Introduction
Ginseng (Panax ginseng CA Meyer) is a
medicinal herb, which has been used in Asia for >1,000 years
(1). Ginseng root, the most
commonly used region of the plant, contains bioactive constituents
with complex and multiple pharmacological effects (2). Previous reports have demonstrated
that ginseng grown for longer durations shows improved efficacy and
a greater concentration of bioactive components, including
ginsenosides (3–6). Various studies have focused on the
genetics underlying these findings, particularly on marker gene
identification or authentication, and on key enzymes involved in
the ginsenoside biosynthetic pathway (7,8).
However, the molecular mechanisms remain to be fully
elucidated.
Gene expression analysis is an effective and widely
used approach to identify marker genes and elucidate biological
mechanisms. Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis is the preferred method for gene
expression analysis owing to its rapidity, sensitivity and
specificity (9). Measurements of
the expression levels of genes of interest are normalized against a
consistently expressed reference gene to improve the accuracy of
the RT-qPCR results. However, reference genes are usually selected
based solely on convention, and reference gene levels have been
found to vary substantially among samples (10–13).
In previous studies analyzing gene expression in ginseng, reference
genes, predominantly actin 1 (ACT1) and 18S rRNA, have been
selected based on previous studies of various plant species
(14–18). However, it has been shown that the
expression levels of these two genes are not consistent in
different ginseng organs (19).
Thus, the selection of suitable reference genes is an important
prerequisite for gene expression analysis in ginseng.
RNA sequencing (RNA-Seq) is an ideal method to
identify the most consistently expressed genes for use as reference
genes (20), as large-scale gene
expression data can be generated at the same time and gene
expression values can be converted to reads per kilobase of
transcript per million (RPKM) (21) for direct comparisons between gene
data sets. Publically available RNA-Seq data have been used
previously to identify superior reference genes (21–24).
In the present study, RNA-Seq data was obtained from our previous
ginseng RNA-Seq sequencing project, which included a panel of six
ginseng transcriptome databases, and were used to identify
reference genes with lower variations across multiple developmental
stages in ginseng root. Statistical methods were implemented in
geNorm (25), NormFinder (26) and BestKeeper (27), and the effectiveness of the
candidate genes for RT-qPCR normalization were then compared with
traditional reference genes.
Materials and methods
Ginseng samples
P. ginseng CA Meyer plants were used in the
present study. The P. ginseng samples, which had been grown
for 3, 5, 7, 10, 15 and 20 years, were originally collected from
Fu-song County (longitude, 127.28; latitude, 42.33) of Jilin,
China. A single sample was harvested for each growth period. The
primary roots were collected, immediately frozen in liquid nitrogen
and stored at −80°C until used for library construction.
Selection of candidate reference genes
from ginseng RNA-Seq data
The RNA-Seq data were generated on an Illumina
sequencing platform (HiSeq 2,000; Illumina, Inc., San Diego, CA,
USA), as described previously (28). Briefly, the samples from the six
growing stages were processed according to the manufacturer's
protocol and used for transcriptome analysis, including cDNA
library construction, sequencing, assembly and gene expression
analyses.
Gene expression levels were expressed as RPKM using
the following formula: RPKM=109C/NL, where C is the number of
mappable reads uniquely aligning to a unigene, N is the total
number of mappable reads that uniquely align to all unigenes, and L
is the length of a unigene in base pairs. Candidate reference genes
were selected by calculating the coefficient of variation (CV) and
the maximum fold change (MFC) across multiple samples within each
data set, where CV represents the standard deviation (SD) divided
by the mean RPKM, and MFC represents the maximum RPKM divided by
the minimum RPKM value.
RT-qPCR analysis
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The RT-qPCR analyses were
performed using the One Step SYBR PrimeScript PLUS RT-qPCR kit
(Takara Biotechnology, Co., Ltd. Dalian, China, TaKaRa code:
DRR096A). The PCR amplification was performed in a 25 µl mixture
containing 2.0 µl cDNA, 0.5 µl each primer, 12.5 µl SYBR Premix Ex
Taq, 0.5 µl ROX reference dye II and 9 µl distilled water. Data
were collected using an ABI Prism 7500 real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal
cycling conditions comprised an initial denaturation step at 95°C
for 30 sec and 40 cycles at 95°C for 5 sec and 65°C for 34 sec,
followed by a dissociation stage at 95°C for 15 sec, 60°C for 1 min
and 95°C for 15 sec. All samples were amplified in triplicate and
the mean was used for RT-qPCR analysis. Relative gene expression
was calculated using the 2−ΔΔCq method (29). The primer sequences were designed
using Primer 6.0 software (www.premierbiosoft.com/primerdesign/index.html)
(30).
Stability analysis of candidate
reference genes
The mRNA expression profiling data sets were
prepared and generated from the RNA-Seq data. To compare the
stability of the candidate reference genes, the following three
Visual Basic for Applications were used for Microsoft Excel: GeNorm
(https://genorm.cmgg.be/), NormFinder (http://moma.dk/normfinder-software) and
BestKeeper (http://www.gene-quantification.de/bestkeeper.html).
Expression analysis of
pathogenesis-related (PR) proteins
To determine whether RT-qPCR normalization with
different reference genes altered the expression profiles, PR
proteins were used to validate candidate reference genes. The genes
and their primers are listed in Table
I. The ΔCq values for each sample were calculated using either
a traditional reference gene (ACT1) or a novel reference gene
(UDP-N-acetylgalactosamine transporter; UDP) or the combination of
UDP and nuclear transport factor 2 (NTF2), as identified by geNorm.
All these analyses were performed in compliance with Minimum
Information for Publication of Quantitative Real-Time PCR
Experiments guidelines (31).
 | Table I.Primer sequences and amplicon sizes
of PR proteins. |
Table I.
Primer sequences and amplicon sizes
of PR proteins.
| Symbol | Gene name | Primer sequence
(5′→3′) | Amplicon size
(bp) |
|---|
| PR1 |
Pathogenesis-related protein 1 |
TGTTTCCTTCCTCCCTCG | 145 |
|
|
|
CCCCTTCGCTGATTGGT |
|
| PR2 |
Pathogenesis-related protein 2 |
GCTCCATCCTCAGTCCCA | 132 |
|
|
|
GGTTCCAACTCCACCATCTC |
|
| PR5 |
Pathogenesis-related protein 5 |
CCATTTTCCTTTTCATTTCCA | 147 |
|
|
|
CGTTAATGGTCCAGGTTTGG |
|
| PR10 |
Pathogenesis-related protein 10 |
TTGAAGCACTGGATTGATGAG | 134 |
|
|
|
CCACCATTGGATGATGCC |
|
Results
Construction of RNA-Seq databases
The RNA-Seq data used in the present study were
obtained from our ginseng project, which covered six growing stages
between 3 and 20 years. The data included >39,000,000
high-quality sequencing reads for each sample. Following reads
clustering, >80,000 unigenes were obtained in each data set,
which comprises the gene sequence, gene expression level,
annotation and other information for each unigene.
CV and MFC values were used to estimate the
stability of the RPKM values in order to select candidate reference
genes expressed at moderate or high levels in all six data sets,
based on the following three criteria: %CV <25, MFC <5, mean
RPKM >100. As a result, 21 candidate reference genes were
identified; these comprised eight traditional reference genes:
ACT1, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 18s rRNA,
ubiquitin (UBQ), tubulin, β-tubulin, cyclophilin (CYP), and
PP2Ac-3-phosphatase 2A isoform 3 (PP2A), and 13 non-traditional
reference genes: Ubiquitin conjugating enzyme isoform 2 (UBE2),
GAGA-binding transcriptional activator (GAGA) protein disulfide
isomerase (PDI), mitochondrial-processing peptidase (MPP),
glucose-6-phosphate (G6P); UDP, probable prefoldin subunit 5 (PPS);
auxin response factor 1 (ARF1), putative 3-isopropylmalate
dehydrogenase (3-IPMDH), δ(3,5)-δ(2,4)-dienoyl-CoA isomerase, mitochondrial
(ECH1), eukaryotic translation initiation factor 4E-1 (EIF-4E1),
SKP1 and NTF2. A summary of the sequence information for these
genes is provided in Table
II.
 | Table II.Panax ginseng candidate
reference genes, primers and amplicon sizes. |
Table II.
Panax ginseng candidate
reference genes, primers and amplicon sizes.
| Symbol | Gene name | Primer sequence
(5′→3′) | Amplicon size
(bp) |
|---|
| UBE2 |
Ubiquitin-conjugating enzyme isoform
2 |
AGTGCTGGACCTGTTGGTGAAG | 112 |
|
|
|
CTGGTGGGAAATGAATGGATAC |
|
| GAGA | GAGA-binding
transcriptional activator BBR/BPC |
AATGAGTAGCGGGGTTGATGAC | 132 |
|
|
|
CCTCCATTTCCCCATTTGTAGC |
|
| PDI | Protein disulfide
isomerase |
GCAGACAAAGATAGCCCATTCC | 173 |
|
|
|
AAGGCAACAAAGCAGATGGCAG |
|
| MPP |
Mitochondrial-processing peptidase |
CGACCTAAGGAACCACAATCAG | 121 |
|
|
|
CTTCCTTCACATTATGCCAGCC |
|
| G6P |
Glucose-6-phosphate |
TGAAGGGGAAGTCTGTTAGTGG | 121 |
|
|
|
TTCCATCCAAGTGCCCACATCT |
|
| UDP |
UDP-N-acetylgalactosamine dual
transporter |
CGGCAAGCAGAGATAAGACACT | 95 |
|
|
|
CGGCAAGCAGAGATAAGACACT |
|
| PPS | Probable prefoldin
subunit 5 |
AGCAGTAAAGGAACAAACCGAT | 159 |
|
|
|
ACATAAAGCGACGCCGTAAGAG |
|
| ARF1 | Auxin response
factor 1 |
GAGCGTGGAGAAAAAGGTATTG | 142 |
|
|
|
GCTTCAACTGATAAATGCGACC |
|
| 3-IPMDH | Putative
3-isopropylmalate dehydrogenase |
TCCCGCTATCTTCGTGTCTTCT | 105 |
|
|
|
GGATAGGTTGGGAAATGAAGGT |
|
| ECH1 | δ(3,5)-δ(2,4)-dienoyl-CoA isomerase,
mitochondrial |
AATCTCTTCCTCAATCGCCCAT | 130 |
|
|
|
ATTAGGGTTTTGGTCAAGGGAG |
|
| EIF-4E1 | Eukaryotic
translation initiation factor 4E-1 |
TATTCCACATCCACTTGAGCAC | 111 |
|
|
|
GAAGAGAAAGTGTAGATGGGGC |
|
| SKP1 | Skp1 |
CGCTAACACCAGTATTCCCCTT | 214 |
|
|
|
GATGTTGAGGTAGTTTGCTGCC |
|
| NTF2 | Nuclear transport
factor 2 |
AGAACATCGTTGCCAAACTCAC | 112 |
|
|
|
CTGACAAAGACGAGCATACCAC |
|
| ACT1 | Actin 1 |
TGGCATCACACTTTCTACAACG | 109 |
|
|
|
TTTGTGTCATCTTCTCCCTGTT |
|
| GAPDH |
Glyceraldehyde-3-phosphate dehydrogenase,
cytosolic |
GAGAAGGAATACACACCTGACC | 106 |
|
|
|
CAGTAGTCATAAGCCCCTCAAC |
|
| 18s rRNA | 18S rRNA |
TTCACACCAAGTATCGCATTTC | 145 |
|
|
|
CCAAGGAAATCAAACTGAACTG |
|
| UBQ | Ubiquitin,
putative |
AACCAACTGATACCATTGACCG | 120 |
|
|
|
CTTTTGCTGTTTTGTCATCTCC |
|
| Tublin | Tubulin α-1
chain |
CTCTGTTGTTGGAACGCTTGTC | 144 |
|
|
|
CTGTGTGCTCAAGAAGGGAATG |
|
| β-Tublin | β-tubulin |
TGTTGTGAGGAAAGAAGCCGAG | 165 |
|
|
|
GGAGAAGGGAAGACAGAGAAAG |
|
| CYP | Cyclophilin |
CAGGCAAAGAAAAAGTCAAGTG | 108 |
|
|
|
AAAGAGACCCATTACAATACGC |
|
| PP2A | PP2Ac-3-phosphatase
2A isoform 3 |
GCTCCAAACTACTGTTACCGCT | 141 |
|
|
|
ATAATCAGGTGTCTTGCGGGTG |
|
Expression profiles of the candidate
reference genes
The expression profiles of the 21 candidate
reference genes in the RNA-Seq data sets across the six growth
stages were analyzed. The 21 genes were ranked from lowest to
highest CV values based on the RPKMs (Fig. 1), which allowed direct comparisons
within and between samples with no bias for short genes. The
results showed that the non-traditional reference genes, UDP, NTF2
and UBE2, were the most stably expressed genes, whereas traditional
reference genes, including ACT1, GAPDH and 18s rRNA, were less
consistently expressed in the six growth stages.
Subsequently, the expression profiles of the 21
candidate reference genes were determined using RT-qPCR analysis.
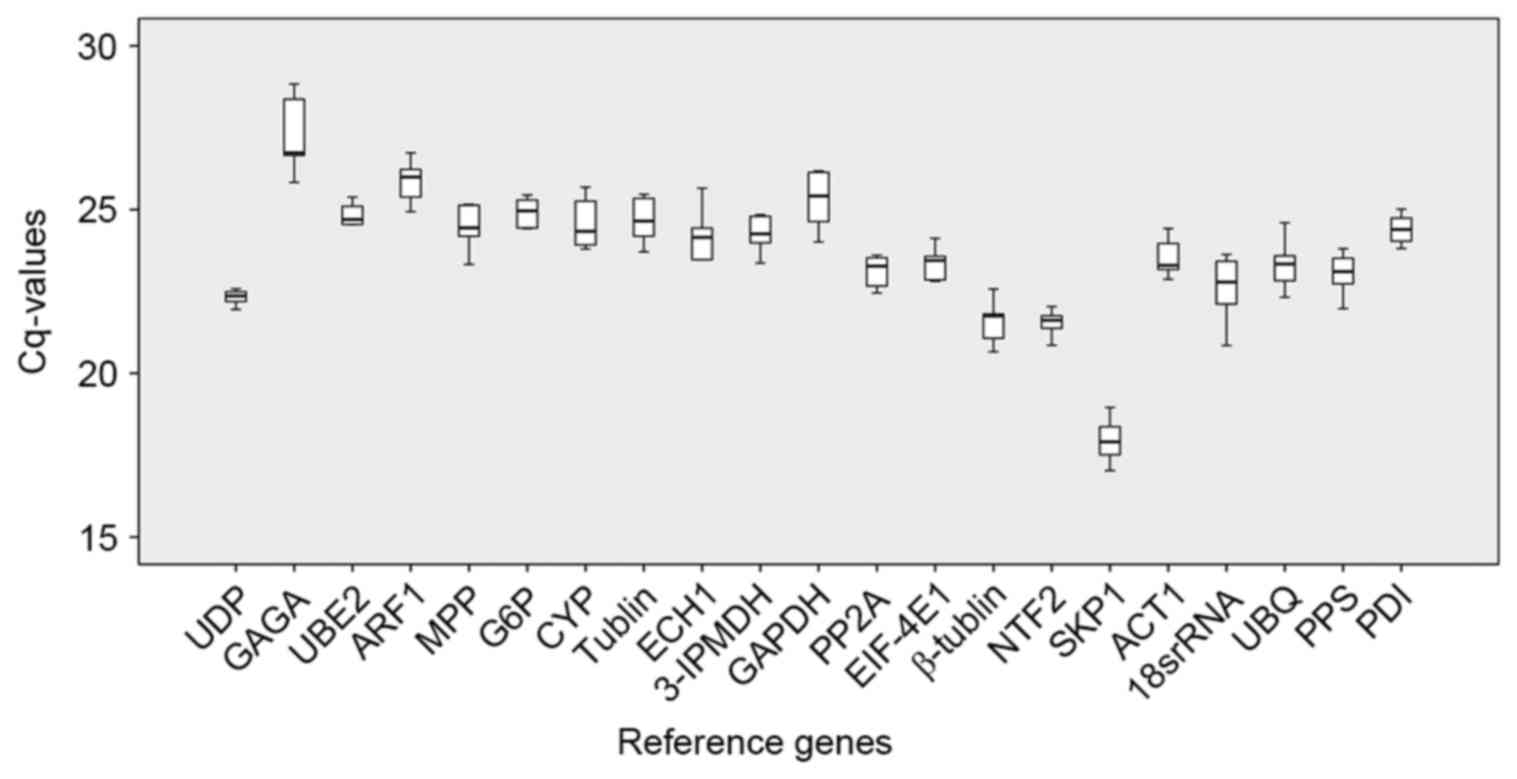
The Cq values for individual genes reflect the actual mRNA levels
in the samples and can be compared directly. The Cq distribution is
shown as a box-plot in Fig. 2. The
average Cq values for the 21 genes ranged between 15 and 29 cycles,
with the majority falling between 20 and 26 cycles. Consistent with
the results from the analysis of the RNA-Seq data, the
non-traditional UDP, NTF2, and UBE2 reference genes had more
consistent Cq values, compared with the traditional reference
genes.
Statistical analysis of RT-qPCR data
using geNorm, NormFinder and BestKeeper
The consistency of the expression levels of each
reference gene was analyzed using the geNorm, NormFinder and
BestKeeper software packages.
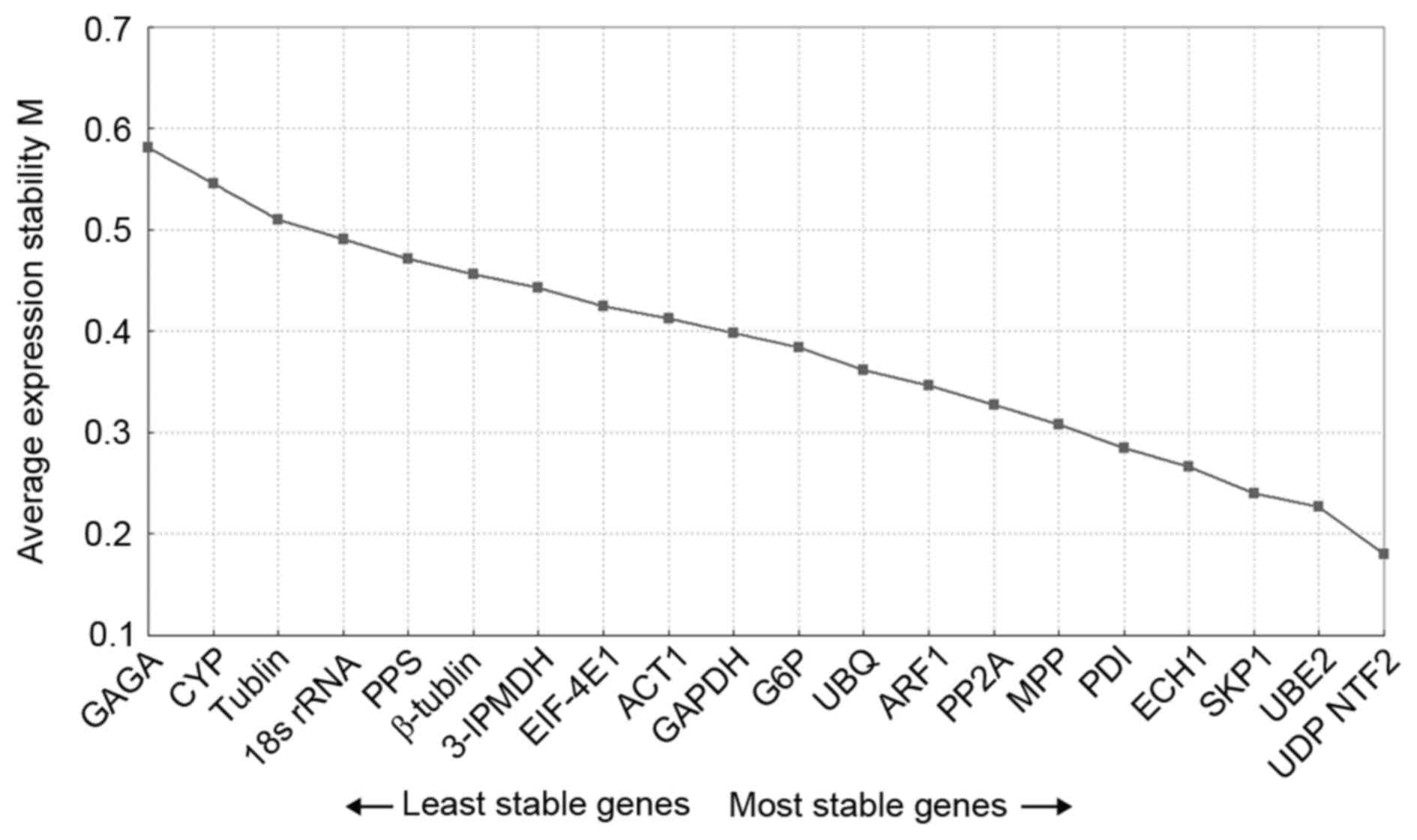
geNorm was designed to analyze the expression
stability of candidate reference genes based on the assumption that
the ratio of the expression levels of two ideal reference genes is
constant in all samples. The average expression stability (M
value), for each reference gene is calculated using the average of
pairwise variations, according to which the expression stability of
all reference genes is ranked. The least stable gene, which has the
highest M value, is then excluded and the M value is recalculated
in a stepwise manner until the two most stably expressed genes are
identified (32). The geNorm
analyses of all six samples revealed that the UDP and NTF2
combination had the lowest M value (0.18), whereas GAGA had the
highest M value (0.58; Fig. 3).
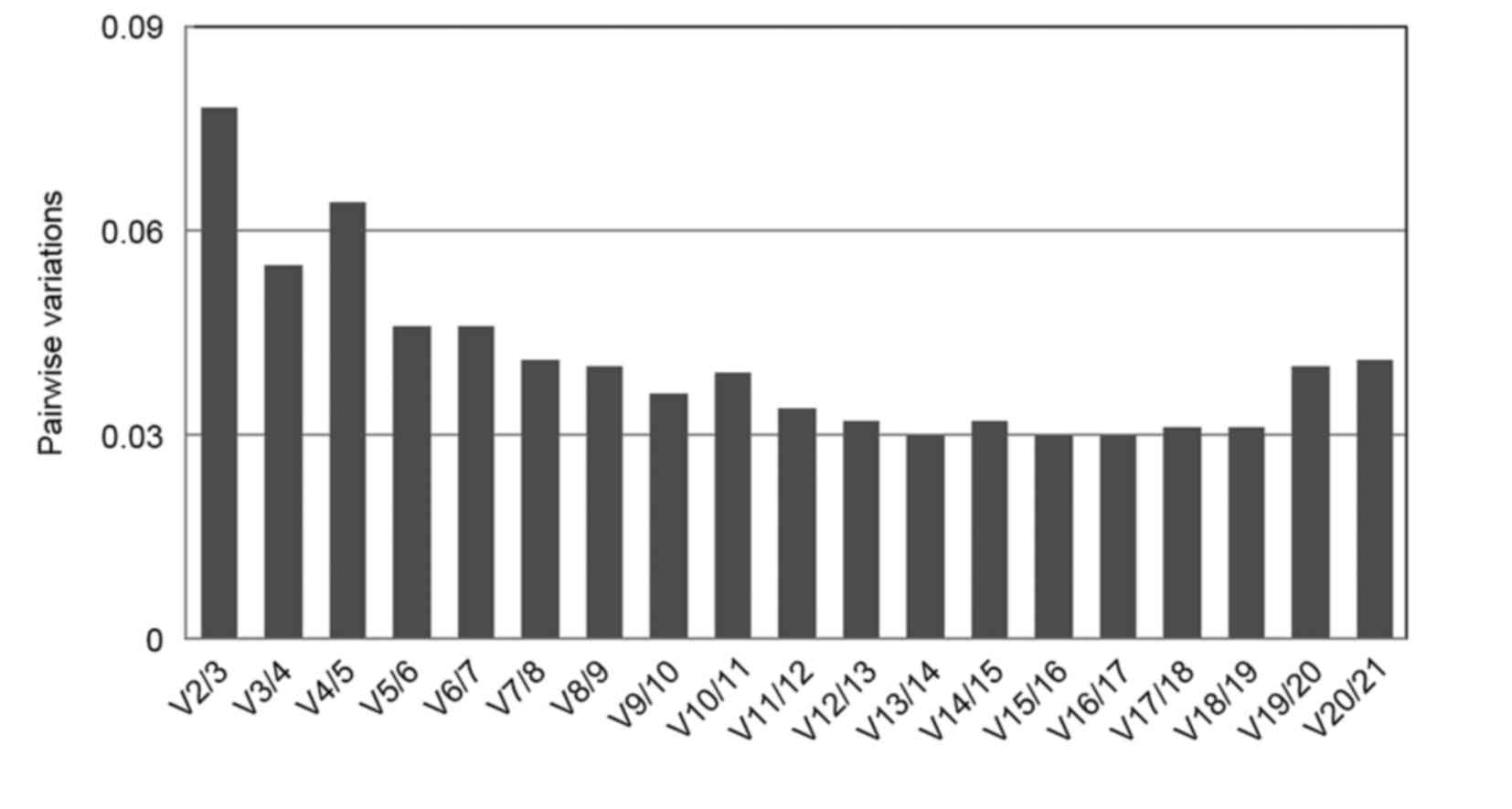
geNorm also calculates the pairwise variation (Vn/Vn+1) between two
sequential normalization factors, NFn and NFn+1, to determine the
optimum number of reference genes. As a general rule, the stepwise
inclusion of reference genes is performed until Vn/Vn+1 falls below
a theoretical threshold of 0.15, when the benefit of adding another
gene (n+1) is limited (25,33).
In the present study, the pairwise variation V2/3 was below the
default cut-off value of 0.15, which indicated that the inclusion
of a third reference gene was not necessary. Thus, UDP/NTF2 may be
the most suitable combination of reference genes for gene
expression analyses in ginseng at different growth stages (Fig. 4).
The present study also used NormFinder to rank the
expression stability of the 21 candidate reference genes.
NormFinder uses an analysis of variance-based model to estimate
intra- and inter-group variations, and combines these estimates to
provide a direct measure of the variations in expression for each
gene (26). Genes with lower
average expression stability values are more stably expressed.
NormFinder analyses of all six samples revealed that UDP was the
most stable gene, followed by ECH1, which surpassed that of UBE2,
whereas GAGA was the least stable (Table III).
 | Table III.Ranking of candidate reference genes
using Norm-Finder. |
Table III.
Ranking of candidate reference genes
using Norm-Finder.
| Rank | Tissue (Stability
value) |
|---|
| 1 | UDP (0.101) |
| 2 | ECH1 (0.105) |
| 3 | UBE2 (0.139) |
| 4 | NTF2 (0.143) |
| 5 | ARF1 (0.145) |
| 6 | MPP (0.157) |
| 7 | SKP1 (0.188) |
| 8 | PP2A (0.223) |
| 9 | EIF-4E1
(0.229) |
| 10 | PDI (0.244) |
| 11 | G6P (0.253) |
| 12 | GAPDH (0.272) |
| 13 | UBQ (0.276) |
| 14 | ACT1 (0.280) |
| 15 | PPS (0.309) |
| 16 | 3-IPMDH
(0.334) |
| 17 | β-tublin
(0.348) |
| 18 | Tublin (0.391) |
| 19 | 18srRNA
(0.420) |
| 20 | CYP (0.546) |
| 21 | GAGA (0.591) |
The BestKeeper program analyzes the stability of a
candidate reference gene based on the CV and standard deviation
(SD) of Cq values using the average Cq value of each duplicate
reaction (27). Reference genes,
which exhibit the lowest CV±SD, are determined as the most stable
genes. The BestKeeper analyses revealed that UBE2 and UDP showed
the highest expression stability in all six samples (Table IV), whereas GAGA and tubulin
showed the least stable expression. Although the preferred
reference genes differed marginally for each program, UDP
consistently ranked high in expression stability.
 | Table IV.Ranking of candidate reference genes
using BestKeeper. |
Table IV.
Ranking of candidate reference genes
using BestKeeper.
| Rank | Tissues
(CV%±SD) |
|---|
| 1 | UBE2
(0.87±0.19) |
| 2 | UDP
(0.93±0.20) |
| 3 | NTF2
(1.08±0.26) |
| 4 | PDI
(1.11±0.28) |
| 5 | PP2A
(1.61±0.39) |
| 6 | SKP1
(1.65±0.38) |
| 7 | ECH1
(1.72±0.42) |
| 8 | MPP
(1.85±0.48) |
| 9 | G6P
(1.92±0.47) |
| 10 | UBQ
(1.95±0.46) |
| 11 | GAPDH
(2.06±0.44) |
| 12 | β-tublin
(2.24±0.52) |
| 13 | ACT1
(2.28±0.49) |
| 14 | 3-IPMDH
(2.44±0.59) |
| 15 | PPS
(2.49±0.61) |
| 16 | ARF1
(2.53±0.62) |
| 17 | EIF-4EI
(2.96±0.75) |
| 18 | CYP
(2.97±0.68) |
| 19 | 18s rRNA
(3.00±0.54) |
| 20 | GAGA
(3.46±0.94) |
| 21 | Tublin
(3.91±0.88) |
Consensus list of candidate reference
genes
To provide a consensus result from the outputs of
the three statistical programs, an arithmetic mean ranking value
was calculated for each gene to obtain the final gene stability
ranking order (Table V). The
results revealed that UDP, NTF2 and UBE2 were the most stable
reference genes, whereas tubulin, CYP and GAGA were the least
stable.
 | Table V.Comprehensive ranking order. |
Table V.
Comprehensive ranking order.
| Ranking order | Genorm | Normfinder | Bestkeeper | Comprehensive
ranking (mean) |
|---|
| 1 | UDP | UDP | UBE2 | UDP (1.67) |
| 2 | NTF2 | ECH1 | UDP | NTF2 (3.67) |
| 3 | UBE2 | UBE2 | NTF2 | UBE2 (3.83) |
| 4 | SKP1 | NTF2 | PDI | PDI (4.67) |
| 5 | ECH1 | ARF1 | PP2A | ECH1 (5.33) |
| 6 | PDI | MPP | SKP1 | SKP1 (5.57) |
| 7 | MPP | SKP1 | ECH1 | MPP (6.23) |
| 8 | PP2A | PP2A | MPP | PP2A (6.58) |
| 9 | ARF1 | EIF-4E1 | G6P | ARF1 (9.17) |
| 10 | UBQ | PDI | UBQ | UBQ (10.31) |
| 11 | G6P | G6P | GAPDH | G6P (10.99) |
| 12 | GAPDH | GAPDH | β-tublin | GAPDH (11.65) |
| 13 | ACT1 | UBQ | ACT1 | ACT1 (12.98) |
| 14 | EIF-4E1 | ACT1 | 3-IPMDH | EIF-4E1
(13.31) |
| 15 | 3-IPMDH | PPS | PPS | 3-IPMDH
(14.98) |
| 16 | β-tublin | 3-IPMDH | ARF1 | β-tublin
(15.00) |
| 17 | PPS | β-tublin | EIF-4E1 | PPS (15.67) |
| 18 | 18s rRNA | Tublin | CYP | 18s rRNA
(18.67) |
| 19 | Tublin | 18sr RNA | 18sr RNA | Tublin (19.33) |
| 20 | CYP | CYP | GAGA | CYP (19.34) |
| 21 | GAGA | GAGA | Tublin | GAGA (20.67) |
Validation of the usefulness of the
selected reference genes
Validation of the sets of candidate reference genes
involved normalizing the RT-qPCR expression levels of the genes
encoding four PR proteins (PR1, PR2, PR5 and PR10) in the six
growing stages. To survive under different environmental stresses,
ginseng has developed mechanisms to perceive external signals,
which trigger adaptive responses and appropriate physiological
alterations, with the induction of PR proteins being one such
response (34). PR proteins have
been classified into 17 families on the basis of structural
differences, serological associations and biological activity
(35).
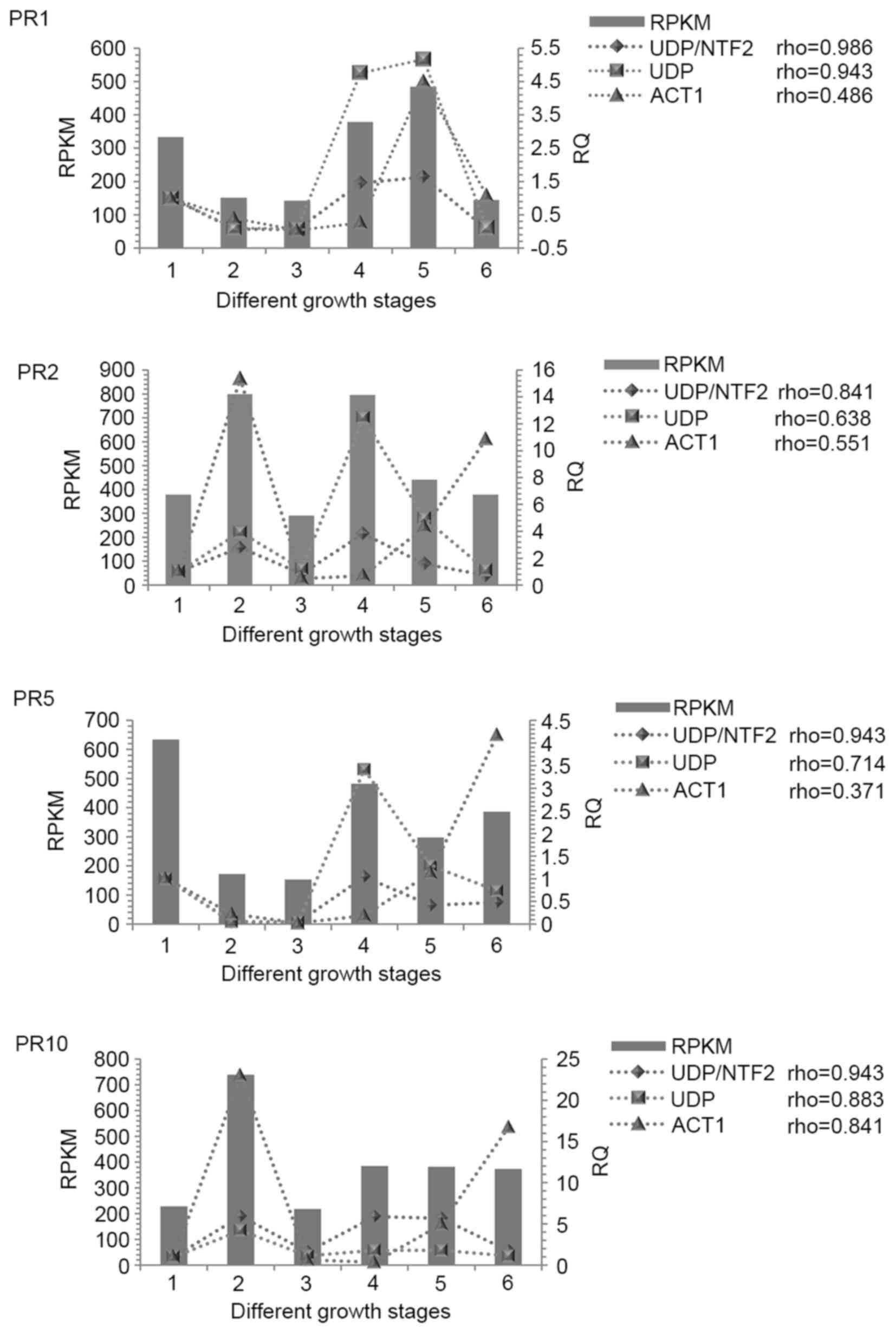
In accordance with the results obtained from the
RNA-Seq data sets, the alterations in gene expression levels of the
PR proteins showed similar patterns when the UDP/NTF2 combination
(from geNorm) and the most consistent reference gene, UDP, were
used for normalization. However, significantly different gene
expression levels were observed for the PR proteins when the
traditional reference gene, ACT1, was used for normalization.
Spearman's correlation analysis also demonstrated a high degree of
correlation between RPKM and relative quantification when the
UDP/NTF2 combination or UDP were used as reference genes, and a low
degree of correlation when ACT1 was used (Fig. 5).
These results showed that the choice of reference
gene had a considerable effect on the normalization results, and
that using inappropriate reference genes may introduce bias to the
analysis and cause misleading results.
Discussion
Using inaccurate reference genes for normalization
can lead to conflicting results in gene expression investigations
based on RT-qPCR analysis, particularly when transcription rate
variations between sample groups are small (36). Increasing evidence indicates that
traditional reference genes do not show stable expression under all
conditions (27,37). Therefore, it is important to
validate the expression stability of a reference gene under
specific experimental conditions prior to use in RT-qPCR
normalization.
The present study performed systematic analysis of
the stability of mRNA expression levels of 21 candidate reference
genes, including eight traditional reference genes from six ginseng
transcriptome data sets. RNA-Seq data and three independent
methods, geNorm, NormFinder and BestKeeper, were used to identify
suitable reference genes for differential gene expression analyses
during ginseng growth years. Among the 21 candidate reference genes
analyzed, UDP and NTF2 were determined to be the optimal
combination of reference genes for analyzing expression.
Microarray and large-scale sequencing technologies
have been used to identify stably expressed reference genes
(38). RNA-Seq technology is
considered a method technology for the following reasons: i)
RNA-Seq reads are digital rather than analog; ii) there is low
background signal; and iii) there is virtually no upper limit for
detection results in a substantially larger dynamic range (20,21,39–42).
A higher degree of technical reproducibility with RNA-Seq, compared
with microarrays has been reported, and RNA-Seq expression data
correlate well with RT-qPCR data, regardless of the sequencing
platform used (39,41).
As a single software package may introduce bias,
three statistical approaches, geNorm, NormFinder and BestKeeper,
were used in the present study to determine the stability of the
expression of the 21 candidate reference genes. GeNorm and
BestKeeper identified UDP, NTF2 and UBE2 as the genes with the
least variation, and NormFinder identified UDP, ECH1 and UBE2 as
the genes with the highest expression stabilities. Inconsistencies
between the three methods can be expected, as they use different
statistical algorithms (43). To
summarize the results, a comprehensive ranking order of each
reference gene was calculated, and it was found that the reference
gene with the highest stability was the combination of UDP and
NTF2.
UDP, a novel nucleotide sugar transporter with dual
substrate specificity, is important in the development of plants,
and may also be involved in glucuronidation and chondroitin sulfate
biosynthesis (44). NTF2 is
indispensable in plants, as it facilitates protein transport into
the nucleus. It may be a component of a multicomponent system of
cytosolic factors, which assemble at the pore complex during
nuclear import (45,46). These two reference genes exhibited
similar expression patterns in the six growth stages of ginseng,
possibly due to them being involved in basic cell metabolism and
cellular functions. In addition to their high expression stability,
the superiority of UDP and NTF2 over the traditional reference
genes was based on their lower expression levels. The use of
reference genes with low expression levels similar to the target
genes has been recommended in order for the comparisons to fall on
the same linear scale (47). The
data obtained in the present study supported the unsuitability of
the traditional reference genes, including ACT1, for normalization,
which was in accordance with other studies (48,49).
The reference genes selected in the present study may be superior
reference genes for the normalization of a wide range of genes,
particularly weakly expressed genes. This result is significant as
the majority of transcripts in tissues are expressed at low levels
(50).
The results of the present study revealed that the
expression levels normalized by a single top-ranked reference gene
were less accurate, compared with expression levels normalized
using two reference genes. Therefore, for investigations of ginseng
development and growth, it is recommended that two reference genes
are used for reliable quantification.
In conclusion, the present study used RNA-Seq data
to identify 21 candidate reference genes in ginseng root grown for
different durations, and identified UDP and NTF2 as the most
suitable reference genes using geNorm, NormFinder and BestKeeper.
These genes were validated using RT-qPCR analysis for use as
reference genes in ginseng investigations. The results showed that
the use of unsuitable reference genes for normalization may result
in biased expression levels. These findings are useful for further
gene expression analyses of ginseng growth, particular associated
with marker identification, environmental stress and the
characterization of gene function. In addition, the results of the
present study provide useful guidelines for reference gene
selection in investigations of other species.
Acknowledgements
This study was supported by grants from the National
Natural Foundation of China (grant nos. 81373937 and 81503212), the
Scientific and Technological Development Program of Jilin, China
(grant nos. 20140622003JC and 20150520139JH) and the Strategic
Adjustment of the Economic Structure of Jilin Province to Guide the
Capital Projects (grant no. 2014N155).
Glossary
Abbreviations
Abbreviations:
|
UDP
|
UDP-N-acetylgalactosamine
transporter
|
|
NTF2
|
nuclear transport factor 2
|
|
RT-qPCR
|
reverse transcription- quantitative
polymerase chain reaction
|
|
RNA-Seq
|
RNA sequencing
|
|
RPKM
|
reads per kilobase of transcript per
million
|
|
UBE2
|
ubiquitin conjugating enzyme isoform
2
|
|
GAGA
|
GAGA-binding transcriptional
activator
|
|
PDI
|
protein disulfide isomerase
|
|
MPP
|
mitochondrial-processing peptidase
|
|
G6P
|
glucose-6-phosphate
|
|
PPS
|
probable prefoldin subunit 5
|
|
ARF1
|
auxin response factor 1
|
|
3-IPMDH
|
putative 3-isopropylmalate
dehydrogenase
|
|
ECH1
|
δ(3,5)-δ(2,4)-dienoyl-CoA isomerase,
mitochondrial
|
|
EIF-4E1
|
eukaryotic translation initiation
factor 4E-1
|
|
ACT1
|
actin 1
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
UBQ
|
ubiquitin
|
|
CYP
|
cyclophilin
|
|
PP2A
|
PP2Ac-3-phosphatase 2A isoform 3
|
References
|
1
|
Xiang YZ, Shang HC, Gao XM and Zhang BL: A
comparison of the ancient use of ginseng in traditional chinese
medicine with modern pharmacological experiments and clinical
trials. Phytother Res. 22:851–858. 2008. View Article : Google Scholar
|
|
2
|
James AD: The Green Pharmacy Herbal
Handbook: Your comprehensive reference to the best herbs for
healing. Rodale: Emmaus, USA. 115–116. 2000.
|
|
3
|
Dan M, Xie G, Gao X, Long X, Su M, Zhao A,
Zhao T, Zhou M, Qiu Y and Jia W: A rapid ultra-performance liquid
chromatography-electrospray Ionisation mass spectrometric method
for the analysis of saponins in the adventitious roots of Panax
notoginseng. Phytochem Anal. 20:68–76. 2009. View Article : Google Scholar
|
|
4
|
Shan SM, Luo JG, Huang F and Kong LY:
Chemical characteristics combined with bioactivity for
comprehensive evaluation of Panax ginseng C.A. Meyer in different
ages and seasons based on HPLC-DAD and chemometric methods. J Pharm
Biomed Anal. 89:76–82. 2014. View Article : Google Scholar
|
|
5
|
Wan JY, Fan Y, Yu QT, Ge YZ, Yan CP,
Alolga RN, Li P, Ma ZH and Qi LW: Integrated evaluation of malonyl
ginsenosides, amino acids and polysaccharides in fresn and
processed ginseng. J Pharm Biomed Anal. 107:89–97. 2015. View Article : Google Scholar
|
|
6
|
He JM, Zhang YZ, Luo JP, Zhang WJ and Mu
Q: Variation of ginsenoside in ginseng of different ages. Nat Prod
Commun. 11:739–740. 2016.
|
|
7
|
Sathiyaraj G, Srinivasan S, Subramanium S,
Kim YJ, Kim YJ, Kwon WS and Yang DC: Polygalacturonase inhibiting
protein: Isolation, developmental regulation and pathogen related
expression in Panax ginseng C.A. Meyer. Mol Biol Rep. 37:3445–3454.
2010. View Article : Google Scholar
|
|
8
|
Han JY, Kim HJ, Kwon YS and Choi YE: The
Cyt P450 enzyme CYP716A47 catalyzes the formation of
protopanaxadiol from dammarenediol-II duringginsenoside
biosynthesis in Panax ginseng. Plant Cell Physiol. 52:2062–2073.
2011. View Article : Google Scholar
|
|
9
|
Bustin SA: Quantification of mRNA using
real-time reverse transcription PCR (RT-PCR): Trends and problems.
J Mol Endocrinol. 29:23–39. 2002. View Article : Google Scholar
|
|
10
|
Thellin O, Zorzi W, Lakaye B, De Borman B,
Coumans B, Hennen G, Grisar T, Igout A and Heinen E: Housekeeping
genes as internal standards: Use and limits. J Biotechnol.
75:291–295. 1999. View Article : Google Scholar
|
|
11
|
Bustin SA: Absolute quantification of mRNA
using real-time reverse transcription polymerase chain reaction
assays. J Mol Endocrinol. 25:169–193. 2000. View Article : Google Scholar
|
|
12
|
Schmittgen TD and Zakrajsek BA: Effect of
experimental treatment on housekeeping gene expression: Validation
by real-time, quantitative RT-PCR. J Biochem Biophys Methods.
46:69–81. 2000. View Article : Google Scholar
|
|
13
|
Lee PD, Sladek R, Greenwood CM and Hudson
TJ: Control genes and variability: Absence of ubiquitous reference
transcripts in diverse mammalian expression studies. Genome Res.
12:292–297. 2002. View Article : Google Scholar :
|
|
14
|
Huang Z, Lin J, Cheng Z, Xu M, Guo M,
Huang X, Yang Z and Zheng J: Production of oleanane-type sapogenin
in transgenic rice via expression of β-amyrin synthase gene from
Panax japonicas C. A. Mey. BMC Biotechnol. 15:452015. View Article : Google Scholar :
|
|
15
|
Lim W, Shim MK, Kim S and Lee Y: Red
ginseng represses hypoxia-induced cyclooxygenase-2 through sirtuin1
activation. Phytomedicine. 22:597–604. 2015. View Article : Google Scholar
|
|
16
|
Oh GS, Yoon J, Lee GG, Oh WK and Kim SW:
20 (S)-protopanaxatriol inhibits liver X receptor α-mediated
expression of lipogenic genes in hepatocytes. J Pharmacol Sci.
128:71–77. 2015. View Article : Google Scholar
|
|
17
|
Qi J, Sun P, Liao D, Sun T, Zhu J and Li
X: Transcriptomic analysis of american ginseng seeds during the
dormancy release process by RNA-Seq. PLoS One. 10:e01185582015.
View Article : Google Scholar :
|
|
18
|
Zhu L, Li J, Xing N, Han D, Kuang H and Ge
P: American ginseng regulates gene expression to protect against
premature ovarian failure in rats. Biomed Res Int. 2015:7671242015.
View Article : Google Scholar :
|
|
19
|
Liu J, Wang Q, Sun M, Zhu L, Yang M and
Zhao Y: Selection of reference genes for quantitative real-time PCR
normalization in Panax ginseng at different stages of growth and in
different organs. PLoS One. 9:e1121772014. View Article : Google Scholar :
|
|
20
|
Mortazavi A, Williams BA, McCue K,
Schaeffer L and Wold B: Mapping and quantifying mammalian
transcriptomes by RNA-Seq. Nat Methods. 5:621–628. 2008. View Article : Google Scholar
|
|
21
|
Wang Z, Gerstein M and Snyder M: RNA-Seq:
A revolutionary tool for transcriptomics. Nat Rev Genet. 10:57–63.
2009. View
Article : Google Scholar :
|
|
22
|
Macrae T, Sargeant T, Lemieux S, Hébert J,
Deneault E and Sauvageau G: RNA-Seq reveals spliceosome and
proteasome genes as most consistent transcripts in human cancer
cells. PLoS One. 8:e728842013. View Article : Google Scholar :
|
|
23
|
Cankorur-Cetinkaya A, Dereli E, Eraslan S,
Karabekmez E, Dikicioglu D and Kirdar B: A novel strategy for
selection and validation of reference genes in dynamic
multidimensional experimental design in yeast. PLoS One.
7:e383512012. View Article : Google Scholar :
|
|
24
|
de Jonge HJ, Fehrmann RS, de Bont ES,
Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te
Meerman GJ and ter Elst A: Evidence based selection of housekeeping
genes. PLoS One. 2:e8982007. View Article : Google Scholar :
|
|
25
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:research0034. 2002. View Article : Google Scholar :
|
|
26
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: A model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar
|
|
27
|
Pfaffl MW, Tichopad A, Prgomet C and
Neuvians TP: Determination of stable housekeeping genes,
differentially regulated target genes and sample integrity:
BestKeeper--Excel-based tool using pair-wise correlations.
Biotechnol Lett. 26:509–515. 2004. View Article : Google Scholar
|
|
28
|
Yao B, Zhao Y, Zhang H, Zhang M, Liu M,
Liu H and Li J: Sequencing and de novo analysis of the Chinese Sika
deer antler-tip transcriptome during the ossification stage using
Illumina RNA-Seq technology. Biotechnol Lett. 34:813–822. 2012.
View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Rozen S and Skaletsky H: Primer3 on the
WWW for general users and for biologist programmers. Methods Mol
Biol. 132:365–386. 2000.
|
|
31
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar
|
|
32
|
Pombo-Suarez M, Calaza M, Gomez-Reino JJ
and Gonzalez A: Reference genes for normalization of gene
expression studies in human osteoarthritic articular cartilage. BMC
Mol Biol. 9:172008. View Article : Google Scholar :
|
|
33
|
Warzybok A and Migocka M: Reliable
reference genes for normalization of gene expression in cucumber
grown under different nitrogen nutrition. PLoS One. 8:e728872013.
View Article : Google Scholar :
|
|
34
|
Gibbs GM, Roelants K and O'Bryan MK: The
CAP superfamily: Cysteine-rich secretory proteins, antigen 5, and
pathogenesis-related 1 proteins-roles in reproduction, cancer and
immune defense. Endocr Rev. 29:865–897. 2008. View Article : Google Scholar
|
|
35
|
Sels J, Mathys J, De Coninck BM, Cammue BP
and De Bolle MF: Plant pathogenesis-related (PR) proteins: A focus
on PR peptides. Plant Physiol Biochem. 46:941–950. 2008. View Article : Google Scholar
|
|
36
|
Etschmann B, Wilcken B, Stoevesand K, von
der Schulenburg A and Sterner-Kock A: Selection of reference genes
for quantitative real-time PCR analysis in canine mammary tumors
using the GeNorm algorithm. Vet Pathol. 43:934–942. 2006.
View Article : Google Scholar
|
|
37
|
Huis R, Hawkins S and Neutelings G:
Selection of reference genes for quantitative gene expression
normalization in flax (Linum usitatissimum L.). BMC Plant Biol.
10:712010. View Article : Google Scholar :
|
|
38
|
Hamalainen HK, Tubman JC, Vikman S, Kyrölä
T, Ylikoski E, Warrington JA and Lahesmaa R: Identification and
validation of endogenous reference genes for expression profiling
of T helper cell differentiation by quantitative real-time RT-PCR.
Anal Biochem. 299:63–70. 2001. View Article : Google Scholar
|
|
39
|
Oshlack A, Robinson MD and Young MD: From
RNA-seq reads to differential expression results. Genome Biol.
11:2202010. View Article : Google Scholar :
|
|
40
|
Mane SP, Evans C, Cooper KL, Crasta OR,
Folkerts O, Hutchison SK, Harkins TT, Thierry-Mieg D, Thierry-Mieg
J and Jensen RV: Transcriptome sequencing of the microarray quality
control (MAQC) RNA reference samples using next generation
sequencing. BMC Genomics. 10:2642009. View Article : Google Scholar :
|
|
41
|
Wilhelm BT and Landry JR:
RNA-Seq-quantitative measurement of expression through massively
parallel RNA-sequencing. Methods. 48:249–257. 2009. View Article : Google Scholar
|
|
42
|
Shendure J: The beginning of the end for
microarrays? Nat Methods. 5:585–587. 2008. View Article : Google Scholar
|
|
43
|
Jian B, Liu B, Bi Y, Hou W, Wu C and Han
T: Validation of internal control for gene expression study in
soybean by quantitative real-time PCR. BMC Mol Biol. 9:592008.
View Article : Google Scholar :
|
|
44
|
Muraoka M, Kawakita M and Ishida N:
Molecular characterization of human UDP-glucuronic
acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide
sugar transporter with dual substrate specificity. FEBS Lett.
495:87–93. 2001. View Article : Google Scholar
|
|
45
|
Bayliss R, Ribbeck K, Akin D, Kent HM,
Feldherr CM, Görlich D and Stewart M: Interaction between NTF2 and
xFxFG-containing nucleoporins is required to mediate nuclear import
of RanGDP. J Mol Biol. 293:579–593. 1999. View Article : Google Scholar
|
|
46
|
Goepfert S, Vidoudez C, Rezzonico E,
Hiltunen JK and Poirier Y: Molecular identification and
characterization of the Arabidopsis delta(3,5),
delta(2,4)-dienoyl-coenzyme A isomerase, a peroxisomal enzyme
participating in the beta-oxidation cycle of unsaturated fatty
acids. Plant Physiol. 138:1947–1956. 2005. View Article : Google Scholar :
|
|
47
|
Czechowski T, Stitt M, Altmann T, Udvardi
MK and Scheible WR: Genome-wide identification and testing of
superior reference genes for transcript normalization in
Arabidopsis. Plant Physiol. 139:5–17. 2005. View Article : Google Scholar :
|
|
48
|
Goidin D, Mamessier A, Staquet MJ, Schmitt
D and Berthier-Vergnes O: Ribosomal 18S RNA prevails over
glyceraldehyde-3-phosphate dehydrogenase and beta-actin genes as
internal standard for quantitative comparison of mRNA levels in
invasive and noninvasive human melanoma cell subpopulations. Anal
Biochem. 295:17–21. 2001. View Article : Google Scholar
|
|
49
|
Selvey S, Thompson EW, Matthaei K, Lea RA,
Irving MG and Griffiths LR: Beta-actin: An unsuitable internal
control for RT-PCR. Mol Cell Probes. 15:307–311. 2001. View Article : Google Scholar
|
|
50
|
Warrington JA, Nair A, Mahadevappa M and
Tsyganskaya M: Comparison of human adult and fetal expression and
identification of 535 housekeeping/maintenance genes. Physiol
Genomics. 2:143–147. 2000.
|