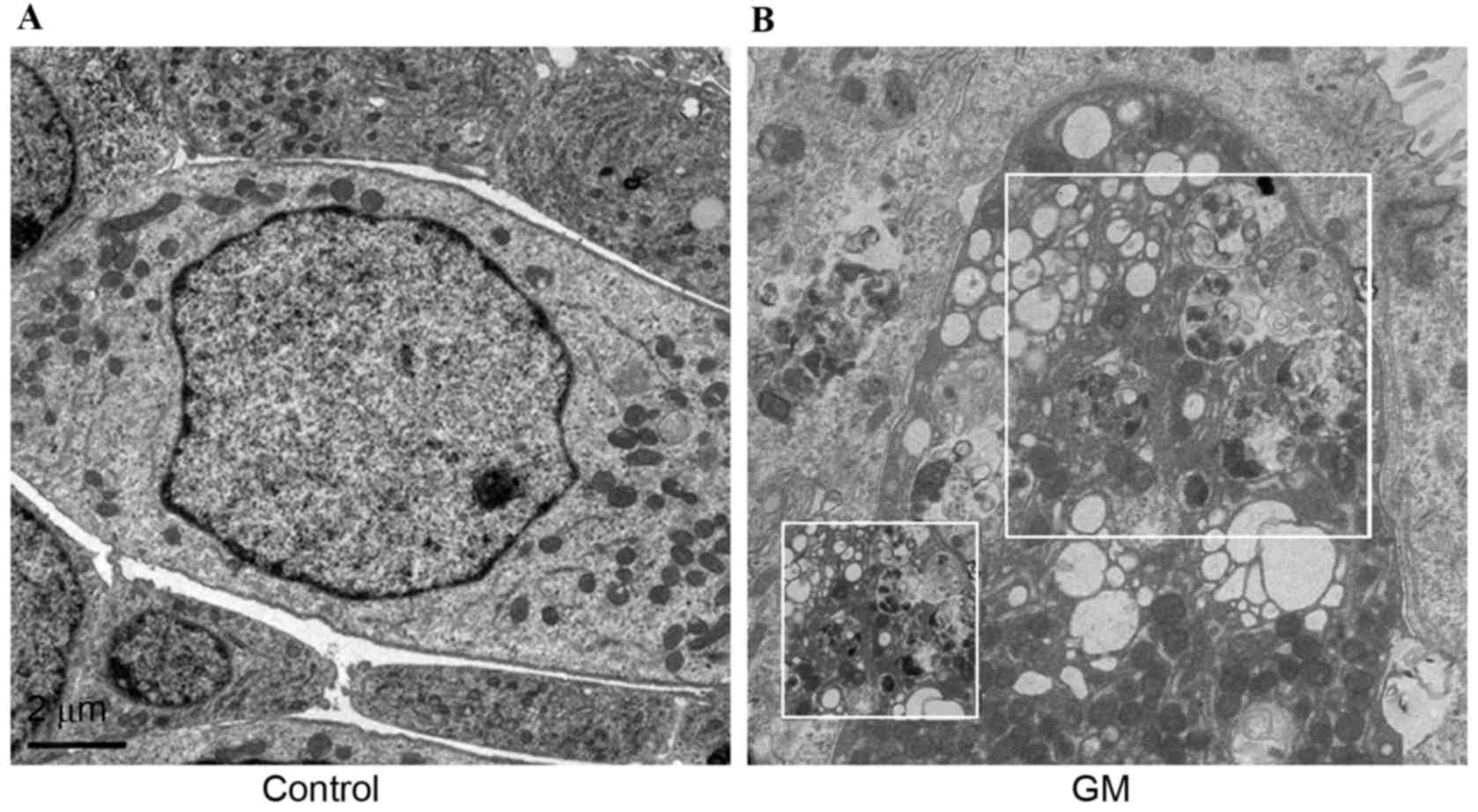
TEM analysis of autophagy in GM
GM-induced autophagy in the cochlea was evaluated by
TEM, and the morphology of hair cells in GM-treated cochlea was
investigated. As presented by representative micrographs, compared
with the control (Fig. 1A),
GM-treated cells (Fig. 1B) had
autophagosomes with characteristic double or multiple membranes
after 24 h. In addition, numerous autophagic vacuoles or vesicles
were observed.
Protective role of 3-MA on GM-induced
hair cell loss
In order to assess cochleotoxicity and the
protective underlying mechanism of 3-MA, cells were double-stained
with FITC-phalloidin (green) to label hair cells, and DAPI (blue)
as a nuclear stain (Fig. 2A).
Previously, cochlear cultures from mice were incubated in medium
containing 10 mmol/l 3-MA and 0.1 mM GM for 24 h. Loss of hair
cells indirectly reflects cochleotoxicity; percentage hair cell
loss was increased in the GM-treated group compared with the
control group. However, the addition of 3-MA attenuated this
effect, particularly in the middle region of the cochlea (Fig. 2B). Compared with untreated
controls, GM severely distorted the anatomy of the organ of Corti
in the GM-treated group, where the hair cells were deformed and
disordered, and gaps were clearly observed. Following 3-MA
treatment, the number and alignment of cells in the organ of Corti
was improved compared with the GM group. FITC-phalloidin-labeled
hair cells were counted in each group after 24 h. No significant
differences were observed in the number of hair cells in the apex
between groups. Conversely, in the GM-treated group, OHC and IHC
counts were significantly decreased in the middle region of the
cochlea. However, in GM + 3-MA-treated group, 3-MA significantly
protected against OHC and IHC loss in the middle region of the
cochlea. In summary, these results suggested that 3-MA treatment
significantly protects against GM-induced hair cell loss in the
middle region of the cochlea.
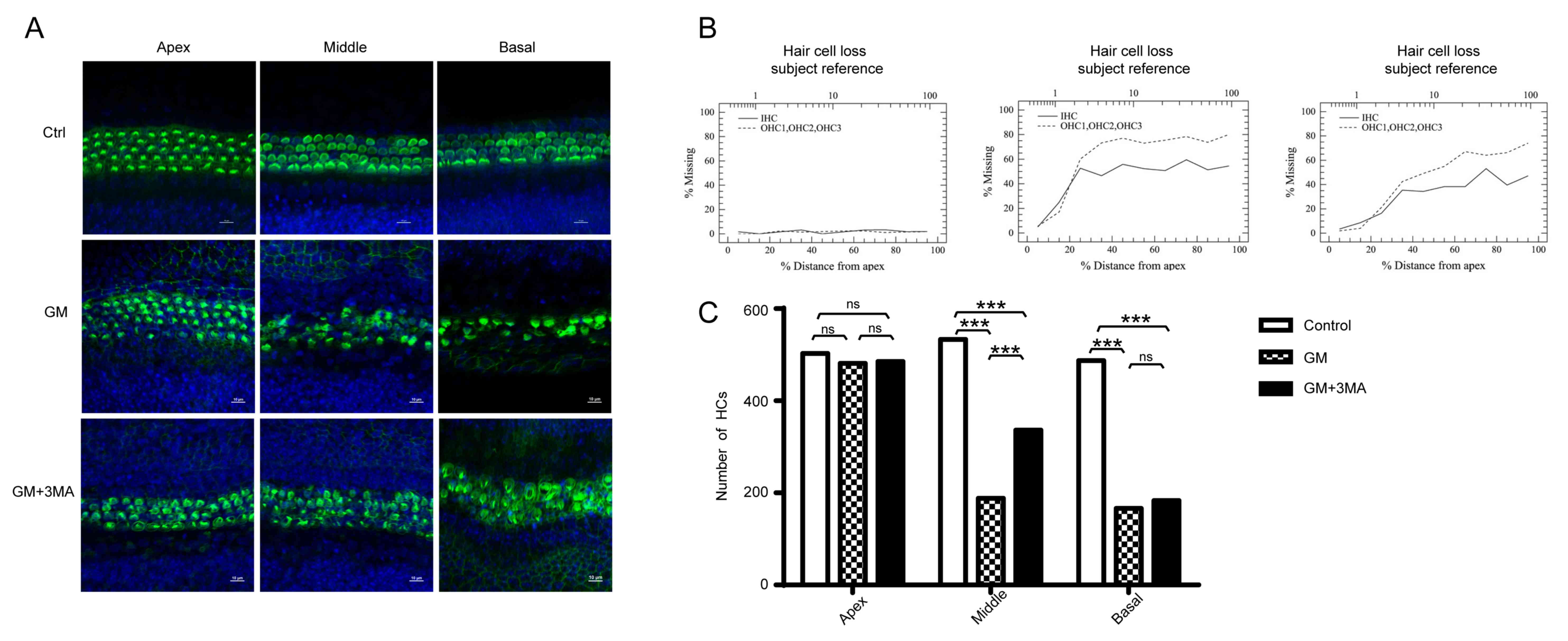 | Figure 2.Morphological and quantitative
analysis of hair cells in the different groups. (A) Explant
cultures treated with GM, GM + 3-MA and control (no drug) stained
with fluorescein isothyanate-phalloidin and DAPI exhibited
variations in loss of hair cells in the apical, middle and basal
regions. (B) Cytocochleograms representing the percentage of IHC
and OHC loss as a percentage of total distance from the apex of the
cochlea (n=6/group). (C) Hair cell density in the apical, middle
and basal regions of the cultured cochlea treated with 100 µM GM
alone or 100 µM GM + 10 mM 3-MA, compared with non-treated
controls. OHC and IHC counts were significantly decreased in the
middle region of the cochlea. Data are presented as the mean ±
standard deviation, all experiments were repeated at least three
times. ***P<0.001. GM, gentamicin; IHC, inner hair cell; OHC,
outer hair cell; 3-MA, 3-methyladenine; HCs, hair cells; Ctrl,
control; ns, not significant. |
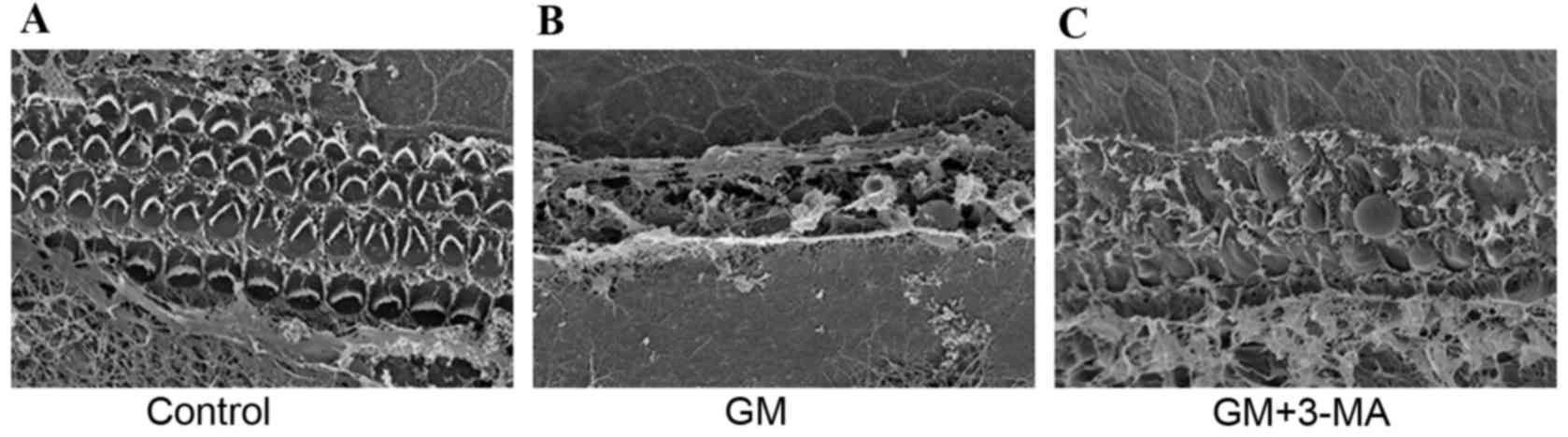
SEM examination of the cochlear
surface structure
SEM was performed to examine the surface structure
of the cochlea. Examination of the organ of Corti of the control
group (Fig. 3A) indicated three
rows of OHCs and one row of IHCs. Conversely, cochleae from the
GM-induced ototoxic group (Fig.
3B) exhibited loss of the well-known architecture of hair cells
of the organ of Corti. The stereocilia of the OHCs and IHCs were
disrupted, disarrayed, fused, had focal loss or were completely
absent. Treatment with 3-MA preserved the majority of hair cells;
however, the stereocilia became more dispersed (Fig. 3C).
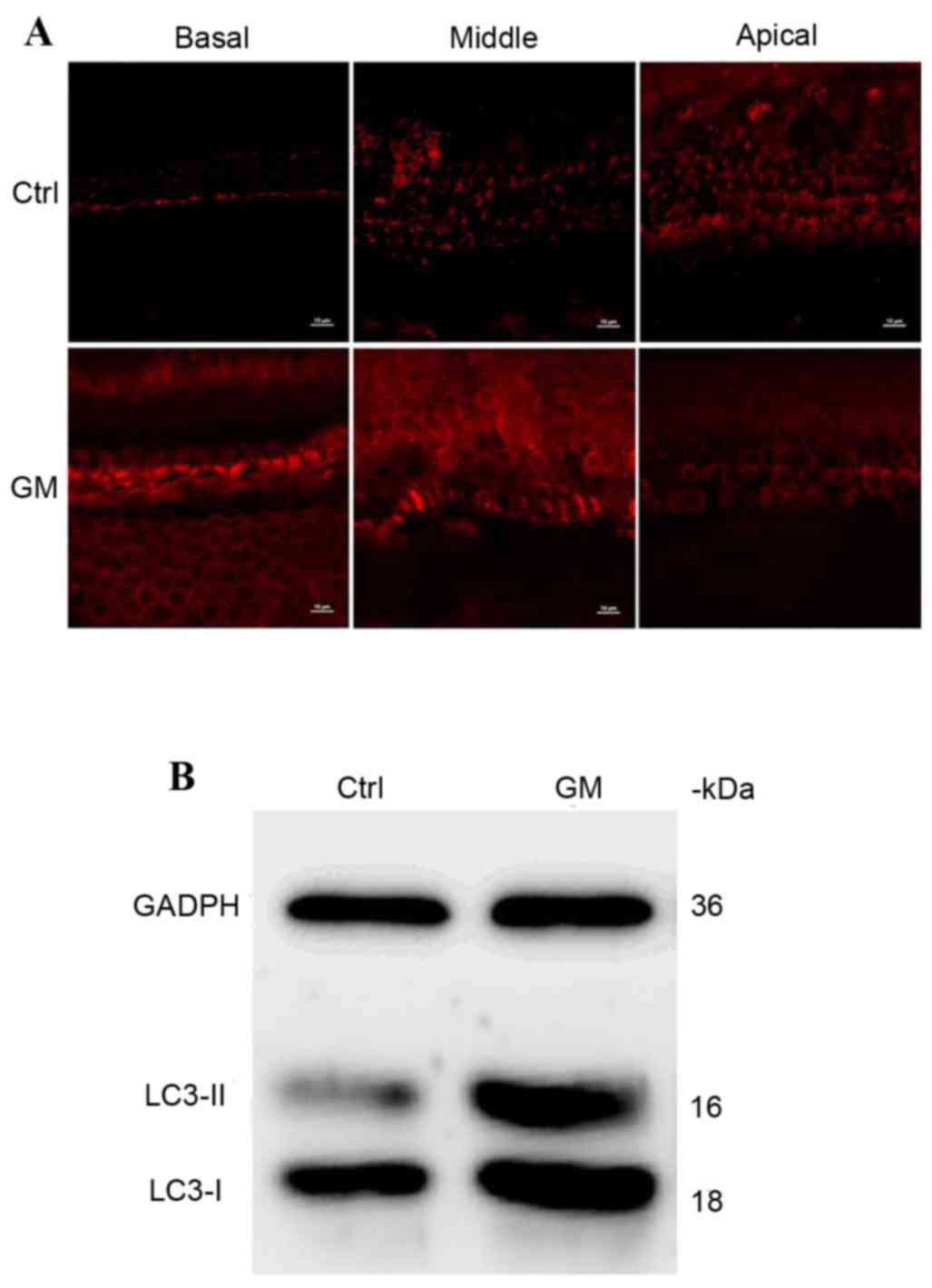
Upregulation of LC3 protein expression
levels following GM-induced injury
To examine whether GM injury affects protein
expression levels of LC3, immunostaining and western blotting were
performed on each group. LC3 was detected in the control and
GM-treated groups; however, expression levels were markedly
upregulated in the GM-treated group (Fig. 4A). In western blots, LC3 protein
presents with double bands; one is unconjugated and the other is
conjugated with phosphotidylethanolamine to form the LC3-II
complex. This is recruited to the autophagosome membrane; increased
expression levels reflects the level of autophagy in cells. LC3
protein levels were overexpressed in the GM-treated group compared
with the control group (Fig.
4B).
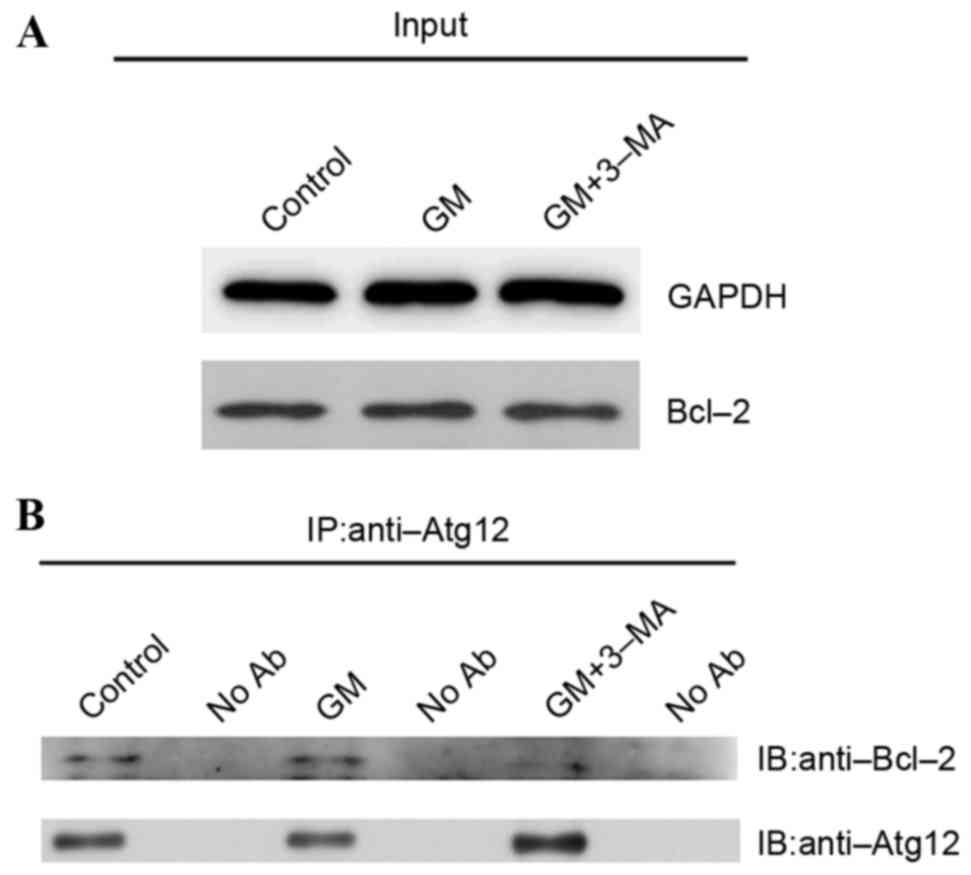
Interaction of Atg12 with Bcl-2 to
promote apoptosis in GM-treated cochleae
To assess the interaction between Atg12 and Bcl-2 in
cochleae, western blotting was performed to observe protein
expression levels of Bcl-2 in the GM-, GM + 3-MA-treated and
control groups. Bcl-2 was equally expressed in all three groups
(Fig. 5A). Co-immunoprecipitation
was performed using a rabbit anti-Atg12 antibody to observe the
protein complexes involved in ototoxicity and further analyze the
interaction between Atg12 and Bcl-2 (Fig. 5B). Bcl-2 and Atg12 were
co-expressed in the GM-treated and control groups, indicating that
they interact in the cochlea. However, when autophagy was inhibited
by 3-MA, Bcl-2 protein expression levels were reduced,
demonstrating that 3-MA interrupts the Atg12 and Bcl-2 interaction
in GM-induced cochleotoxicity. As a result, the apoptotic function
of Atg12 is inhibited, reflecting an increase in the number of hair
cells. In summary, these results suggested that following
GM-induced cochleotoxicity, Atg12 and Bcl-2 interact to promote
cochlear apoptosis, and 3-MA rescues this effect by inhibiting the
Atg12-Bcl-2 complex.
Discussion
Aminoglycosides, including GM, are primarily used
for the treatment of aerobic systemic infection caused by
gram-negative bacilli (26).
However, GM treatment may induce hearing loss and balance disorders
by damaging IHC structure in the cochlea (27). The clinical use of aminoglycosides
including GM is limited due to their potential ototoxicity and
nephrotoxicity (28). In recent
years, a variety of cephalosporins and quinolones have been widely
used in clinics because of their longer post-antibiotic effects
against Pseudomonas aeruginosa, Klebsiella pneumoniae
and Escherichia coli; however, aminoglycosides are used for
the treatment of severe infections from aerobic gram-negative
bacillus, including meningitis, trauma, burns and respiratory
tract, urinary tract, skin soft tissue, joint and gastrointestinal
tract infections (29). Previous
studies have reported that the GM-induced hearing loss is
associated with damage of cilia and hair cells (1,30).
An important goal of preventing the ototoxicity of
GM is to protect the hair cells from apoptosis; a well-known
primary death mode following ototoxicity (7). Numerous drugs have been demonstrated
to reduce apoptosis induced by GM ototoxicity (31–33),
and apoptosis has been reported to be closely associated with
autophagy (8). Notably, autophagy
may cause cells to survive or may lead to cell death, although it
is unclear which factors influence these transitional states
(14,34). Recent research on autophagy and the
underlying mechanisms has developed; however, little is understood
about the regulation and physiology of autophagy in cochlear
tissue. The present study investigated GM-induced hair cell loss in
neonatal mouse cochleae culture and demonstrated evidence of
autophagy. Notably, it was revealed that inhibition of autophagy
may influence the ototoxicity induced by GM.
The experiments in the present study were conducted
in an in vitro model, where cultures of the organ of Corti
were incubated with GM to induce cell stress injury that leads to
hair cells death. This model has been used and characterized in
numerous studies (35–37). The present study demonstrated the
occurrence of autophagy in this model using multiple techniques,
including examination of autophagosomes by electron microscopy,
immunofluorescence and immunoblot analysis of LC3-II formation. In
the current study, it was demonstrated that autophagic expression
was increased following GM treatment; protein expression levels of
the autophagy marker LC3 were upregulated.
Numerous previous studies have suggested that the
interaction between autophagy and apoptosis extends to ‘core
machinery’ proteins, which serve as essential components of one
signaling pathway and regulators of the other (34,38,39).
Another important autophagy regulator identified in the present
study was Bcl-2. Bcl-2 is well recognized as an anti-apoptotic
protein. In previous studies, Bcl-2 has been indicated to inhibit
autophagy by binding to Beclin-1 (40–42).
Bcl-2 binding to Beclin-1 may disrupt a protein complex (involving
Beclin-1, ultra violet radiation resistance-associated gene
protein, serine/threonine-protein kinase VPS15 and others) that is
critical to vesicle nucleation, the start point of autophagosome
formation (43). In certain cells,
Atg12 is crucial for apoptosis, in addition to serving a role in
autophagy (39). The autophagic
function of Atg12 does not necessarily interfere with its role in
apoptosis; the dual nature of these proteins suggests that
interaction between the two signaling pathways is important for the
activation and inhibition of apoptosis.
Atg12 contains a region with a sequence similar to
Bcl-2 homology 3-domain that is required for its interaction with
Bcl-2 to induce apoptosis (18).
Co-immunoprecipitation studies have indicated that Atg12 interacts
with Bcl-2 in the cochlea. In conclusion, the results of the
present study suggested that Atg12 has an apoptotic function via
binding to Bcl-2 (Fig. 6). This
binding may subsequently function as a molecular switch to activate
apoptosis. These results implicate Atg12 as a potential therapeutic
target for the treatment of GM-induced cochlear hair loss.
Acknowledgements
The present study was supported by the Nanjing
Science and Technology Development Foundation (grant no. 201303003)
and the Nanjing Medical Science and Technique Development
Foundation (grant no. QRX11079). The authors would like to thank
Professor Junfeng Zhang and Lei Dong from the State Key Laboratory
of Pharmaceutical Biotechnology of Nanjing University (Nanjing,
China), for providing technical assistance.
References
|
1
|
Bertolaso L, Bindini D, Previati M,
Falgione D, Lanzoni I, Parmeggiani A, Vitali C, Corbacella E,
Capitani S and Martini A: Gentamicin-induced cytotoxicity involves
protein kinase C activation, glutathione extrusion and
malondialdehyde production in an immortalized cell line from the
organ of corti. Audiol Neurootol. 8:38–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nordang L and Anniko M: Nitro-L-arginine
methyl ester: A potential protector against gentamicin ototoxicity.
Acta Otolaryngol. 125:1033–1038. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wargo KA and Edwards JD:
Aminoglycoside-induced nephrotoxicity. J Pharm Pract. 27:573–577.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hahn H, Salt AN, Schumacher U and Plontke
SK: Gentamicin concentration gradients in scala tympani perilymph
following systemic applications. Audiol Neurootol. 18:383–391.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salt AN, King EB, Hartsock JJ, Gill RM and
O'Leary SJ: Marker entry into vestibular perilymph via the stapes
following applications to the round window niche of guinea pigs.
Hear Res. 283:14–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
King EB, Salt AN, Eastwood HT and O'Leary
SJ: Direct entry of gadolinium into the vestibule following
intratympanic applications in Guinea pigs and the influence of
cochlear implantation. J Assoc Res Otolaryngol. 12:741–751. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forge A and Li L: Apoptotic death of hair
cells in mammalian vestibular sensory epithelia. Hear Res.
139:97–115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marino G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deter RL, Baudhuin P and De Duve C:
Participation of lysosomes in cellular autophagy induced in rat
liver by glucagon. J Cell Biol. 35:C11–C16. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klionsky DJ: Autophagy: From phenomenology
to molecular understanding in less than a decade. Nat Rev Mol Cell
Biol. 8:931–937. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki K and Ohsumi Y: Molecular machinery
of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS
Lett. 581:2156–2161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Periyasamy-Thandavan S, Jiang M, Wei Q,
Smith R, Yin XM and Dong Z: Autophagy is cytoprotective during
cisplatin injury of renal proximal tubular cells. Kidney Int.
74:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levine B and Yuan J: Autophagy in cell
death: An innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reggiori F and Klionsky DJ: Autophagy in
the eukaryotic cell. Eukaryot Cell. 1:11–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. Embo J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD,
Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M
and Ohsumi Y: A unified nomenclature for yeast autophagy-related
genes. Dev Cell. 5:539–545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lautermann J, Dehne N, Schacht J and
Jahnke K: Aminoglycoside- and cisplatin-ototoxicity: From basic
science to clinics. Laryngorhinootologie. 83:317–323. 2004.(In
German). PubMed/NCBI
|
|
21
|
Chi le NU, Tabuchi K, Nakamagoe M,
Nakayama M, Nishimura B and Hara A: Ceramide/sphingomyelin cycle
involvement in gentamicin-induced cochlear hair cell death. Arch
Toxicol. 89:415–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding D, Stracher A and Salvi RJ: Leupeptin
protects cochlear and vestibular hair cells from gentamicin
ototoxicity. Hear Res. 164:115–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding DL, Wang J, Salvi R, Henderson D, Hu
BH, McFadden SL and Mueller M: Selective loss of inner hair cells
and type-I ganglion neurons in carboplatin-treated chinchillas.
Mechanisms of damage and protection. Ann N Y Acad Sci. 884:152–170.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hofstetter P, Ding D, Powers N and Salvi
RJ: Quantitative relationship of carboplatin dose to magnitude of
inner and outer hair cell loss and the reduction in distortion
product otoacoustic emission amplitude in chinchillas. Hear Res.
112:199–215. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morell M, Lenoir M, Shadwick RE, Jauniaux
T, Dabin W, Begeman L, Ferreira M, Maestre I, Degollada E,
Hernandez-Milian G, et al: Ultrastructure of the Odontocete organ
of Corti: Scanning and transmission electron microscopy. J Comp
Neurol. 523:431–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gomółka M and Niemczyk S: How to safely
and effectively administer aminoglycoside antibiotics. Pol Merkur
Lekarski. 36:225–258. 2014.(In Polish). PubMed/NCBI
|
|
27
|
Theopold HM: Comparative surface studies
of ototoxic effects of various aminoglycoside antibiotics on the
organ of Corti in the guinea pig. A scanning electron microscopic
study. Acta Otolaryngol. 84:57–64. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Forge A and Schacht J: Aminoglycoside
antibiotics. Audiol Neurootol. 5:3–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Chen Y, Wu P and Chen B: Update on
new medicinal applications of gentamicin: Evidence-based review. J
Formos Med Assoc. 113:72–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maudonnet EN, de Oliveira JA, Rossato M
and Hyppolito MA: Gentamicin attenuates gentamicin-induced
ototoxicity-self-protection. Drug Chem Toxicol. 31:11–25. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du XF, Song JJ, Hong S and Kim J: Ethanol
extract of Piper longum L. attenuates gentamicin-induced hair cell
loss in neonatal cochlea cultures. Pharmazie. 67:559–563.
2012.PubMed/NCBI
|
|
32
|
Choung YH, Kim SW, Tian C, Min JY, Lee HK,
Park SN, Lee JB and Park K: Korean red ginseng prevents
gentamicin-induced hearing loss in rats. Laryngoscope.
121:1294–1302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang J, Jung HH, Yang JY, Choi J, Im GJ
and Chae SW: Protective role of antidiabetic drug metformin against
gentamicin induced apoptosis in auditory cell line. Hear Res.
282:92–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bas E, Van De Water TR, Gupta C, Dinh J,
Vu L, Martínez-Soriano F, Láinez JM and Marco J: Efficacy of three
drugs for protecting against gentamicin-induced hair cell and
hearing losses. Br J Pharmacol. 166:1888–1904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yadav MK, Choi J and Song JJ: Protective
effect of hexane and ethanol extract of piper longum L. On
gentamicin-induced hair cell loss in neonatal cultures. Clin Exp
Otorhinolaryngol. 7:13–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choung YH, Taura A, Pak K, Choi SJ, Masuda
M and Ryan AF: Generation of highly-reactive oxygen species is
closely related to hair cell damage in rat organ of Corti treated
with gentamicin. Neuroscience. 161:214–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rubinstein AD, Eisenstein M, Ber Y, Bialik
S and Kimchi A: The autophagy protein Atg12 associates with
antiapoptotic Bcl-2 family members to promote mitochondrial
apoptosis. Mol Cell. 44:698–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Erlich S, Mizrachy L, Segev O, Lindenboim
L, Zmira O, Adi-Harel S, Hirsch JA, Stein R and Pinkas-Kramarski R:
Differential interactions between Beclin 1 and Bcl-2 family
members. Autophagy. 3:561–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Germain M, Nguyen AP, Le Grand JN, Arbour
N, Vanderluit JL, Park DS, Opferman JT and Slack RS: MCL-1 is a
stress sensor that regulates autophagy in a developmentally
regulated manner. Embo J. 30:395–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pattingre S and Levine B: Bcl-2 inhibition
of autophagy: A new route to cancer? Cancer Res. 66:2885–2889.
2006. View Article : Google Scholar : PubMed/NCBI
|