Introduction
Damage to the central nervous system, including
brain injury and spinal cord injury, is common in the clinic.
Traumatic brain injury (TBI) remains a public health problem and
patients aged ≥75 years tend to have a highest rate of
TBI-associated deaths in the United States (1). Furthermore, traumatic brain injury
and spinal cord injury may result in gastrointestinal dysfunction.
Head injury predominantly delays gastric emptying in patients with
short injury duration (2,3). Similarly, spinal cord injury is known
to contribute to prolong gastric emptying of solid meals (4).
The gastrointestinal tract is involved in double
innervation: To the central nervous system and the enteric nervous
system (ENS). The effects of the central nervous system are
transmitted to the ENS predominantly via cholinergic fibers in the
vagus nerve. Vagal activities are positively associated with
gastric emptying, and electroacupuncture alleviates the delayed
gastric emptying through a vagal mechanism (5). In addition, hyperglycemia damages
vagal afferents, which inhibits gastric motility, while
hyperglycemia activates the vagal efferent pathway to modulate
gastric relaxation (6). Therefore,
increasing attention has been dedicated to the potential mechanisms
underlying gastrointestinal dysmotility resulting from the injury
of central nervous system.
The ENS is the system that dominates the
gastrointestinal activities independently by the central nervous
system (7). The ENS is comprised
of neurons and glial cells which are distributed in the myenteric
and the submucosal plexus (8).
Enteric glial cells (EGCs) are more abundant than enteric neurons
(9) and are tightly packed around
enteric neurons. Injured EGC lose functions of neuronal maintenance
and survival and have been linked with ENS abnormalities (10). EGCs have an influence on mast
cells, which participate in obstructed defecation (11). Previous studies have indicated that
the reduced EGCs attenuate Ca2+-mediated responses
through connexin-43 causing gastrointestinal motility disorder
(12).
The digestive tract loses control of the central
nervous system following vagotomy, which is partly analogous to
brain injury or spinal cord injury (13). The effects of vagotomy on gastric
motility and EGCs are unknown. Whether SGES improves the gastric
motility disorder and restore the change of EGCs resulting from
vagotomy remains elusive. SGES may be able to alleviate
gastrointestinal dysfunction. Therefore, the aims of the present
study were to investigate the changes of gastric emptying and EGCs
in the stomach with a course of subdiaphragmatic vagotomy in rats
prior to and following SGES for further study on the underlying
mechanism of the effect of EGCs in gastrointestinal motility.
Materials and methods
Animals
A total of 52 adult male Sprague Dawley rats
(weighing 250–350 g) were used in the study. They were housed under
normal laboratory conditions of 22°C and 12 h dark/light cycle, and
were given food and water ad libitum. All animal protocols
were approved by the Animals Care and Use Committee of Huazhong
University of Science and Technology (Wuhan, China). The rats were
obtained from the Experimental Center of Tongji Medical College
(Wuhan, China). They were randomly divided into two groups: The
control group, which involved incisions of the abdomen; and the
subdiaphragmatic vagotomy group. These groups were further divided
into subgroups. The control group consisted of early control group
(ECN, 7 days, n=6) and terminal control group (TCN, 56 days, n=6).
The experimental group contained the early subdiaphragmatic
vagotomy group (ESDV, 7 days, n=20) and the terminal
subdiaphragmatic vagotomy group (TSDV, 56 days, n=20). Finally, the
ESDV and TSDV were divided into three groups, which were either
treated with sham gastric electrical stimulation (n=6), short-term
SGES (30 min/day, 7 days, n=7) or long-term SGES (30 min/day, 21
days, n=7), respectively.
Surgical procedure
Following fasting the subdiaphragmatic vagotomized
rats for 24 h, the rats were anesthetized with an intraperitoneal
injection of 1% pentobarbital sodium (40 mg/kg; Boster Biological
Technology, Ltd., Wuhan, China). Under aseptic conditions, the
stomach and subdiaphragmatic esophagus were exposed. Ventral and
dorsal subdiaphagmatic vagi were cut completely, in addition to
surrounding mesenteries. Simultaneously, a pair of temporary
cardiac pacing wires (Medtronic, Dublin, Ireland) was placed on the
serosal surface of the stomach. The pair was mounted in the middle
of the greater curvature for stimulation. The implanted stimulating
electrodes travelled separately from the subcutaneous layer of the
backside to the top of the head. Penicillin (Boster Biological
Technology, Ltd.) was used locally to prevent infection. The
muscular layer was stimulated following one week of recovery.
SGES
SGES was performed as previously described by Yang
et al (14). Briefly, each
stimulation consisted of a long pulse (300 msec, 4 mA) followed
with five short pulses (0.33 msec, 100 Hz, 4 mA). The stimulation
was synchronized with the peak of gastric intrinsic slow waves.
Gastric emptying test
The authors used a modified gastric emptying model,
as described previously (15).
Phenol red (0.5 mg/ml) and carboxymethylcellulose (15 mg/ml) were
thoroughly mixed as test meal. At the scheduled time, the rats were
fasted for 24 h before 2 ml phenol red solution was administered to
their stomachs. The stomachs were removed with the gastroesophageal
junction and the pylorus harvested after 30 min. Gastric content
was rinsed in physiological saline up to 20 ml; then 20 ml NaOH
(0.5 M) was added. The solution was mixed and allowed to stand for
1 h at room temperature. Following this, 5 ml supernatant was
removed and placed in a centrifuge tube with 0.5 ml trichloroacetic
acid (20%, w/v). Centrifugation (1,050 × g, 4°C, 10 min) separated
the phenol red solution (supernatant) from solution containing 5 ml
supernatant and 0.5 ml trichloroacetic acid. The absorption value
of phenol red was identified using a spectrophotometer at 560 nm.
Meanwhile, 2 ml phenol red solution, 18 ml physiological saline, 20
ml NaOH (0.5 mol/l) and 4 ml trichloroacetic acid (20%, w/v)
represented a standard sample. Absorption value of the standard
sample was measured as above. The gastric emptying rate was
obtained by: 1-(phenol red absorption value from the stomach of
animals sacrificed 30 min after the test meal/standard sample
absorption value).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was used to measure the expression levels of
the S100B/glial fibrillary acidic protein (GFAP) gene. RNA was
isolated from stomach tissue using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Single-stranded cDNA was
synthesized with PrimeScript™ RT Master Mix (Takara Biotechnology
Co., Ltd., Dalian, China). Primer sequences were as follows: S100B,
forward 5′-GAGCAGGAAGTGGTGGACAAA-3′ and reverse
5′-CACTCCCCATCCCCATCTT-3′; GFAP, forward
5′-TGACCGCTTTGCTAGCTACATC-3′ and reverse 5′-GCGCCTTGTTTTGCTGTTC-3′;
and GAPDH, forward 5′-GTATGACTCTACCCACGGCAAGT-3′ and reverse
5′-TTCCCGTTGATGACCAGCTT-3′. GAPDH acted as an internal control. A
total of 10 µl PCR reaction volume was used, and included: 0.5 µl
upstream primer (Invitrogen; Thermo Fisher, Scientific, Inc.), 0.5
µl downstream primer (Invitrogen; Thermo Fisher Scientific, Inc.),
5 µl SYBR-Green (Qiagen GmbH, Hilden, Germany), 1 µl cDNA, 3 µl
ddH2O. All PCR reactions following the established
procedure were quantified using the ABI-OneStep real-time system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Gene relative
expression levels were calculated with the 2−∆∆Ct method
(16).
Double-labeling
immunofluorescence
Gastric tissues were double-labeled for enteric
glial markers, S100B and GFAP protein. The acquired tissues were
fixed in 4% paraformaldehyde for 6–24 h and cut into small segments
of ~5 mm. The segments were then embedded into paraffin in a vacuum
and sliced into at a thickness of 5 µm. Sections were boiled 1–2
min in citrate buffer for antigen retrieval after dewaxed in xylene
and hydrated in the graded ethanol solutions. Following this,
nonspecific binding sites were blocked by 5% bovine serum albumin
(Boster Biological Technology, Ltd.) for 30 min at room
temperature. The sections were then incubated with the following
primary antibodies: Rabbit anti-S100B (1:300; cat. no. sc-136061;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and goat anti-GFAP
(1:300; cat. no. ab53554; Abcam, Cambridge, MA, USA). Each section
was incubated at 4°C overnight. Sections were washed 3 times in PBS
following re-warming to room temperature for 60 min the following
day. Sections were then incubated with secondary antibodies for
Alexa Fluor 488-donkey anti-rabbit IgG (1:100 dilution; AntGene
Biotech Co., Ltd, Wuhan, China) and AlexaFluor 594-donkey anti-goat
IgG (1:200 dilution; AntGene Biotech Co., Ltd.) and kept in the
dark at 37°C for 90 min. Following washing 3 times in PBS, sections
were treated with Hoechst stain (1:1,500) at 37°C for 10 min.
Sections were sealed with fluorescence quenching agent (Boster
Biological Technology, Ltd.) and observed using laser scanning
confocal microscopy (Nikon Corporation, Tokyo, Japan). Image
analysis was performed using Image Pro5.1 (Media Cybernetics, Inc.,
Rockville, MD, USA). Quantification was reported as mean signal per
field, from 6 random fields per sample.
Transmission electron microscopy
The specimens of the antrum were fixed in 2.5%
glutaraldehyde (pH 7.4) for 2 h at 4°C and rinsed with 0.1 M PBS
twice and immersed in 1% OsO4 (pH 7.4) for 1
h. Then they were dehydrated with graded alcohol, embedded in Epon
(Boster Biological Technology, Ltd.), cut into ultra-thin sections
with an ultramicrotome (Leica Microsystems GmbH, Wetzlar, Germany),
stained with lead citrate for 10 min. Finally, these sections were
viewed using a transmission electron microscope (Tecnai G2 12, FEI;
Thermo Fisher Scientific, Inc.).
Statistical analysis
The mean value was used for statistical analysis and
the results were presented as mean ± standard error. One-way
analysis of variance, followed by Bonferroni post hoc test, was
performed to evaluate the difference between normal control groups
and the subgroups of subdiaphragamtic vagotomy. P<0.05 was
considered to indicate a statistically significant difference. All
calculations were performed using SPSS software (version 17.0;
SPSS, Inc., Chicago, IL, USA).
Results
Assessment of gastric motility
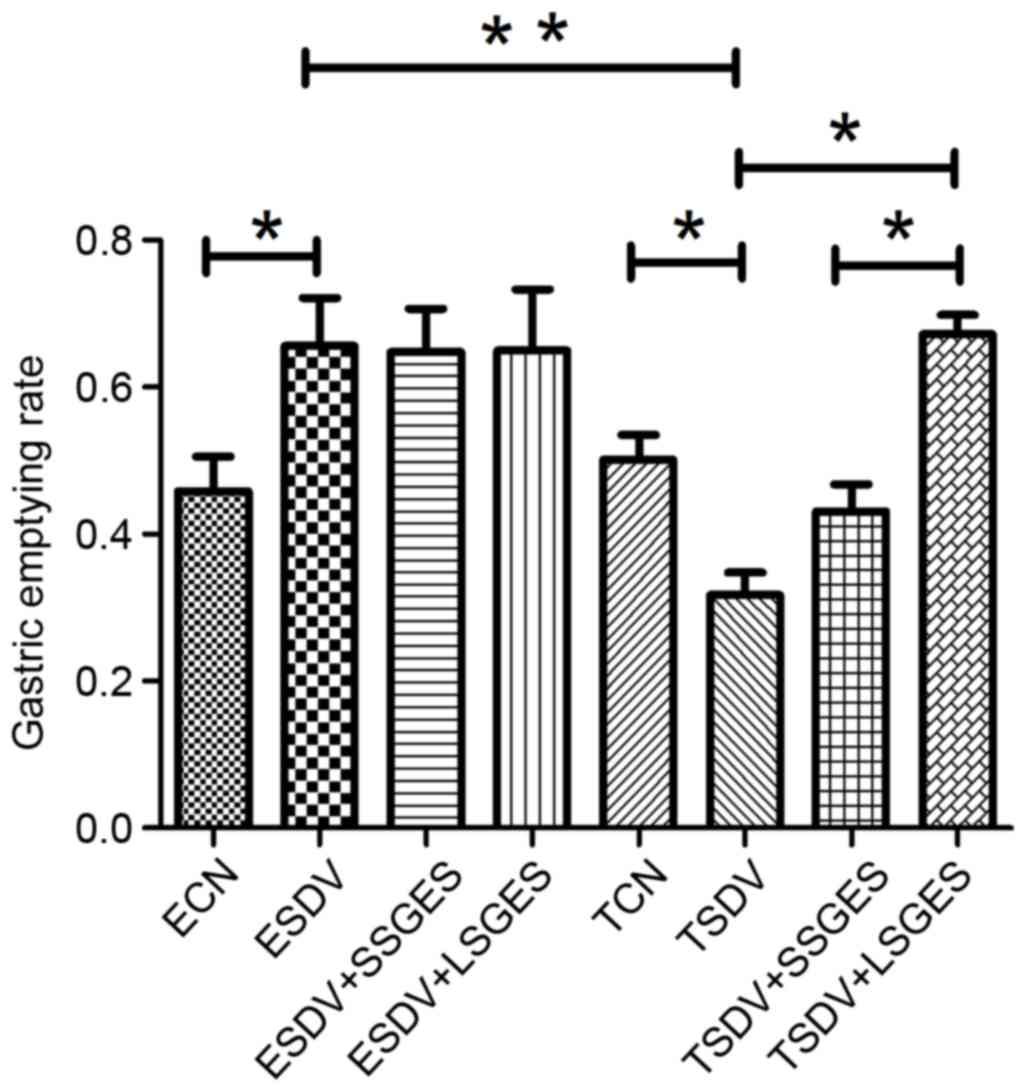
Gastric emptying rates in different subdiaphragmatic
vagotomy groups are presented in Fig.
1. In the early groups, ESDV demonstrated a faster gastric
emptying when compared with the ECN group (P<0.05). In the
terminal groups, gastric emptying was decreased in TSDV, when
compared with TCN (P<0.05) and long-term SGES significantly
improved delayed gastric emptying compared with TSDV (P<0.05).
In addition, long-term SGES was more effective in accelerating
delayed gastric emptying than short-term SGES (P<0.05).
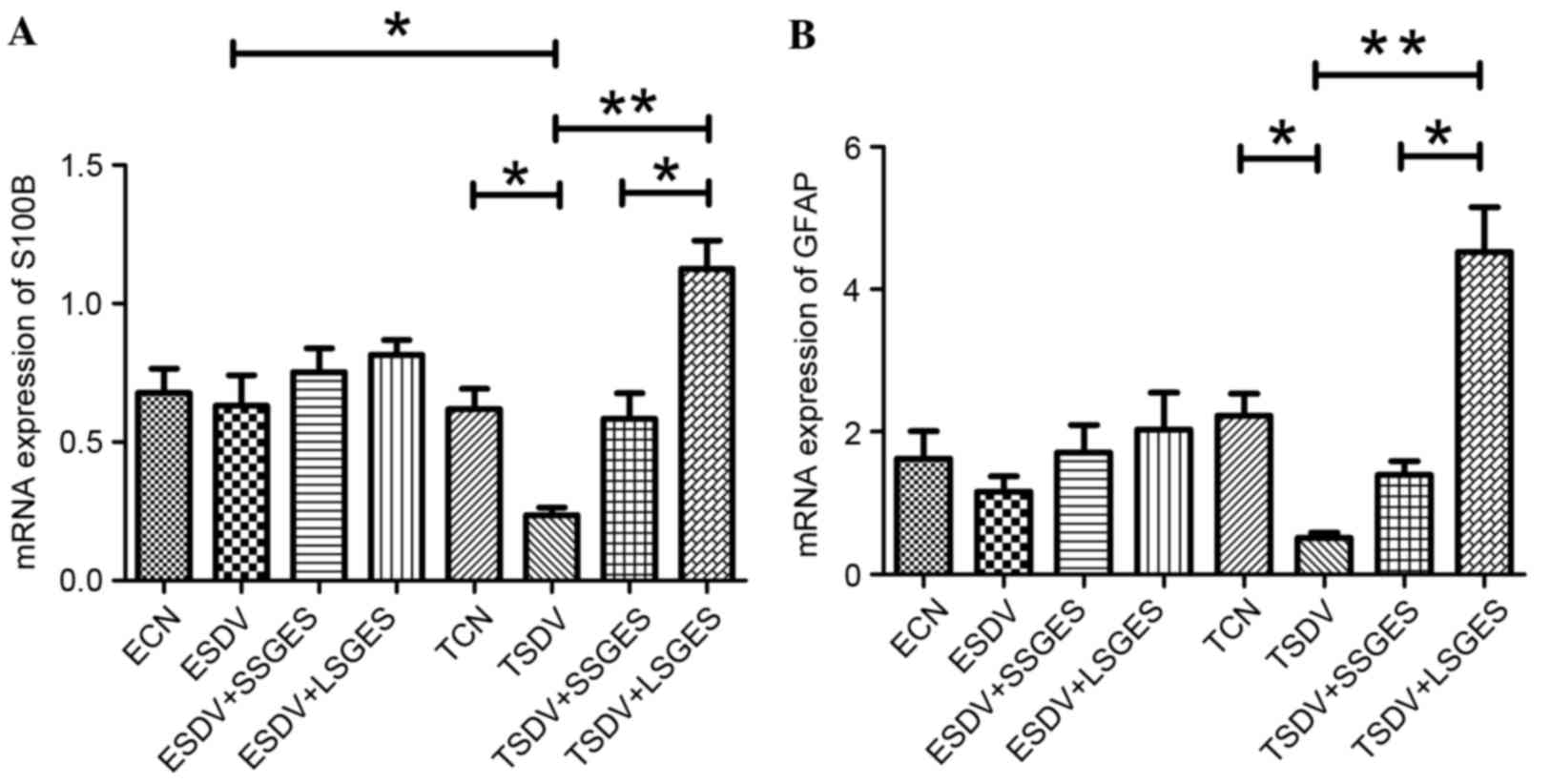
Analysis of S100B and GFAP mRNA
expression
The mRNA expression of S100B and GFAP was measured
by RT-qPCR (Fig. 2). During the
course of subdiaphragmatic vagotomy, expression levels of S100B and
GFAP were decreased gradually. However, mRNA expression of S100B
and GFAP decreased significantly in the TSDV group compared with
the TCN group (P<0.05). In the terminal groups, long-term SGES
increased mRNA expression of S100B and GFAP and expression levels
were higher compared with short-term SGES (P<0.05).
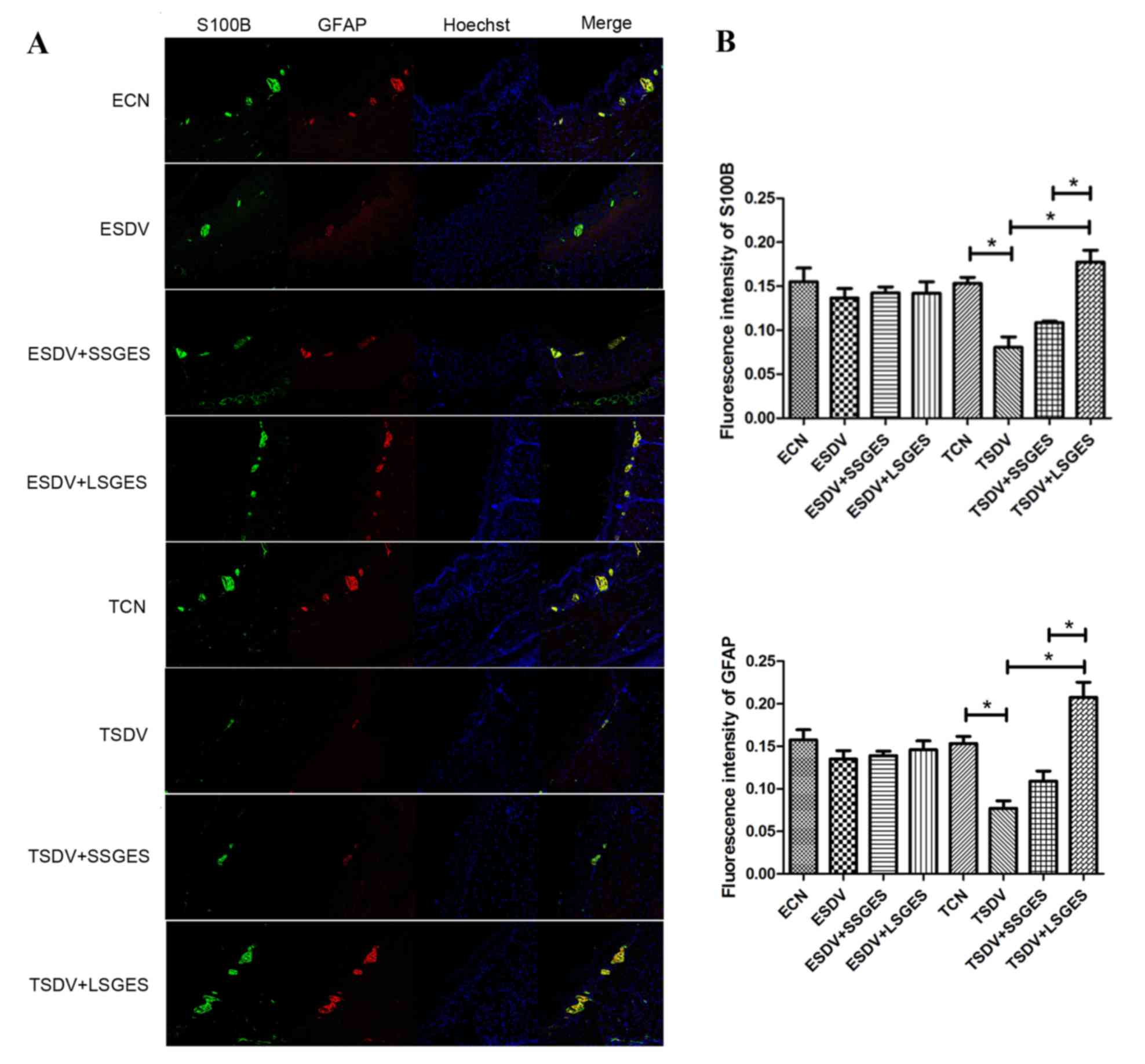
Assessment of double-labeling
immunofluorescence
S100B and GFAP proteins were evaluated by
double-labeling immunofluorescence (Fig. 3A). There was no significant change
between the early groups (P>0.05; Fig. 3B). S100B and GFAP protein
expression was decreased gradually in the course of
subdiaphragmatic vagotomy (Figs.
3B). The protein expression decreased significantly in the TSDV
group, when compared with those of the TCN group (P<0.05;
Fig. 3B). Long-term SGES
significantly upregulated S100B and GFAP protein expression
(P<0.05) and was more effective at S100B and GFAP than
short-term SGES. (P<0.05; Figs.
3B).
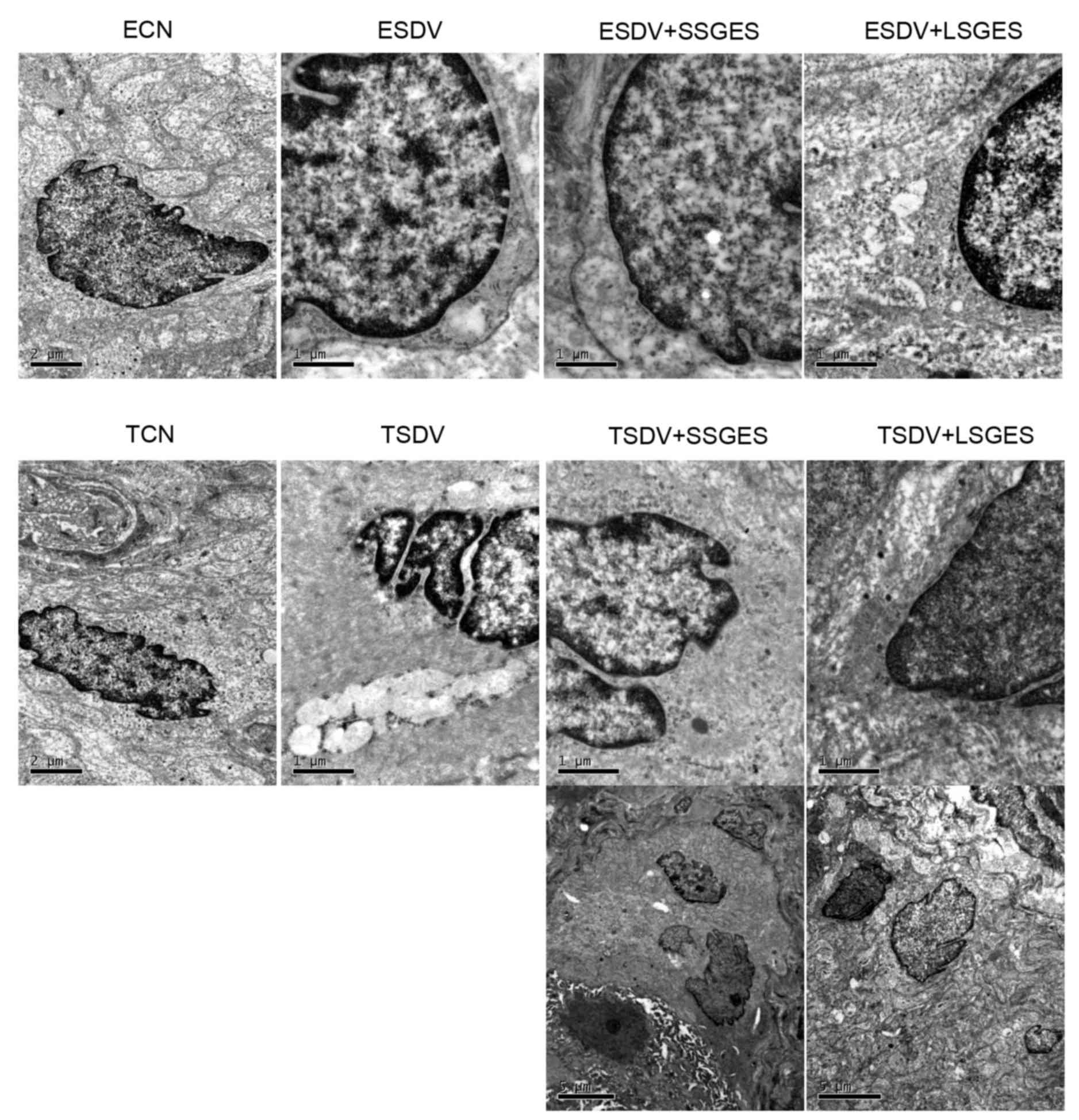
Assessment of ultrastructure of
EGCs
The ultrastructure of EGC in the myenteric plexus
was demonstrated by transmission electron microscopy (Fig. 4). There were abundant cell
organelles containing mitochondria, filaments and the smooth and
rough endoplasmic reticulum in the cytoplast of EGCs in the ECN and
TCN groups (Fig. 4). The damaged
ultrastructural features involved swelling of mitochondria,
dilation of the endoplasmic reticulum, decreased numbers of
filaments and condensed chromatin, and were identified in the ESDV
and TSDV groups (Fig. 4) and the
latter was marked than the former. In the short-term or long-term
SGES, the numbers of mitochondria and filaments increased (Fig. 4). Unexpectedly, an apoptotic
enteric neuron was observed in the TSDV group (Fig. 4).
Discussion
The results of the present study clearly
demonstrated that EGCs were severely damaged and gastric emptying
was delayed following subdiaphragmatic vagotomy in rats. Meanwhile,
the data indicate that SGES may activate injured EGCs and improve
gastric motility. In addition, EGCs had a certain reserve capacity
in the early subdiaphragmatic vagotomy for the expression of S100B
and GFAP mostly the same amount compared with the early control
group.
Vagal activities have clear influence on
gastrointestinal motility. Vagal innervation regulates the
contractile pattern and contractile activity of stomach (17) and vagal nerve stimuli promotes
activation of EGCs in a burn-induced intestinal injury mouse model
(18). In the current study,
gastric emptying was delayed in the TSDV group, while it remained
unchanged in the ESDV group. The underlying mechanism may be that
the EGCs initiated a compensatory function modulating gastric
motility in the ESDV group and beyond its ability in the TSDV
group. Therefore, SGES improved the symptoms of the
vagotomy-induced delayed gastric motility, which is consistent with
previous literature (7,14). To the best of the authors'
knowledge, the vagal fibers primarily promoted gastrointestinal
movement and secretion, while sympathetic fibers have the opposite
effect. There are conflicting results concerning which one has the
main role in the ENS. A previous paper reported that only
sympathetic fibers released ATP to activate EGCs, and then EGCs
selectively responded to sympathetic activities in the guinea pig
distal colon (19). The possible
explanation for the discordance may be that sympathetic fibers and
vagal fibers innervate the specific regions of gastrointestinal
tract respectively and different species may also affect the
results.
EGCs have been previously reported to respond to
pro-inflammatory stimuli by producing nitric oxide or increasing
GFAP+ enteric glia (20). In
addition, glia cell lined-derived neurotrophic factor (GDNF) has
been demonstrated to promote the survival of enteric neurons, and
the decrease of GDNF may be involved in the damage to enteric
neurons in diabetics (21).
Glial-derived s-nitrosoglutathione restored the function of the
mucosal barrier through upregulating F-actin and tight junction
associated proteins (22). EGCs
act as glutamatergic neurotransmitter receptors, and expression of
glial metabotropic glutamate receptor subtype 5 is decreased during
colitis (23). EGCs have been
associated with the modulation of the intestinal epithelial barrier
in acute intestinal ischemia reperfusion injury, leading to
activation of EGCs as barrier protection (24). In addition, nicotinic cholinergic
agonists promote EGC activation, and the activated EGCs modulate
intestinal barrier integrity by inhibiting the nuclear factor-κB
pathway (22,25). EGCs serve an indispensable role in
maintaining the intestinal epithelial barrier and participate in
the inflammatory response. Therefore, activation of EGCs by
different stimulating factors is necessary. In the present study,
EGCs were damaged seriously in the TSDV group, but SGES activated
EGCs. Nevertheless, how the activated EGCs regulate
gastrointestinal barrier function in subdiaphragamtic vagotomy is
yet to be investigated.
EGCs participate in regulating gastrointestinal
motility. The John Cunningham (JC) virus, a polymavirus, can affect
glial cells in the brain, resulting in fatal diseases. In a
previous study, EGCs in the myenteric plexus were demonstrated to
be infected with the JC virus in chronic idiopathic intestinal
pseudo-obstruction, which may be associated with the lesion of
intestinal propulsive motility (26). However, an additional study
indicated that mucosa-associated glial cell networks decline in
diabetics caused by a high-fat diet, while myenteric glial cells
saw no change in the early and the late disease periods (27). A previous study demonstrated that
EGCs in the myenteric plexus of the stomach were decreased in
terminal diabetic rats (14).
EGCs, together with enteric ganglion cells and interstitial cells
of Cajal, were significantly decreased in idiopathic slow transit
constipated patients (28).
EGCs communicate with enteric neurons dynamically
which has been recognized increasingly important. Neuronal activity
may be detected by EGCs through neurotransmitters (29). Likewise, EGCs may be activated by
specific neurotransmitters or receptors. Mechanical stimulation and
ATP activate EGCs, resulting in the increasing of intracellular
calcium levels; these increases propagate to neighboring cells
partly via gap junctions (30).
Gulbransen and Sharkey (31)
reported that EGCs express P2Y4 receptors, which predominantly
mediate ATP detection. In addition, EGCs respond to serotonergic
and cholinergic signaling (32).
In the presented subdiaphragmatic vagotomy rat model, it was
verified that EGCs were activated by SGES. However, the reasons
underlying the alterations to Ca2+ levels, together with
K+ and Na+ in activated EGCs, remain unclear.
There are certain difficulties to overcome, for example, how to
acquire viable EGCs sufficiently. In the future, more studies
should be conducted on ion changes in EGCs.
S100B is a Ca2+ binding protein, which
acts as a marker of EGC activation. The expression of S100B can
have a trophic or toxic effect in EGCs, depending on its
extracellular concentration (33).
Pathogens cause a dramatic upregulation of S100B expression and NO
products in human-derived EGCs in a host-bacteria interaction model
(34). This indicates that
glial-derived S100B is involved in the pro-inflammatory response,
which agrees with previous research (35,36).
The present study demonstrated the activation of EGCs through S100B
gene/protein upregulation, which was involved in improving gastric
emptying.
EGCs contain a dense intermediate filament and GFAP,
which is another marker for EGCs in the gut. The distortion of EGCs
was associated with the alterations to the position and form of
GFAP; the distorted EGCs then induced damage to neighboring enteric
neurons (37). Furthermore, GFAP
was upregulated and dephosphorylated in the EGCs of patients with
Parkinson's disease (38). The
activation of EGCs led to GFAP overexpression in the
gastrointestinal tract; the same result as the present finding.
Based on the expression of GFAP, von Boyen et al (20) divided EGCs into two different
types: GFAP- and GFAP+. The significantly increased GFAP+ EGCs
respond to pro-inflammatory cytokines, which participate in
modulating the integrity of the inflamed gut.
In conclusion, the authors displayed that SGES was
concerned with increased activation of EGCs and promoted gastric
motility in rats with subdiaphragmatic vagotomy. However, further
evidence is required to indicate that how EGCs communicate with
enteric neurons and with interstitial cells of Cajal and smooth
muscle cells to regulate the gastrointestinal functions. Finally,
the ultimate aim is to make EGCs accessible as a potential
therapeutic target point in the diseases of enteric neuropathy.
Acknowledgements
The current study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81170342).
References
|
1
|
Coronado VG, Xu L, Basavaraju SV, McGuire
LC, Wald MM, Faul MD, Guzman BR and Hemphill JD; Centers for
Disease Control and Prevention (CDC), : Surveillance for Traumatic
Brain Injury-Deaths-United States, 1997–2007. MMWR Surveill Summ.
60:1–32. 2011.PubMed/NCBI
|
|
2
|
Kao CH, ChangLai SP, Chieng PU and Yen TC:
Gastric emptying in head-injured patients. Am J Gastroenterol.
93:1108–1112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pinto TF, Rocha R, Paula CA and de Jesus
RP: Tolerance to enteral nutrition therapy in traumatic brain
injury patients. Brain Inj. 26:1113–1117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fynne L, Worsoe J, Gregersen T, Schlageter
V, Laurberg S and Krogh K: Gastric and small intestinal dysfunction
in spinal cord injury patients. Acta Neurol Scand. 125:123–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song J, Yin J, Sallam HS, Bai T, Chen Y
and Chen JD: Electroacupuncture improves burn-induced impairment in
gastric motility mediated via the vagal mechanism in rats.
Neurogastroenterol Motil. 25:807–e635. 2013.PubMed/NCBI
|
|
6
|
Zhou SY, Lu YX and Owyang C: Gastric
relaxation induced by hyperglycemia is mediated by vagal afferent
pathways in the rat. Am J Physiol Gastrointest Liver Physiol.
294:G1158–G1164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Koothan T and Chen JD:
Synchronized gastric electrical stimulation improves
vagotomy-induced impairment in gastric accommodation via the
nitrergic pathway in dogs. Am J Physiol Gastrointest Liver Physiol.
296:G310–G318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furness JB: The Enteric Nervous System.
Blackwell Publishing; Oxford: pp. 2742006
|
|
9
|
Gulbransen BD: Enteric glia. Morgan &
Claypool Publishers; San Rafael, CA: 2014, PubMed/NCBI
|
|
10
|
De Giorgio R, Giancola F, Boschetti E,
Abdo H, Lardeux B and Neunlist M: Enteric glia and neuroprotection:
Basic and clinical aspects. Am J Physiol Gastrointest Liver
Physiol. 303:G887–G893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bassotti G, Villanacci V, Nascimbeni R,
Cadei M, Manenti S, Antonelli E, Fanini L and Salerni B: Increase
of colonic mast cells in obstructed defecation and their
relationship with enteric glia. Dig Dis Sci. 57:65–71. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McClain JL, Grubišić V, Fried D,
Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V and Gulbransen
BD: Ca2+ responses in enteric glia are mediated by connexin-43
hemichannels and modulate colonic transit in mice. Gastroenterol.
146:497–507.e1. 2014. View Article : Google Scholar
|
|
13
|
Collares EF and Vinagre AM: Evidence of
the effect of dipyrone on the central nervous system as a
determinant of delayed gastric emptying observed in rats after its
administration. Braz J Med Biol Res. 36:1375–1382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang W, Wang N, Shi X and Chen J:
Synchronized dual pulse gastric electrical stimulation induces
activation of enteric glial cells in rats with diabetic
gastroparesis. Gastroenterol Res Pract. 2014:9640712014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Marzo V, Capasso R, Matias I, Aviello
G, Petrosino S, Borrelli F, Romano B, Orlando P, Capasso F and Izzo
AA: The role of endocannabinoids in the regulation of gastric
emptying: Alterations in mice fed a high-fat diet. Br J Pharmacol.
153:1272–1280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka T, Kendrick ML, Zyromski NJ, Meile
T and Sarr MG: Vagal innervation modulates motor pattern but not
initiation of canine gastric migrating motor complex. Am J Physiol
Gastrointest Liver Physiol. 281:G283–G292. 2001.PubMed/NCBI
|
|
18
|
Costantini TW, Bansal V, Krzyzaniak M,
Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP and
Coimbra R: Vagal nerve stimulation protects against burn-induced
intestinal injury through activation of enteric glia cells. Am J
Physiol Gastrointest Liver Physiol. 299:G1308–G1318. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gulbransen BD, Bains JS and Sharkey KA:
Enteric glia are targets of the sympathetic innervation of the
myentericplexus in the guinea pig distal colon. J Neurosci.
30:6801–6809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
von Boyen GB, Steinkamp M, Reinshagen M,
Schäfer KH, Adler G and Kirsch J: Proinflammatory cytokines
increase glial fibrillary acidic protein expression in enteric
glia. Gut. 53:222–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du F, Wang L, Qian W and Liu S: Loss of
enteric neurons accompanied by decreased expression of GDNF and
PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil.
21:1229–e114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Savidge TC, Newman P, Pothoulakis C, Ruhl
A, Neunlist M, Bourreille A, Hurst R and Sofroniew MV: Enteric glia
regulate intestinal barrier function and inflammation via release
of S-nitrosoglutathione. Gastroenterol. 132:1344–1358. 2007.
View Article : Google Scholar
|
|
23
|
Nasser Y, Keenan CM, Ma AC, McCafferty DM
and Sharkey KA: Expression of a functional metabotropic glutamate
receptor 5 on enteric glia is altered in states of inflammation.
Glia. 55:859–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao W, Wang W, Chen W, Sun L, Li X, Zhang
C and Yang H: GDNF is involved in the barrier-inducing effect of
enteric glial cells on intestinal epithelial cells under acute
ischemia reperfusion stimulation. Mol Neurobiol. 50:274–289. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheadle GA, Costantini TW, Bansal V,
Eliceiri BP and Coimbra R: Cholinergic signaling in the gut: A
novel mechanism of barrier protection through activation of enteric
glia cells. Surg Infect (Larchmt). 15:387–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Selgrad M, De Giorgio R, Fini L,
Cogliandro RF, Williams S, Stanghellini V, Barbara G, Tonini M,
Corinaldesi R, Genta RM, et al: JC virus infects the enteric glia
of patients with chronic idiopathic intestinal pseudo-obstruction.
Gut. 58:25–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stenkamp-Strahm C, Patterson S, Boren J,
Gericke M and Balemba O: High-fat diet and age-dependent effects on
enteric glial cell populations of mouse small intestine. Auton
Neurosci. 177:199–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bassotti G, Villanacci V, Maurer CA,
Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G
and Salerni B: The role of glial cells and apoptosis of enteric
neurones in the neuropathology of intractable slow transit
constipation. Gut. 55:41–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gulbransen BD, Bains JS and Sharkey KA:
Enteric glia are targets of the sympathetic innervation of the
myenteric plexus in the guinea pig distal colon. J Neurosci.
30:6801–6809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gomes P, Chevalier J, Boesmans W, Roosen
L, van den Abbeel V, Neunlist M, Tack J and Berghe P Vanden:
ATP-dependent paracrine communication between enteric neurons and
glia in a primary cell culture derived from embryonic mice.
Neurogastroenterol Motil. 21:870–e62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gulbransen BD and Sharkey KA: Purinergic
neuron-to-glia signaling in the enteric nervous system.
Gastroenterol. 136:1349–1358. 2009. View Article : Google Scholar
|
|
32
|
Boesmans W, Cirillo C, Van den Abbeel V,
Van den Haute C, Depoortere I, Tack J and Berghe P Vanden:
Neurotransmitters involved in fast excitatory neurotransmission
directly activate enteric glial cells. Neurogastroenterol Motil.
25:e151–e160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cirillo C, Sarnelli G, Esposito G, Turco
F, Steardo L and Cuomo R: S100B protein in the gut: The evidence
for enteroglial-sustained intestinal inflammation. World J
Gastroenterol. 17:1261–1266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Turco F, Sarnelli G, Cirillo C, Palumbo I,
De Giorgi F, D'Alessandro A, Cammarota M, Giuliano M and Cuomo R:
Enteroglial-derived S100B protein integrates bacteria-induced
Toll-like receptor signalling in human enteric glial cells. Gut.
63:105–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cirillo C, Sarnelli G, Turco F, Mango A,
Grosso M, Aprea G, Masone S and Cuomo R: Proinflammatory stimuli
activates human-derived enteroglial cells and induces autocrine
nitric oxide production. Neurogastroenterol Motil. 23:e372–e382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Esposito G, Cirillo C, Sarnelli G, De
Filippis D, D'Armiento FP, Rocco A, Nardone G, Petruzzelli R,
Grosso M, Izzo P, et al: Enteric glial-derived S100B protein
stimulates nitric oxide production in celiac disease.
Gastroenterol. 133:918–925. 2007. View Article : Google Scholar
|
|
37
|
Thacker M, Rivera LR, Cho HJ and Furness
JB: The relationship between glial distortion and neuronal changes
following intestinal ischemia and reperfusion. Neurogastroenterol
Motil. 23:e500–e509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clairembault T, Kamphuis W,
Leclair-Visonneau L, Rolli-Derkinderen M, Coron E, Neunlist M, Hol
EM and Derkinderen P: Enteric GFAP expression and phosphorylation
in Parkinson's disease. J Neurochem. 130:805–815. 2014. View Article : Google Scholar : PubMed/NCBI
|