Introduction
The worldwide prevalence of diabetes mellitus is
expected to reach 642 million people by 2,040 (1). Diabetic nephropathy (DN), which
develops in 40% of diabetic patients, is the leading cause of
end-stage renal disease in the majority of developed countries
(1). DN is characterized by renal
hypertrophy, glomerulosclerosis and renal fibrosis, which involves
extracellular matrix (e.g. collagen and fibronectin) accumulation
(2,3). Hyperglycemia, transforming growth
factor-β1 (TGF-β1) and deficient endothelial nitric oxide synthase
(eNOS) are among the key pathogenic mechanisms of diabetic renal
fibrosis (4). However, current
supplementary pharmacological treatments, other than glycemic
control and renin-angiotensin system blockade, have had limited
success (1). Thus, the development
of novel pharmacologic agents is required.
A previous study of the authors indicated that
nitric oxide (NO)-cyclic guanosine monophosphate protein kinase
(cGMP) inducers attenuate advanced glycation end-product-induced
effects in renal fibroblasts (5).
KMUP-1 is a synthetic xanthine-based derivative which enhances
soluble guanylate cyclase (sGC), eNOS and cGMP (6). A previous study indicated that KMUP-1
attenuates rat diabetic glomerulosclerosis while increasing eNOS
levels (7). However, the
anti-fibrotic mechanisms of KMUP-1 in DN regarding cell biology and
TGF-β1 remain unclear.
As a result, the present study focused on
elucidating the effects of KMUP-1 on high glucose (HG) or
TGF-β1-induced pro-fibrotic proteins in mouse mesangial (MES13)
cells, as well as the effects of KMUP-1 on streptozotocin
(STZ)-induced diabetic rats.
Materials and methods
Reagents
Cell culture media, Dulbecco's modified Eagle medium
(DMEM) and F12, were obtained from Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Recombinant human TGF-β1 was
obtained from PeproTech, Inc. (Rocky Hill, NJ, USA). KMUP-1 was
synthesized in the laboratory of the authors, the stock solution
(10 mM) was prepared by dissolving KMUP-1 in the solvent (10%
absolute alcohol, 10% propylene glycol and 2% 1 N HCl), according
to previous studies (8–10).
Cells
Mouse kidney mesangial cells (MES13) were purchased
from the American Type Culture Collection (cat. no. CRL-1927;
Manassas, VA, USA). Cells were grown in DMEM/F12 (3:1) medium (with
6.67 mM glucose) supplemented with 5% fetal bovine serum (FBS) and
1% penicillin/streptomycin in a humidified 5% CO2
incubator at 37°C. Cells were starved in serum-free (0.5% FBS)
media for 24 h prior to experiments in 5% FBS medium. All cell
culture materials were obtained from Gibco (Thermo Fisher
Scientific, Inc.).
Cell viability
MES13 cells were cultured in 24-well plates
(5×103/well). After 24 h, cells were treated with the
control (KMUP-1 solvent at a final concentration of 1%) or KMUP-1
for 72 h. MTT (0.5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was incubated at 37°C for 4 h prior to harvesting.
Following removal of the culture medium, cells were dissolved in
dimethylsulfoxide (DMSO) and shaken for 10 min. Formazan was
dissolved by DMSO and the assay was quantified by determining the
absorbance at 540 nm using an ELISA reader (Dynex Technologies
GmbH, Denkendorf, Germany).
TGF-β1 promoter activity and
bioactivity
Human TGF-β1 promoter activity was detected using
the phTG5 plasmid, which was donated by Dr Jean-Louis Virelizier
(Unité d'Immunologie Virale, Institut Pasteur, Paris, France)
(11). The TGF-β1 bioactivity
reporter p3TP-lux, which contains the plasminogen activator
inhibitor-1 promoter, was donated by Dr. Joan Massagué (Memorial
Sloan Kettering Cancer Center, New York, USA) (12). Cells were cultured in 6-well plates
at a density of 1.5×104 cells/well and transfected with
1 µg of the phTG5 or p3TP-lux plasmid using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. Following 6 h of
transfection, cells were treated with HG (30 mM) or combined with
KMUP-1 (10 µM) for 72 h. Luciferase activity was measured using the
Dynatech ML1,000 luminometer (Dynatech Laboratories, Inc.,
Chantilly, VA, USA).
Immunoblotting
Briefly (13),
MES13 cells were lysed using radioimmunoprecipitation assay (RIPA)
buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 1 mM
Na3VO4, 50 mM NaF and 10 mM β-glycerol
phosphate) containing 0.1% protease inhibitor cocktail (Merck
KGaA). Cell proteins were extracted and quantified with a DC™
protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA).
Proteins (50 µg/well) were separated by 10% SDS-PAGE and
transferred to PolyScreen polyvinylidene difluoride membranes
(PerkinElmer, Inc., Waltham, MA, USA). Following blocking for 2 h
at room temperature with 5% non-fat milk and rinsing with PBS, the
membranes were probed with the primary antibodies overnight at 4°C
and washed with 0.1% PBS-Tween-20 (PBST) 3 times (5 min each). The
primary antibodies used were phosphorylated-Smad2/3 (p-Smad2/3;
1:1,000; cat. no. 8828), Smad2/3 (1:2,000; cat. no. 3102), Suv39h1
(1:1,500; cat. no. 8729) and H3K9me3 (1:2,000; cat. no. 9754),
antibodies were obtained from Cell Signaling Technology, Inc.,
Danvers, MA, USA). Fibronectin (1:10,000; cat. no. AJ1297b) and
collagen IV (1:2,000; cat. no. AP7369a) antibodies were obtained
from Abgent, Inc. (San Diego, CA, USA), GAPDH (1:5,000; cat. no.
sc-25778) and α-tubulin (1:5,000; cat. no. MS-581-P) were obtained
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and
Labvision/NeoMarkers (Thermo Fisher Scientific, Inc.),
respectively. The membranes were then incubated in horseradish
peroxidase (HRP)-conjugated anti-rabbit (1:5,000; cat. no. AP132P)
or anti-mouse (1:5,000; cat. no. AP124P) secondary antibodies
(Merck KGaA) for 1 h at room temperature and washed with 0.1% PBST
5 times (5 min each). The protein bands were detected by using the
enhanced chemiluminescence ECL system (PerkinElmer, Inc.) and the
bands were quantified by densitometric analysis (Image Studio Lite
5.25; LI-COR Biosciences, Lincoln, NE, USA).
Cell hypertrophy
Cell hypertrophy was determined by the ratio of
whole cell protein lysates to total cell numbers (14). Briefly, MES13 cells were cultured
in 6-well plates to 50% confluence and then treated with HG (30 mM)
or HG + KMUP-1 (10 µM) for 3 days. Following trypsinization, cells
were washed twice with PBS and cell numbers were counted using a
hemocytometer. Equal numbers of cells were lysed in RIPA buffer
containing the protease inhibitor cocktail. Total protein content
was measured by using the DC™ protein assay kit (Bio-Rad
Laboratories, Inc.).
Animal experiments
Male Sprague-Dawley rats (n=23) weighing 200–250 g
(aged 7 weeks) were purchased from BioLASCO Taiwan Co., Ltd.
(Taipei, Taiwan) and were housed (3 per cage) in a
temperature-(22±2°C) and humidity (50±10%)-controlled room with a
12 h light/dark cycle in the Animal Center of Kaohsiung Medical
University (Kaohsiung, Taiwan). Rats were divided into three
groups: Control, streptozotocin (STZ)-diabetic and diabetic +
KMUP-1. Briefly (15), after
overnight fasting, rats received a single intraperitoneal injection
of 55 mg/kg STZ (Sigma-Aldrich; Merck KGaA) in 0.1 M citrate buffer
(diabetic, n=9; diabetic + KMUP-1, n=9) or citrate buffer (control,
n=5). Thereafter, rats were given free access to food and water.
Diabetic rats received Lantus insulin (Sanofi S.A., Paris, France)
to maintain non-fasting blood glucose levels between 19.4 and 27.8
mmol/l. The diabetic + KMUP-1 treatment group was intraperitoneally
injected with KMUP-1 (dissolved in distilled water, 5 mg/kg/day)
daily (16). Rats were perfused
with normal saline and anesthetized intraperitoneally with sodium
pentobarbital (50 mg/kg; Abbott Pharmaceutical Co., Ltd., Lake
Bluff, IL, USA) at week 8. Kidneys were removed and kidney slices
were immersed in 4% paraformaldehyde at room temperature for 24 h.
Kidney slices were embedded in the paraffin block and cut into
sections (thickness, 4 µm) for immunohistochemical studies. All
animal procedures were conducted in accordance with the national
guidelines (17) and were approved
by the Kaohsiung Medical University Animal Experiment
Committee.
Immunohistochemistry
Briefly (15),
tissue sections were rehydrated and deparaffinized in xylene and
ethanol. The sections then underwent antigen retrieval in 10 mM
sodium citrate buffer by microwaving for 30 min. Following this,
preincubation of tissue sections with the blocking buffer was
conducted for 30 min prior to incubation with primary antibodies
overnight at 4°C. Primary antibodies used were fibronectin (1:400,
cat. no. AJ1297b) obtained from Abgent, Inc. and collagen IV
antibodies (1:500, cat. no. ab6586) obtained from Abcam (Cambridge,
UK). Following washing with PBST, sections were stained and
incubated with DAB+ and the ready-to-use (undiluted) HRP-conjugated
anti-rabbit secondary antibodies contained in the Dako REAL™
EnVision™ Detection system (cat. no. K5007; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) for 30 min at room
temperature, according to the manufacturer's instructions, and
counterstained with hematoxylin.
Statistical analysis
Statistical analyses were performed by using Stata
13.1 software (StataCorp LP, College Station, TX, USA). Data were
expressed as the mean ± standard error. Unpaired Student's
t-tests were used for the comparison between two groups.
P<0.05 was considered to be statistically significant.
Results
KMUP-1 attenuated HG-induced TGF-β1
bioactivity and gene transcriptional activity in MES13 cells
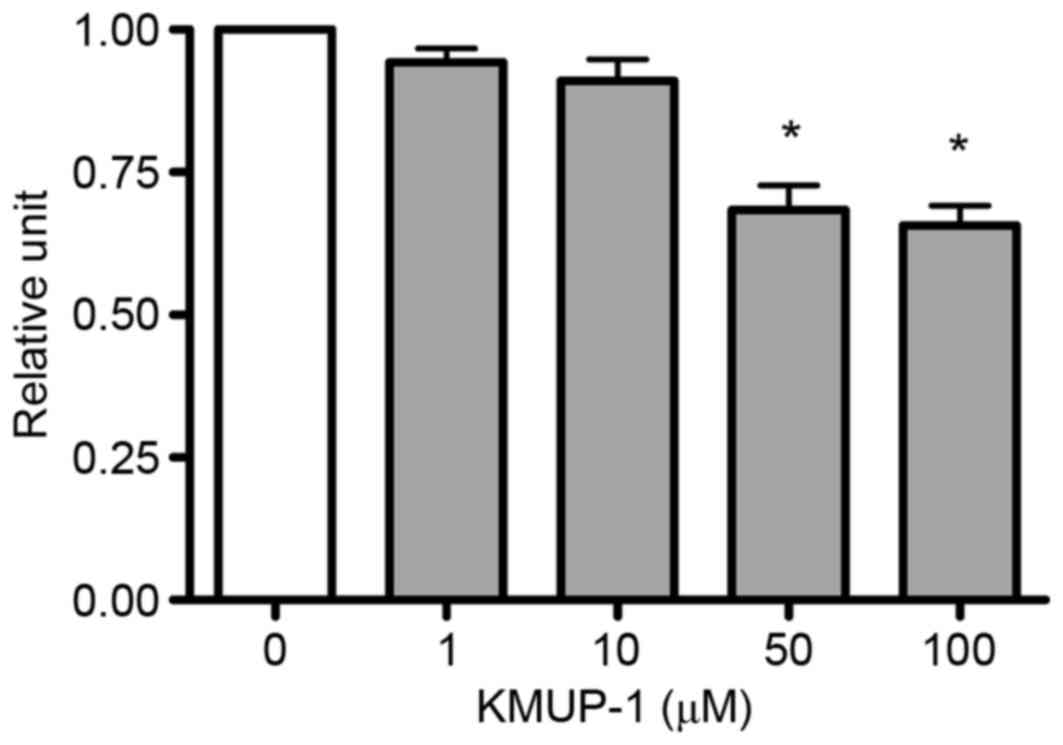
The present study investigated the effects of KMUP-1
on cell viability of MES13 cells to determine the optimum
concentration of KMUP-1. MES13 cells were treated with KMUP-1
(1–100 µM) for 72 h and cell viabilities were measured using an MTT
assay. The optimum concentration of KMUP-1 was determined to be 10
µM (Fig. 1).
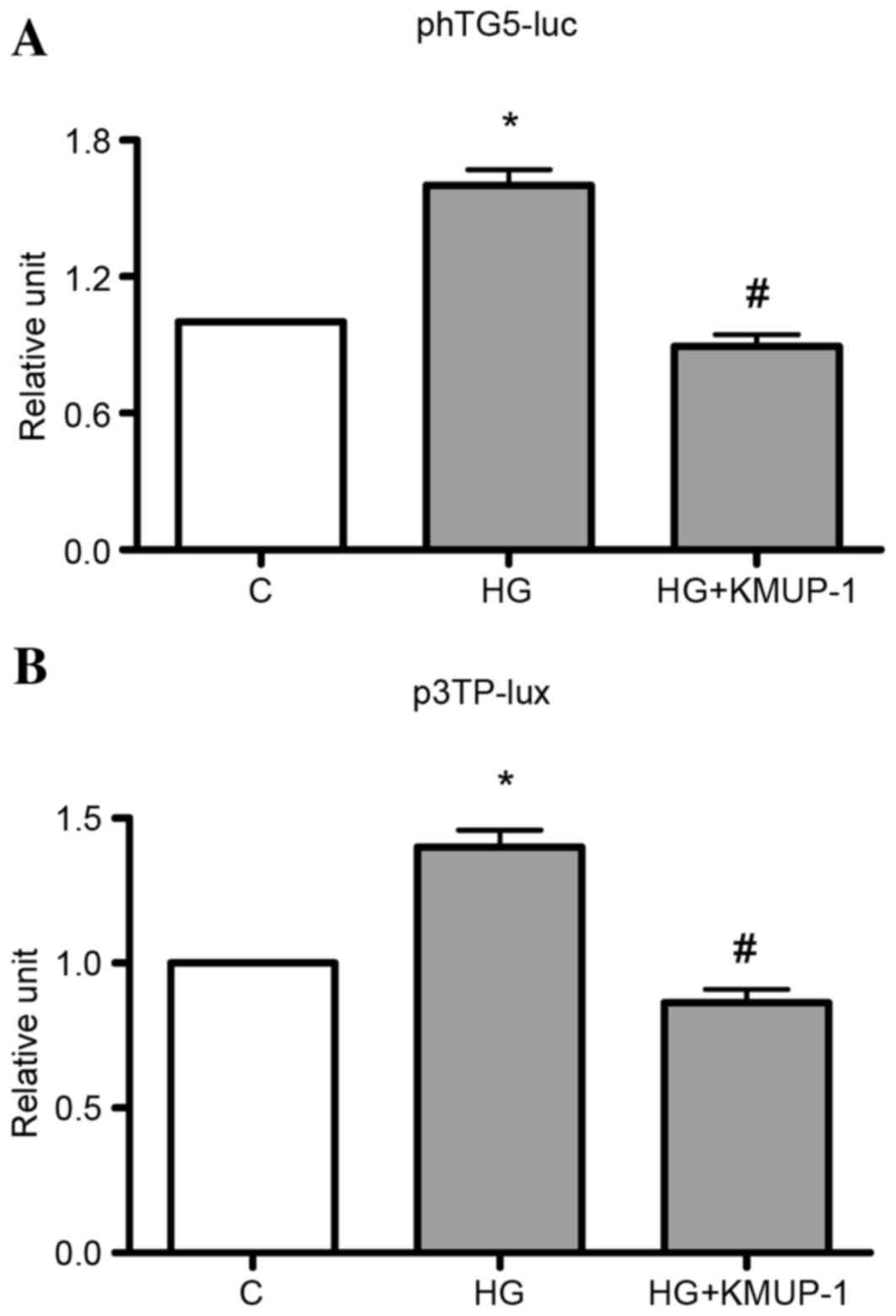
TGF-β1 gene transcriptional activity or TGF-β1
bioactivity was measured by transient transfection of phTG5 or
p3TP-lux, respectively. It was identified that KMUP-1 (10 µM)
attenuated HG (30 mM)-induced TGF-β1 gene transcriptional activity
or TGF-β1 bioactivity at 72 h (Fig.
2).
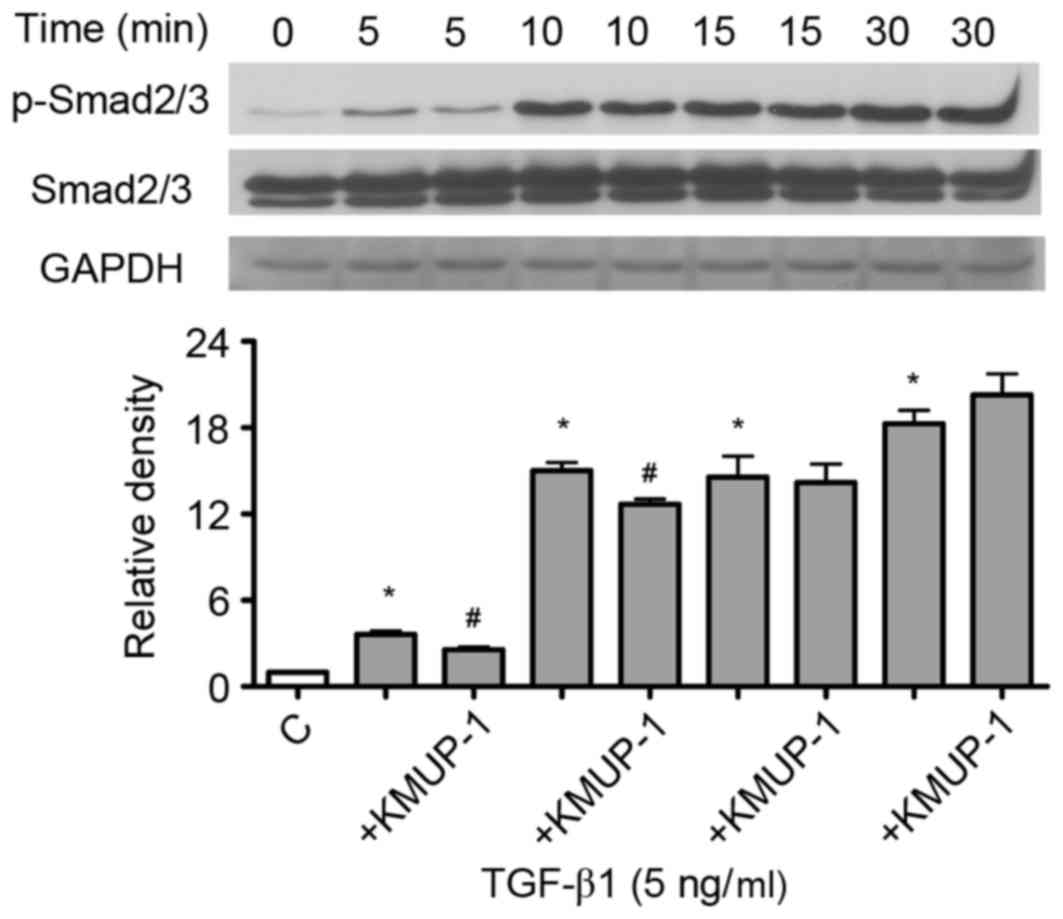
KMUP-1 attenuated TGF-β1-induced
smad2/3 phosphorylation
Since TGF-β1 gene transcriptional activity and
TGF-β1 bioactivity were attenuated by KMUP-1, the present study
examined whether KMUP-1 also attenuated TGF-β1-Smad signaling
pathway. TGF-β1 (5 ng/ml) increased phosphorylation of Smad2/3 in a
time-dependent manner (5–30 min; Fig.
3). Furthermore, KMUP-1 (10 µM) attenuated TGF-β1-induced
p-Smad2/3 expression at 5–10 min (5 ng/ml; Fig. 3).
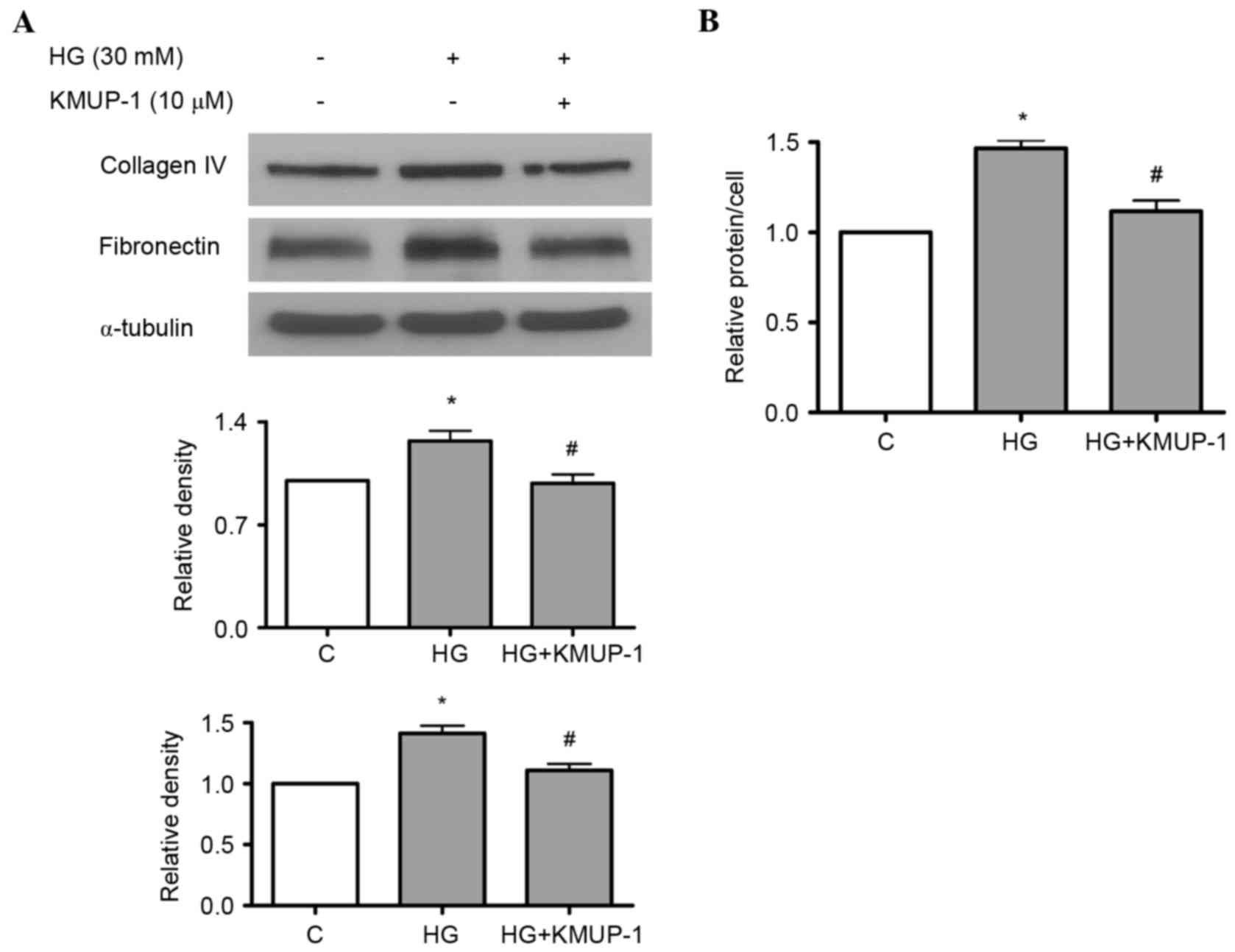
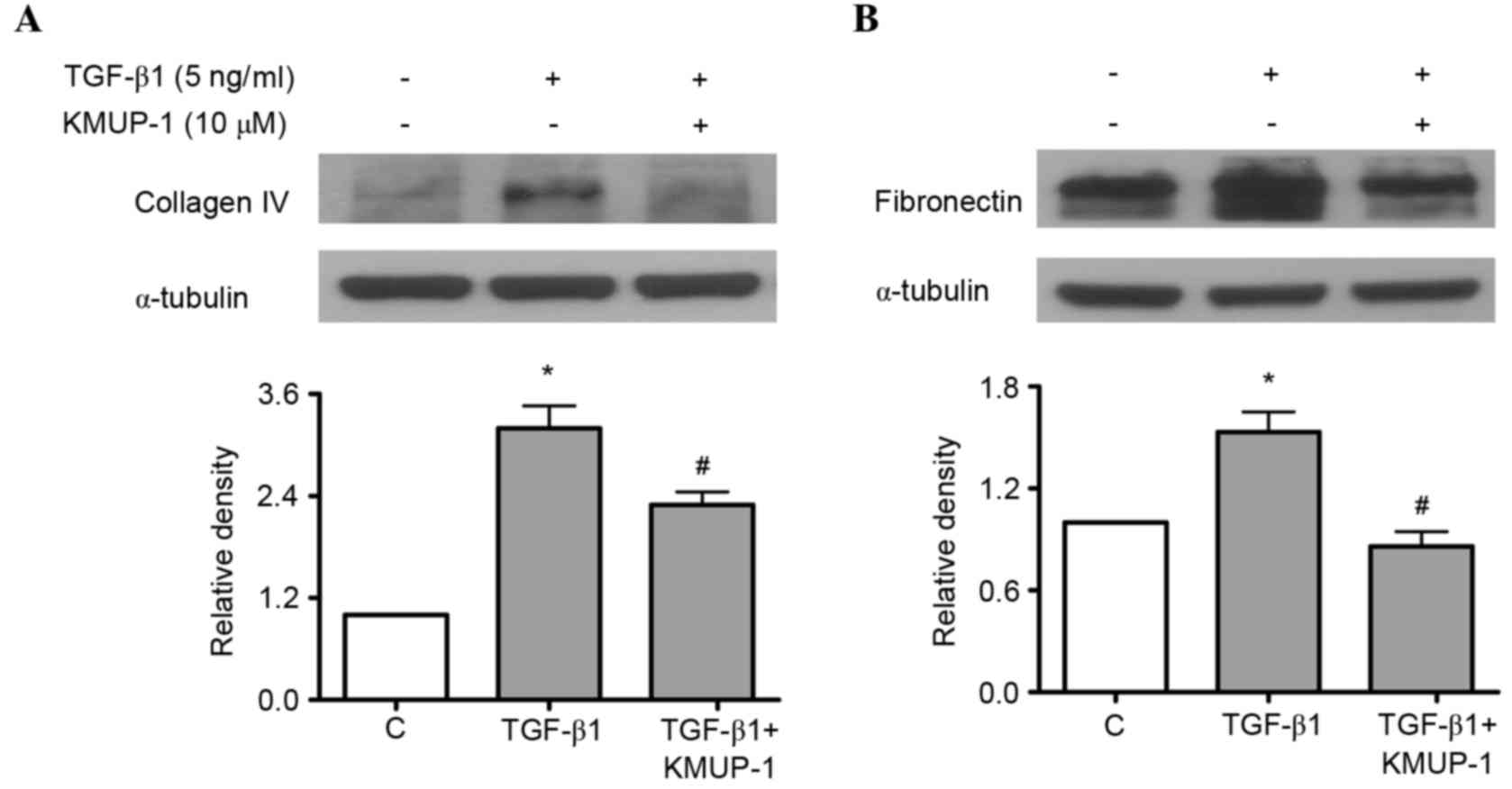
KMUP-1 attenuated HG or TGF-β1-induced
collagen IV or fibronectin protein expression
Both HG and TGF-β1 have been previously reported to
increase fibronectin and collagen IV protein expression in
mesangial cells (18,19). As a result, the present study
investigated the effects of KMUP-1 on HG or TGF-β1-induced
fibronectin and collagen IV protein expression. KMUP-1 (10 µM) was
demonstrated to attenuate HG (Fig.
4A) or TGF-β1 (5 ng/ml)-induced fibronectin and collagen IV
protein expression at 72 h (Fig.
5).
KMUP-1 attenuated HG-induced cell
hypertrophy
A previous study identified that KMUP-1 decreased
cardiac hypertrophy via the NO/cGMP pathway (20). In addition to extracellular matrix
accumulation, DN is characterized by renal (including mesangial
cell) hypertrophy (21). In
addition, DN is associated with NO deficiency (22). For example, eNOS knockout mice
develop diabetic renal hypertrophy while soluble guanylate cyclase
enhancers attenuate DN (23,24).
Therefore, the authors studied the effects of KMUP-1 on HG-induced
cell hypertrophy. KMUP-1 (10 µM) attenuated HG-induced cell
hypertrophy at 72 h (Fig. 4B).
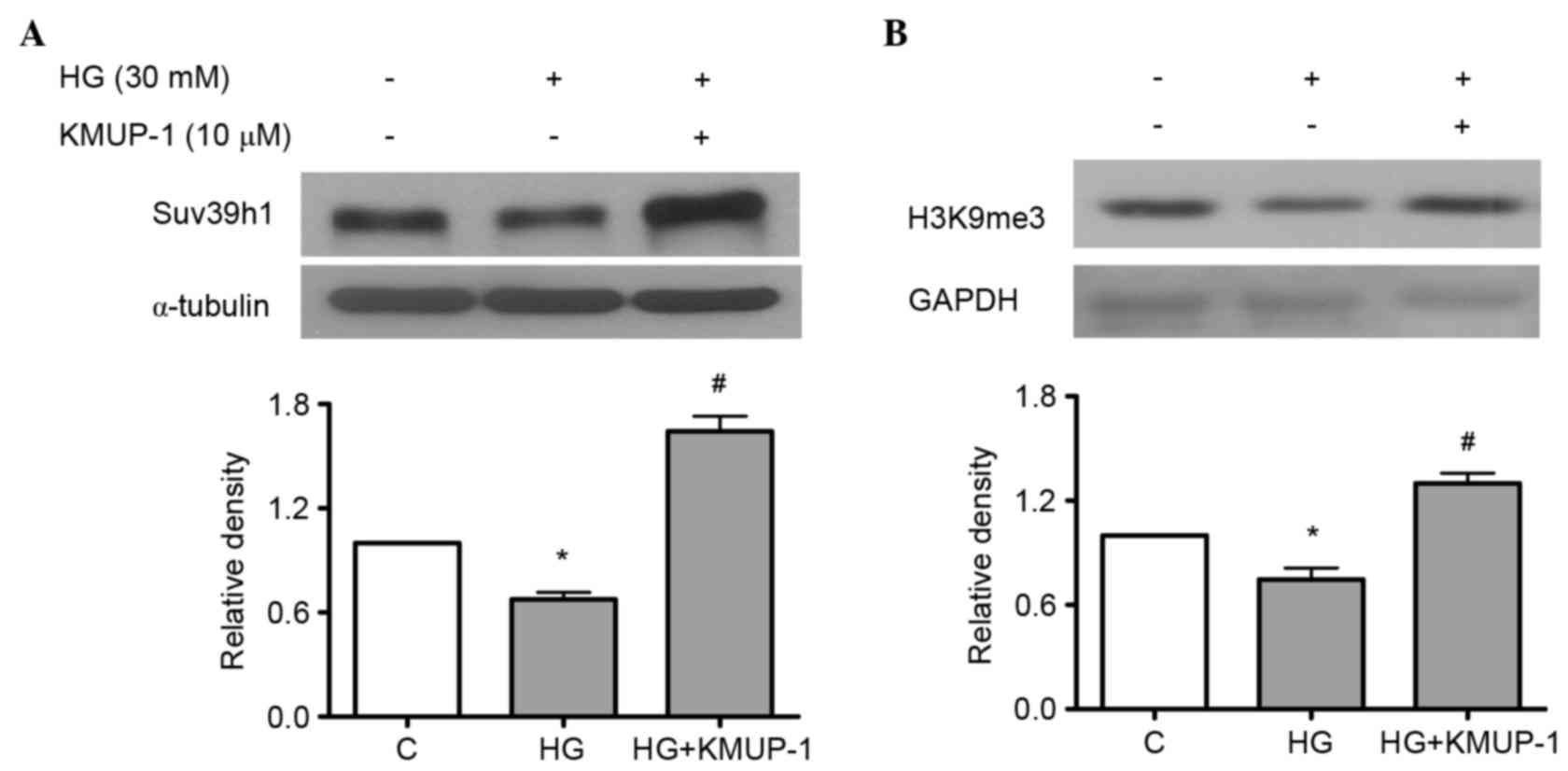
KMUP-1 attenuated HG-reduced histone
methyltransferase Suv39h1
Suv39h1 is a histone lysine methyltransferase, which
induces the gene-silencing H3K9me3, while HG increases
pro-inflammatory genes concomitantly with decreased levels of
H3K9me3 and Suv39h1 at their promoters in vascular smooth muscle
cells (25). However, the roles of
Suv39h1 in diabetic nephropathy remain unclear. As a result, the
study investigated whether HG-induced pro-fibrotic collagen IV and
fibronectin is associated with reduced Suv39h1 levels and the
effects of KMUP-1 on HG-decreased Suv39h1 levels. KMUP-1 (10 µM)
was demonstrated to attenuate HG-decreased Suv39h1 (Fig. 6A) and H3K9me3 (Fig. 6B) levels at 72 h.
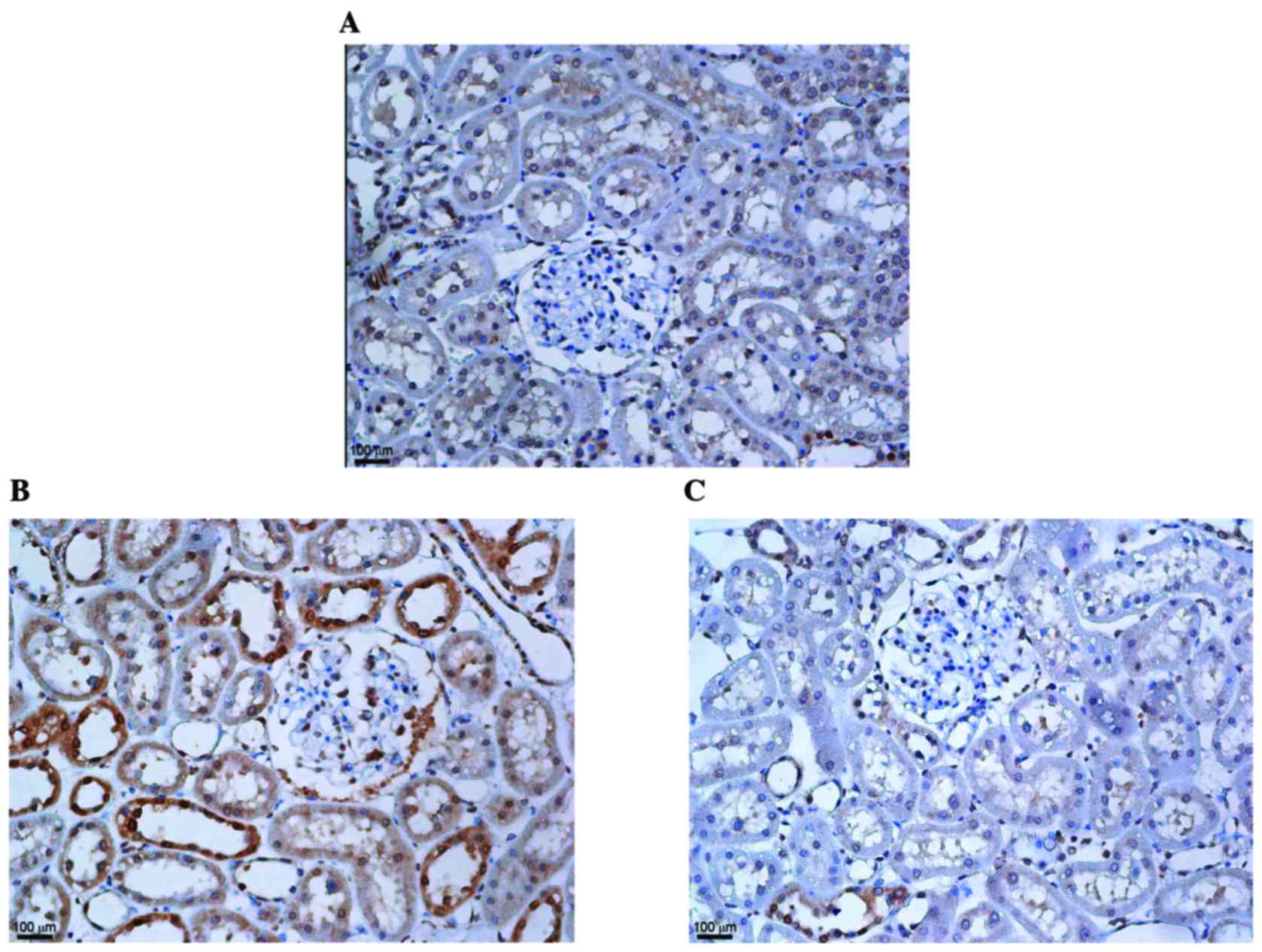
KMUP-1 attenuated collagen type IV and
fibronectin expression in STZ-diabetic rats
To corroborate the in vitro findings, the
effects of intraperitoneal KMUP-1 (5 mg/kg/day) treatment on
STZ-diabetic rats were investigated at 8 weeks. Increased
glomerular and tubular expression of collagen IV were both
attenuated by KMUP-1 in diabetic rats (Fig. 7). Furthermore, increased
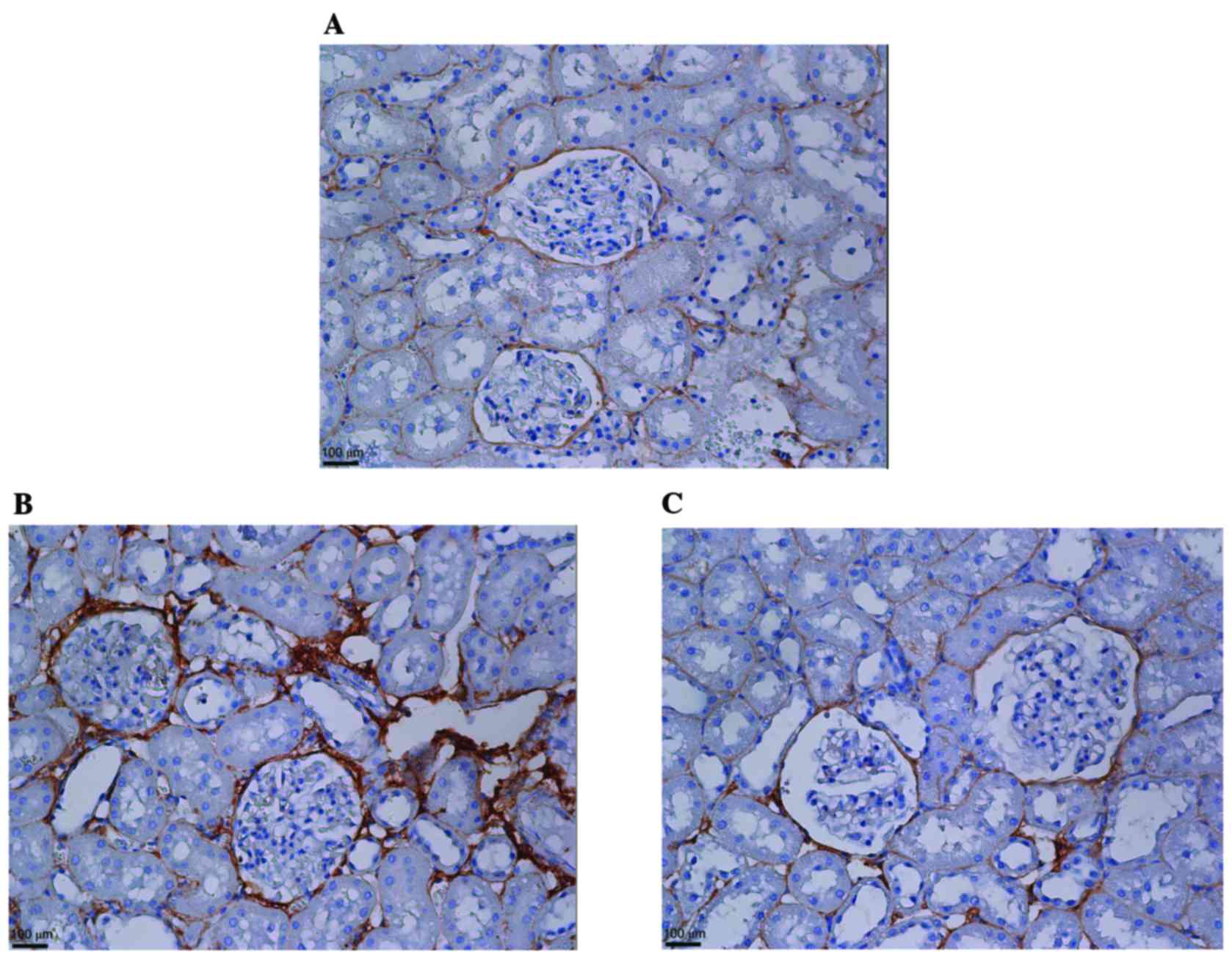
peri-glomerular and tubulointerstitial expression of fibronectin
was attenuated by KMUP-1 in diabetic rats (Fig. 8).
Discussion
KMUP-1 was demonstrated to attenuate HG-induced
TGF-β1 and TGF-β1-induced Smad2/3 signaling in mesangial cells. In
addition, KMUP-1 attenuated HG or TGF-β1-induced collagen IV and
fibronectin expression and HG-induced cell hypertrophy while
attenuating HG-decreased Suv39h1 expression in mesangial cells. In
addition, KMUP-1 attenuated collagen IV and fibronectin expression
in STZ-diabetic rats. The observations provide mechanistic insights
into the role of KMUP-1 in the attenuation of diabetic rat
glomerulosclerosis.
The finding that KMUP-1, an eNOS-NO-sGC-cGMP
enhancer (6), attenuated
HG-induced TGF-β1 and its Smad2/3 signaling corroborates the notion
that DN is associated with a deficiency of NO (22). Notably, it was identified KMUP-1
attenuated TGF-β1-induced p-Smad2/3 expression only at 5–10 min.
Similarly, NO has been identified to delay (however not abolish)
TGF-β1-induced p-Smad2/3 expression from 15–60 min in endothelial
cells (26). Previous studies
indicated that HG induces TGF-β1 by decreasing NO and cGMP
(27).
In the present study, KMUP-1 attenuated HG- or
TGF-β1-induced collagen IV and fibronectin expression while
attenuating cell hypertrophy. These observations corroborate with a
previous study demonstrating that overexpression of cGMP-dependent
protein kinase attenuates HG-induced TGF-β1 and expression of
collagen or fibronectin in mesangial cells (28). In addition, sGC enhancers decrease
DN in eNOS-knockout mice in combination with angiotensin II type I
receptor blockade (29). Thus, sGC
enhancers may be renoprotective in DN (24) by attenuating TGF-β1-induced
effects. Similarly, a previous study identified that eNOS
deficiency induces diabetic renal hypertrophy and
glomerulosclerosis in mice (23).
The present study presented that KMUP-1 attenuated
HG-decreased Suv39h1 and H3K9me3 expression. Similarly, additional
studies indicated that Suv39h1 is decreased in diabetic mouse
vascular smooth muscle cells (30), while occupancy of H3K9me3 on some
pro-fibrotic genes is decreased in diabetic mouse glomeruli
(31). Thus, the decreased
gene-silencing activity of Suv39h1 may be one of the pro-fibrotic
mechanisms in DN. Notably, Suv39h1 protects from myocardial
ischemia-reperfusion injury in diabetic rats (32).
The in vitro results were corroborated with
the in vivo results that KMUP-1 attenuated increased
expression of collagen IV and fibronectin in STZ-diabetic rats.
This is in agreement with a previous study observing that KMUP-1
attenuates rat DN while increasing glomerular eNOS and decreasing
matrix metalloproteinase-9 (MMP-9) expression (7). The attenuation of MMP-9 expression by
KMUP-1 (7) may be an additional
anti-fibrotic mechanism in that MMP-9 inhibitors attenuate
HG-induced TGF-β1 (33) and
attenuate DN (34).
In conclusion, KMUP-1 attenuates HG- and
TGF-β1-induced pro-fibrotic proteins within mesangial cells. In
addition, attenuation of TGF-β1-induced Smad2/3 signaling and
attenuation of HG-decreased Suv39h1 expression may be two of the
anti-fibrotic mechanisms of KMUP-1. These observations provide
novel mechanistic insight into the previously observed attenuation
of diabetic rat glomerulosclerosis by KMUP-1.
Acknowledgements
The authors would like to thank the staff of the
Department of Pharmacology from the Kaohsiung Medical University
(Kaohsiung, Taiwan) for the assistance with the pharmaceutical
properties of KMUP-1. We also thank Dr Jean-Louis Virelizier (Unité
d'Immunologie Virale, Institut Pasteur, Paris, France) and Dr Joan
Massagué (Memorial Sloan Kettering Cancer Center, New York, NY,
USA) for the plasmids.
References
|
1
|
Perkovic V, Agarwal R, Fioretto P,
Hemmelgarn BR, Levin A, Thomas MC, Wanner C, Kasiske BL, Wheeler DC
and Groop PH: Conference Participants: Management of patients with
diabetes and CKD: Conclusions from a ‘Kidney disease: Improving
global outcomes’ (KDIGO) controversies conference. Kidney Int.
90:1175–1183. 2016. View Article : Google Scholar
|
|
2
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012. View Article : Google Scholar :
|
|
3
|
Kanwar YS, Sun L, Xie P, Liu FY and Chen
S: A glimpse of various pathogenetic mechanisms of diabetic
nephropathy. Annu Rev Pathol. 6:395–423. 2011. View Article : Google Scholar :
|
|
4
|
Lan HY: Transforming growth factor-β/Smad
signalling in diabetic nephropathy. Clin Exp Pharmacol Physiol.
39:731–738. 2012. View Article : Google Scholar
|
|
5
|
Huang JS, Chuang LY, Guh JY, Chen CJ, Yang
YL, Chiang TA, Hung MY and Liao TN: Effect of nitric
oxide-cGMP-dependent protein kinase activation on advanced
glycation end-product-induced proliferation in renal fibroblasts. J
Am Soc Nephrol. 16:2318–2329. 2005. View Article : Google Scholar
|
|
6
|
Wu BN, Chen CW, Liou SF, Yeh JL, Chung HH
and Chen IJ: Inhibition of proinflammatory tumor necrosis
factor-{alpha}-induced inducible nitric-oxide synthase by
xanthine-based
7-[2-[4-(2-chlorobenzene)piperazinyl]ethyl]-1,3-dimethylxanthine
(KMUP-1) and 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1,
3-dimethylxanthine (KMUP-3) in rat trachea: The involvement of
soluble guanylate cyclase and protein kinase G. Mol Pharmacol.
70:977–985. 2006. View Article : Google Scholar
|
|
7
|
Hong TY, Guh JY, Wu BN, Chai CY, Huang HT
and Chen IJ: KMUP-1 protects kidney from Streptozotocin-induced
proinflammation in early diabetic nephropathy by restoring
eNOS/PPARgamma and inhibiting MMP-9. European Journal of
Inflammation. 12:89–100. 2014. View Article : Google Scholar
|
|
8
|
Wu BN, Lin RJ, Lin CY, Shen KP, Chiang LC
and Chen IJ: A xanthine-based KMUP-1 with cyclic GMP enhancing and
K(+) channels opening activities in rat aortic smooth muscle. Br J
Pharmacol. 134:265–274. 2001. View Article : Google Scholar :
|
|
9
|
Lin RJ, Wu BN, Lo YC, Shen KP, Lin YT,
Huang CH and Chen IJ: KMUP-1 relaxes rabbit corpus cavernosum
smooth muscle in vitro and in vivo: Involvement of cyclic GMP and
K(+) channels. Br J Pharmacol. 135:1159–1166. 2002. View Article : Google Scholar :
|
|
10
|
Wu BN, Lin RJ, Lo YC, Shen KP, Wang CC,
Lin YT and Chen IJ: KMUP-1, a xanthine derivative, induces
relaxation of guinea-pig isolated trachea: The role of the
epithelium, cyclic nucleotides and K+ channels. Br J Pharmacol.
142:1105–1114. 2004. View Article : Google Scholar :
|
|
11
|
Michelson S, Alcami J, Kim SJ, Danielpour
D, Bachelerie F, Picard L, Bessia C, Paya C and Virelizier JL:
Human cytomegalovirus infection induces transcription and secretion
of transforming growth factor beta 1. J Virol. 68:5730–5737.
1994.
|
|
12
|
Wrana JL, Attisano L, Cárcamo J, Zentella
A, Doody J, Laiho M, Wang XF and Massagué J: TGF beta signals
through a heteromeric protein kinase receptor complex. Cell.
71:1003–1014. 1992. View Article : Google Scholar
|
|
13
|
Huang JS, Guh JY, Hung WC, Yang ML, Lai
YH, Chen HC and Chuang LY: Role of the Janus kinase (JAK)/signal
transducters and activators of transcription (STAT) cascade in
advanced glycation end-product-induced cellular mitogenesis in
NRK-49F cells. Biochem J. 342:231–238. 1999. View Article : Google Scholar :
|
|
14
|
Huang JS, Chuang LY, Guh JY, Huang YJ and
Hsu MS: Antioxidants attenuate high glucose-induced hypertrophic
growth in renal tubular epithelial cells. Am J Physiol Renal
Physiol. 293:F1072–F1082. 2007. View Article : Google Scholar
|
|
15
|
Shin SJ, Lai FJ, Wen JD, Hsiao PJ, Hsieh
MC, Tzeng TF, Chen HC, Guh JY and Tsai JH: Neuronal and endothelial
nitric oxide synthase expression in outer medulla of
streptozotocin-induced diabetic rat kidney. Diabetologia.
43:649–659. 2000. View Article : Google Scholar
|
|
16
|
Dai ZK, Lin TC, Liou JC, Cheng KI, Chen
JY, Chu LW, Chen IJ and Wu BN: Xanthine derivative KMUP-1 reduces
inflammation and hyperalgesia in a bilateral chronic constriction
injury model by suppressing MAPK and NFκB activation. Mol Pharm.
11:1621–1631. 2014. View Article : Google Scholar
|
|
17
|
Tsai CW: A Guidebook for the Care and Use
of Laboratory Animals. 3rd. Taiwan Council of Agriculture; Taipei,
Taiwan: 2010, (In Chinese).
|
|
18
|
Yano N, Suzuki D, Endoh M, Cao TN, Dahdah
JR, Tseng A, Stabila JP, McGonnigal BG, Padbury JF and Tseng YT:
High ambient glucose induces angiotensin-independent AT-1 receptor
activation, leading to increases in proliferation and extracellular
matrix accumulation in MES-13 mesangial cells. Biochem J.
423:129–143. 2009. View Article : Google Scholar
|
|
19
|
Jiang T, Che Q, Lin Y, Li H and Zhang N:
Aldose reductase regulates TGF-beta1-induced production of
fibronectin and type IV collagen in cultured rat mesangial cells.
Nephrology (Carlton). 11:105–112. 2006. View Article : Google Scholar
|
|
20
|
Yeh JL, Hsu JH, Wu PJ, Liou SF, Liu CP,
Chen IJ, Wu BN, Dai ZK and Wu JR: KMUP-1 attenuates
isoprenaline-induced cardiac hypertrophy in rats through
NO/cGMP/PKG and ERK1/2/calcineurin A pathways. Br J Pharmacol.
159:1151–1160. 2010. View Article : Google Scholar :
|
|
21
|
Goruppi S, Bonventre JV and Kyriakis JM:
Signaling pathways and late-onset gene induction associated with
renal mesangial cell hypertrophy. EMBO J. 21:5427–5436. 2002.
View Article : Google Scholar :
|
|
22
|
Tessari P: Nitric oxide in the normal
kidney and in patients with diabetic nephropathy. J Nephrol.
28:257–268. 2015. View Article : Google Scholar
|
|
23
|
Nakagawa T, Sato W, Glushakova O, Heinig
M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ
and Croker B: Diabetic endothelial nitric oxide synthase knockout
mice develop advanced diabetic nephropathy. J Am Soc Nephrol.
18:539–550. 2007. View Article : Google Scholar
|
|
24
|
Stasch JP, Schlossmann J and Hocher B:
Renal effects of soluble guanylate cyclase stimulators and
activators: A review of the preclinical evidence. Curr Opin
Pharmacol. 21:95–104. 2015. View Article : Google Scholar
|
|
25
|
Reddy MA, Zhang E and Natarajan R:
Epigenetic mechanisms in diabetic complications and metabolic
memory. Diabetologia. 58:443–455. 2015. View Article : Google Scholar
|
|
26
|
Saura M, Zaragoza C, Herranz B, Griera M,
Diez-Marqués L, Rodriguez-Puyol D and Rodriguez-Puyol M: Nitric
oxide regulates transforming growth factor-beta signaling in
endothelial cells. Circ Res. 97:1115–1123. 2005. View Article : Google Scholar
|
|
27
|
Wang S, Shiva S, Poczatek MH, Darley-Usmar
V and Murphy-Ullrich JE: Nitric oxide and cGMP-dependent protein
kinase regulation of glucose-mediated thrombospondin 1-dependent
transforming growth factor-beta activation in mesangial cells. J
Biol Chem. 277:9880–9888. 2002. View Article : Google Scholar
|
|
28
|
Wang S, Wu X, Lincoln TM and
Murphy-Ullrich JE: Expression of constitutively active
cGMP-dependent protein kinase prevents glucose stimulation of
thrombospondin 1 expression and TGF-beta activity. Diabetes.
52:2144–2150. 2003. View Article : Google Scholar
|
|
29
|
Ott IM, Alter ML, von Websky K, Kretschmer
A, Tsuprykov O, Sharkovska Y, Krause-Relle K, Raila J, Henze A,
Stasch JP and Hocher B: Effects of stimulation of soluble guanylate
cyclase on diabetic nephropathy in diabetic eNOS knockout mice on
top of angiotensin II receptor blockade. PLoS One. 7:e426232012.
View Article : Google Scholar :
|
|
30
|
Villeneuve LM, Reddy MA, Lanting LL, Wang
M, Meng L and Natarajan R: Epigenetic histone H3 lysine 9
methylation in metabolic memory and inflammatory phenotype of
vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA.
105:pp. 9047–9052. 2008; View Article : Google Scholar :
|
|
31
|
Reddy MA, Sumanth P, Lanting L, Yuan H,
Wang M, Mar D, Alpers CE, Bomsztyk K and Natarajan R: Losartan
reverses permissive epigenetic changes in renal glomeruli of
diabetic db/db mice. Kidney Int. 85:362–373. 2014. View Article : Google Scholar
|
|
32
|
Yang B, Yang J, Bai J, Pu P, Liu J, Wang F
and Ruan B: Suv39h1 protects from myocardial ischemia-reperfusion
injury in diabetic rats. Cell Physiol Biochem. 33:1176–1185. 2014.
View Article : Google Scholar
|
|
33
|
Wu L and Derynck R: Essential role of
TGF-beta signaling in glucose-induced cell hypertrophy. Dev Cell.
17:35–48. 2009. View Article : Google Scholar :
|
|
34
|
Williams JM, Zhang J, North P, Lacy S,
Yakes M, Dahly-Vernon A and Roman RJ: Evaluation of metalloprotease
inhibitors on hypertension and diabetic nephropathy. Am J Physiol
Renal Physiol. 300:F983–F998. 2011. View Article : Google Scholar :
|