Introduction
The development of diabetes mellitus (DM) is a
chronic inflammatory response process, and chemokines serve an
important role in the course of events (1). Hyperglycemia is a major factor
leading to diabetic nephropathy (DN), which may lead to renal
fibrosis. Drugs are usually administered to regulate blood glucose
levels and to help prevent the progression of renal disease;
however, drugs are not able to completely delay the development of
DN. Consequently, novel therapeutic agents for the treatment and
prevention of DN progression are required. During the
epithelial-mesenchymal transition (EMT) process, cellular polarity
is removed from the epithelial cells and the transdifferentiation
of epithelial cells into mesenchymal cells occurs. E-cadherin and
cytokeratin are markers of epithelial cells, whereas a-SMA and
vimentin are markers of mesenchymal cells (2). Kalluri and Neilson (3) demonstrated that during the course of
kidney fibrosis in mice, ~12% of fibroblasts are derived from the
bone marrow and ~30% are derived due to EMT of tubular epithelial
cells in the kidney. E-cadherin and α-SMA are the most important
proteins involved in EMT (4).
During the EMT process, a significant decrease in
E-cadherin and an increase in α-SMA expression has long been
demonstrated, along with an increase in Snail protein (5). Certain transdifferentiating cells
express α-SMA, which is completely absent in tubular epithelial
cells. The appearance of α-SMA in transdifferentiating cells during
EMT is a characteristic of myofibroblast formation (6). The loss of E-cadherin expression
together with the simultaneous upregulation of vimentin expression
is known to be a marker of EMT changes in epithelial cells
(7). Vimentin is a type III
intermediate filament protein that is normally found in mesenchymal
cells, although it is occasionally expressed in migratory
epithelial cells during embryogenesis and wound healing (8).
Tanshinone IIA
(C19H18O3) is a fat-soluble,
pharmacologically active component of the herb Salvia
miltiorrhiza, which has long been used for the treatment of
renal disease in China. Previous studies (9–12)
have demonstrated that tanshinone IIA inhibits the proliferation
and migration of arterial smooth muscle cells, reduces pulmonary
artery pressure, ameliorates hypoxia-induced pulmonary artery
remodeling and attenuates interleukin-17A-induced systemic
sclerosis in patient-derived dermal vascular smooth muscle cell
activation (13). In addition, it
has been demonstrated that tanshinone IIA effectively inhibits the
proliferation and phenotypic transformation of rat cardiac
fibroblasts, delays the progression of myocardial fibrosis and
attenuates bleomycin-induced pulmonary fibrosis in rats (14,15).
The present study used an in vitro model to
elucidate the effects of tanshinone IIA on renal fibrosis and
identified that tanshinone IIA serves an important role in the
regulation of renal tubular epithelial cell fibrosis. Tanshinone
IIA has also been shown to inhibit high glucose (HG)-induced renal
tubular epithelial cell fibrosis possibly through the EMT
pathway.
Materials and methods
Cell culture
The HK-2 cell line (CRL-2190; American Type Culture
Collection, Manassas, VA, USA) consists of proximal tubular cells
derived from a normal human kidney. The cells (5×105)
were seeded in 25T flasks (Corning Incorporated, Corning, NY, USA)
and cultured with Dulbecco's modified Eagle's medium (DMEM)
nutrient mixture (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 2% (v/v)
penicillin/streptomycin (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) and 1% (v/v) L-glutamine at 37°C in an atmosphere
containing 95% environmental air and 5% CO2. The cells
were trypsinized using 0.05% trypsin-EDTA (Gibco; Thermo Fisher
Scientific, Inc.). Prior to exposure to high glucose, cells were
cultured in a minimum starvation media (0.1% FBS) for 24 h. And
then the cells were cultured in DMEM containing 5.5 mM D-glucose
(normal glucose, NG) as control group, 30 mM mannitol (Harbin
Pharmaceutical Group, Harbin, China) or 30 mM D-glucose (Gibco;
Thermo Fisher Scientific, Inc.) in culture flasks for 48 h to
induce cellular fibrogenesis, and were subsequently treated with
various concentrations of tanshinone IIA (98.0% pure; Xi'an
Hongsheng Biotech Company, Xi'an, China) over 48 h. Three wells
were allocated for each treatment, including a negative control
(untreated cells). Tanshinone IIA was prepared as a 1-mM stock
solution in dimethyl sulfoxide and stored in the dark at −20°C.
Cell viability assay
To detect apoptosis in vitro, TUNEL assays
were performed using a one-step TUNEL fluorescent kit (Beyotime
Institute of Biotechnology, Haimen, China). HK-2 cells were
permeabilized with 0.1% Triton X-100, followed by fluorescein
isothiocyanate-labeled TUNEL staining for 1 h at 37°C.
TUNEL-positive cells were imaged under a fluorescent microscope and
quantified as the number of green spots in each image (x100
magnification); three images were counted using Cellsens Imaging
software version 1.4.1 (Olympus Corporation, Tokyo, Japan) in each
group. In addition, treated cells were lysed using a lysis buffer
[10 mM Tris, 1 mM EDTA, 1% Triton X-100, 1 mM
Na3VO4, 20 µg/ml aprotinin, 20 µg/ml
leupeptin, 1 mM dithiothreitol and 50 mM phenylmethane sulfonyl
fluoride (PMSF)] and a bicinchoninic acid protein assay kit (cat.
no. P0010S; Beyotime Institute of Biotechnology) was used to detect
protein content.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNAiso Plus reagent (Takara Biotechnology Co., Ltd.,
Dalian, China) was used to isolate total RNA from the cultured
cells in accordance with the manufacturer's protocol. RNA (1 µg)
from each sample was then reverse transcribed using an RNA PCR kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol. The resulting cDNAs were processed using RT-qPCR with
SYBR-Green technology (Takara Biotechnology Co., Ltd.) according to
manufacturer's protocol. Briefly, reactions were conducted in a
StepOnePlus real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) for 40 cycles (95°C for 5 sec and 60°C for 40
sec) following an initial 10 min incubation at 95°C in a 20 µl
volume. Subsequently, the quantification cycle (Cq) was determined
and the relative expression levels of Snail, fibronectin and
E-cadherin were calculated based on the Cq values that were
normalized to GAPDH in each sample. The relative gene expression
levels were calculated using a comparative Cq method formula
2−∆∆Cq method (16).
The primers for the RT-qPCR reaction were purchased from Sangon
Biotech Co., Ltd. (Shanghai, China). The sequences were as follows:
Snail, sense 5′-CATTCCACGCCCAGCTACCC-3′, antisense
5′-CGCCCAGGCTCACATATTCC-3′; fibronectin, sense
5-GTGATCTACGAGGGACAGC-3, antisense 5-GCTGGTGGTGAAGTCAAAG-3;
E-cadherin, sense 5-GGGCTTGGATTTTGAGGC-3, antisense
5-AGATGGGGGCTTCATTCAC-3; GAPDH, sense 5-ATGCTGGTGCTGAGTATGTC-3,
antisense 5-AGTTGTCATATTTCTCGTGG-3; and α-SMA sense
5′-AACTGTGAATGTCCTGTG-3′ and antisense
5′-CATAGGTAACGAGTCAGAG-3′.
Western blot analysis
Western blot analysis was used to evaluate the
protein expression levels of fibronectin, α-SMA, vimentin, Snail
and E-cadherin. The HK-2 cells were lysed using a lysis buffer (10
mM Tris, 1 mM EDTA, 1% Triton X-100, 1 mM
Na3VO4, 20 µg/ml aprotinin, 20 µg/ml
leupeptin, 1 mM dithiothreitol and 50 mM PMSF). Bicinchoninic acid
protein assay kit (cat. no. P0010S; Beyotime Institute of
Biotechnology) was used to detect protein content. Equal quantities
of protein (30 µg) from each sample were separated by 8 or 10%
SDS-PAGE, the protein was transferred onto a polyvinylidene
difluoride (PVDF) membrane and the PVDF membrane was blocked with
10% (w/v) non-fat milk in Tris-buffered saline with 0.1% Tween-20
for 1 h at room temperature. The blots were probed overnight at 4°C
with the following primary antibodies (all purchased from Abcam,
Cambridge, UK) diluted to 1:2,000 (v/v): E-cadherin (ab133597),
vimentin (ab184631), Snail (ab167609), fibronectin (ab194395) and
α-SMA (ab124964). Following hybridization, the blots were washed
and hybridized with 1:6,000 (v/v) dilutions of a goat anti-rabbit
immunoglobulin G (IgG) or goat anti-mouse IgG horseradish
peroxidase-conjugated secondary antibody (cat. no. A0208 and A0216;
Beyotime Institute of Biotechnology) at room temperature for 1 h.
Immunodetection was performed using enhanced chemiluminescence
reagents (Beyotime Institute of Biotechnology). The blots were
scanned and the intensity of each band was quantified using Image J
software version 1.48u (National Institutes of Health, Bethesda,
MD, USA). Signals were normalized against β-actin or GAPDH diluted
to 1:1,500 (v/v) (cat. no. sc-130301 and sc-47724; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and the results were
expressed as a relative expression of the control signal.
Immunocytochemistry
Following different treatments, HK-2 cells were
plated onto 6-well plates with polylysine-coated coverslips at a
density of 1.6×105 cells/well. Cells were fixed in 4%
paraformaldehyde for 15 min at 4°C and stained with
anti-fibronectin antibodies diluted to 1:50 (v/v). Briefly, HK-2
cells were fixed, blocked with 10% skimmed milk, washed with
phosphate-buffered saline (PBS), inactivate endogenous peroxidase
with 0.3% H2O2 for 15 min and then incubated
for 1 h with anti-fibronectin, washed with PBS, incubated for 1 h
with secondary antibody 1:50 (v/v) (cat. no. A0216; Beyotime
Institute of Biotechnology), Dako Real™
Envision™ detection system (cat. no. P0203; Beyotime
Institute of Biotechnology) was used for detection. All procedures
were performed at room temperature.
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± standard deviation. Data were
analyzed using SPSS software version 13.0 (SPSS, Inc., Chicago, IL,
USA). Statistical significance was assessed using analysis of
variance followed by the least significant difference test for
multiple comparisons and P<0.05 was considered to indicate a
statistically significant difference.
Results
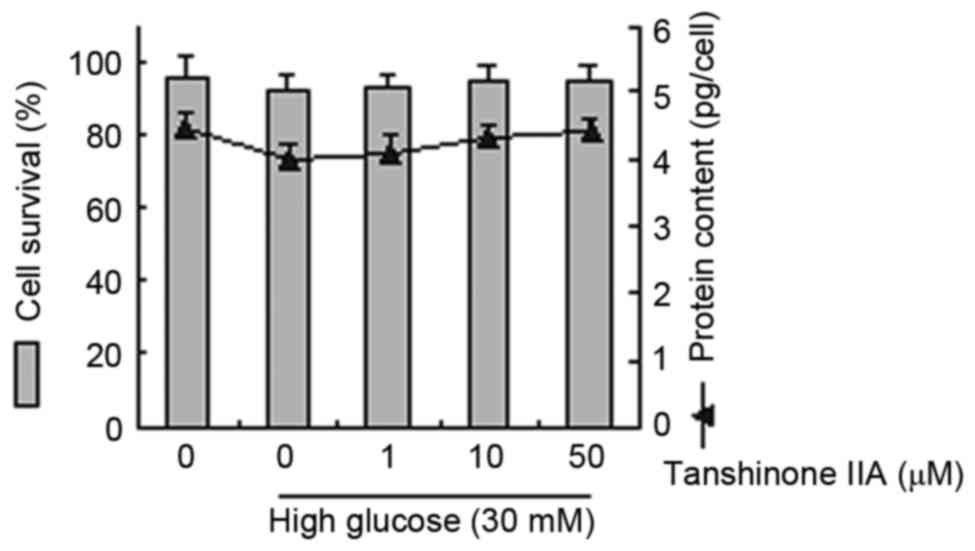
Dose-dependent effects of tanshinone
IIA and HG on cell survival rates and protein content in HK-2
The underlying effects of tanshinone IIA and HG on
glucose-induced pharmacology were evaluated. Cell survival rates
and protein content were analyzed in cells treated with glucose (30
mM) and tanshinone IIA (1, 10 and 50 µM). These observations
demonstrated that treatment with tanshinone IIA or HG did not
statistically affect cellular survival or protein content (Fig. 1).
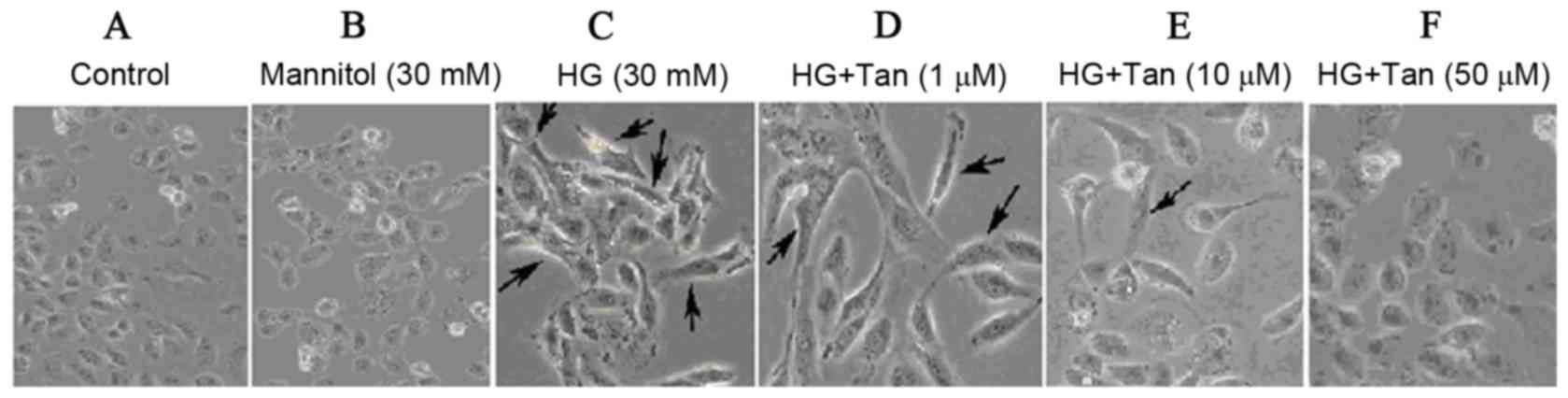
HG-induced EMT in HK-2 cells
To investigate whether HG could induce EMT in HK-2
cells, cells were treated with DMEM containing 5.5 mM D-glucose, 30
mM mannitol or HG (30 mM glucose). Cells treated with 5.5 mM
glucose exhibited a typical epithelial cuboidal shape with a
characteristic confluent monolayer and cobblestone morphology;
however, cells that were exposed to HG exhibited distinct
morphological alterations and possessed an elongated, spindle shape
(Fig. 2C). The expression of the
epithelial phenotypic marker E-cadherin was significantly decreased
in the HG group (P<0.05; Fig.
3A) and mesenchymal phenotypic markers, α-SMA and vimentin,
were markedly increased (P<0.05; Fig. 3B and C). In addition, the
expression of fibronectin was enhanced following treatment with HG,
according to immunocytochemistry and western blot analysis
(Fig. 3B and D). No significant
differences were identified between the mannitol and NG groups. The
downregulation of E-cadherin and the concomitant upregulation of
α-SMA, vimentin and fibronectin in the tubular epithelial cells
strongly supported the case for HG as a potent stimulus of EMT in
HK-2 cells.
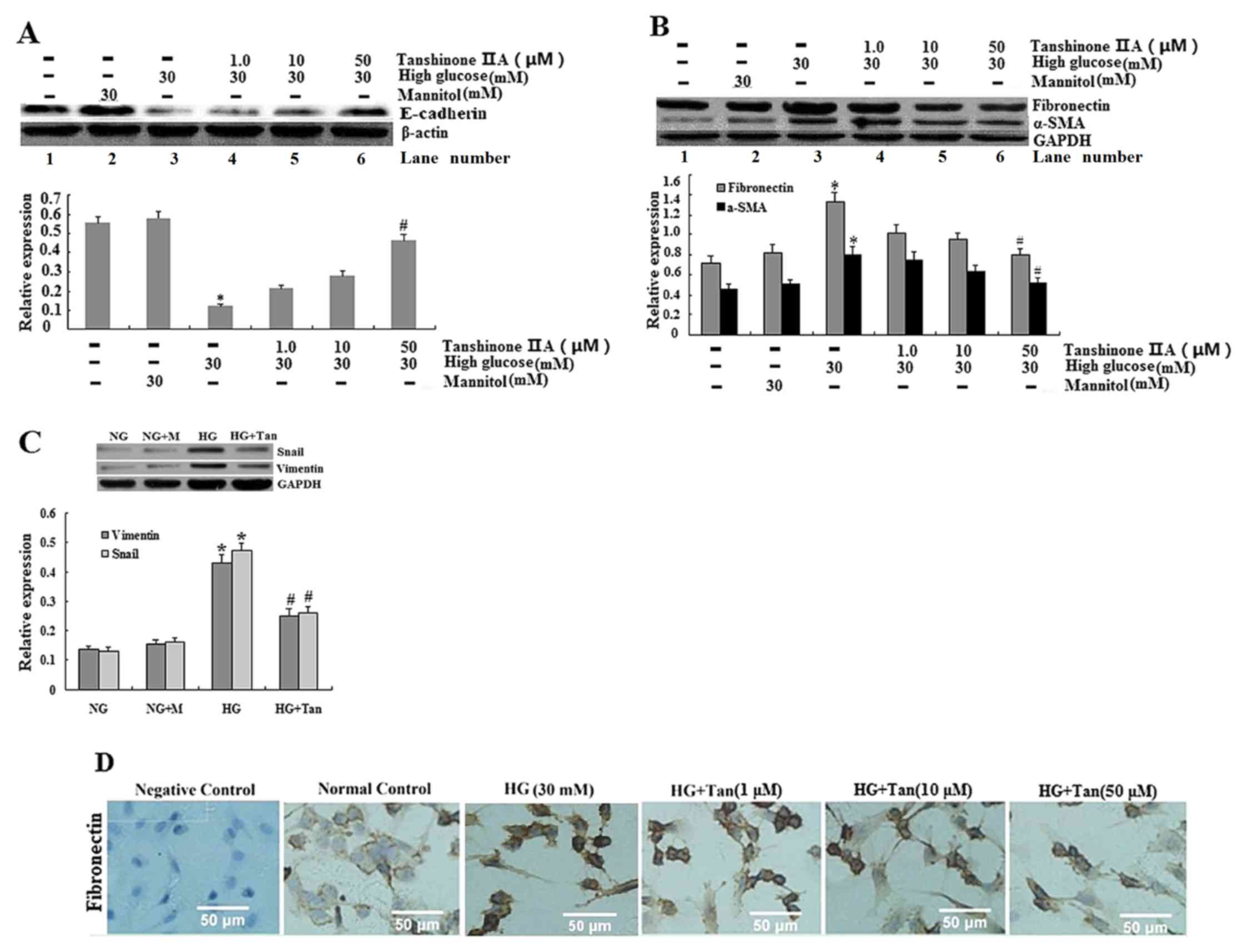 | Figure 3.Expression of E-cadherin, α-SMA,
fibronectin, vimentin and Snail in HK-2 cells. (A and B)
E-cadherin, α-SMA and fibronectin expression was analyzed using
western blotting for HK-2 cells cultured in 5.5 mM glucose (lane
1), 30 mM mannitol (lane 2), 30 mM glucose (lane 3), 30 mM
glucose+1 µM tanshinone IIA (lane 4), 30 mM glucose+10 µM
tanshinone IIA (lane 5) and 30 mM glucose+50 µM tanshinone IIA
(lane 6). *P<0.05 vs. 5.5 mM glucose-treated cells;
#P<0.05 vs. 30 mM glucose-treated cells. (C) Vimentin
and Snail expression was analyzed using western blotting for HK-2
cells cultured in 5.5 mM glucose (NG group), 30 mM mannitol (NG+M
group), 30 mM glucose (HG group) and 30 mM glucose+50 µM tanshinone
IIA (HG+Tan group). *P<0.05 vs. NG group; #P<0.05
vs. HG group. (D) Expression of fibronectin in HK-2 cells, as
determined using immunocytochemistry; HK-2 cells were starved
(without FBS) and treated with glucose (30 mM) for 48 h with
tanshinone IIA (1, 10 and 50 µM) for 48 h. Values are presented as
the mean ± standard deviation. α-SMA, α-smooth muscle actin; HG,
high glucose; NG, normal glucose. |
Effects of tanshinone IIA on
glucose-induced changes in HK-2 cell shape
To examine whether tanshinone IIA inhibits
glucose-induced changes in HK-2 cells, cells were pretreated with
glucose or mannitol for 48 h, followed by tanshinone IIA at the
indicated concentrations (1, 10 and 50 µM) for 48 h. To demonstrate
the osmotic effects of glucose, mannitol was used as a control in
the present study (Fig. 3). It is
evident that 30 mM glucose, but not mannitol, statistically
increased the expression of protein markers. Therefore, it may be
proposed that glucose (not osmosis) serves a pivotal role on the
regulation of HK-2 cell fibrosis. HK-2 cells produced a confluent
monolayer with cobblestone morphology in the control group
(Fig. 2A and B). Following
treatment with 30 mM glucose, a marked number of cells exhibited an
elongated, spindle shape (Fig. 2C,
as indicated by arrows). As presented in Fig. 2D-F, cells were incubated with 30 mM
glucose for 48 h and treated with tanshinone IIA to investigate
cellular morphology. It is evident that tanshinone IIA
significantly reversed HG-induced changes. In addition, tanshinone
IIA appeared to be able to restore HK-2 cell epithelial
morphology.
Tanshinone IIA suppresses HG-induced
EMT in HK-2 epithelial cells
The expression of EMT marker proteins (α-SMA,
vimentin and fibronectin) was examined. HG (30 mM) induced a
notable increase in α-SMA, vimentin and Snail expression, and a
decrease in E-cadherin (Fig. 3).
The aforementioned effects were significantly reversed by 50 µM
tanshinone IIA. Subsequently, α-SMA, vimentin and Snail were
decreased and E-cadherin was increased following treatment with
tanshinone IIA in proximal tubule cells.
Effects of tanshinone IIA on Snail and
E-cadherin synthesis in HK-2 cells
In the HK-2 cells treated with HG (30 mM), the
expression of Snail was significantly greater than that of cells
treated with 5.5 mM glucose or 30 mM mannitol; however, the
expression of Snail following pretreatment with tanshinone IIA was
significantly decreased, as compared with HG treatment (Fig. 3C). It is evident that 30 mM
glucose, but not mannitol, significantly increased the expression
of Snail. In addition, the mRNA expression levels of Snail were
increased in HK-2 cells following treatment with HG; however, the
expression of Snail mRNA was reduced following treatment with 50 µM
tanshinone IIA (Fig. 4), which
concomitantly increased E-cadherin protein expression, as the
protein expression of E-cadherin is increasing in HG+Tan (50 µM)
group (Fig. 3A).
Effects of tanshinone IIA on EMT in
HK-2 cells
EMT marker proteins (including α-SMA, vimentin and
fibronectin), epithelial phenotypic markers (E-cadherin) and
transcriptional factors for EMT (Snail) were assessed using
immunocytochemistry, western blotting and RT-qPCR. HG (30 mM)
significantly increased extracellular fibronectin, α-SMA and
vimentin in HK-2 cells, compared with in the control group
(Fig. 3; P<0.05). Notably,
tanshinone IIA dose dependently (1, 10 and 50 µM) and significantly
suppressed HG-induced expression of fibronectin, α-SMA, vimentin
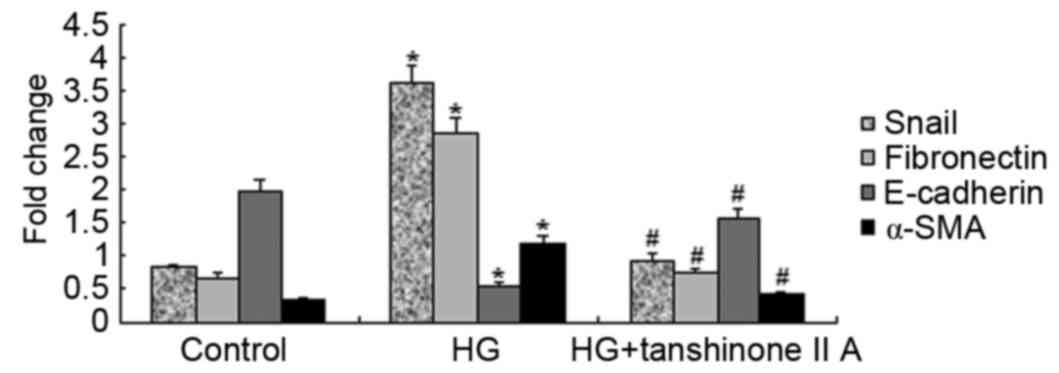
and Snail. Detection of mRNA expression was performed using
RT-qPCR. E-cadherin, fibronectin, α-SMA and Snail mRNA expression
was assessed in the control, HG and HG+tanshinone IIA groups
(Fig. 4). E-cadherin mRNA
expression was markedly reduced in the HG group, whereas Snail,
fibronectin and α-SMA mRNA expression was significantly increased.
However, following treatment with 50 µM tanshinone IIA, the mRNA
expression levels were reversed, resulting in levels similar to
those in the control group. HG increased the expression of EMT
marker proteins, whereas tanshinone IIA significantly reversed the
increase of EMT marker proteins in HK-2 cells. The aforementioned
observation indicated that tanshinone IIA has the potential to
downregulate HG-induced EMT in HK-2 cells.
Discussion
DN is a serious microvascular complication
associated with DM. The degree of renal interstitial fibrosis is
more closely associated with the progressive loss of renal
functions, compared with diabetic glomerulopathy (1). Urinary glucose, proteinuria and
cytokines stimulate renal tubular epithelial cells in patients with
DN, causing the cells to be prone to structural and functional
damage, and phenotypic modulation, which are considered to be the
initiating factors for renal fibrosis. As for renal tubular
epithelial cells, EMT is an important mechanism by which renal
interstitial fibrosis occurs (5).
EMT describes a process by which cells lose their relatively
differentiated epithelial characteristics and demonstrate increased
migratory or synthetic properties. This transition is evidenced by
the loss of proteins involved in cell-cell junctions and an
increase in proteins, including vimentin, fibronectin and α-SMA
(17). In the EMT process, a
significant decrease in E-cadherin expression and an increase in
α-SMA expression have been demonstrated, along with an increase in
Snail protein (18,19). The present study demonstrated that
HG significantly increased extracellular fibronectin, α-SMA and
vimentin expression in HK-2 cells, compared with in the control
group (Fig. 3; P<0.05). Changes
in E-cadherin were rapidly accompanied by an upregulation of
mesenchymal markers. The loss of E-cadherin expression is the most
common biochemical alteration associated with EMT. These results
indicated that HG induced EMT in HK-2 cells.
In previous studies, tanshinone IIA has been
reported to modulate growth, anti-inflammatory effects,
proliferation and migration of numerous cells, including
keratinocytes, RAW 264.7 cells, cardiac fibroblasts,
cardiomyocytes, tumor cells and human aortic smooth muscle cells
(10–12,20–23).
The present study identified that the expression levels of
fibronectin, α-SMA, vimentin and Snail were markedly suppressed
following treatment of HG-stimulated HK-2 cells with 50 µM
tanshinone IIA. Therefore, tanshinone IIA may be considered an
effective treatment for HK-2 fibrosis.
It has previously been demonstrated that stimulating
HK-2 with HG resulted in increased expression of fibronectin
(24). Elevated glucose levels
have been detected in renal fibrosis animal models and in patients
with DN (25). Glucose also
induced accumulation of extracellular matrix components, including
fibronectin, in mesangial cells (26). These findings suggested that
fibronectin may serve an important role in renal fibrosis.
In the present study, in HK-2 cells, the expression
of fibronectin, α-SMA and vimentin was significantly increased
following treatment with HG (30 mM). Tanshinone IIA dose
dependently (1, 10 and 50 µM) and significantly, suppressed
HG-induced increases in the secretion levels of fibronectin, α-SMA
and vimentin (Fig. 3). The present
study demonstrated that tanshinone IIA serves a pivotal role in the
regulation of renal tubular fibrosis. Therefore, tanshinone IIA may
be considered an effective therapeutic supplement for the treatment
of renal tubular fibrosis by inhibiting fibronectin, α-SMA and
vimentin expression.
Snail is a zinc finger transcription factor that
functions as a regulator to suppress the expression of adhesion
molecules during EMT (27). The
most common biochemical change associated with EMT is the loss of
E-cadherin expression. According to Medici et al (28), Snail suppresses E-cadherin. In the
present study, Snail expression was promoted in HK-2 cells under HG
conditions; however, Snail expression was reversed by treatment
with tanshinone IIA, which concomitantly increased E-cadherin
protein. These results indicated that tanshinone IIA completely
reversed HG-induced increase in α-SMA and decrease in E-cadherin.
Therefore, tanshinone IIA may be considered an effective
therapeutic compound for the treatment of proximal tubular
fibrosis, since it regulates Snail expression and the EMT
process.
In renal tubular epithelial cells,
hyperglycemia-induced EMT may contribute to renal fibrosis. The
present study used an in vitro model to elucidate the
effects of tanshinone IIA on renal fibrosis. The results
demonstrated that HG induced the EMT process in HK-2 cells; and
notably, tanshinone IIA suppressed HG-induced EMT in HK-2 cells.
The present study demonstrated that tanshinone IIA may decrease
HG-induced HK-2 fibrosis by downregulating the expression of Snail
to promote E-cadherin expression in HK-2 cells. These findings
suggested that tanshinone IIA may be an effective therapeutic
supplement for the treatment of renal tubular fibrosis via its
ability to inhibit the EMT process.
In conclusion, the results of the present study
demonstrated that tanshinone IIA serves a protective role against
HG-induced renal tubular epithelial cell fibrosis. Tanshinone IIA
antagonized HG-induced EMT signals, possibly by downregulating the
expression of Snail in renal cells. Tanshinone IIA also increased
the expression of E-cadherin and decreased the expression of α-SMA,
fibronectin and vimentin. Therefore, tanshinone IIA may exert the
ability to inhibit HG-induced renal tubular epithelial cell
fibrosis possibly by regulating the EMT pathway. Tanshinone IIA may
be considered a renoprotective agent for the treatment of renal
fibrosis in DN.
Acknowledgements
The present study was supported by research grants
from the Fujian Science and Technology Project of Nature Science
Foundation (grant no. 2012J01435), a special fund from the Fujian
Medical University for Scientific and Technological Development
(grant no. FZS13022Y) and the Youth Foundation from the Health
Department of Fujian province (grant no. 2013-1-50).
References
|
1
|
Phillips AO: The role of renal proximal
tubular cells in diabetic nephropathy. Curr Diab Rep. 3:491–496.
2003. View Article : Google Scholar
|
|
2
|
Câmara J and Jarai G:
Epithelial-mesenchymal transition in primary human bronchial
epithelial cells is Smad-dependent and enhanced by fibronectin and
TNF-alpha. Fibrogenesis Tissue Repair. 3:22010. View Article : Google Scholar :
|
|
3
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar :
|
|
4
|
Fragiadaki M and Mason RM:
Epithelial-mesenchymal transition in renal fibrosis-evidence for
and against. Int J Exp Pathol. 92:143–150. 2011. View Article : Google Scholar :
|
|
5
|
Carew RM, Wang B and Kantharidis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116. 2012.
View Article : Google Scholar
|
|
6
|
Sebe A, Leivonen SK, Fintha A, Masszi A,
Rosivall L, Kähäri VM and Mucsi I: Transforming growth
factor-beta-induced alpha-smooth muscle cell actin expression in
renal proximal tubular cells is regulated by p38beta
mitogen-activated protein kinase, extracellular signal-regulated
protein kinase1,2 and the Smad signalling during
epithelial-myofibroblast transdifferentiation. Nephrol Dial
Transplant. 23:1537–1545. 2008. View Article : Google Scholar
|
|
7
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar :
|
|
8
|
Liu LK, Jiang XY, Zhou XX, Wang DM, Song
XL and Jiang HB: Upregulation of vimentin and aberrant expression
of E-cadherin/beta-catenin complex in oral squamous cell
carcinomas: Correlation with the clinicopathological features and
patient outcome. Mod Pathol. 23:213–224. 2010. View Article : Google Scholar
|
|
9
|
Wu WY, Yan H, Wang XB, Gui YZ, Gao F, Tang
XL, Qin YL, Su M, Chen T and Wang YP: Sodium tanshinone IIA silate
inhibits high glucose-induced vascular smooth muscle cell
proliferation and migration through activation of AMP-activated
protein kinase. PLoS one. 9:e949572014. View Article : Google Scholar :
|
|
10
|
Zhang HH, Chen YC, Liang L and Zeng Z:
Tanshinone IIA inhibits in vitro cellular proliferation and
migration of vascular smooth muscle cell of rabbit. Sichuan Da Xue
Xue Bao Yi Xue Ban. 39:188–192. 2008.(In Chinese).
|
|
11
|
Luo Y, Xu DQ, Dong HY, Zhang B, Liu Y, Niu
W, Dong MQ and Li ZC: Tanshinone IIA inhibits hypoxia-induced
pulmonary artery smooth muscle cell proliferation via
Akt/Skp2/p27-associated pathway. PLoS one. 8:e567742013. View Article : Google Scholar :
|
|
12
|
Li X, Du JR, Yu Y, Bai B and Zheng XY:
Tanshinone IIA inhibits smooth muscle proliferation and intimal
hyperplasia in the rat carotid balloon-injured model through
inhibition of MAPK signaling pathway. J Ethnopharmacol.
129:273–279. 2010. View Article : Google Scholar
|
|
13
|
Liu M, Yang J and Li M: Tanshinone IIA
attenuates interleukin-17A-induced systemic sclerosis
patient-derived dermal vascular smooth muscle cell activation via
inhibition of the extracellular signal-regulated kinase signaling
pathway. Clinics (Sao Paulo). 70:250–256. 2015. View Article : Google Scholar :
|
|
14
|
Zhan CY, Tang JH, Zhou DX and Li ZH:
Effects of tanshinone IIA on the transforming growth factor β1/Smad
signaling pathway in rat cardiac fibroblasts. Indian J Pharmacol.
46:633–638. 2014. View Article : Google Scholar :
|
|
15
|
Zhou D, Li Z, Zhang L and Zhan C:
Inhibitory effect of tanshinone IIA on TGF II-β1-induced cardiac
fibrosis. J Huazhong Univ Sci Technolog Med Sci. 32:829–833. 2012.
View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Galichon P and Hertig A: Epithelial to
mesenchymal transition as a biomarker in renal fibrosis: Are we
ready for the bedside? Fibrogenesis Tissue Repair. 4(11)2011.
|
|
18
|
Yu H, Shen Y, Hong J, Xia Q, Zhou F and
Liu X: The contribution of TGF-β in Epithelial-Mesenchymal
Transition (EMT): Down-regulation of E-cadherin via snail.
Neoplasma. 62:1–15. 2015. View Article : Google Scholar
|
|
19
|
He L, Lou W, Ji L, Liang W, Zhou M, Xu G,
Zhao L, Huang C, Li R, Wang H, et al: Serum response factor
accelerates the high glucose-induced Epithelial-to-Mesenchymal
Transition (EMT) via snail signaling in human peritoneal
mesothelial cells. PLoS one. 9:e1085932014. View Article : Google Scholar :
|
|
20
|
Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu
LM, Su YF, Kang LY and Zhang BL: The anti-inflammatory activities
of Tanshinone IIA, an active component of TCM, are mediated by
estrogen receptor activation and inhibition of iNOS. J Steroid
Biochem Mol Biol. 113:275–280. 2009. View Article : Google Scholar
|
|
21
|
Chen J, Shi DY, Liu SL and Zhong L:
Tanshinone IIA induces growth inhibition and apoptosis in gastric
cancer in vitro and in vivo. Oncol Rep. 27:523–528. 2012.
|
|
22
|
Li FL, Xu R, Zeng QC, Li X, Chen J, Wang
YF, Fan B, Geng L and Li B: Tanshinone IIA inhibits growth of
keratinocytes through cell cycle arrest and apoptosis: Underlying
treatment mechanism of psoriasis. Evid Based Complement Alternat
Med. 2012:9276582012.
|
|
23
|
Chan P, Liu JC, Lin LJ, Chen PY, Cheng TH,
Lin JG and Hong HJ: Tanshinone IIA inhibits angiotensin II-induced
cell proliferation in rat cardiac fibroblasts. Am J Chin Med.
39:381–394. 2011. View Article : Google Scholar
|
|
24
|
Hsieh PF, Liu SF, Lee TC, Huang JS, Yin
LT, Chang WT, Chuang LY, Guh JY, Hung MY and Yang YL: The role of
IL-7 in renal proximal tubule epithelial cells fibrosis. Mol
Immunol. 50:74–82. 2012. View Article : Google Scholar
|
|
25
|
Haneda M, Koya D, Isono M and Kikkawa R:
Overview of glucose signaling in mesangial cells in diabetic
nephropathy. J Am Soc Nephrol. 14:1374–1382. 2003. View Article : Google Scholar
|
|
26
|
Lan T, Liu W, Xie X, Huang K, Peng J,
Huang J, Shen X, Liu P, Yang H and Huang H: Berberine suppresses
high glucose-induced TGF-β1 and fibronectin synthesis in mesangial
cells through inhibition of sphingosine kinase 1/AP-1 pathway. Eur
J Pharmacol. 697:165–172. 2012. View Article : Google Scholar
|
|
27
|
Zhu LF, Hu Y, Yang CC, Xu XH, Ning TY,
Wang ZL, Ye JH and Liu LK: Snail overexpression induces an
epithelial to mesenchymal transition and cancer stem cell-like
properties in SCC9 cells. Lab Invest. 92:744–752. 2012. View Article : Google Scholar
|
|
28
|
Medici D, Hay ED and Olsen BR: Snail and
Slug promote epithelial-mesenchymal transition through
beta-catenin-T-cell factor-4-dependent expression of transforming
growth factor-beta3. Mol Biol Cell. 19:4875–4887. 2008. View Article : Google Scholar :
|