Introduction
Pancreatic cancer is a fatal disease and remains
consistently lethal with a 5-year survival rate of <5% (1). The disease generally causes few or no
symptoms during early stages, thus the majority of patients are
diagnosed at an advanced or terminal stage and few are suitable for
curative surgical resection. Gemcitabine-based chemotherapy is the
only option for treatment of advanced disease. Development of
resistance to gemcitabine is a major concern. FLFIRINOX is an
emerging treatment that offers a better survival outcome compared
with gemcitabine. However, this chemotherapy regimen induces
increased toxicity and exhibits only modest results (2,3).
Thus, the development of novel methods for treating pancreatic
cancer is urgently required.
Replication-selective oncolytic viruses (OVs) have
emerged as promising therapeutic tools for cancer treatment with
attractive advantages, including tumor-selective amplification and
replication, thus inducing cancer cells lysis with minimal affect
on normal tissues (4–9). Recently, >12 different oncolytic
viruses are undergoing phase I–III clinical trials targeting
different types of cancer (10).
Oncolytic adenovirus is the first and most intensively investigated
OV to date and H101 (a mutant with E1B55K gene deleted) is
now authorized in China for treatment of head and neck cancer
(Oncorine; Shanghai Sunway Biotech Co., Ltd., Shanghai, China) Our
previous study developed a cancer-targeting dual-gene virotherapy
(CTGVT-DG) strategy and generated a novel E1B55K gene
deleted oncolytic adenovirus ZD55-TRAIL-IETD-Smac (ZD55-TIS)
harboring tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL) and the second mitochondria-derived activator of caspase
(Smac) genes joined by the four-amino acid
isoleucine-aspartate-threonine-glutamate (IETD) linker (11). ZD55-TIS co-expressed TRAIL and Smac
genes simultaneously and produced broad antitumor activity in
vitro. Following intratumoral injection, the ZD55-TIS
completely eradicated hepatocellar carcinoma cell xenograft tumors.
However, the antitumor effects of ZD55-TIS against pancreatic
cancer have not been evaluated.
The cyclin-dependent kinases (CDKs) are a family of
serine/threonine kinases that control cell cycle events, and
certain members are associated with transcriptional regulation. The
tumor-specific deregulation makes the CDKs a major target for
therapy (12,13). SNS-032 is a effective and selective
inhibitor of CDK2, −7, and −9 (13). It has been reported that SNS-032
has antitumor activity in various tumors (14–17).
Based on the report which suggesting that aberrant activation of
CDKs and dysregulation of cell cycle progression is a feature of
pancreatic cancer (18), the
current study investigated whether SNS-032 is able to enhance the
anticancer activity of ZD55-TRAIL-IETD-Smac against pancreatic
carcinoma.
The present study examined whether the CDK inhibitor
SNS-032 may enhance the antitumor activities of ZD55-TIS against
pancreatic cancer. To the best of our knowledge, the present study
is the first to demonstrate that ZD55-TRAIL-IETD-Smac
co-operatively act on pancreatic cancer in vitro and in
vivo for the first time. The present study indicated that
combination therapy with ZD55-TRAIL-IETD-Smac and SNS-032 may be a
practical novel strategy against pancreatic cancer in the
future.
Materials and methods
Cell lines and viruses
Human pancreatic cancer cell lines PANC-1 and BxPC-3
and human embryonic kidney cell line HEK293 were obtained from the
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat inactivated fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.). Cells were incubated in a 5%
CO2 humidified incubator at 37°C.
Construction and production of recombinant oncolytic
adenovirus ZD55-TIS were described previously (11). The amplification of recombinant
adenovirus was performed by infecting HEK293 cells.
Cytotoxicity assay and quantitative
analysis of synergy in vitro
SNS-032 (Selleck Chemicals, Houston, TX, USA) was
prepared at 1 mg/ml in dimethyl sulfoxide, stored at −20°C, and
then diluted as needed in cell culture medium. Cell viability was
estimated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. Briefly, PANC-1 and BxPC-3 cells were plated
in 96-well plates (1×104 density) in 100 µl culture
medium. Cells were treated with different treatments (ZD55-TIS and
SNS-032) at the indicated concentrations. After 48 or 72 h, MTT
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) solution (10 µl, 5
g/l) was added to the cells which were then cultured for a further
4 h. Absorbance (570 nm) was measured using a DNA microplate reader
(GENios model; Tecan, Maennedorf, Switzerland).
Hoechst 33342 staining assay
Hoechst 33342 staining was used to detect
morphological features of cell apoptosis. PANC-1 cells were treated
with SNS-032 or ZD55-TRAIL-IETD-Smac, or the combination of SNS-032
and ZD55-TRAIL-IETD-Smac. After treatment for 72 h, 1 mg/ml (5 µl)
Hochest 33342 (Sigma-Aldrich, Merck KGaA) was added in the cells
for 30 min and morphology was observed under an inverted
fluorescence microscope. Untreated cells served as a control.
Flow cytometry analysis
PANC-1 cells were treated with ZD55-TRAIL-IETD-Smac
[8 multiplicity of infection (MOI)], SNS-032 (160 ng/ml), or
ZD55-TRAIL-IETD-Smac (8 MOI) plus SNS-032 (160 ng/ml). After 48 h,
apoptotic cells were detected by using Annexin V- fluorescein
isothiocyanate (FITC) and propidium iodide (PI) double staining or
PI staining alone following the manufacturer's instructiona. Cell
apoptosis and cell cycle were examined using the FACStar
cytofluorometer (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
PANC-1 cells were collected and washed twice with
PBS, then lysed in radioimmunoprecipitation assay buffer (4°C, 30
min). Protein concentrations were determined by bicinchoninic acid
assay (Thermo Fisher Scientific, Inc.). Protein samples (10 µg)
were separated by 12% SDS polyacrylamide-gel and transferred to
polyvinylidene fluoride membranes. Membranes were blocked with 5%
bovine serum albumin (cat. no. P0007; Beyotime Institute of
Biotechnology, Haimen, China), in a 10 mmol/l Tris-HCl pH 8.0, 150
mmol/l NaCl and 0.05% Tween-20 buffer overnight at 4°C, and then
incubated with the corresponding primary antibodies at 1:1,000
dilution overnight at 4°C. Following incubation with horseradish
peroxidase-conjugated secondary antibodies for 2 h at room
temperature, signals were detected by enhanced chemiluminescence
(ECL) with BeyoECL reagents (cat. no. P0018; Beyotime Institute of
Biotechnology). Antibodies targeting Adenovirus-5 E1A (cat. no.
sc-374663), caspase-3 (cat. no. sc-271759), poly (ADP-ribose)
polymerase (PARP; cat. no. sc-56197), caspase-8 (cat. no.
sc-166596) and GAPDH (cat. no. sc-47724) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies targeting
CDK-2 (cat. no. 2546S), CDK-7 (cat. no. 2916), CDK-9 (cat. no.
2316S), BCL2 apoptosis regulator (Bcl-2; cat. no. 4223S), BCL2
family apoptosis regulator (Mcl-1; cat. no. 4572), X-linked
inhibitor of apoptosis (XIAP; cat. no. 2042), TRAIL (cat. no.
3219S) and Smac (cat. no. 2954S) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The secondary antibodies (cat.
nos. 70-GAR007 for anti-rabbit and 70-GAM007 for anti-mouse) were
purchased from MultiSciences (Hangzhou, China).
Animal experiments
Animal experiments were performed according to the
Guide for the Care and Use of Laboratory Animals (National
Institutes of Health, Bethesda, MD, USA) and in accordance with
institutional standards. Male BALB/C nude mice (32 total) aged 5
weeks were purchased from the Shanghai Experimental Animal Center
of Chinese Academy of Science (Shanghai, China). The mice were
adapted to animal housing at 25°C. BxPC-3 cells (7×106
cells in 100 µl serum-free DMEM/mouse) were subcutaneously injected
into the right flank of male nude mice. Mice were checked three
times per week for tumor development. Once subcutaneous tumors
reached ~100-150 mm3, the nude mice were randomly
divided into four groups (n=7 per group). Subsequently, mice were
injected with ZD55-TIS, SNS-032, a combination of the virus and the
drug, or PBS. ZD55-TIS (1×109 plaque-forming units per
mouse) was delivered via an intratumor injection, while SNS-032 was
intraperitoneally injected into the mice at a dose of 30 mg/kg body
weight or 100 µl PBS as control by intratumor injection for three
times of continuous injection once every other day. Tumor volume
was measured with a vernier caliper every 4 days as calculated as
follows: Tumor volume (mm3)=(AxB2)/2; A and B
are the tumor length and width (in mm), respectively. The tumor
volumes were used the produce tumor volume growth curves. The mice
were sacrificed at 60 days post-tumor cell injection.
Hematoxylin and eosin (H&E)
staining, immunohistochemistry and terminal deoxynucleotidyl
transferase-mediated dUTP-biotin nick end labeling (TUNEL)
assay
Tumor tissues were fixed in 4% paraformaldehyde and
embedded in paraffin wax. The sections from tumors were stained
with H&E for histological analysis. For immunohistochemistry,
formalin-fixed, paraffin-embedded tissue sections were cut at a
thickness of 4 µm. For heat-induced epitope retrieval, tissue
sections were deparaffinized and rehydrated in 0.01 M pH 6.0
citrate buffer 3 times at 90°C for 5 min using a microwave oven.
Immunohistochemical staining was performed using the
streptavidin-biotin immunoperoxidase technique. Endogenous
peroxidase activity was blocked by incubation with 0.3%
H2O2 in methanol for 15 min, and nonspecific
immunoglobulin binding was blocked by incubation with 5% normal
rabbit serum (cat. no. ZLI-9025; ZSGB-BIO Beijing, China) for 10
min. Sections were incubated at 4°C overnight with primary
antibodies at 1:50 dilution. Antibodies targeting CDK-2 (cat. no.
2546S), CDK-7 (cat. no. 2916), and CDK-9 (cat. no. 2316S) were from
Cell Signaling Technology, Inc., and antibodies targeting E1A (cat.
no. sc-374663), TRAIL (cat. no. sc-4547), and Smac (cat. no.
sc-136302) were from Santa Cruz Biotechnology, Inc. The sections
were rinsed and incubated for 30 min at room temperature with
biotinylated secondary antibody at 1:100 dilution (cat. no. PV8000;
ZSGB-BIO). Following washing, the sections were incubated for
another 30 min with horseradish peroxidase-conjugated streptavidin
(cat. no. ZLI-9017; ZSGB-BIO), and finally treated with
3,3′-diaminobenzidine tetrahydrochloride as a substrate for 10 min.
For the TUNEL assay, an in situ cell apoptosis detection kit
(Roche Diagnostics, Indianapolis, IN, USA) was used, according to
the manufacturer's instructions. PBS-treated tissue sections were
used as a negative control. Hematoxylin was used as counterstain.
Bright field microscopy was used to examine sections.
Statistical analysis
The experimental results are expressed as the mean ±
standard deviation. Statistical significance was analyzed by
GraphPad 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
For comparison of two groups, two-tailed unpaired t-test was
used. For comparison of more than two groups, one-way analysis of
variance followed by Tukey's post hoc test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
SNS-032 enhances
ZD55-TRAIL-IETD-Smac-mediated growth inhibition in pancreatic
cancer cells
The oncolytic adenovirus vector, ZD55, was
constructed by deleting the E1B 55-kDa gene of adenovirus 5, which
can selectively replicate in various tumor cells. Based on the
Cancer Targeting Gene-Viro-Therapy, ZD55 was used to package the
dual therapeutic gene TRAIL and Smac linked via a caspase-8
cleavage site (IETD) to obtained the novel recombinant oncolytic
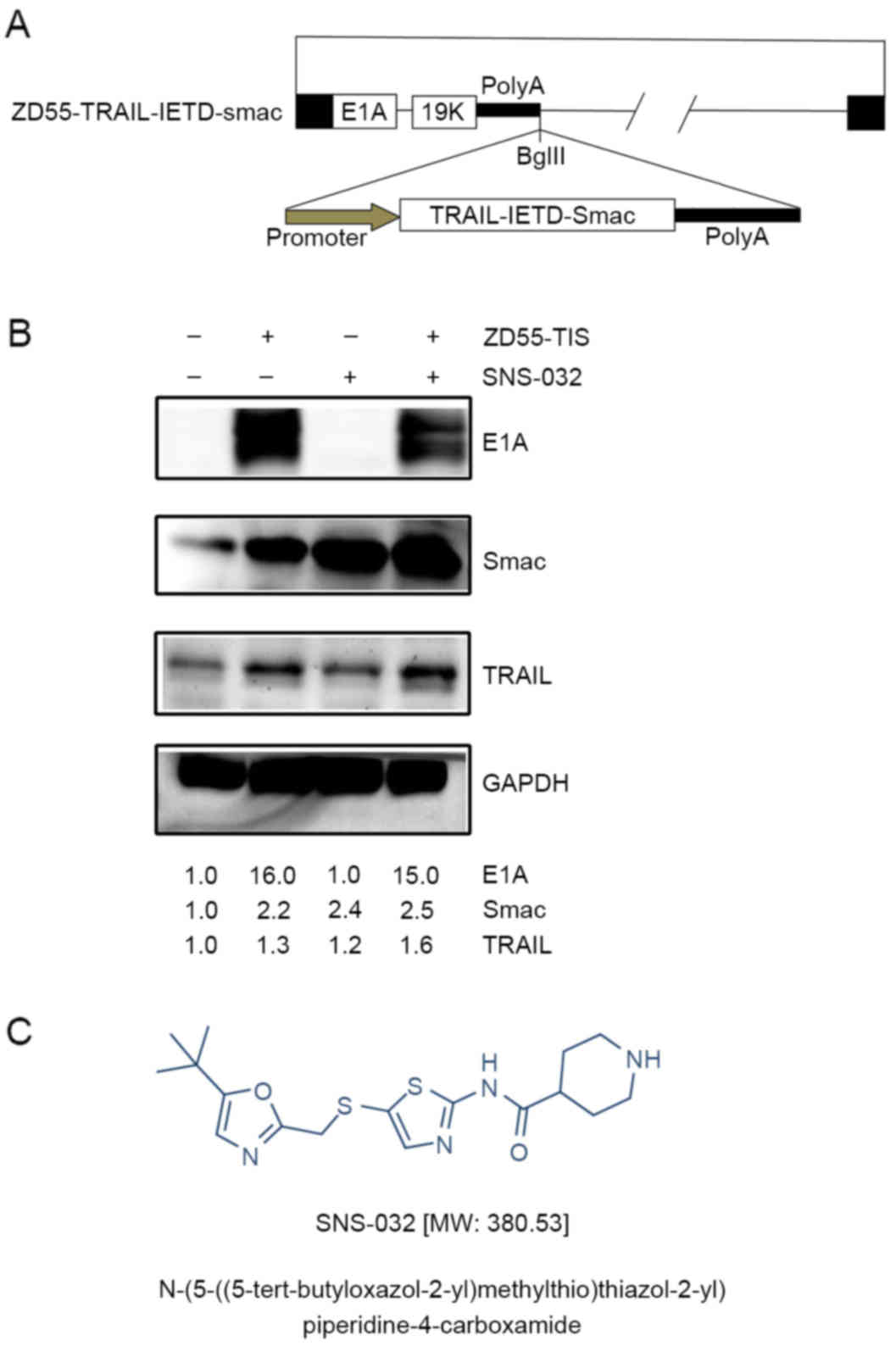
adenovirus, ZD55-TRAIL-IETD-Smac (Fig.
1A). To validate the characterization of ZD55-TRAIL-IETD-Smac,
western blot analysis was performed to detected the protein
expression of the adenovirus E1A, and therapeutic genes TRAIL and
Smac. The strong expression of E1A, TRAIL and Smac in PANC-1 cells
infected with ZD55-TRAIL-IETD-Smac indicated that
ZD55-TRAIL-IETD-Smac can selectively replicate at high levels in
pancreatic cancer cells (Fig.
1B).
To assess whether the CDK inhibitor SNS-032
(Fig. 1C) enhances
ZD55-TRAIL-IETD-Smac-induced cell death in pancreatic cancer cells,
MTT assay was performed. PANC-1 and BxPC-3 pancreatic cancer cell
lines were infected with ZD55-TRAIL-IETD-Smac and/or treated with
SNS-032. The results suggest that the combination of
ZD55-TRAIL-IETD-Smac and SNS-032 has an enhanced cytotoxic effect
on pancreatic cancer cells compared with either
ZD55-TRAIL-IETD-Smac or SNS-032 alone (Fig. 2A and B).
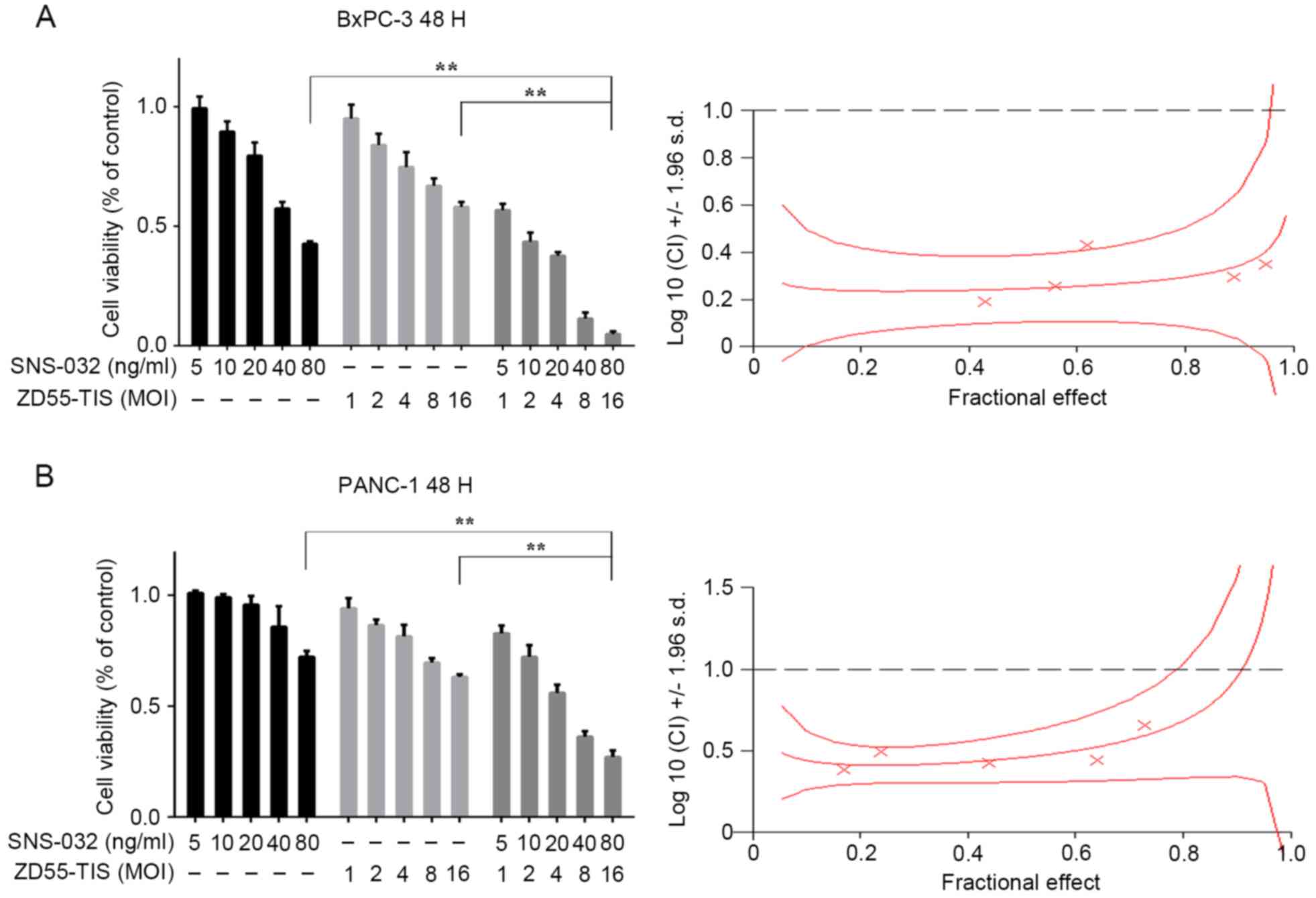 | Figure 2.Combination of ZD55-TIS and SNS-032
enhances suppression of tumor cell proliferation. Pancreatic cancer
cells (A) PANC-1 and (B) BxPC-3 were treated with ZD55-TIS (1, 2,
4, 8 and 16 MOI), SNS-032 (5, 10, 20, 40 and 80 ng/ml), or ZD55-TIS
plus SNS-032 for 48 h. The image represents three independent
experiments. Cell viability was evaluated by MTT assay and the
synergistic effect of ZD55-TIS combined with SNS-032 on PANC-1 and
BxPC-3 was quantified by CI analysis and expressed as log (CI) vs.
fractional effect. Where calculable, 95% confidence intervals are
shown. **P<0.05. ZD55-TIS, ZD55-TRAIL-IETD-Smac; TRAIL, tumor
necrosis factor-related apoptosis-inducing ligand; IETD,
isoleucine-aspartate-threonine-glutamate; Smac, second
mitochondria-derived activator of caspase; CI, combination
index. |
SNS-032 enhances
ZD55-TRAIL-IETD-Smac-induced apoptosis and cell cycle arrest in
pancreatic cancer cells
Subsequently, Hoechst 33342 staining was performed
to observe the morphological alterations of PANC-1 cells treated
with ZD55-TRAIL-IETD-Smac and/or SNS-032. The results in Fig. 3A demonstrated that compared with
ZD55-TRAIL-IETD-Smac treatment alone, co-treatment with SNS-032 led
to marked apoptosis characterized by chromatin condensation,
nuclear fragmentation and apoptotic body formation.
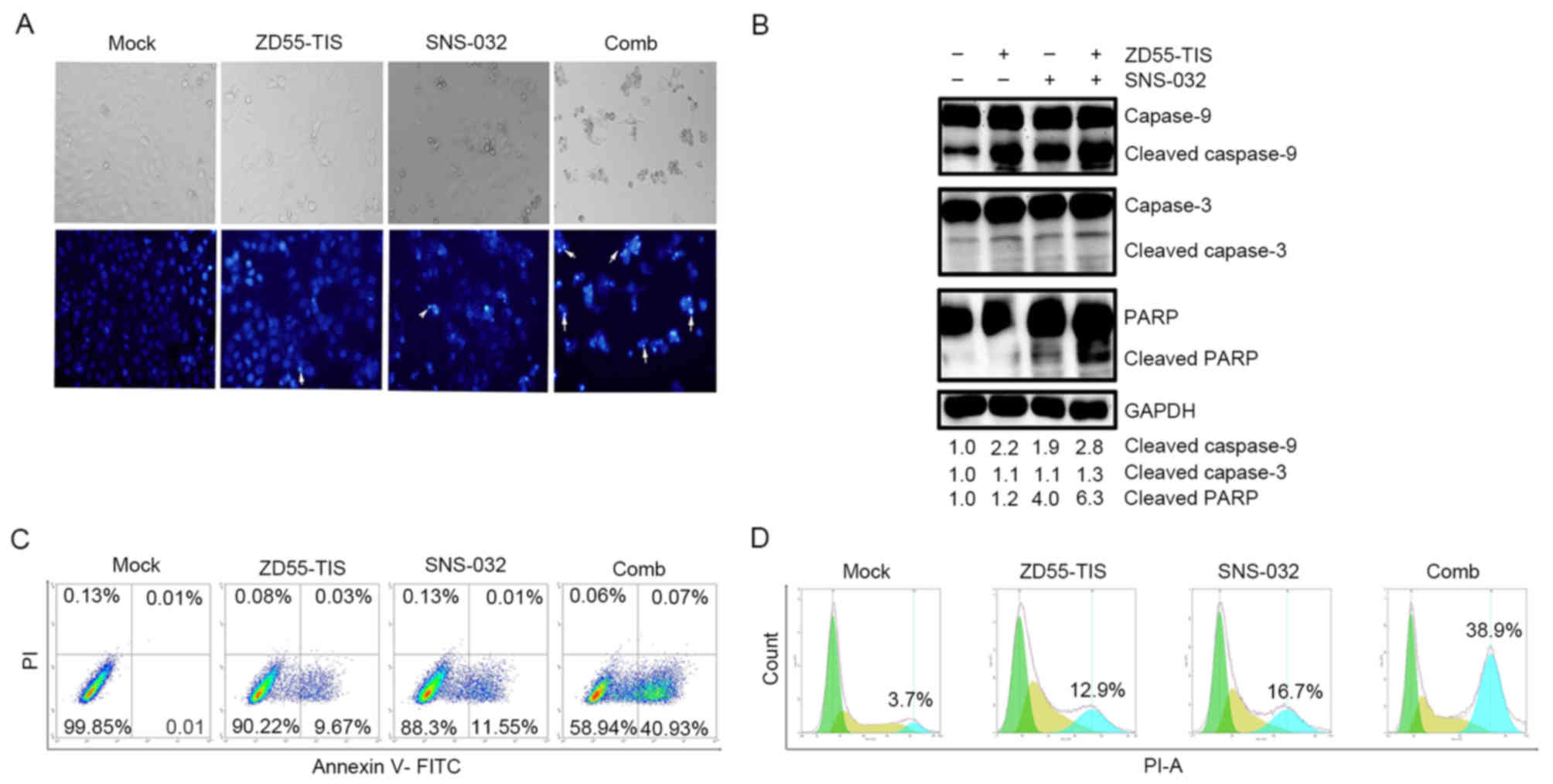 | Figure 3.SNS-032 enhances ZD55-TIS-induced
apoptosis and cell cycle arrest in pancreatic cancer cells. PANC-1
were infected with ZD55-TIS (8 MOI), treated with SNS-032 (160
ng/ml) or ZD55-TIS (8 MOI) plus SNS-032 (160 ng/ml). (A) After 72
h, PANC-1 cells were stained with Hoechst 33342 and observed under
an inverted fluorescence microscope. White arrows indicate positive
apoptotic cells. Original magnification, ×200. (B) Western blotting
was performed to detect cleaved caspase-9 and −3, and PARP. GAPDH
was used as the loading control and the level in the untreated
group was set as 1. At 48 h after treatment, (C) apoptosis and (D)
the cell cycle were analyzed by flow cytometry and the % of cells
in the G2 phase was calculated. Uninfected cells served as control.
ZD55-TIS, ZD55-TRAIL-IETD-Smac; TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand; IETD,
isoleucine-aspartate-threonine-glutamate; Smac, second
mitochondria-derived activator of caspase; Comb, combination. PI,
propidium iodide; FITC, fluorescein isothiocyanate; PARP,
poly(ADP-ribose) polymerase. |
Western blot analysis demonstrated that
ZD55-TRAIL-IETD-Smac activated the caspase-dependent pathway,
including activation of caspase-9 and caspase-3, and cleavage of
PARP, and this effect was further enhanced by co-treatment with
SNS-032 and ZD55-TRAIL-IETD-Smac compared with either treatment
alone (Fig. 3B).
To quantify the effects of SNS-032 on
ZD55-TRAIL-IETD-Smac-induced apoptosis, Annexin V-FITC/PI double
staining was used to analyze cell apoptosis (Fig. 3C). The results demonstrated that
the apoptotic rate of PANC-1 cells co-treated with
ZD55-TRAIL-IETD-Smac and SNS-032 was 40.93%, which was nearly
4-fold more than that of ZD55-TRAIL-IETD-Smac treatment alone
(9.67%).
Furthermore, to determine whether the
anti-proliferative effects of ZD55-TRAIL-IETD-Smac and SNS-032 may
also cause cell cycle arrest, cell cycle analyses were performed on
PANC-1 cells treated for 48 h. As demonstrated in Fig. 3D, when used individually, treatment
with ZD55-TRAIL-IETD-Smac or SNS-032, 12.9 or 16.7% cells,
respectively, were in the G2 cell cycle phase. However, treatment
with a combination of ZD55-TRAIL-IETD-Smac and SNS-032 arrested a
greater number of cells in the G2 phase (38.9%).
SNS-032 synergized the anti-pancreatic
cancer effect of ZD55-TRAIL-IETD-Smac by changing the expression of
cell apoptosis signaling elements
In order to further investigate the potential
mechanism of the synergistic effect of SNS-032 combined with
ZD55-TRAIL-IETD-Smac, CDK-2, −7 and −9, and other
apoptosis-associated proteins (Mcl-1, XIAP and Bcl-2) were analyzed
by western blotting. The results are presented in Fig. 4. Compared with the control, the
expression levels of CDK-2, −7 and −9, and Mcl-1, XIAP and Bcl-2
were downregulated by treatment with SNS-032 (160 ng/ml) or
ZD55-TRAIL-IETD-Smac (8 MOI) treatment alone in PANC-1 cells.
Downregulation of CDK-2, −7, and −9 were not notable when treated
with ZD55-TRAIL-IETD-Smac alone, the effects were marked when
combined treatment with SNS-032 (160 ng/ml) was performed. These
results indicated that SNS-032 has the synergistic effect on
ZD55-TRAIL-IETD-Smac-induced apoptosis in pancreatic cells by
affecting anti-apoptotic regulators, including CDK-2, CDK-9, Mcl-1
and XIAP.
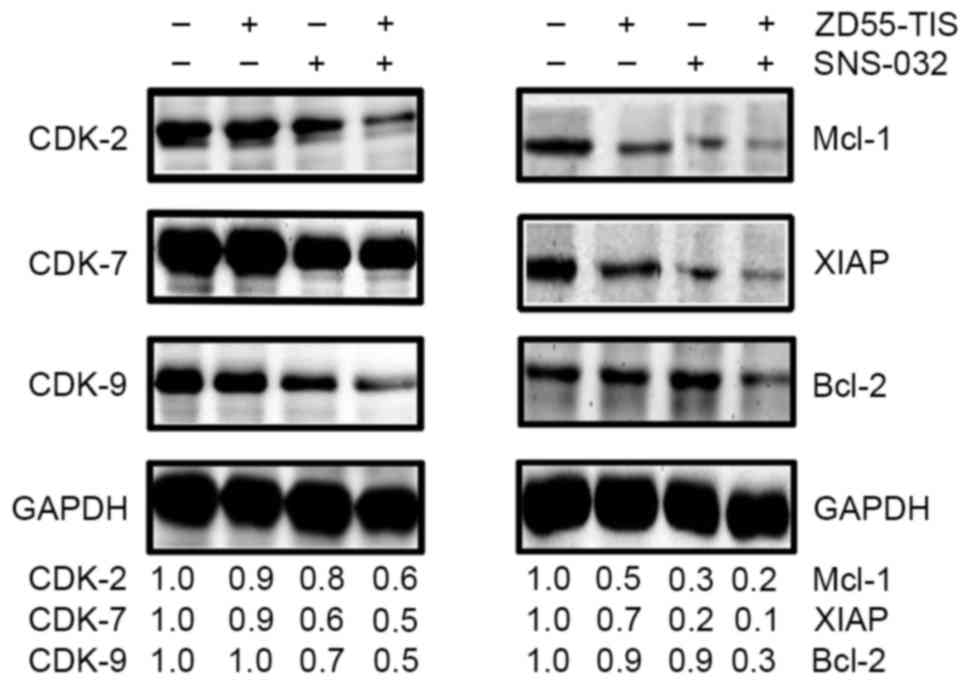 | Figure 4.Effect of SNS-032 combined with
ZD55-TIS on pro-apoptotic signaling elements in PANC-1 cells.
PANC-1 cells were treated with ZD55-TIS (8 MOI) or/and SNS-032 (160
ng/ml). The combination of ZD55-TIS and SNS-032 downregulated the
protein expression of CDK-2, −7, and −9, and XIAP, Mcl-1 and Bcl-2.
ZD55-TIS, ZD55-TRAIL-IETD-Smac; TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand; IETD,
isoleucine-aspartate-threonine-glutamate; Smac, second
mitochondria-derived activator of caspase; CDK, cyclin-dependent
kinase; Mcl-1, Bcl-2 family apoptosis regulator; XIAP, X-linked
inhibitor of apoptosis; Bcl-2, Bcl-2 apoptosis regulator. |
SNS-032 enhances
ZD55-TRAIL-IETD-Smac-mediated pancreatic tumor growth suppression
in vivo
To determine the therapeutic effects of combination
treatment with SNS-032 and ZD55-TRAIL-IETD-Smac in vivo,
animal experiments were performed using a pancreatic tumor
xenograft model established using BxPC-3 cells. Compared with the
PBS group, SNS-032 group or ZD55-TRAIL-IETD-Smac group, SNS-032
plus ZD55-TRAIL-IETD-Smac significantly suppressed tumor growth
(P<0.0001 compared with ZD55-TRAIL-IETD-Smac; P<0.0001
compared with SNS-032) in Fig. 5A.
The average tumor volume in the mice receiving combination therapy
was 682 mm3 at the end of the experiment (day 60).
Whereas, the average volumes of mice injected with
ZD55-TRAIL-IETD-Smac, SNS-032 and PBS were 1129.07 mm3,
1394.18 mm3 and 2805.36 mm3, respectively
(Fig. 5A). Additionally, all the
tumors masses were removed for imaging and to compare the volume
changes among different treatment groups (Fig. 5B).
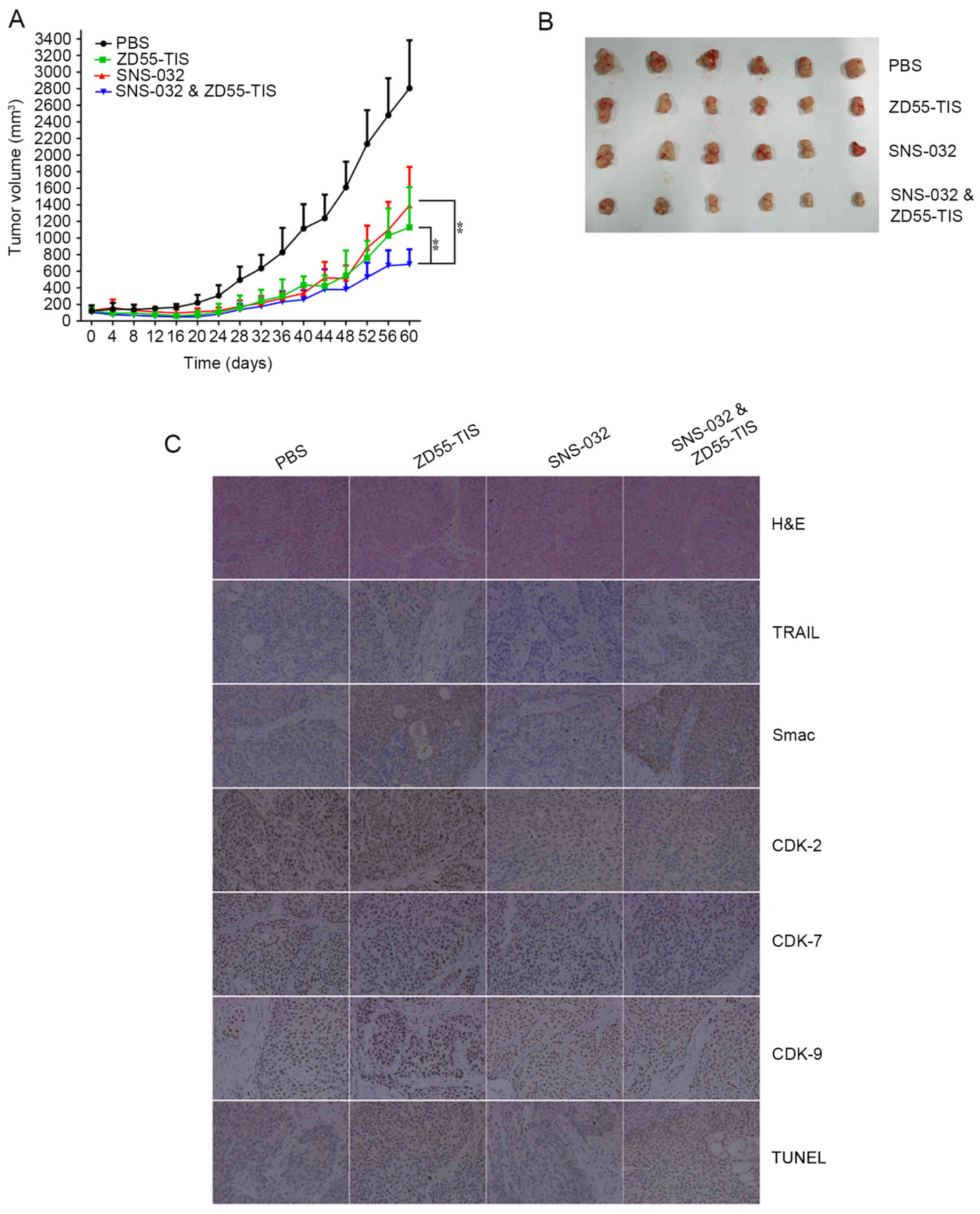 | Figure 5.Synergistic effects of ZD55-TIS and
SNS-032 in vivo. (A) Tumor volume was measured at different
times after treatment. Data are presented as the mean ± standard
deviation (n=7). **P<0.05, with comparisons indicated by lines.
(B) The image presents inhibitory effect of each group on tumor
growth at the last time point (day 60) when the mice were
sacrificed. (C) Subcutaneous BxPC-3 tumors were collected 7 days
after injection, and sections were analyzed by different methods.
H&E staining showed that tumor tissues treated with the
combination of ZD55-TIS and SNS-032 exhibited the strongest cell
death (original magnification, ×400). Immunohistochemistry analysis
demonstrated there was strong expression of TRAIL and Smac in
xenografts treated with ZD55-TIS and the combined therapy group.
However, the combined therapy group had a more obvious
downregulation of CDK-2, −7 and −9 expression compared with SNS-032
alone. TUNEL assay revealed enhanced cell apoptosis in the group
treated with ZD55TIS plus SNS-032. ZD55-TIS, ZD55-TRAIL-IETD-Smac;
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand;
IETD, isoleucine-aspartate-threonine-glutamate; Smac, second
mitochondria-derived activator of caspase; H&E, hematoxylin and
eosin; CDK, cyclin-dependent kinase; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick end labeling. |
Cytopathic effect of combination
therapy on tumor growth inhibition
H&E staining verified that treatment of
ZD55-TRAIL-IETD-Smac plus SNS-032 resulted in marked cell death and
tumor mass necrosis, whereas minimal or no death/necrosis was
observed in tumor tissue from the SNS-032, ZD55-TRAIL-IETD-Smac or
PBS groups.
Furthermore, analysis of tumors by
immunohistochemical staining using anti-TRAIL, anti-Smac,
anti-CDK-2, anti-CDK-7 and anti-CDK-9 antibodies revealed that
there was a strong expression of TRAIL and Smac in xenografts
treated with ZD55-TRAIL-IETD-Smac and the combined therapy group.
However, there was a downregulation of CDK-2, −7 and −9 in
xenografts treated with SNS-032 and in the combined therapy
group.
To investigate the potential mechanisms underlying
tumor growth inhibition induced by ZD55-TRAIL-IETD-Smac combined
with SNS-032, a TUNEL assay was used to verify whether the action
of the combination therapy can lead to a pro-apoptotic effect. As
demonstrated in Fig. 5C,
ZD55-TRAIL-IETD-Smac plus SNS-032 caused marked cell death in the
tumor.
Discussion
TRAIL, a member of the tumor necrosis factor super
family, can selectively induce apoptosis in various tumor cells,
but almost no toxicity in normal cells (19). The TRAIL apoptotic signaling
pathway inhibitory regulation has several levels. Inhibitors of
apoptosis (IAPs) can inhibit caspase activation. XIAP can directly
bind and inhibits caspases-3, −7 and −9 (20,21).
IAP overexpression has been demonstrated to be associated with
tumor resistance to apoptosis-inducing agents (22,23).
Hence, reducing IAP inhibition of apoptosis may be pivotal for
sensitizing cancer cells to anti-cancer drugs. Additionally, by
eliminating the IAP inhibition of caspases 12 and −13, Smac and the
murine homolog DIABLO promote caspase activation. In response to
apoptotic stimuli, Smac is released from mitochondria into the
cytosol and binds to XIAP, thereby reduced the XIAP inhibition of
caspases (24). Our previous
studies have demonstrated that overexpressed Smac rapidly enhances
sensitivity and promotes apoptosis of hepatocellular carcinoma
(HCC) cells to TRAIL and complete regression of HCC could only be
achieved by the combined treatment of Smac and TRAIL (25). Furthermore, our previous study
generated a novel E1B-55 K deleted oncolytic adenovirus,
ZD55-TRAIL-IETD-Smac, that harbors both TRAIL and Smac genes
(11). In this vector a caspase-8
cleavage site (IETD) was introduced between the genes, allowing the
production of TRAIL and Smac following activation of caspase-8 in
virus-infected cells. Several in vitro and in vivo
experiments have demonstrated the antitumor effects of
ZD55-TRAIL-IETD-Smac in models of hepatocellular, cervical, lung,
breast and colorectal cancer.
Combination targeted therapy is necessary for cancer
treatment because tumors are genetically diverse and resistance
seems inevitable (26,27). Therefore, it is important to
identify combinations of two or more therapeutic agents, which
function by different mechanisms with synergistic effects without
increasing adverse effects. Oncolytic virotherapy for cancer is a
novel treatment strategy. However, oncolytic virotherapy has not
been effective in preclinical animal tumor models and clinical
trials (28). Several clinical
studies have illustrated that combination of oncolytic adenoviruses
with chemotherapy (29–31) or radiation therapy (32) may enhance and have synergistic
antitumor activity. Oncolytic adenoviruses combined with
cytotoxicitic chemotherapies may enhance the potential of oncolytic
adenovirus and optimize treatment.
The aim of this study was to investigate the
possibility of combining ZD55-TRAIL-IETD-Smac with SNS-032, a CDK
inhibitor that may be a novel therapeutic agent for patients with
pancreatic carcinoma (18,33,34),
and thereby enhance their antitumor activities. The MTT analysis
demonstrated that combination of SNS-032 with ZD55-TRAIL-IETD-Smac
was obviously superior to SNS-032 or ZD55-TRAIL-IETD-Smac
alone.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that SNS-032 sensitizes
human pancreatic cancer cells to ZD55-TRAIL-IETD-Smac-induced cell
death in vitro and in vivo. These findings indicate
that the combined treatment with SNS-032 and ZD55-TRAIL-IETD-Smac
could represent a rational approach for anti-pancreatic cancer
therapy.
Acknowledgements
This study was supported by the National Science
Foundation of China (grant nos. 81602706, 81372463 and 81472210),
the Zhejiang Provincial Natural Science Foundation of China (grant
nos. LY13H080005, LY15H160051 and LY14H160041), the Funds of
CONBA-ZSTU Sino-American Academician Laboratory (grant no.
14040365-J), the Funds of Science Technology Department of Zhejiang
Province (grant nos. 2014C37101 and 2015C37035), the Zhejiang
Province Bureau of Health (grant no. 2015ZA009) and the Open Fund
of Zhejiang Provincial Top Key Discipline of Biology.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burris H and Storniolo AM: Assessing
clinical benefit in the treatment of pancreas cancer: Gemcitabine
compared to 5-fluorouracil. Eur J Cancer. 33 Suppl 1:S18–S22. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
4
|
Lei W, Liu HB, Wang SB, Zhou XM, Zheng SD,
Guo KN, Ma BY, Xia YL, Tan WS, Liu XY and Wang YG: Tumor suppressor
in lung cancer-1 (TSLC1) mediated by dual-regulated oncolytic
adenovirus exerts specific antitumor actions in a mouse model. Acta
Pharmacol Sin. 34:531–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hermiston TW and Kuhn I: Armed therapeutic
viruses: Strategies and challenges to arming oncolytic viruses with
therapeutic genes. Cancer Gene Ther. 9:1022–1035. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH,
Sun LY, Qian QJ and Liu XY: An armed oncolytic adenovirus system,
ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res.
13:481–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong F, Wang L, Davis JJ, Hu W, Zhang L,
Guo W, Teraishi F, Ji L and Fang B: Eliminating established tumor
in nu/nu nude mice by a tumor necrosis factor-alpha-related
apoptosis-inducing ligand-armed oncolytic adenovirus. Clin Cancer
Res. 12:5224–5230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qian W, Liu J, Tong Y, Yan S, Yang C, Yang
M and Liu X: Enhanced antitumor activity by a selective
conditionally replicating adenovirus combining with
MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of
apoptosis. Leukemia. 22:361–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan QW, Zhong SY, Liu BS, Liu J, Cai R,
Wang YG, Liu XY and Qian C: Enhanced sensitivity of hepatocellular
carcinoma cells to chemotherapy with a Smac-armed oncolytic
adenovirus. Acta Pharmacol Sin. 28:1996–2004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Bonifati S, Hristov G, Marttila T,
Valmary-Degano S, Stanzel S, Schnölzer M, Mougin C, Aprahamian M,
Grekova SP, et al: Synergistic combination of valproic acid and
oncolytic parvovirus H-1PV as a potential therapy against cervical
and pancreatic carcinomas. EMBO Mol Med. 5:1537–1555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang SB, Tan Y, Lei W, Wang YG, Zhou XM,
Jia XY, Zhang KJ, Chu L, Liu XY and Qian WB: Complete eradication
of xenograft hepatoma by oncolytic adenovirus ZD55 harboring
TRAIL-IETD-Smac gene with broad antitumor effect. Hum Gene Ther.
23:992–1002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Senderowicz AM: Inhibitors of
cyclin-dependent kinase modulators for cancer therapy. Prog Drug
Res. 63:183–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dickson MA and Schwartz GK: Development of
cell-cycle inhibitors for cancer therapy. Curr Oncol. 16:36–43.
2009.PubMed/NCBI
|
|
14
|
Meng H, Jin Y, Liu H, You L, Yang C, Yang
X and Qian W: SNS-032 inhibits mTORC1/mTORC2 activity in acute
myeloid leukemia cells and has synergistic activity with perifosine
against Akt. J Hematol Oncol. 6:182013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Y, Chen C, Sun X, Shi X, Jin B, Ding K,
Yeung SC and Pan J: Cyclin-dependent kinase 7/9 inhibitor SNS-032
abrogates FIP1-like-1 platelet-derived growth factor receptor a and
bcr-abl oncogene addiction in malignant hematologic cells. Clin
Cancer Res. 18:1966–1978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walsby E, Lazenby M, Pepper C and Burnett
AK: The cyclin-dependent kinase inhibitor SNS-032 has single agent
activity in AML cells and is highly synergistic with cytarabine.
Leukemia. 25:411–419. 2012. View Article : Google Scholar
|
|
17
|
Xie G, Tang H, Wu S, Chen J, Liu J and
Liao C: The cyclin-dependent kinase inhibitor SNS-032 induces
apoptosis in breast cancer cells via depletion of Mcl-1 and
X-linked inhibitor of apoptosis protein and displays antitumor
activity in vivo. Int J Oncol. 45:804–812. 2014.PubMed/NCBI
|
|
18
|
Feldmann G, Mishra A, Bisht S, Karikari C,
Garrido-Laguna I, Rasheed Z, Ottenhof NA, Dadon T, Alvarez H,
Fendrich V, et al: Cyclin-dependent kinase inhibitor Dinaciclib
(SCH727965) inhibits pancreatic cancer growth and progression in
murine xenograft models. Cancer Biol Ther. 12:598–609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chai J, Shiozaki E, Srinivasula SM, Wu Q,
Datta P, Alnemri ES and Shi Y: Structural basis of caspase-7
inhibition by XIAP. Cell. 104:769–780. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC
and Takahashi R: X-linked inhibitor of apoptosis protein (XIAP)
inhibits caspase-3 and −7 in distinct modes. J Biol Chem.
276:27058–27063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tamm I, Kornblau SM, Segall H, Krajewski
S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al:
Expression and prognostic significance of IAP-family genes in human
cancers and myeloid leukemias. Clin Cancer Res. 6:1796–1803.
2000.PubMed/NCBI
|
|
23
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Srinivasula SM, Hegde R, Saleh A, Datta P,
Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y
and Alnemri ES: A conserved XIAP-interaction motif in caspase-9 and
Smac/DIABLO regulates caspase activity and apoptosis. Nature.
410:112–116. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R,
Gu J, Qian C and Liu X: An oncolytic adenoviral vector of Smac
increases antitumor activity of TRAIL against HCC in human cells
and in mice. Hepatology. 39:1371–1381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levinson AD: Cancer therapy reform.
Science. 328:1372010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ehrhardt H, Häcker S, Wittmann S, Maurer
M, Borkhardt A, Toloczko A, Debatin KM, Fulda S and Jeremias I:
Cytotoxic drug-induced, p53-mediated upregulation of caspase-8 in
tumor cells. Oncogene. 27:783–793. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu RL, Post DE, Khuri FR and Van Meir EG:
Use of replicating oncolytic adenoviruses in combination therapy
for cancer. Clin Cancer Res. 10:5299–5312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kruyt FA and Curiel DT: Toward a new
generation of conditionally replicating adenoviruses: Pairing tumor
selectivity with maximal oncolysis. Hum Gene Ther. 13:485–495.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Post DE, Khuri FR, Simons JW and Van Meir
EG: Replicative oncolytic adenoviruses in multimodal cancer
regimens. Hum Gene Ther. 214:933–946. 2003. View Article : Google Scholar
|
|
31
|
Libertini S, Iacuzzo I, Ferraro A, Vitale
M, Bifulco M, Fusco A and Portella G: Lovastatin enhances the
replication of the oncolytic adenovirus dl1520 and its
antineoplastic activity against anaplastic thyroid carcinoma cells.
Endocrinology. 148:5186–5194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan JJ, Zhang SW, Chen CB, Xiao SW, Sun Y,
Liu CQ, Su X, Li DM, Xu G, Xu B and Lu YY: Effect of recombinant
adenovirus-p53 combined with radiotherapy on long-term prognosis of
advanced nasopharyngeal carcinoma. J Clin Oncol. 27:799–804. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Subramaniam D, Periyasamy G, Ponnurangam
S, Chakrabarti D, Sugumar A, Padigaru M, Weir SJ, Balakrishnan A,
Sharma S and Anant S: CDK-4 inhibitor P276 sensitizes pancreatic
cancer cells to gemcitabine-induced apoptosis. Mol Cancer Ther.
11:1598–1608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu C, Dadon T, Chenna V, Yabuuchi S,
Bannerji R, Booher R, Strack P, Azad N, Nelkin BD and Maitra A:
Combined inhibition of cyclin-dependent kinases (Dinaciclib) and
AKT (MK-2206) blocks pancreatic tumor growth and metastases in
patient-derived xenograft models. Mol Cancer Ther. 14:1532–1539.
2015. View Article : Google Scholar : PubMed/NCBI
|