Introduction
Ovarian cancer, one of the most common gynecologic
malignancies, is an insidious cancer with a 5-year survival rate of
merely 20–30% (1). Currently, the
pathogenesis of ovarian cancer remains incompletely understood.
Apoptosis is a highly regulated, crucial biological process leading
to cell death: Regulatory imbalance can cause excessive cell
proliferation or apoptosis, leading to diseases including ovarian
cancer. Several studies have demonstrated that abnormal apoptosis
regulation in ovarian cells contributes to the development of
ovarian cancer (2,3). Wild-type p53-induced phosphatase 1
[Wip1; official name: protein phosphatase, Mg2+/Mn2+ dependent 1D
(PPM1D)], a p53-dependent proto-oncogene first identified in 1997
(4), is widely involved in the
regulation of multiple cell signaling pathways, and is important in
the pathogenesis of cancers, including ovarian cancer (5–7).
Previous studies have demonstrated that Wip1 is
closely associated with apoptosis. Ma et al (8) revealed that manganese (Mn) exposure
led to neuronal necrosis in rats, accompanied by a significant
increase in neuronal apoptosis and a notable reduction in Wip1
expression in nerve tissues and cells. Sun et al (9,10)
reported that Wip1 expression was significantly higher in
nasopharyngeal cancer and renal cancer tissues than in normal
tissues. Wip1 silencing led to a markedly accelerated apoptosis in
these types of cancer cells, indicating involvement of Wip1 in
suppressing apoptosis. By contrast, elevated Wip1 expression
exhibits an inhibitory effect on apoptosis (8–10).
To the best of our knowledge, the mechanism by which Wip1 regulates
apoptosis in ovarian cancer cells has not been reported to
date.
The present study aimed to investigate in
vitro the role of Wip1 in apoptosis of ovarian cancer SKOV3
cells and its potential mechanism of action.
Materials and methods
Cell culture
The human ovarian cancer cell lines SKOV3, CAOV3,
AZ780, ES2 and the normal ovarian epithelial cell line were
purchased from Cell Center, Peking Union Medical College (Beijing,
China). They were cultured in Dulbecco's modified eagle's
medium-1640 supplemented with 5% fetal bovine serum (FBS), 2 mM
l-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin in an
atmosphere containing 95% air, 5% CO2. Cells were plated (1
×103 cells/well) in 96-well plates for 24 h and
incubated at 37°C for 4 h in
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
which was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). The medium was removed, 50 µl DMSO was added to each well
and incubated at room temperature for 45 min while shaking.
Absorbance was measured at a wavelength of 570 nm, using a
SynergyMx microplate reader (Bio Tek Instruments, Inc., Winooski,
VT, USA) to determine the viable cell fraction.
Cells at a 75–85% confluence were either left
untreated, transfected with Wip1 siRNA plasmid or control siRNA
plasmid which was performed with Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol, and then collected for
experimental assay 48 h following transfection.
Antibodies and siRNAs
Antibodies towards Wip1 (cat. no. D4F7), p38
mitogen-activated protein kinase (p38 MAPK; cat. no. 9212),
phosphorylated (p-) p38 MAPK (Thr180/Tyr182; catalog no. 3D7),
tumor protein 53 (p53; cat. no. 7F5), mitogen-activated protein
kinase 1 (ERK; cat. no. 137F5), phosphorylated (p-) ERK
(Thr202/Tyr204; cat. no. D13.14.4E), mitogen-activated protein
kinase 8 (JNK; cat. no. 56G8), phosphorylated (p-) JNK
(Thr183/Tyr185; cat. no. G9) and cleaved caspase-3 (cat. no. 9661)
and the MAPK inhibitor SB203580 were purchased from Cell Signaling
Technology, Inc. (1:1,000; Danvers, MA, USA). Mouse anti-BCL2 (cat.
no. ab7923) associated X (Bax; cat. no. ab77566) monoclonal
antibody, rabbit anti-BCL2 apoptosis regulator (Bcl-2; cat. no.
ab7973), caspase-3 (cat. no. ab32499) antibody were diluted at
1:1,000 and purchased from Abcam, Cambridge, UK. Pro-Light
horseradish peroxidase chemiluminescence detection reagents were
purchased from Tiangen Biotech Co., Ltd. (Beijing, China). siRNAs
were purchased from Sigma-Aldrich; Merck KGaA. siRNA sequences were
as follows: Wip1 siRNA-1, 5′-UUGUGAGUGAGUCGAGGUCGUUUCC-3′; Wip1
siRNA-2, 5′-UAUCCUUAAAGUCAGGGCUUUAGCG-3′; Wip1 siRNA-3,
5′-CCTCACAGCGAAAGAACTCTGTTAA-3′; and control non-targeting N-siRNA,
5′-GAGUGGGUCUGGGUCUUCCCGUAGA-3′.
Apoptosis analysis by Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
staining
Apoptotic cells in different groups were determined
using an Annexin V/PI apoptosis detection kit according to the
manufacturer's protocol (Multi Sciences Biotech Co., Ltd.,
Hangzhou, China). Briefly, the cell pellet was resuspended in 1x
binding buffer followed by incubation with 5 ml of Annexin V
(conjugated with FITC) and 10 ml of PI, in the dark for 5 min. Cell
fluorescence was then analyzed using a flow cytometer (Epics-XLII,
Becman Coulter, Inc., Brea, CA, USA). This test discriminates
intact cells (Annexin V-/PI-), early apoptotic cells (Annexin
V+/PI-) and late apoptotic cells (Annexin V+/PI+).
Western blot
Cells were lysed in lysis buffer for 2 h on ice.
Confluent cell layers were washed with PBS and lysed for 30 min at
4°C with 1% Nonidet P-40, 0.1% Triton X-100, 30 mM sodium phosphate
(pH 7.4) containing 1 mM sodium orthovanadate, 2.5 mM Tris-HCl (pH
7.5), 100 mM NaCl, and 10 µg/ml of leupeptin, aprotinin. Following
centrifugation at 13,000 × g for 20 min at 4°C, the supernatant was
collected. Protein concentration was measured using a Lowry protein
assay. Total cell lysates (40 µg protein) were separated by
polyacrylamide 10% SDS-PAGE gels, and electrotransferred to a
polyvinylidene fluoride membrane. The membrane was blocked by
incubation with 0.05% nonfat milk powder in TBST for 2 h at 37°C,
and then primary antibodies to cleaved caspase-3, caspase-3, p53,
p38 MAPK, p-p38 MAPK, Bax and Bcl-2 were added for incubation
overnight at 4°C. Following washing, the membrane was incubated
with horseradish peroxidase-labeled goat anti-mouse or rabbit
secondary antibody (1:5,000 dilution in TBST containing 1% bovine
serum albumin; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for
120 min at room temperature and then washed three times with TBST
at 37°C. Finally, blots were visualized by chemiluminescence and
autoradiography. LabWorks 4.5 analysis software (UVP, Inc., Upland,
CA, USA) was used to perform quantification of bands in the western
blots.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and reverse transcribed. Total
RNA (2 µg) was reverse transcribed using random primers and M-MLV
at 42°C for 1 h and then heated to 94°C for 5 min in a total
reaction volume of 20 µl. Equal amounts of the product of the
reverse transcription reaction were subjected to PCR amplification.
qPCR was performed at a final volume of 20 µl, with 20 µl SYBR
Premix Ex TaqTM II (2X; Takara Bio, Inc., Otsu, Japan), 0.4 µl ROX
Reference Dye (50X; Takara Bio, Inc.), 2 µl reverse transcription
cDNA, 0.8 µl of mixed forward and reverse primers (at a final
concentration of 1 ng/ml) and 6 µl double-distilled water. The
reaction was performed for 40 cycles as follows: 95°C for 30 sec,
95°C for 5 sec, 55°C for 30 sec and 72°C for 30 sec. Relative fold
changes in mRNA expression were calculated using the
2−ΔΔCq method (11).
Primer sequences were as follows: Bax, forward
5′-GCTTGCTTCAGGGTTTCATCCA-3′ and reverse
5′-TGTCCACGGCGGCAATCATC-3′; Bcl-2, forward
5′-GGCGGAGAACAGGGTACGATAAC-3′ and reverse
5′-CGGGATGCGGCTGGATGGGG-3′; p53, forward
5′-CTAACCGCGGTCCCTTCCCAGAAAACCTAC-3′ and reverse
5′-TACAGTCAGAGCCAACCTCAGGCG-3′; caspase-3, forward
5′-AATGTCATCTCGCTCTTGGT-3′ and reverse 5′-GCTTAGAATCACACACAC-3′;18S
ribosomal RNA, forward 5′-ACACGGACAGGATTGACAGA-3′ and reverse
5′-GGACATCTAAGGGCATCACAG-3′.
Statistical analysis
Experimental data were expressed as the mean +
standard deviation. Statistical analysis was performed with the
SPSS 13.0 statistical software (SPSS, Inc. Chicago, IL, USA).
One-way analysis of variance was followed by Dunnett's test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
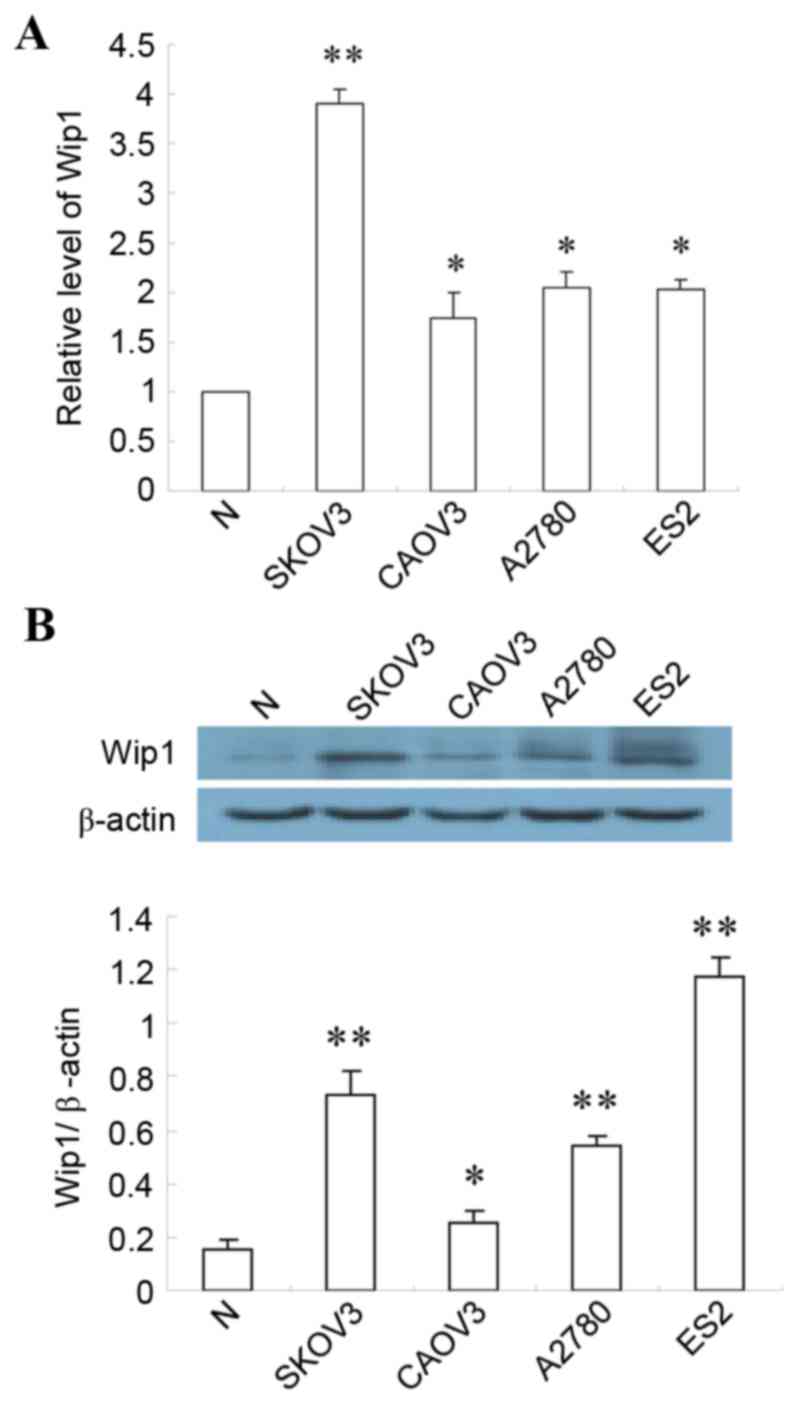
Screening of ovarian cancer cell
lines
Wip1 expression was analyzed in various ovarian
cancer cells lines by RT-qPCR and western blotting. Both mRNA and
protein expression levels of Wip1 were significantly elevated in
SKOV3, CAOV3, AZ780 and ES2 cells ovarian cancer cell lines
compared with normal (ovarian epithelial cell line), indicating
that overexpression of Wip1 is a feature of ovarian cancer cells
(Fig. 1). Expression of Wip1 mRNA
and protein was significantly higher in SKOV3 cells than in the
other three cell lines (Fig. 1).
Consequently, SKOV3 cells were selected to further explore the
relationship between Wip1 and ovarian cancer.
Wip1 silencing by siRNA on SKOV3
cells
In order to efficiently silence expression of Wip1
in SKOV3 cells, three different siRNA sequences were tested, as
well as various conditions for the transfection. As demonstrated in
Fig. 2A, reduced Wip1 protein
expression was observed in SKOV3 cells following siRNA
transfection, compared with untransfected cells, with siRNA-2 being
the most efficient. Wip1 protein expression was decreased in Wip1
siRNA-2 transfected cells compared with control, reaching the
lowest level at 80 nmol/l siRNA (Fig.
2B) and at 48 h following transfection (Fig. 2C). Assessment of cell viability by
MTT assay demonstrated that SKOV3 cells were still viable following
Wip1 siRNA transfection, proliferation was significantly decreased
compared to control (P<0.01, Fig.
2D).
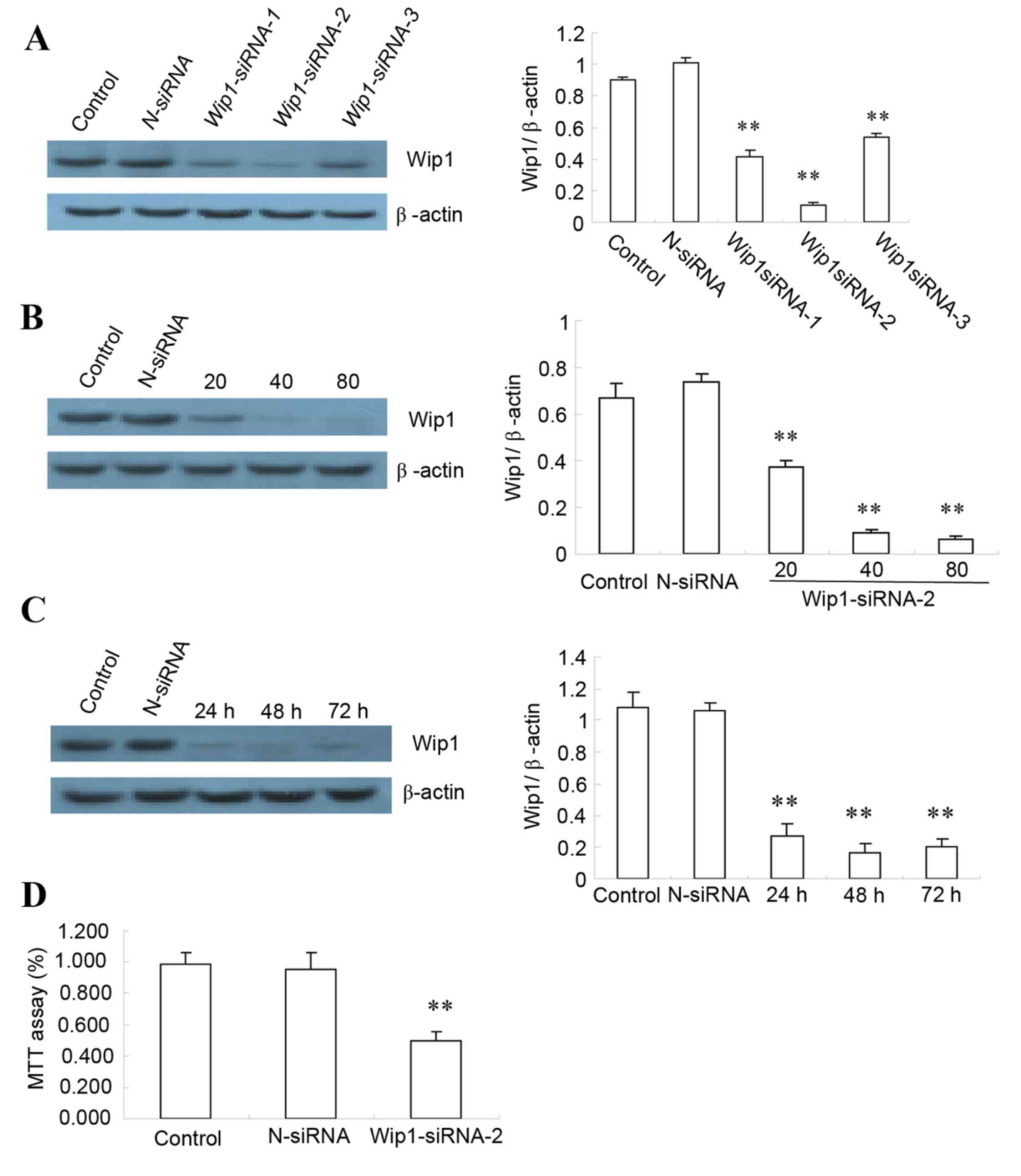 | Figure 2.Wip1 silencing by siRNA in SKOV3
ovarian cancer cells. (A) Wip1 protein expression levels were
assessed by western blotting in untransfected cells (Control) and
cells transfected with either N-siRNA or one of the three different
siRNA sequences (siRNA-1, −2 or −3), with quantification relative
to β-actin. (B) SKOV3 cells were either left untransfected
(Control) or transfected with either N-siRNA or with 20, 40, or 80
nmol/l Wip1-siRNA-2. The effect on Wip1 expression was analyzed by
western blot. Representative images and quantification is reported
here as fold ratio to β-actin. (C) SKOV3 cells were either left
untransfected (Control) or transfected with either N-siRNA or
Wip1-siRNA-2 (80 nmol/l Wip1-siRNA-2), for 24, 48 or 72 h. The
effect on Wip1 expression was analyzed by western blot.
Representative images and quantification is reported here as fold
ratio to β-actin. (D) SKOV3 cells were either left untransfected
(Control) or transfected with either N-siRNA or Wip1-siRNA-2 (80
nmol/l Wip1-siRNA-2 for 48 h), and the proliferation rate was
analyzed by MTT assay (n=4 repeats). **P<0.01 vs. Control. Wip1,
wild-type p53-induced phosphatase 1; siRNA, small interfering RNA;
N-siRNA, non-targeting siRNA. |
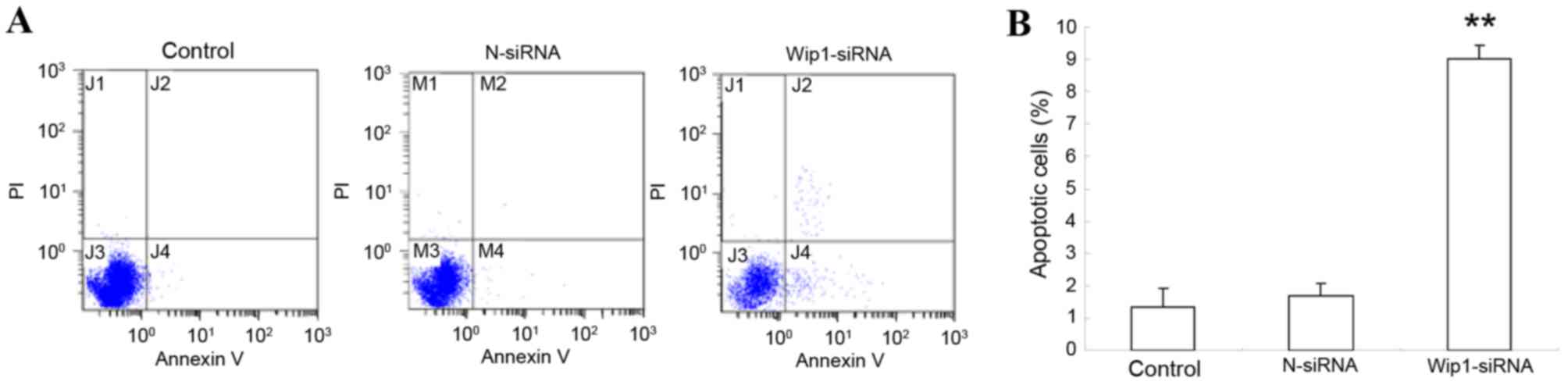
Effect of Wip1 silencing on apoptosis
of SKOV3 cells
Assessment of apoptosis by Annexin V/PI staining
revealed a significant increase in SKOV3 apoptosis following Wip1
silencing by siRNA, compared with control untransfected cells
(Control; P<0.01, Fig. 3), or
with non-targeting siRNA transfected cells (N-siRNA; P<0.01,
Fig. 3). The apoptosis rate of
SKOV3 cells was similar in both the control untransfected cells and
the non-targeting siRNA transfected cells.
Effect of Wip1 silencing on mRNA
expression of apoptosis-related proteins
To gain insight into the mechanism of SKOV3
apoptosis caused by Wip1 silencing, the expression of
apoptosis-related proteins Bax, caspase-3, p53 and Bcl-2 was
examined. Upon Wip1 silencing, relative expression of Bax
(2.5±0.034), caspase-3 (2.32±0.032) and p53 (1.87±0.068) mRNA was
significantly higher in SKOV3 cells transfected with Wip1 siRNA,
compared with control (P<0.01; Fig.
4A). The relative expression of Bcl-2 mRNA (0.47±0.035) was
significantly reduced compared with control (P<0.05; Fig. 4A), resulting in an increased
Bax/Bcl-2 ratio. The mRNA expression of apoptosis-related proteins
did not differ significantly in the control untransfected cells and
the cells transfected with the non-targeting siRNA (data not
shown). The above findings suggested that silencing Wip1 promoted
expression of pro-apoptotic markers in SKOV3 cells.
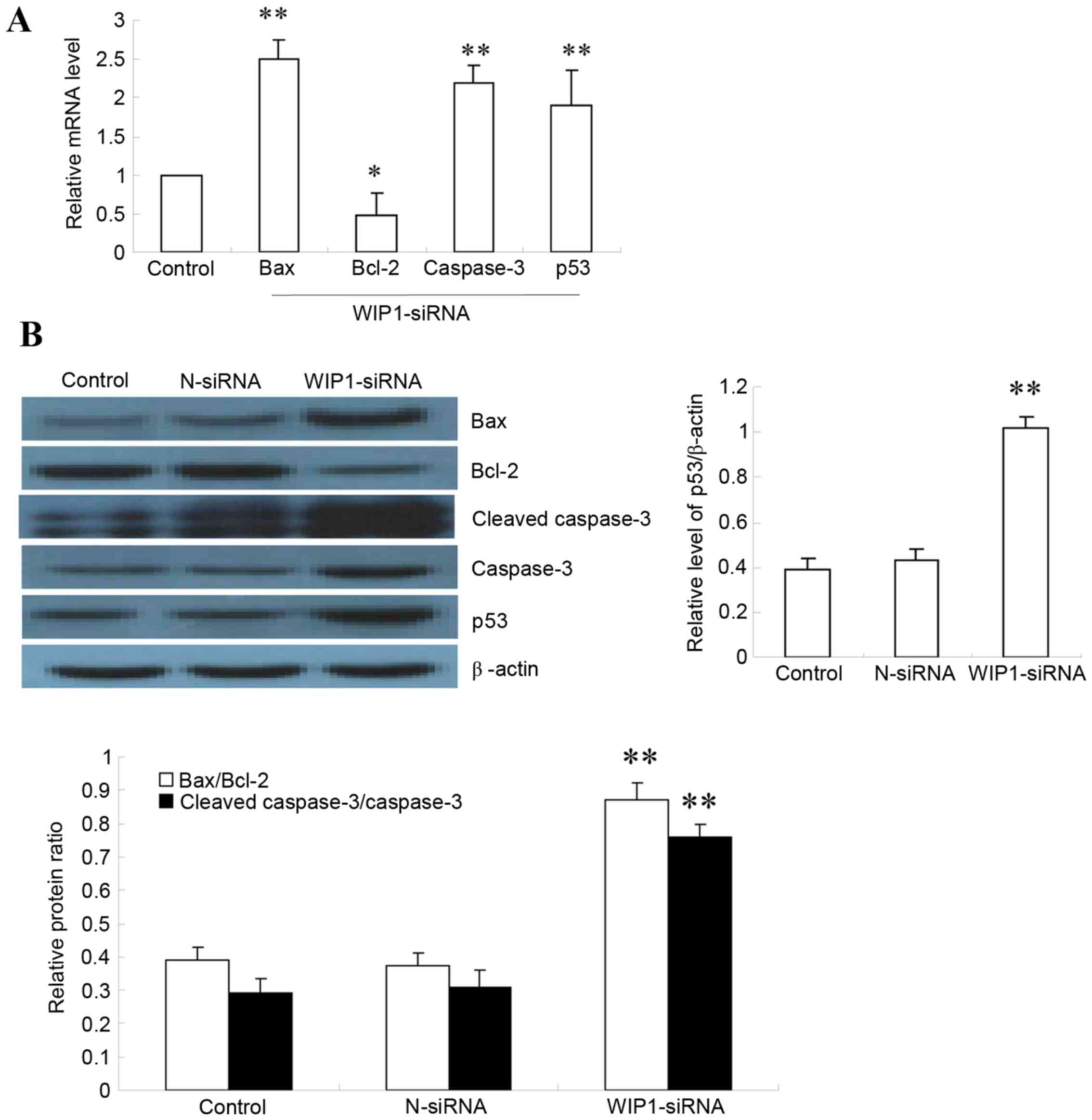 | Figure 4.Effect of Wip1 siRNA on expression of
apoptotic markers in SKOV3 cells. (A) The mRNA expression levels of
Bax, Bcl-2, caspase-3 and p53 were analyzed by reverse
transcription-quantitative polymerase chain reaction in SKOV3 cells
transfected with Wip1 siRNA and control untransfected cells (n=6).
Results are plotted relative to the control. (B) The protein levels
of Bax, Bcl-2, cleaved caspase-3, caspase-3 and p53 were analyzed
by western blot (n=6 repeats). Results for p53 are plotted relative
to β-actin, and for the other proteins as ratios of Bax/Bcl-2
expression and of cleaved caspase-3/caspase-3 expression.
*P<0.05 and **P<0.01 vs. Control. Wip1, wild-type p53-induced
phosphatase 1; siRNA, small interfering RNA; Bax, BCL2 associated
X; Bcl-2, BCL2 apoptosis regulator; p53, tumour protein 53;
N-siRNA, non-targeting siRNA. |
Effect of Wip1 silencing on SKOV3
apoptosis-related protein expression
Protein expression of apoptosis-related markers Bax,
cleaved caspase-3, caspase-3, p53 and Bcl-2 was detected by western
blotting (Fig. 4B). In SKOV3
cells, expression of Bax/Bcl-2 protein ratio (0.86±0.041), cleaved
caspase-3/caspase-3 protein ratio (0.78±0.031) and p53 protein
(1.02±0.051) was significantly higher in Wip1 siRNA cells than
control (0.38±0.059; P<0.01; Fig.
4B). There were no significant differences in expression of
apoptosis-related proteins in the control untransfected cells
(Control) compared with the cells transfected with the
non-targeting siRNA (N-siRNA; Fig.
4B). These results indicated that silencing Wip1 promoted
expression of pro-apoptotic proteins in SKOV3 cells.
Wip1 acts through the p38 MAPK
signaling pathway
Following Wip1 silencing, expression of the major
signaling molecules ERK, p-ERK, JNK, p-JNK, p38 MAPK and p-p38 MAPK
was analyzed by western blot. The results demonstrated a
significant increase in p-p38 MAPK protein (P<0.05, Fig. 5A), suggesting involvement of p38
MAPK signaling in the Wip1-mediated apoptosis effect in SKOV3
cells. To confirm this, the p38 MAPK inhibitor SB203580 was
utilized to block p38 MAPK signaling: Treatment with SB203580
significantly reduced p38 MAPK phosphorylation (P<0.01, Fig. 5B). Assessment of cell proliferation
by MTT assay revealed that SB203580 treatment completely reversed
the proliferation-inhibition effect of Wip1 silencing in SKOV3
cells, suggesting that p38 MAPK is the main mediator of this effect
(P<0.01, Fig. 5C). Based on
these findings, Wip1 silencing promoted SKOV3 apoptosis by
regulating the p38 MAPK pathway.
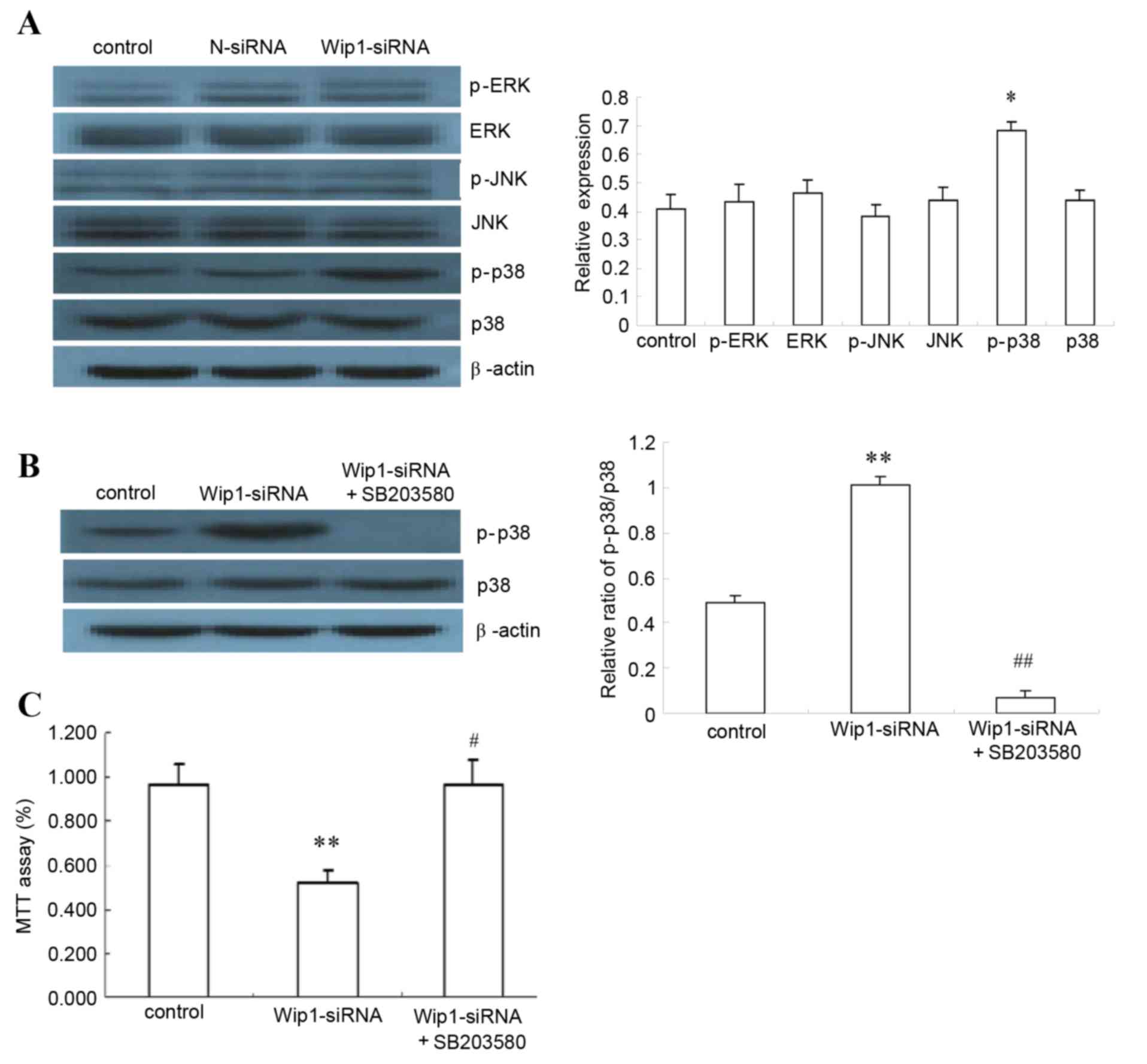 | Figure 5.Effects of p38 MAPK inhibitor on
Wip1-silenced SKOV3 cells. (A) SKOV3 cells were either left
untransfected (Control, SKOV3 cells), or were transfected with
N-siRNA or Wip1-siRNA. The protein expression of p-p38 MAPK, p38
MAPK, p-ERK, ERK, p-JNK and JNK were analyzed by western blot
(n=6). Representative images and quantification are reported here,
as relative level to β-actin. (B) Effects of p38 MAPK and p-p38
MAPK were analyzed by western blot, following transfection with
Wip1 siRNA and treatment with p38 MAPK inhibitor SB203580 for 48 h
(10 µM) (n=6). Representative images and quantification are
reported here, as the ratio of p-p38/p38. (C) The proliferation
rate of SKOV3 cells was analyzed by MTT assay, following
transfection with Wip1 siRNA and treatment with p38 MAPK inhibitor
SB203580 for 48 h (10 µM) (n=4). Results are plotted as % ratio
relative to control (SKOV3 cells). *P<0.05 and **P<0.01 vs.
Control (SKOV3 cells). #P<0.01 and
##P<0.01 vs. Wip1-siRNA. Wip1, wild-type p53-induced
phosphatase 1; siRNA, small interfering RNA; N-siRNA, non-targeting
siRNA; p-, phosphorylated; p38 MAPK, p38 mitogen-activated protein
kinase; ERK, mitogen-activated protein kinase 1; JNK,
mitogen-activated protein kinase 8. |
Discussion
Previous studies have suggested that Wip1 may be
important in regulation of treatment response in malignant tumors.
Lee et al (12) revealed a
significant increase in UV-induced apoptosis by Wip1 knockout in
mice or human keratinocytes. A Wip1 inhibitor, CCT007093, has also
been reported to increase apoptosis in breast cancer cells and skin
keratinocytes (12). A previous
study by Yang et al (13)
assessed tumor specimens derived from breast cancer patients for
Wip1 mRNA and protein expression using semi-quantitative RT-PCR,
immunohistochemistry and western blotting, and demonstrated that
expression of Wip1 was significantly higher in breast cancer
tissues than in adjacent, normal breast tissues. High expression of
Wip1 mRNA and protein may therefore promote breast cancer growth,
suggesting that may be a novel target for breast cancer therapy.
Thus, regulation of Wip1 levels may also be a potential treatment
of ovarian cancer.
Wip1, a serine/threonine protein phosphatase located
in human chromosome region 17q23/q24, is encoded by the gene
protein phosphatase Mg2+/Mn2+ dependent 1D (PPM1D). The Wip1 gene
is commonly overexpressed or amplified in human cancers, including
primary breast cancers (14–16),
gastric cancers (17),
medulloblastomas (18–20), neuroblastomas (21), and ovarian clear cell
adenocarcinomas (5,22). Previous studies have demonstrated a
close association between Wip1 genes and apoptosis (23,24).
Apoptosis is a process of programmed cell death, regulated by a
balance of anti-apoptotic factors (such as Bcl-2) and pro-apoptotic
factors (such as p53, Bax and caspase-3). The interaction between
pro-apoptotic and anti-apoptotic factors is closely associated with
the pathogenesis and development of tumors. Song et al
(25) reported a significant
increase in Wip1 levels following γ-irradiation in prostate cancer
cells. In these cells, Wip1 interacted with and dephosphorylated
Bax, resulting in Bax translocating to the mitochondria and
inhibiting apoptosis. The study also indicated that the apoptosis
inhibiting effect of Wip1 could be reverted by its inhibitors. In a
Mn neurotoxicity study by Ma et al (8), Wip1 expression in the neurons of
Mn-exposed rats was progressively decreased, whereas p53 level and
Bax/Bcl-XL ratio were elevated progressively, suggesting that Wip1
downregulation might induce apoptotic cell death. Another study
also demonstrated a close association between Wip1 expression and
apoptosis: In HeLa cells, knockdown of Wip1 significantly decreased
growth and colony formation ability and increased apoptosis, as
evident also by a significant increase in the protein expression
levels of p-p38 MAPK, p53, and p-p53 (26). By contrast, Goloudina et al
(27) demonstrated that
overexpression of Wip1 in p53-negative tumor cells activated the
apoptotic pathway through increased Bax/Bcl-XL ratio, thereby
leading to cancer cell apoptosis. The present study demonstrated
that Wip1 silencing promoted apoptosis in SKOV3 ovarian cancer
cells and upregulated caspase-3 expression and Bax/Bcl-2 ratio.
The MAPK signaling pathway is an important
intracellular signaling system, and is involved in cell growth,
development and apoptosis. The p38 MAPK signaling pathway directly
phosphorylates the key regulator p53, which activates cell cycle
arrest. In addition, activation of the p38 MAPK pathway is
essential for drug-induced tumor cell death. Holnes et al
(28) have studied the mechanism
of vitamin A as a drug for ovarian cancer, and demonstrated that
CD437 (a synthetic analog for vitamin A) induces CAOV3 cell
apoptosis through the p38 MAPK pathway, and p38 MAPK antagonists
could block this effect. Arsenic trioxide (ATO), a potential cancer
chemotherapeutic drug, is known to have a curative effect on acute
promyelocytic leukemia (29,30).
Yoda et al (31) confirmed
that ATO induces phosphorylation of checkpoint kinase 2 (Chk2) and
p38 MAPK and activates the p38 MAPK/p53 pathway, thus leading to
apoptosis. Wip1 was demonstrated to suppress DNA damage-induced
phosphorylation of Chk2 and p38 MAPK in vitro, an effect
that was restored by ATO treatment. Following suppression of Wip1
expression, ATO-induced activation of the p38 MAPK apoptotic
pathway was significantly enhanced (31). Wip1 was therefore identified as a
direct target of ATO in repression of tumor cell apoptosis. In the
present study, silencing Wip1 significantly enhanced p38 MAPK
phosphorylation, suggesting that Wip1 acts through the p38 MAPK
pathway.
In conclusion, silencing Wip1 promoted apoptosis in
SKOV3 ovarian cancer cells, most likely by promoting activation of
the p38 MAPK signaling pathway. Therefore, Wip1 may be a potential
therapeutic target in ovarian cancer.
Acknowledgements
This study was supported by the Key Project of Hebei
Provincial Health and Family Planning Commission Fund (grant no.
20150204).
References
|
1
|
Jemal A, Bray F, Melissa M, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Bieber MM and Teng NN: Hedgehog
signaling regulates drug sensitivity by targeting ABC transporters
ABCB1 and ABCG2 in epithelial ovarian cancer. Mol Carcinog.
53:625–634. 2014.PubMed/NCBI
|
|
3
|
Zhang P, Jia R, Ying L, Liu B, Qian G, Fan
X and Ge S: WWOX-mediated apoptosis in A549 cells mainly involves
the mitochondrial pathway. Mol Med Rep. 6:121–124. 2012.PubMed/NCBI
|
|
4
|
Fiscella M, Zhang H, Fan S, Sakaguchi K,
Shen S, Mercer WE, Woude GF Vande, O'Connor PM and Appella E: Wip1,
a novel human protein phospahtase that is induced in response to
ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci
USA. 94:pp. 6048–6053. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirasawa A, Saito-Ohara F, Inoue J, Aoki
D, Susumu N, Yokoyama T, Nozawa S, Inazawa J and Imoto I:
Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas
with poor prognosis and identification of PPM1D and APPBP2 as
likely amplification targets. Clin Cancer Res. 9:1995–2004.
2003.PubMed/NCBI
|
|
6
|
Richter M, Dayaram T, Gilmartin AG, Ganji
G, Pemmasani SK, Van Der Key H, Shohet JM, Donehower LA and Kumar
R: WIP1 phosphatase as a potential therapeutic target in
neuroblastoma. PLoS One. 10:e01156352015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang YL, Liu X, Gao SY, Feng H, Jiang YP,
Wang SS, Yang J, Jiang J, Ma XR, Tang YJ, et al: WIP1 stimulates
migration and invasion of salivary adenoid cystic carcinoma by
inducing MMP-9 and VEGF-C. Oncotarget. 6:9031–9044. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma X, Han J, Wu Q, Liu H, Shi S, Wang C,
Wang Y, Xiao J, Zhao J, Jiang J and Wan C: Involvement of
dysregulated Wip1 in manganese-induced p53 signaling and neuronal
apoptosis. Toxicol Lett. 235:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun GG, Zhang J, Ma XB, Wang YD, Cheng YJ
and Hu WN: Overexpression of Wild-Type p53-Induced Phosphatase 1
confers poor prognosis of patients with Nasopharyngeal Carcinoma.
Pathol Oncol Res. 21:283–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun GG, Wang YD, Liu Q and Hu WN:
Expression of Wip1 in kidney carcinoma and its correlation with
tumor metastasis and clinical significance. Pathol Oncol Res.
21:219–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JS, Park JR, Kwon OS, Kim H, Fornace
AJ Jr and Cha HJ: Off-target response of a Wip1 chemical inhibitor
in skin keratinocytes. J Dermatol Sci. 73:125–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang DH, He JA, Li J, Ma WF, Hu XH, Xin SJ
and Duan ZQ: Expression of proto-oncogene Wip1 in breast cancer and
its clinical significance. Zhonghua Yi Xue Za Zhi. 90:519–522.
2010.PubMed/NCBI
|
|
14
|
Li J, Yang Y, Peng Y, Austin RJ, Van
Eyndhoven WG, Nguyen KC, Gabriele T, McCurrach ME, Marks JR, et al:
Oncogenic properties of PPM1D located within a breast cancer
amplification epicenter at 17q23. Nat Genet. 31:133–134. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bulavin DV, Demidov ON, Saito S,
Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G,
Nebreda AR, Anderson CW, et al: Amplification of PPM1D in human
tumors abrogates p53 tumor-suppressor activity. Nat Genet.
31:210–215. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rauta J, Alarmo EL, Kauraniemi P, Karhu R,
Kuukasjärvi T and Kallioniemi A: The serine-threonine protein
phosphatase PPM1D is frequently activated through amplification in
aggressive primary breast tumours. Breast Cancer Res Treat.
95:257–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuku T, Semba S, Yutori H and Yokozaki H:
Increased wild-type p53-induced phosphatase 1 (Wip1 or PPM1D)
expression correlated with downregulation of checkpoint kinase 2 in
human gastric carcinoma. Pathol Int. 57:566–571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castellino RC, De Bortoli M, Lu X, Moon
SH, Nguyen TA, Shepard MA, Rao PH, Donehower LA and Kim JY:
Medulloblastomas overexpress the p53-inactivating oncogene
WIP1/PPM1D. J Neurooncol. 86:245–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ehrbrecht A, Müller U, Wolter M, Hoischen
A, Koch A, Radlwimmer B, Actor B, Mincheva A, Pietsch T, Lichter P,
et al: Comprehensive genomic analysis of desmoplastic
medulloblastomas: Identification of novel amplified genes and
separate evaluation of the different histological components. J
Pathol. 208:554–563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mendrzyk F, Radlwimmer B, Joos S,
Kokocinski F, Benner A, Stange DE, Neben K, Fiegler H, Carter NP,
Reifenberger G, et al: Genomic and protein expression profiling
identifies CDK6 as novel independent prognostic marker in
medulloblastoma. J Clin Oncol. 23:8853–8862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saito-Ohara F, Imoto I, Inoue J, Hosoi H,
Nakagawara A, Sugimoto T and Inazawa J: PPM1D is a potential target
for 17q gain in neuroblastoma. Cancer Res. 63:1876–1883.
2003.PubMed/NCBI
|
|
22
|
Tan DS, Lambros MB, Rayter S, Natrajan R,
Vatcheva R, Gao Q, Marchiò C, Geyer FC, Savage K, Parry S, et al:
PPM1D is a potential therapeutic target in ovarian clear cell
carcinomas. Clin Cancer Res. 15:2269–2280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lowe J, Cha H, Lee MO, Mazur SJ, Appella E
and Fornace A J Jr: Regulation of the Wip1 phosphatase and its
effects on the stress response. Front Biosci (Landmark Ed).
17:1480–1498. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang XP, Liu F and Wang W: Two-phase
dynamics of p53 in the DNA damage response. Proc Natl Acad Sci USA.
108:pp. 8990–8995. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song JY, Ryu SH, Cho YM, Kim YS, Lee BM,
Lee SW and Choi J: Wip1 suppresses apoptotic cell death through
direct dephosphorylation of BAX in response to γ-radiation. Cell
Death Dis. 4:e7442013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HY, Liu ZS, Qiu L, Guo J, Li YF,
Zhang J, Wang TJ and Liu XD: Knockdown of Wip1 enhances sensitivity
to radiation in hela cells through activation of p38 MAPK. Oncol
Res. 22:225–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goloudina AR, Mazur SJ, Appella E, Garrido
C and Demidov ON: Wip1 sensitizes p53-negative tumors to apoptosis
by regulating the Bax/Bcl-xL ratio. Cell Cycle. 11:1883–1887. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holnes WF, Soprano DR and Soprano KJ:
Early events in the induction of apoptosis in ovarian carcinoma
cells by CD437: Activation of the p38 MAP kinase signal pathway.
Oncogene. 22:6377–6386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tallman M, Lo-Coco F, Barnes G, Kruse M,
Wildner R, Martin M, Mueller U and Tang B: Cost-effectiveness
analysis of treating acute promyelocytic leukemia patients with
arsenic trioxide and retinoic acid in the United States. Clin
Lymphoma Myeloma Leuk. 15:771–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shepshelovich D, Oniashvili N, Parnes D,
Klein A, Muchtar E, Yeshaya J, Aviram A, Rabizadeh E and Raanani P:
Acute promyelocytic leukemia with isochromosome 17q and cryptic
PML-RARA successfully treated with all-trans retinoic acid and
arsenic trioxide. Cancer Genet. 208:575–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoda A, Toyoshima K, Watanabe Y, Onishi N,
Hazaka Y, Tsukuda Y, Tsukada J, Kondo T, Tanaka Y and Minami Y:
Arsenic trioxide augments Chk2/p53-mediated apoptosis by inhibiting
oncogenic Wip1 phosphatase. J Biol Chem. 283:18969–18979. 2008.
View Article : Google Scholar : PubMed/NCBI
|