Introduction
Human induced pluripotent stem (iPS) cells are a
promising cell source for differentiation to somatic cells that are
transplantable to recipients (1,2).
Liver failure is a fatal disease that is characterized by a
decrease in functioning hepatocytes (3). Transplanted hepatocytes
differentiated from iPS cells may be a potential cure for patients
with liver failure. Currently, hepatocytes are differentiated by
growth factor stimulation or the introduction of transcription
factors (4–9); using current protocols, hepatocytes
remain in an immature state (10).
Glucose is a crucial source of energy for cells.
Unlike iPS cells, hepatocytes can survive in hepatocyte selection
medium (HSM) lacking glucose but supplemented with galactose
(11). Galactokinases (GALKs)
metabolize galactose to galactose-1-phosphate, which enters
glycolysis (12). In humans, there
are two forms: GALK1 and GALK2 (13). The expression levels of GALK1 and
GALK2 increase in iPS cells grown in a modified HSM: hepatocyte
differentiation inducer (HDI) (14). These data suggest that galactose
may be used as an energy source instead of glucose by iPS cells
cultured in HSM. Unexpectedly, the expression levels of
α-fetoprotein (AFP), a marker of immature hepatocytes, were
increased in iPS cells cultured in HSM and HDI (11,14).
These data suggest that hepatocyte differentiation may be initiated
in glucose-free media that is supplemented with galactose.
One major problem is that iPS cells do not survive
in HSM and HDI beyond 3 and 7 days, respectively (14). 2-Deoxy-d-glucose (2DG) is an analogue of
glucose that is taken up by cells but is not metabolized (11,15).
3-Bromopyruvate (3BP) is an analogue of pyruvate, which is the
final product of glycolysis and which enters the citric acid cycle
(16,17). 2DG may be used as a model of
glucose deprivation. The present study therefore investigated the
effects of 2DG and 3BP on iPS cells with respect to hepatocyte
differentiation.
Materials and methods
Cell culture
The human iPS cell line 201B7 was purchased from the
RIKEN Cell Bank (Tsukuba, Japan) and cultured under feeder-free
conditions in ReproFF medium (ReproCELL, Inc., Yokohama, Japan) on
10-cm dishes (Asahi Glass Co. Ltd., Tokyo, Japan) coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). The cells were
incubated at 37°C in a humidified chamber with 5% CO2.
They were then harvested with Accutase (Innovative Cell
Technologies, Inc., San Diego, CA, USA), and spread onto individual
10-cm dishes at a density of 1×106 cells/dish, and
passaged every 4–5 days.
Culture in conventional media with or
without 3BP or 2DG
201B7 cells were harvested and spread onto 6-well
plates (Asahi Glass Co. Ltd.) at a density of 1×106
cells/well and cultured in 5% of carbon dioxide at 37°C in ReproFF,
Leibovitz's-15 (L15), William's E (WE) or Dulbecco's modified
Eagle's medium/nutrient mixture F-12 Ham (DF12). Repro FF was
purchased from ReproCELL Inc., all other media were purchased from
Life Technologies; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). ReproFF, WE, and DF12 all contained glucose, whereas L15
contained. galactose. L15, WE and DF12 media were supplemented with
nicotinamide (1.2 mg/ml), proline (30 ng/ml) and 10% KnockOut Serum
Replacement (Life Technologies; Thermo Fisher Scientific, Inc.)
medium supplement. Nicotinamide (1.2 g/l) and proline (260 mM) were
added as they are necessary for primary hepatocyte proliferation
(18,19). For certain experiments, 3BP (10 µM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or 2DG (10 µM;
Sigma-Aldrich; Merck KGaA) was added. Following 7 days culture, the
cells were observed by light microscopy (CKX41N-31PHP; Olympus
Corporation, Tokyo, Japan) without any treatment or subjected to
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR).
HSM
HSM was prepared from amino acid powders using the
formulation of L15 medium (Life Technologies; Thermo Fisher
Scientific, Inc.), but omitting arginine, tyrosine, glucose and
sodium pyruvate, and with the addition of galactose (900 mg/l),
ornithine (1 mM), glycerol (5 mM) and proline (260 mM; all from
Wako Pure Chemical Industries, Ltd., Osaka, Japan) (11). Knockout Serum Replacement (Life
Technologies; Thermo Fisher Scientific, Inc.) was used in place of
fetal bovine serum to establish defined xeno-free conditions and
was added at a final concentration of 10%.
RT-qPCR
Total RNA (5 µg) was isolated from 201B7 cells using
Isogen (Nippon Gene Co., Ltd., Tokyo, Japan) and subsequently used
for the synthesis of first-strand cDNA with SuperScript III Reverse
Transcriptase and oligo (dT) primers (Life Technologies; Thermo
Fisher Scientific, Inc.), following the manufacturer's protocols.
Total RNA from the human fetal liver was purchased from Clontech
Laboratories, Inc. (Mountain View, CA, USA) to serve as a positive
control. qPCR was performed using Fast SYBR-Green Master Mix (Life
Technologies; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol and the results were analyzed using the
MiniOpticon Real-Time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). qPCR was performed for 40 cycles of two steps,
consisting of 5 sec denaturation (95°C) and 5 sec
annealing-extension (60°C). Primer sequences are presented in
Table I. The constitutively
expressed housekeeping gene ribosomal protein L19 (RPL19)
was used as an endogenous internal control to monitor the levels of
mRNA expression (20). mRNA
expression levels were analyzed automatically using the MiniOpticon
system based on 2−ΔΔCq method (21). The relative expression was
calculated as the expression level of a specific gene divided by
that of RPL19. Experiments were repeated three times.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene (GenBank
accession no.) | Primer name | Sequence (5′→3′) | Product size
(bp) |
|---|
| AFP (NM_001134) | OMC317 | F:
ACACAAAAAGCCCACTCCAG | 147 |
|
| OMC318 | R:
GGTGCATACAGGAAGGGATG | 147 |
| RPL19 (BC095445) | OMC321 | F:
CGAATGCCAGAGAAGGTCAC | 157 |
|
| OMC322 | R:
CCATGAGAATCCGCTTGTTT | 157 |
Plasmid construction
The SacI-HindIII fragments of the
GALK1 and GALK2 promoters, contained within
pLightSwitch Promoter vectors (SwitchGear Genomics, Carlsbad, CA,
USA) were subcloned into the pMetLuc2-reporter vector (Promega
Corporation, Madison, WI, USA) to make pMetLuc2/GALK1 and
pMetLuc2/GALK2 reporter plasmids, respectively. GALK1 or
GALk2 promoters (2 µg) in pLightSwitch Promoter or
pMetLuc2-reporter vector, was digested with 10 U of SacI in
a volume of 10 µl at 37°C for 1 h. The digested samples were mixed
with 10 U of HindIII, the total volume was 50 µl. The
digested fragments were fractionated with gel electrophoresis, and
purified with a gel extraction kit (Qiagen GmbH, Hilden, Germany).
The purified fragments were subcloned into the digested
pMetLuc2-reporter with a ligation kit (Takara Bio. Inc., Otsu,
Japan). The inserted fragments were confirmed by sequencing (Riken
Genesis, Co. Ltd., Tokyo, Japan).
Transfection and Metridia luciferase
activity assay
201B7 cells were plated on 96-well plates (Asahi
Glass Co. Ltd.) coated with Matrigel, at a density of
5×105 cells/well. pMetLuc2-control,
pMetLuc2/GALK1 and pMetLuc2/GALK2 were transfected
with FuGENE HD Transfection Reagent (100 ng/well; Clontech
Laboratories, Inc.) according to the manufacturer's protocol
(22). The reporter plasmids
expressed Metridia luciferase, which is secreted into the
medium; the pMetLuc2-control plasmid expresses Metridia
luciferase driven by the cytomegalovirus (CMV) immediate
early promoter. A luciferase assay was performed following 2 days
of culture in ReproFF, WE or HSM using a Ready-To-Glow Secreted
Luciferase Reporter assay (Clontech Laboratories, Inc.) and a Gene
Light GL-200A luminometer (Microtec Co. Ltd., Funabashi, Japan). To
monitor transfection efficiency, 10 ng of the pSEAP2 control vector
(Clontech Laboratories, Inc.) was added to each well of the black
96-well plates and transcriptional activity was measured using a
Secreted Embryonic Alkaline Phosphatase (SEAP) Chemiluminescence
kit (Clontech Laboratories, Inc.) and the Gene Light luminometer
according to the manufacturer's protocols. Luciferase activity was
calculated as the Metridia luciferase activity divided by
the SEAP activity (relative light units). The Metridia
luciferase activity was normalized against that of the CMV
promoter. The Metridia luciferase activity is presented in
relative light units. The experiments were repeated three
times.
Statistical analysis
Relative expression levels of AFP and
relative light units of Metridia luciferase were analyzed by
a one-way analysis of variance using JMP version 5.0J (SAS
Institute, Inc., Cary, NC, USA), followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
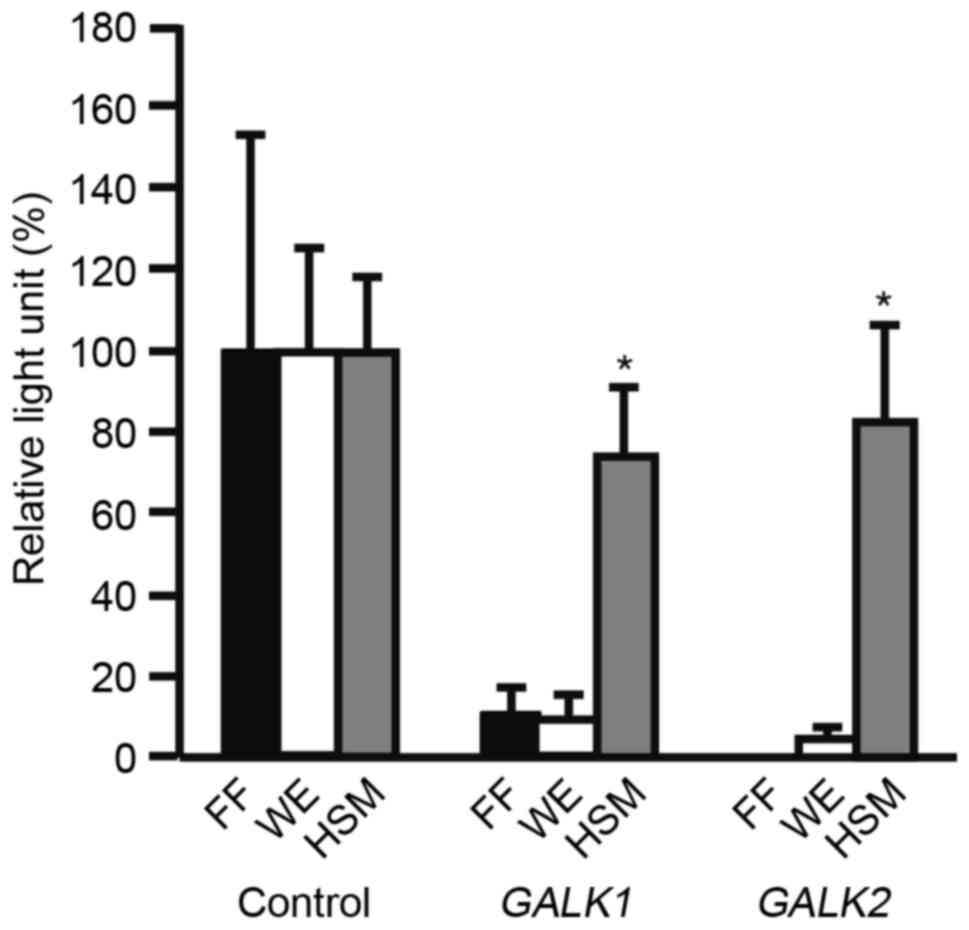
To examine changes in the promoter activities of
GALK1 and GALK2 in medium that lacks glucose and is
supplemented with galactose, HSM was used to culture 201B7 iPS
cells. The 201B7 cells were transfected with pMetLuc2-control,
pMetLuc2/GALK1 or pMetLuc2/GALK2 reporter plasmids,
and cultured in ReproFF, WE or HSM medium. The cells were subjected
to a Metridia luciferase assay (Fig. 1). Metridia luciferase activity of
GALK1 or GALK2 was normalized against that of CMV. WE medium was
used as it was originally established for primary hepatocyte
culture (23,24). Metiridia luciferase activity was
significantly higher in HSM compared with WE (P<0.05).
Metridia luciferase activity was significantly higher in HSM
compared with ReproFF (P<0.05).
The effects of 3BP and 2DG on the morphological
features of 201B7 cells were analyzed by culturing cells in L15, WE
or DF12, with or without 3BP or 2DG (Fig. 2). The morphology of cells grown in
HSM was not assessed as these cells died within three days.
Following 7 days of culture, the cells were observed under a
microscope and imaged. No significant morphological differences
were observed when compared with the morphology of cells cultured
in ReproFF.
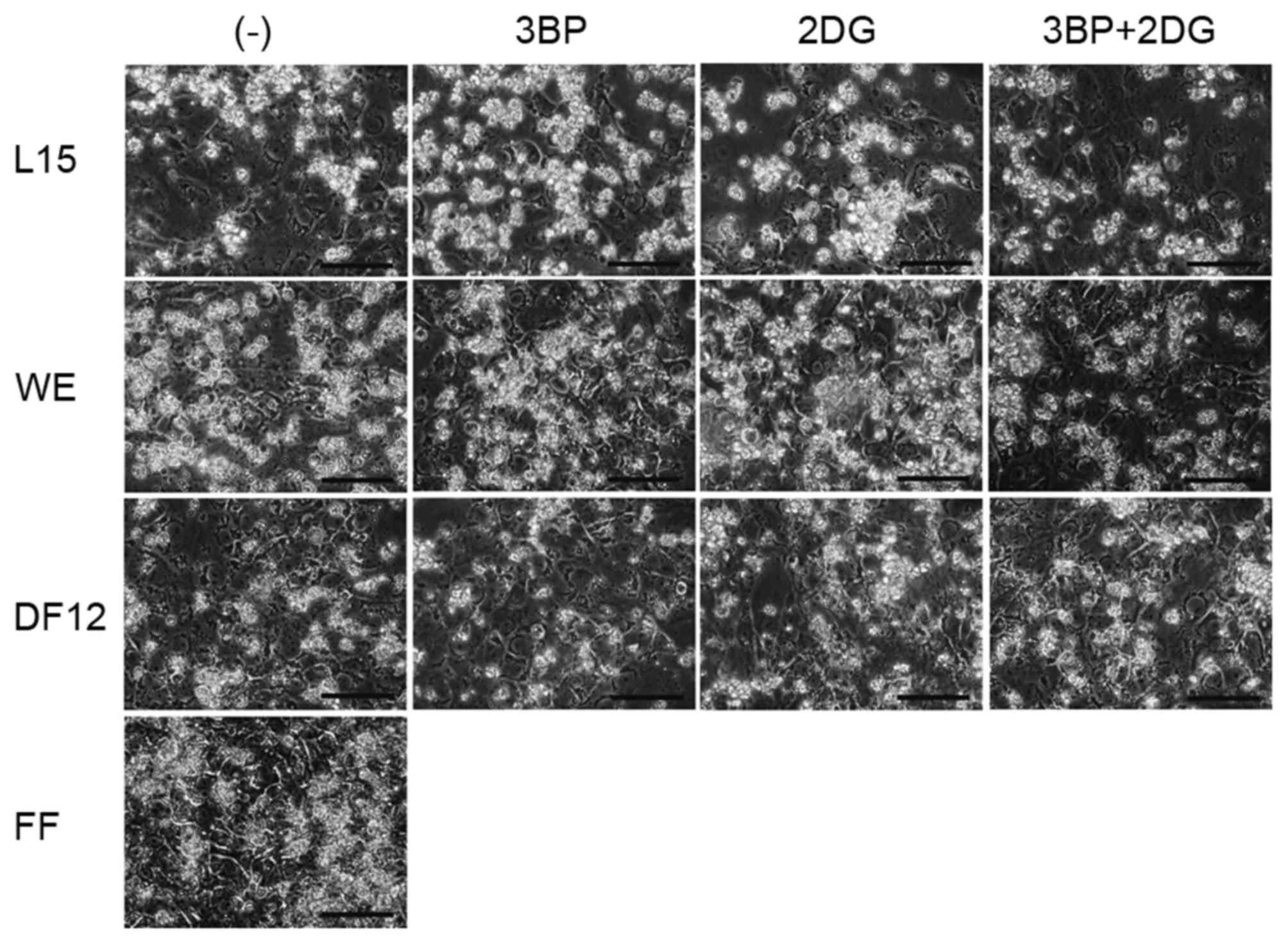 | Figure 2.Morphological analysis of 201B7 human
induced pluripotent stem cells in various culture conditions. 201B7
cells were initially cultured in FF, then the medium was changed to
L15, WE or DF12 medium with or without 3BP (10 µM), 2DG (10 µM) or
a combination of the two (3BP+2DG). Images were captured following
7 days of culture. Original magnification, ×400; scale bar, 100 µm.
2DG, 2-deoxy-d-glucose; 3BP, 3-bromopyruvate;
DF12, Dulbecco's modified Eagle's medium/nutrient mixture F-12 Ham;
FF, ReproFF; L15, Leibovitz's-15; WE, William's E. |
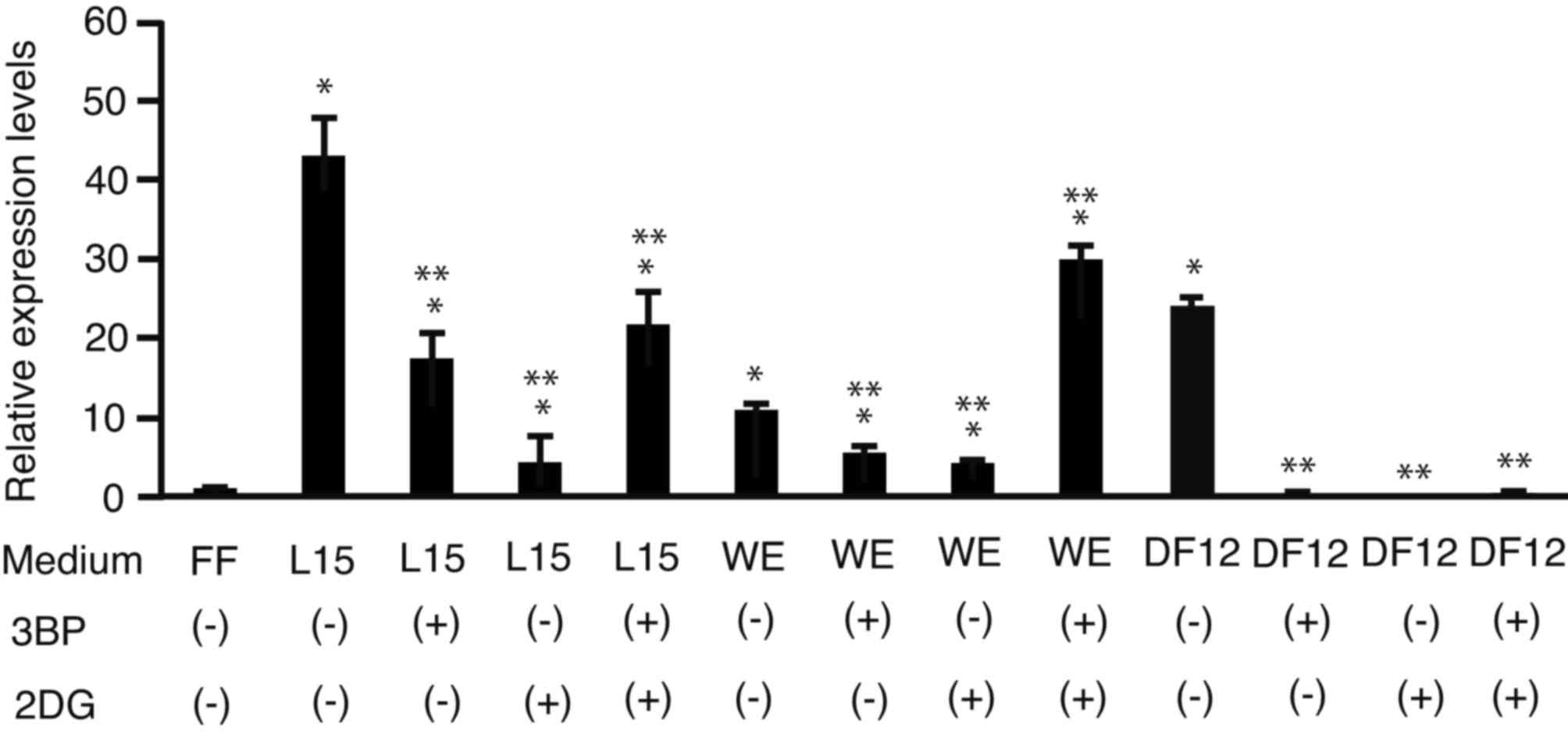
The differentiation of 201B7 cells to the hepatocyte
lineage was examined in cells that were cultured in L15, WE or DF12
with or without 3BP or 2DG. AFP expression in HSM-cultured cells
was not analyzed, because these cells died within three days.
Following 7 days of culture, the cells were subjected to RT-PCR to
analyze AFP mRNA expression (Fig. 3). The expression levels of
AFP were dependent on the culture medium as well as on the
effects of 3BD and 2DG treatment. Treatment with 3BP, 2DG or
3BP+2DG decreased the expression levels of AFP in 201B7
cells cultured in L15 or DF12 media, compared with untreated cells.
The combination of 3BP+2DG treatment resulted in an increase in
AFP expression levels in WE medium. AFP expression
was higher in cells cultured in unsupplemented L15 medium compared
with untreated WE and DF12 media.
Discussion
A previous study demonstrated that the expression
levels of GALK1 and GALK2 are increased in iPS cells cultured in
HDI that lacks glucose and is supplemented with galactose (14). These data suggest that glucose
deprivation and galactose supplementation affect the transcription
of GALK1 and GALK2. However, to the best of our
knowledge, the promoter activities of GALK1 and GALK2
have not been previously analyzed. In the present study, a
Metridia luciferase assay demonstrated that the GALK1
and GALK2 promoters were activated in 201B7 iPS cells
cultured in HSM. Unlike HDI, HSM does not have added growth factors
or small molecules. HSM was suitable for the investigation of the
GALK1 and GALK2 promoter activities in medium without
glucose and supplemented with galactose. The data from the present
study clearly demonstrated that the promoters of GALK1 and
GALK2 were activated in 201B7 cells cultured in a medium
without glucose and supplemented with galactose.
Glucose deprivation and galactose supplementation
were expected to promote the differentiation of iPS cells to the
hepatocyte lineage. One major problem is that iPS cells do not
survive in HSM and HDI beyond 3 and 7 days, respectively (14). To overcome this limitation, the
present study used 3BP and/or 2DG treatment to investigate an
alternative to glucose deprivation and galactose supplementation.
The combination of 3BP+2DG increased AFP mRNA expression
levels in 201B7 cells cultured in WE medium, but not in DF12. It
was not clear why the changes in AFP expression levels
differed in magnitude between the three 3BP and/or 2DG treatments.
Furthermore, it was not clear why AFP was not detected in any of
the DF12 3BP/2DG treatment groups. Cells cultured in L15 medium
without 3BP or 2DG treatment exhibited increased expression levels
of AFP. L15 does not contain glucose, but includes
galactose. Accordingly, these results suggested that glucose
deprivation and supplementation with galactose promoted the
differentiation of iPS cells to the hepatocyte lineage. WE medium
was originally established for primary hepatocyte culture (25). The highest expression was observed
in untreated L15, however, iPS cells decrease in number after seven
days of culture in L15 (26). WE
medium supplemented with 3BP+2DG may be suitable for the
differentiation of iPS cells to the hepatocyte lineage.
One major limitation of the present study was that
the differentiation of iPS cells to a hepatocyte lineage was only
confirmed via the measurement of AFP mRNA expression levels. Future
experiments should verify hepatocyte differentiation using
functional tests, such as indocyanine green up-take. In conclusion,
the promoters of GALK1 and GALK2 were activated in
201B7 cells cultured in HSM. However, a major problem with HSM is
that cultured iPS cells die within three days; 3BP and 2DG in WE
may therefore be suitable for differentiation of iPS cells to
hepatocyte lineages instead of HSM, and future studies should
further investigate cell viability and differentiation into a
hepatocyte lineage, following treatment with these compounds.
Acknowledgements
This study was supported by a Grant-in-Aid for
Scientific Research (C) from the Japan Society for the Promotion of
Science (grant no. 15K09032).
References
|
1
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirschi KK, Li S and Roy K: Induced
pluripotent stem cells for regenerative medicine. Annu Rev Biomed
Eng. 16:277–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugawara K, Nakayama N and Mochida S:
Acute liver failure in Japan: Definition, classification, and
prediction of the outcome. J Gastroenterol. 47:849–861. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeLaForest A, Nagaoka M, Si-Tayeb K, Noto
FK, Konopka G, Battle MA and Duncan SA: HNF4A is essential for
specification of hepatic progenitors from human pluripotent stem
cells. Development. 138:4143–4153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Si-Tayeb K, Noto FK, Nagaoka M, Li J,
Battle MA, Duris C, North PE, Dalton S and Duncan SA: Highly
efficient generation of human hepatocyte-like cells from induced
pluripotent stem cells. Hepatology. 51:297–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo
S, Song X, Guo Y, Zhao Y, Qin H, et al: Efficient generation of
hepatocyte-like cells from human induced pluripotent stem cells.
Cell Res. 19:1233–1242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takayama K, Inamura M, Kawabata K,
Katayama K, Higuchi M, Tashiro K, Nonaka A, Sakurai F, Hayakawa T,
Furue MK and Mizuguchi H: Efficient generation of functional
hepatocytes from human embryonic stem cells and induced pluripotent
stem cells by HNF4α transduction. Mol Ther. 20:127–137. 2012.
View Article : Google Scholar
|
|
8
|
Zaret KS, Watts J, Xu J, Wandzioch E,
Smale ST and Sekiya T: Pioneer factors, genetic competence, and
inductive signaling: Programming liver and pancreas progenitors
from the endoderm. Cold Spring Harb Symp Quant Biol. 73:119–126.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inamura M, Kawabata K, Takayama K, Tashiro
K, Sakurai F, Katayama K, Toyoda M, Akutsu H, Miyagawa Y, Okita H,
et al: Efficient generation of hepatoblasts from human ES cells and
iPS cells by transient overexpression of homeobox gene HEX. Mol
Ther. 19:400–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Single-step protocol for the
differentiation of human-induced pluripotent stem cells into
hepatic progenitor-like cells. Biomed Rep. 1:18–22. 2013.PubMed/NCBI
|
|
11
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Survival of primary human
hepatocytes and death of induced pluripotent stem cells in media
lacking glucose and arginine. PLoS One. 8:e718972013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bayarchimeg M, Ismail D, Lam A, Burk D,
Kirk J, Hogler W, Flanagan SE, Ellard S and Hussain K:
Galactokinase deficiency in a patient with congenital
hyperinsulinism. JIMD Rep. 5:7–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ai Y, Basu M, Bergsma DJ and Stambolian D:
Comparison of the enzymatic activities of human galactokinase GALK1
and a related human galactokinase protein GK2. Biochem Biophys Res
Commun. 212:687–691. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: An optimal medium
supplementation regimen for initiation of hepatocyte
differentiation in human induced pluripotent stem cells. J Cell
Biochem. 116:1479–1489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang D, Li J, Wang F, Hu J, Wang S and
Sun Y: 2-Deoxy-D-glucose targeting of glucose metabolism in cancer
cells as a potential therapy. Cancer Lett. 355:176–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ngo DC, Ververis K, Tortorella SM and
Karagiannis TC: Introduction to the molecular basis of cancer
metabolism and the Warburg effect. Mol Biol Rep. 42:819–823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shoshan MC: 3-Bromopyruvate: Targets and
outcomes. J Bioenerg Biomembr. 44:7–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura T, Teramoto H, Tomita Y and
Ichihara A: L-proline is an essential amino acid for hepatocyte
growth in culture. Biochem Biophys Res Commun. 122:884–891. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mitaka T, Sattler CA, Sattler GL, Sargent
LM and Pitot HC: Multiple cell cycles occur in rat hepatocytes
cultured in the presence of nicotinamide and epidermal growth
factor. Hepatology. 13:21–30. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping, and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tam S, Clavijo A, Engelhard EK and
Thurmond MC: Fluorescence-based multiplex real-time RT-PCR arrays
for the detection and serotype determination of foot-and-mouth
disease virus. J Virol Methods. 161:183–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Sueishi M: Dual gene expression in
embryoid bodies derived from human induced pluripotent stem cells
using episomal vectors. Tissue Eng Part A. 20:3154–3162. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu D, Ramin SA and Cederbaum AI: Effect of
pyridine on the expression of cytochrome P450 isozymes in primary
rat hepatocyte culture. Mol Cell Biochem. 173:103–111. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takeba Y, Matsumoto N, Takenoshita-Nakaya
S, Harimoto Y, Kumai T, Kinoshita Y, Nakano H, Ohtsubo T and
Kobayashi S: Comparative study of culture conditions for
maintaining CYP3A4 and ATP-binding cassette transporters activity
in primary cultured human hepatocytes. J Pharmacol Sci.
115:516–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williams GM, Weisburger EK and Weisburger
JH: Isolation and long-term cell culture of epithelial-like cells
from rat liver. Exp Cell Res. 69:106–112. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Transcription factors and
medium suitable for initiating the differentiation of human-induced
pluripotent stem cells to the hepatocyte lineage. J Cell Biochem.
117:2001–2009. 2016. View Article : Google Scholar : PubMed/NCBI
|