Introduction
Allergic asthma is a type of allergic disease that
is characterized by acute, reversible obstruction of the airway,
airway hyperresponsiveness (AHR) to bronchospasmogenic compounds,
airway inflammation and airway remodeling. It is established that
Thelper 2 cells (Th2) are critical for the induction of allergic
asthma manifestations by producing cytokines and inducing B-cell
class-switching to the isotope immunoglobulin (Ig)E. Neill et
al (1,2) identified a novel interleukin
(IL)-13-producing innate cell, termed nuocytes or group 2 innate
lymphocytes (ILC2s), which are located in fat-associated lymphoid
clusters. These cells were defined as lineage (Lin; lymphocyte,
macrophage, dendritic cell, basophil, eosinophil, mast cell and
natural killer cell)-negative and inducible T cell costimulatory
(ICOS) -positive and additionally express the IL-25 receptor
(IL-17RB) and the IL-33 receptor [T1/ST2)] (3). ILC2s are induced to secrete IL-5 and
IL-13; however, limited IL-4 secretion is induced by IL-25 and
IL-33 (2). In addition, ILC2s
provide an essential source of IL-13 for N. brasiliensis
worm expulsion (1). There is a
limited understanding of the role of ILC2s in allergic asthma
currently; however, IL-13 is essential for various aspects of the
allergic lung response, including AHR. Therefore, investigation
into ILC2s as a non-T-cell source of IL-13 is required. Notably,
the transcription factor RAR related orphan receptor α (RORα) is
critical for the development and functioning of ILC2s (4). ILC2s have an important role in the
process of Th2 response, and the polarization of ILC2s may
contribute to Th2-biased differentiation.
Regulatory T cells (Tregs) oppose effector T cell
activity, and suppress AHR and allergic inflammation following
stimulation with allergens in murine allergic asthma models. For
example, adoptive transfer of Tregs into allergen-sensitized mice
downregulates asthma manifestations (5) and, in contrast, depletion of these
cells exacerbates experimental asthma (6,7). The
triggering of glucocorticoid-induced tumor necrosis factor receptor
(GITR) by its ligand (GITRL) has been demonstrated to prevent the
suppressive function of Tregs, and to increase T cell proliferation
and cytokine production (8). GITR
is a type I transmembrane protein and a member of the tumor
necrosis factor receptor superfamily (9–11)
that has constitutively high expression on the cell surface of
natural Tregs (nTregs) (9,10). However, low expression levels of
GITR are observed on resting naïve cluster of differentiation
(CD)4+ T cells, which are upregulated following activation
(10–15). However, GITR expression is
additionally upregulated following the activation of CD25- effector
T cells (12,13). The ligand of GITR, GITRL, is
expressed on macrophages, dendritic cells and B cells; however, it
is not expressed on T cells (14–18).
GITR stimulation was previously reported to abolish the suppressive
properties of nTreg cells in vitro and in vivo
(12,17–21).
Thus, GITR is an important molecule due to its modulatory role on
regulatory and effector T cell functions. It has been reported that
GITR signaling potentiates murine AHR via enhancing Th2 activity
(11). As ILC2s contribute to
hyperreactivity in asthmatic mice, it was hypothesized that
GITR-GITRL may additionally influence the polarization of ILC2s. To
understand the relevance of GITR-GITRL signaling and ILC2s in a
murine asthma model, the present study detected the number of ILC2s
and GITR or GITRL-expressing cells, analyzed the levels of
cytokines associated with ILC2s in the lung tissue and investigated
the effect of GITR/GITRL expression levels on ILC2s in asthmatic
mice.
Materials and methods
Animals
A total of 96 specific pathogen-free male BALB/c
mice (age, 6–8 weeks; weight, 18±2 g) were purchased from the
Laboratory Animal Center of Yangzhou University (Yangzhou, China)
and housed in plastic cages in a laminar flow cabinet with
sterilized wood-chip bedding. The room temperature was maintained
at 23±2°C and a relative humidity of 55±10% with a 12-h light-dark
cycle. All mice were provided with free access to food and water.
All animal experiments were approved by the Animal Care and Ethics
Committee of Jiangsu University (Jiangsu, China).
Asthma model induction and lung tissue
specimen treatment
As described by Wang et al (22), 50 mice were sensitized
intraperitoneally on days 1 and 11 with 50 µg ovalbumin (OVA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 2 mg aluminum
hydroxide gel in 0.1 ml PBS. Sensitized mice were subsequently
exposed to OVA (10 mg/ml in PBS) inhalation challenges for 30 min
every day between days 22 and 26. Control animals received PBS. The
mice were assessed for allergic inflammation of the lungs 24 h
after the final lung challenge, the peripheral blood and lung
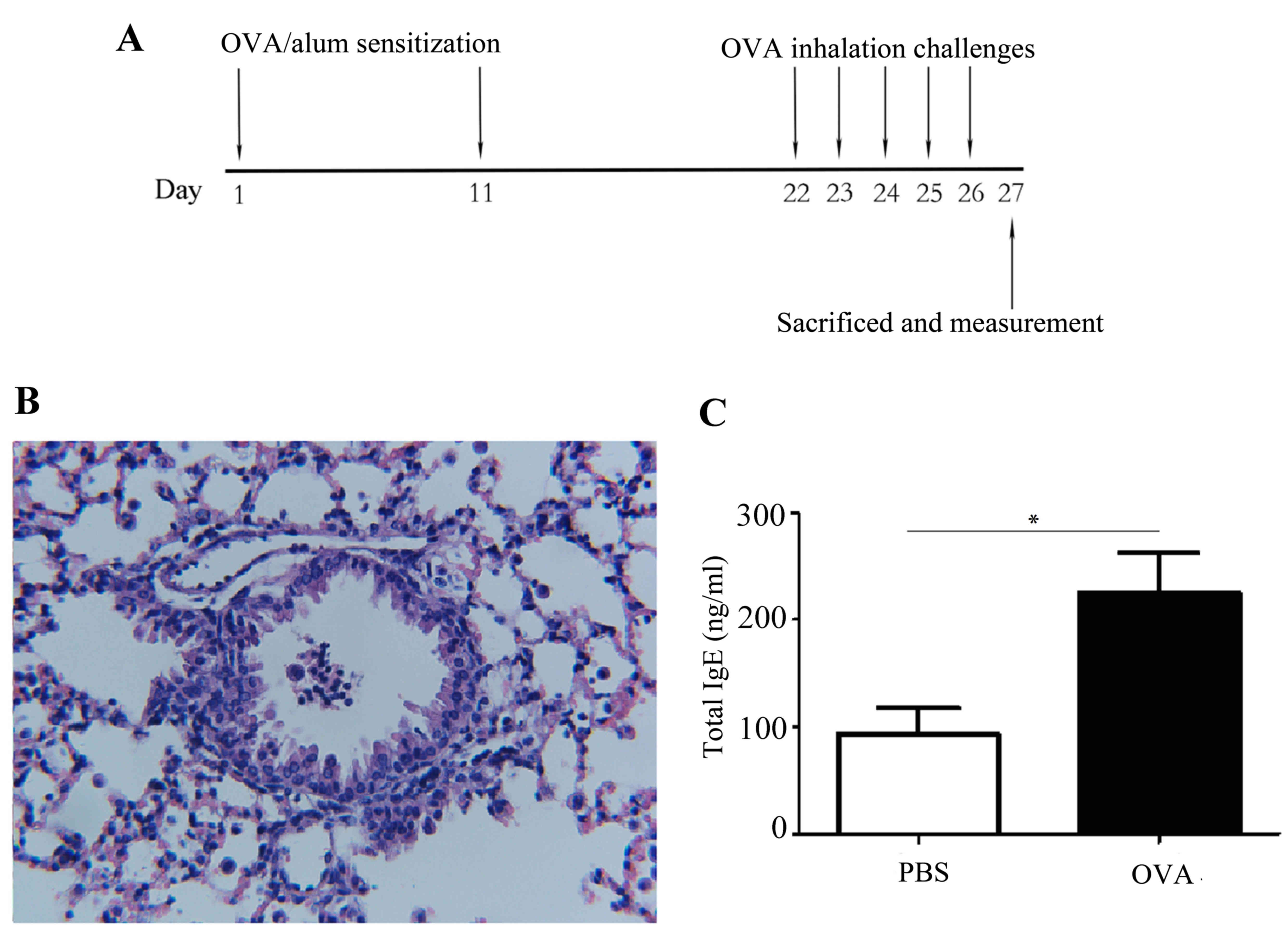
tissue specimens were collected (Fig.
1A). Asthmatic mice were confirmed by the presence of lung
inflammation with HE staining (Fig.
1B) and increased IgE in serum, measured by ELISA (Mouse IgE
ELISA kit; AKRIE-010; Shibayagi, Shibukawa, Japan), according to
the manufacturer's protocol (Fig.
1C). Lung tissue was initially perfused with PBS and
mechanically crushed, followed by digestion at 37°C for 2 h in RPMI
medium with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and collagenase I (0.25%), to
acquire a single cell suspension. The cell suspension was filtrated
through 40 mm filters for flow cytometry analysis.
Flow cytometry analysis
Single-cell suspensions (2×106 cells)
from lung tissues were incubated with fluorescein
isothiocyanate-labeled Lin (22–7770-72; 1:100; eBioscience, Inc.,
San Diego, CA, USA), PerCP/Cy5.5-labeled ICOS (107705; 1:100;
BioLegend, Inc., San Diego, CA, USA) and PE-labeled T1/ST2 (145303;
1:100; BioLegend, Inc.) antibodies at 4°Cfor 30 min, and fixed with
4% paraformaldehyde for flow cytometry analysis. The FITC-IgG1
(401913; 1:200, BioLegend, Inc.), PerCP/Cy5.5-IgG1 (402027, 1:200,
BioLegend, Inc.) and PE-IgG1 (12–4301, 1:200; eBioscience, Inc.)
served as the isotype controls. Stained cells were analyzed with an
Accuri™ C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA)
using FlowJo software 7.6 (TreeStar, Inc., Ashland, OR, USA). ILC2s
were identified as Lin-ICOS+ST2+. GITR+ cells were characterized
with anallophycocyanin-labeled GITR antibody (109101; 1:200;
eBioscience, Inc.) and GITRL+ cells were stained with a
phycoerythrin (PE)-GITRL antibody (120305; 1:200; BioLegend, Inc.).
Mouse spleen cells for flow cytometry analysis were obtained as
previously described (22).
Hematoxylin and eosin (H&E) and
immunofluorescence staining
Freshly obtained lung tissue of asthmatic mice was
fixed in 4% paraformaldehyde for 24 h and embedded in paraffin.
Paraffin-embedded lungs were sectioned (4–6 µm) and stained with
H&E for morphometric analysis. GITRs in the lungs were
identified by immunofluorescence staining. As described previously
(23), sections for
immunofluorescence staining were heated for 3 h in a 37°C incubator
and dewaxed followed by antigen unmasking. Sections were
subsequently blocked with 1% (weight/volume) bovine serum albumin
(Sigma-Aldrich; Merck KGaA) at 37°C for 30 min, stained with a
primary antibody against mouse GITR (14–5874-80; 1:200;
eBioscience, Inc.) and visualized with a goat-anti-rat
PE-conjugated secondary antibody (sc-3738; 1:200, Santa Cruz
Biotechnology, Inc., Dallas, Texas, USA). Finally, slides were
stained in Hoechst 33342 for 10 min. All sections were coverslipped
with neutral resin medium, visualized under an Olympus fluorescence
microscope and analyzed using ImageJ software v2.1.4.7 (https://imagej.nih.gov/ij/).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from frozen tissues or fresh
lung cells using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) and 500 ng total RNA was reverse transcribed
using a PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's protocol. Based on GenBank
sequences, the primers used in this study were designed by Primer
Premier 5.0 software (Premier Biosoft International, Palo Alto, CA,
USA) and synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.). Primer sequences are presented in Table I. Gene-specific PCR products were
amplified with SYBR® Premix Ex Taq (Takara Biotechnology
Co., Ltd., Dalian, China) according to the manufacturer's protocol.
Briefly, 95°C pre-denaturation for 3 min (1 cycle); 95°C
denaturation for 10 sec, 60°C annealing for 30 sec, 72°C extension
for 1 min (30 cycles). The levels of target gene expression were
normalized to β-actin expression using the 2−∆∆Cq method
(24). All samples were performed
in triplicate.
 | Table I.Sequence and length of polymerase
chain reaction primers. |
Table I.
Sequence and length of polymerase
chain reaction primers.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Length, bp |
|---|
| GITR |
CAAGCCAGACGCTACAAGAC |
CCGCTCTCATACACCCACTT | 125 |
| GITRL |
CTACGGCCAAGTGATTCCTGT |
GATGATCCCCCAGTATGTGTT | 220 |
| ICOS |
ACTTGCAGGTGTGACCTCAT |
GGCCAGTGCATAGCTAGAAT | 336 |
| T1/ST2 |
TGGACAGCACCTGTTCAGT |
CAGGACATCAGCCAAGAAGT | 327 |
| RORα |
CTGACGAGGACAGGAGTAGG |
AGCCGAGGTATCTCAGTCAC | 259 |
| β-actin |
TGGAATCCTGTGGCATTCATGAAAC |
TAAAACGCAGCTCAGTAACAGTCCG | 349 |
| IL-5 |
AGGATGCTTCTGCACTTGAG |
CCTCATCGTCTCATTGCTTG | 144 |
| IL-13 |
TGAGCAACATCACACAAGACC |
AGGCCATGCAATATCCTCTG | 157 |
Murine (m) GITRL protein and GITRL
treatment
The murine GITRL fusion protein and control protein
were expressed in E. coli BL-21 with mGITRL-Pet-32a and
eGFP-Pet-32a as previously described (25,26),
respectively. Expression was performed at 30°C, and induced with 1
mmol/lisopropyl β-D-1-thiogalacto-pyranoside (Sigma-Aldrich; Merck
KGaA) for 6 h, fusion proteins were purified by an immobilized
affinity chromatography nickel column (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The endotoxin was removed using a
ToxinEraser™ Endotoxin Removal kit (Genscript,
Piscataway, NJ, USA). A total of 1–5 days prior to the first OVA
challenge, the asthmatic mice were treated intravenously with 20 µg
purified recombinant mGITRL protein and control protein per mice
per day.
Enzyme-linked immunosorbent assay
(ELISA) for plasma cytokines
Plasma IL-5 and IL-13 were measured by ELISA,
according to the manufacturer's protocols (IL-5, LS-F262;
eBioscience, Inc; and IL-13, EM016; Shanghai ExCell Biology,
Shanghai, China). All samples were measured in triplicate using a
microplate reader, and the mean concentration was calculated from
the standard curve.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism Version 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). Data are presented as the mean ± standard
deviation. Comparisons between two groups were performed using
independent-samples t-test. Pearson's correlation was used to
detect the correlation between two continuous variables. P<0.05
was considered to indicate a statistically significant
difference.
Results
Enhanced GITR/GITRL expression in
asthmatic mice
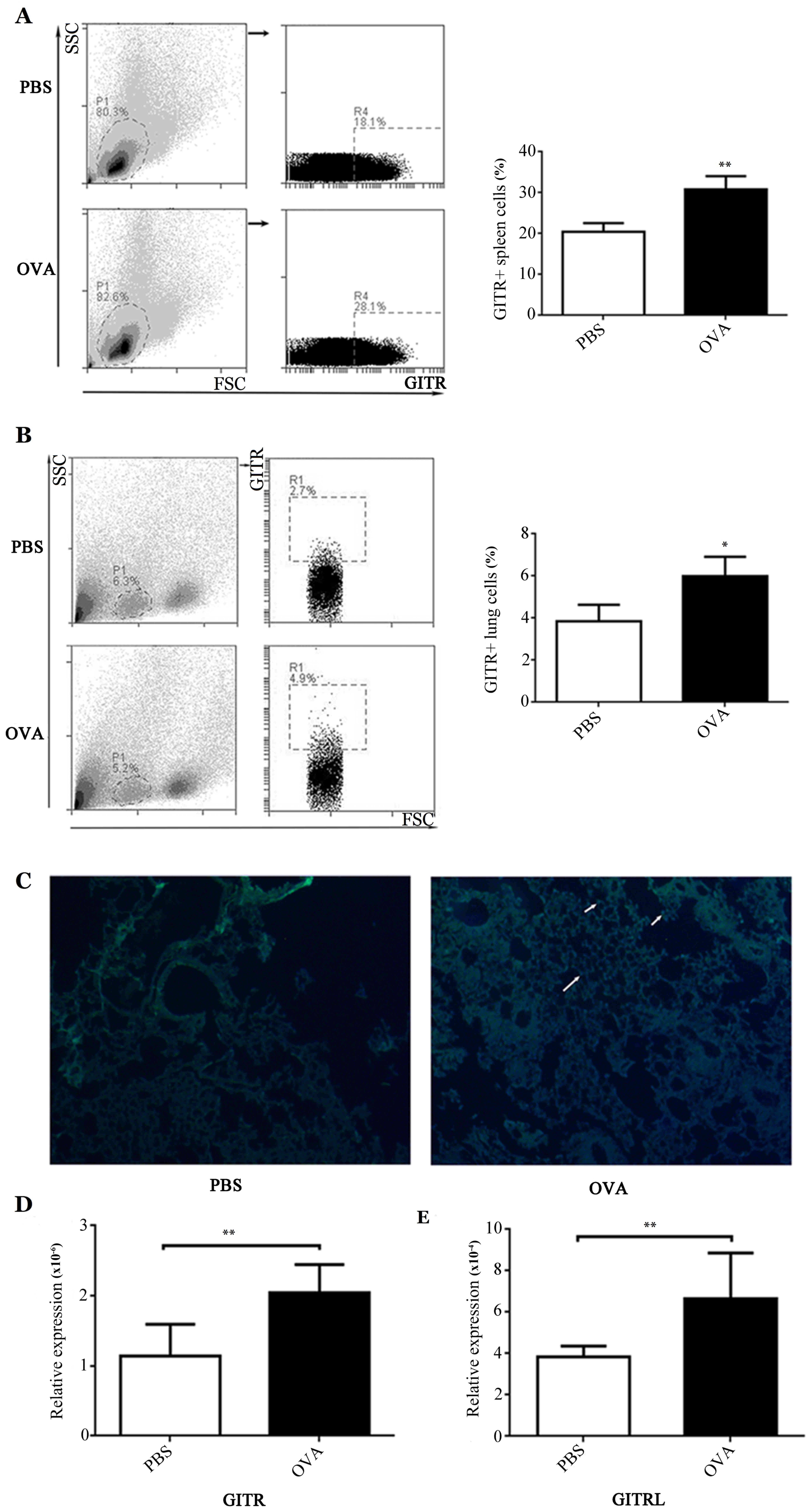
Flow cytometry analysis demonstrated that the
percentage of GITR-positive cells in the spleen (Fig. 2A) and lung tissue (Fig. 2B) were significantly increased in
asthmatic mice. Immunofluorescence staining indicated that
increased GITR-positive cells were present in inflammatory lung
tissue (Fig. 2C). Additionally,
GITR mRNA expression in lung tissues of asthmatic mice was
determined by RT-qPCR, and was demonstrated to be increased 2-fold
compared with control mice (Fig.
2D). GITR and its ligand, GITRL, have been previously
demonstrated to regulate the reactivity of various different cell
types and to influence a variety of immunological conditions
(11). Therefore, the present
study detected the expression level of GITRL in the lung tissue of
asthmatic mice and observed that its expression was additionally
increased compared with control mice (Fig. 2E). The results indicated that
GITR/GITRL signaling may be enhanced in asthmatic mice.
Increased ILC2s and ILC2-associated
factors in the lung tissue of asthmatic mice
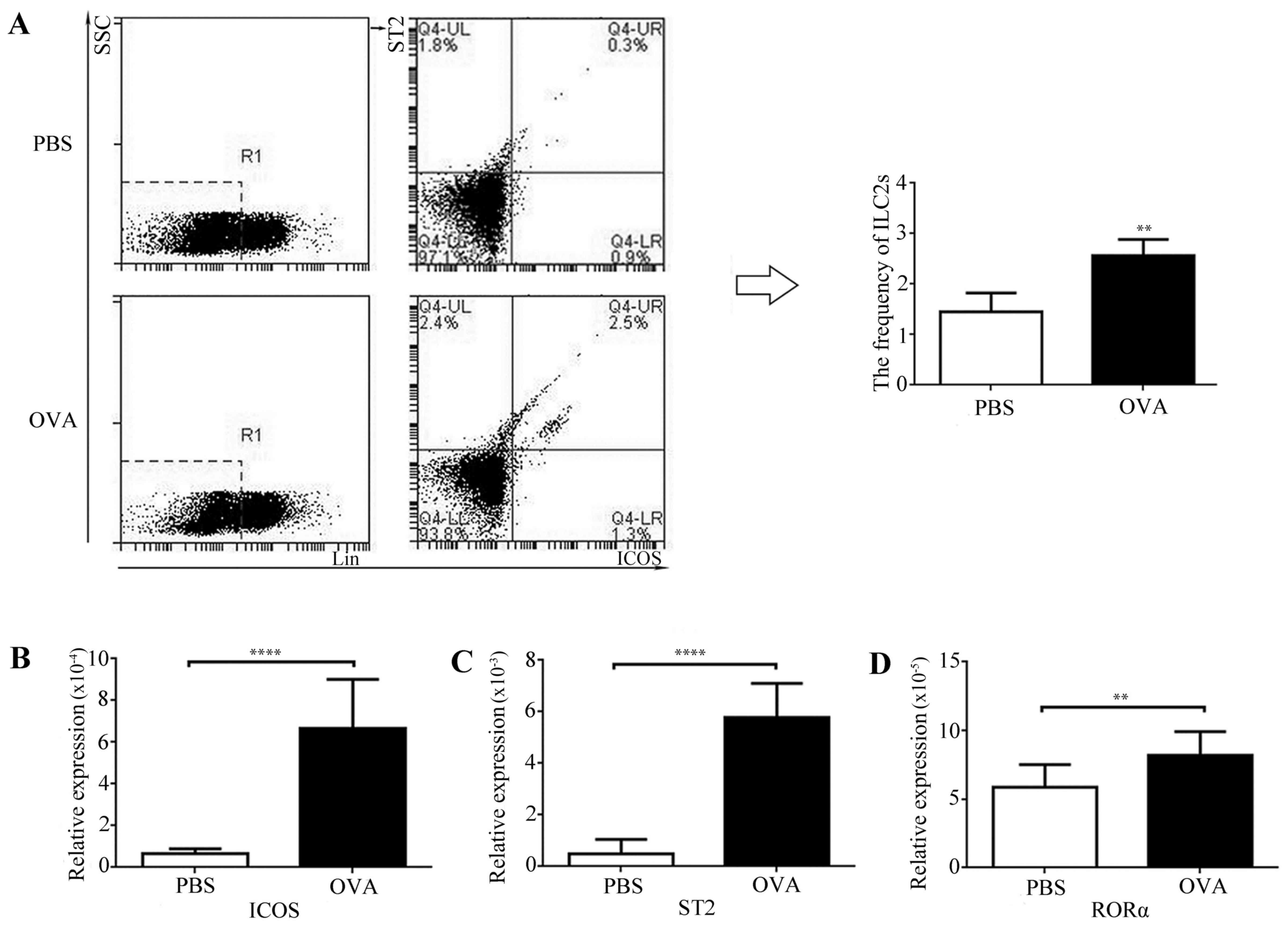
ILC2s were identified as Lin-ICOS+ST2+. The
frequency of ILC2s in lung tissue from asthmatic mice was
significantly increased compared with controls (Fig. 3A). RORα has been previously
demonstrated to have a crucial function in ILC2 development and
function, and ST2 is a surface marker that is expressed by and is
relatively specific to ILC2s. The present study additionally
detected the expression levels of RORα, ICOS and ST2 mRNA by using
RT-qPCR. mRNA expression levels of ICOS (Fig. 3B), ST2 (Fig. 3C) and RORα (Fig. 3D) were significantly increased in
the lung tissues of asthmatic mice compared with control mice. The
results indicated that ILC2s and ILC2-associated molecules were
upregulated in the lung tissue of asthmatic mice.
Enhanced expression of ILC2
function-associated cytokines in asthmatic mice
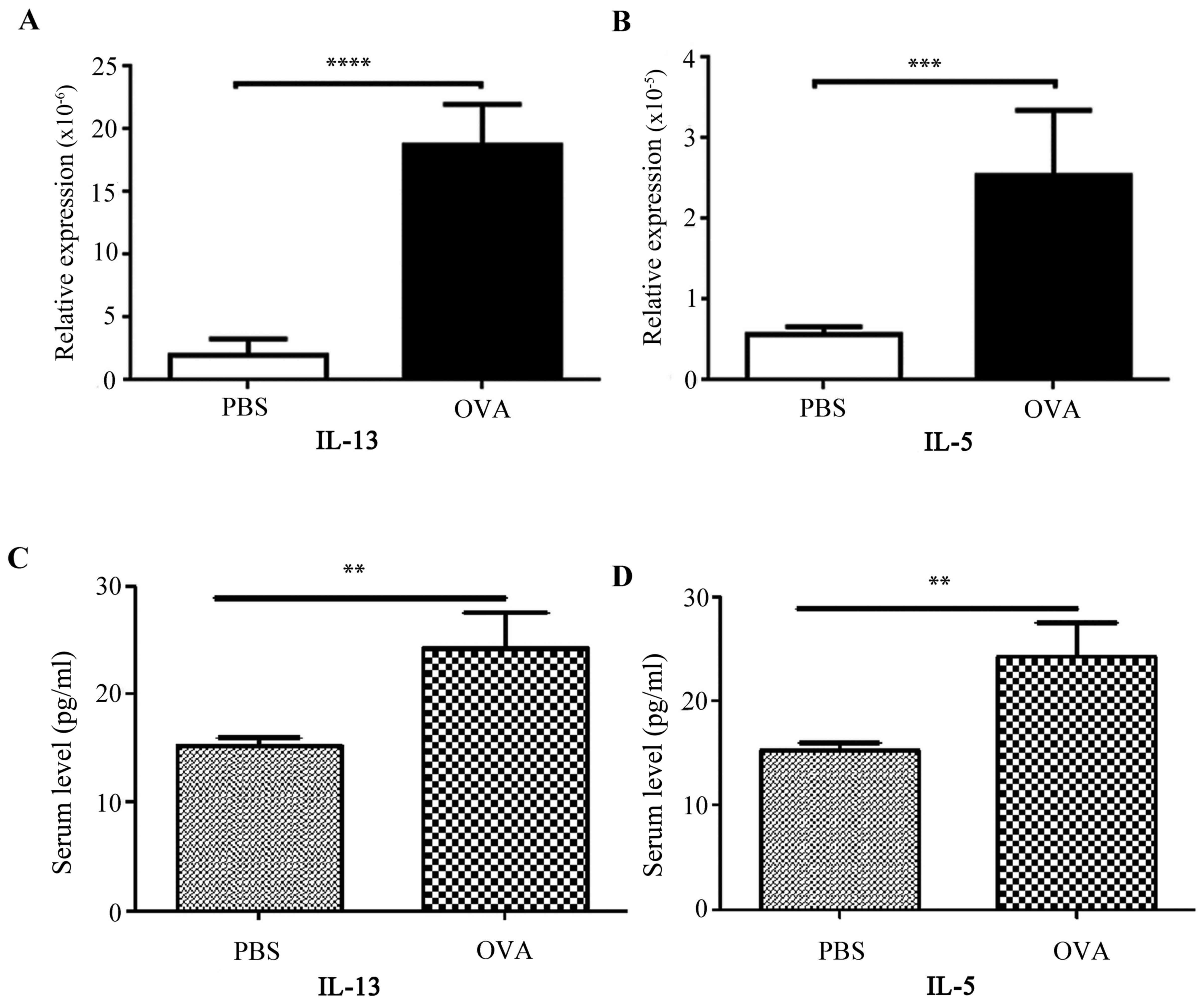
It has previously been demonstrated that IL-25 and
IL-33-responsive ILC2s infiltrate the lung from peripheral blood
and become a primary innate source of IL-13, and additionally
produce large amounts of IL-5 (27). The present study compared gene
expression levels in lung tissues from the asthmatic model and
control mice by RT-qPCR, and demonstrated that the mRNA expression
levels of IL-13 (Fig. 4A) and IL-5
(Fig. 4B) were significantly
increased in asthmatic mice, which was consistent with the results
of serum testing (Fig. 4C and D,
respectively).
Positive correlation between mRNA
levels of GITR/GITRL and ILC2-associated molecules
GITR/GITRL has an important role in regulating
immune polarization conditions. To understand the association
between GITR and ILC2-associated molecules and cytokines in
asthmatic mice, the present study analyzed the correlation between
mRNA expression levels of GITR/GITRL and RORα, T1/ST2, IL-13, and
IL-5. The results indicated that there was a significant, positive
correlation between GITR and ILC2-associated molecules or signature
cytokines in asthmatic mice (Fig.
5).
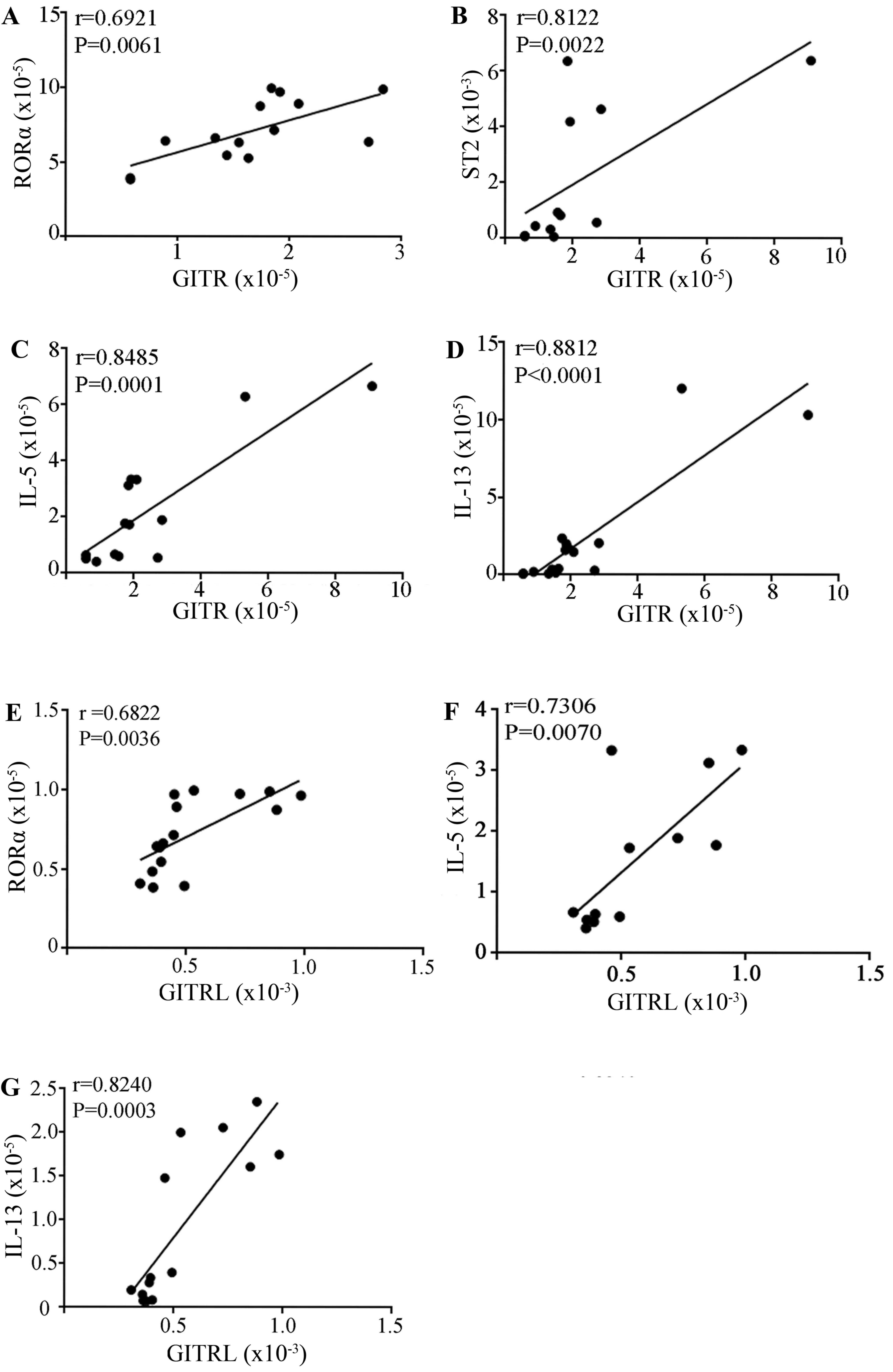 | Figure 5.Correlation analysis of the expression
of GITR/GITRL and ILC2-associated molecules. Correlation between
the expression levels of GITR and (A) RORα, (B) ST2, (C) IL-5 and
(D) IL-13. Correlation between the expression levels of GITRL and
(E) RORα, (F) IL-5, (G) IL-13 expression in lung tissue from
asthmatic mice. GITR, glucocorticoid-induced tumor necrosis factor
receptor; GITRL, GITR ligand; ILC2s, group 2 innate lymphocytes;
RORα, RAR related orphan receptor 2; IL, interleukin; ST2, IL-33
receptor; ICOS, inducible T cell costimulator. |
Expression of GITR on lung ILC2s
As presented in Fig.
3, ILC2s were located in the native lung tissue of mice and
were induced by intranasal OVA administration. Once lung cells from
PBS-and OVA-treated mice had been obtained, lung ILC2s were
identified as Lin-ICOS+ST2+ cells and the expression of GITR and
GITRL expression on resting and activated lung ILC2s was
investigated by flow cytometry. As presented in Fig. 6A, different expression levels of
GITR amongst control, PBS-treated and OVA-treated groups was
observed; however, GITRL expression levels were more consistent in
the three groups.
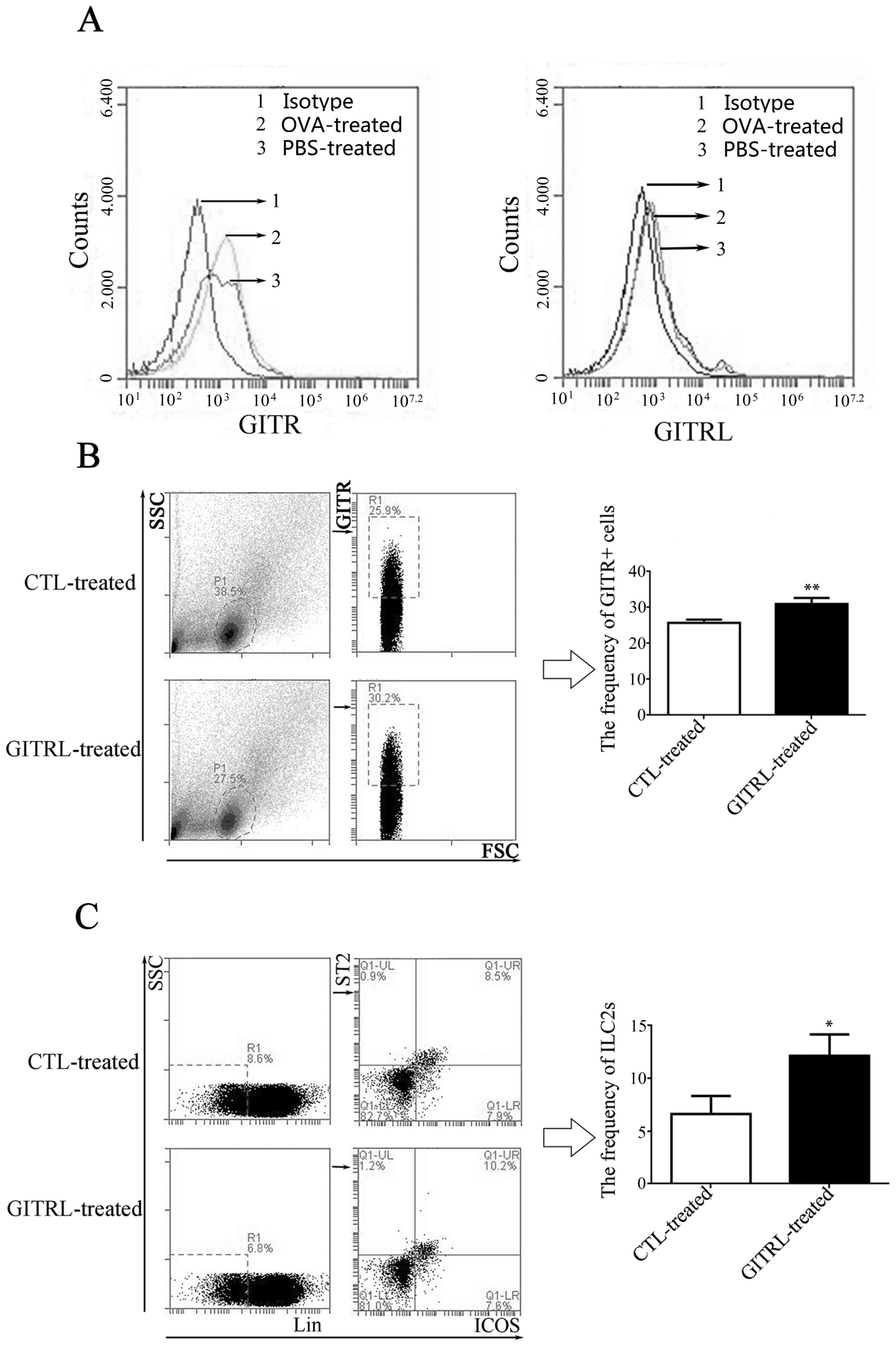 | Figure 6.Frequency of GITR+ cells and ILC2s in
lung tissue from GITRL-treated asthmatic mice. (A) GITR expression
in lung tissue from OVA-treated mice. Left, frequency of
GITR-positive cells in lung tissue from asthmatic or control mice;
1, isotype control; 2, OVA-treated mice; 3, PBS-treated mice.
Increased frequency of GITR-positive cells was observed in lung
tissue from asthmatic mice. Right, frequency of GITRL-positive
cells in lung tissue from asthmatic or control mice; 1, isotype
control; 2, OVA-treated mice; 3, PBS-treated mice. There was no
difference between the 3 groups. Flow cytometry analysis indicated
that the percentage of (B) GITR+ cells and (C) ILC2s
(ST2+ICOS+ cells) was increases in lung
tissue from GITRL-treated asthmatic mice compared with CTL
protein-treated asthmatic mice. *P<0.05 and **P<0.01. GITR,
glucocorticoid-induced tumor necrosis factor receptor; ILC2s, group
2 innate lymphocytes; GITRL, GITR ligand; OVA, ovalbumin; CTL,
control; SSC, side scatter; FSC, forward scatter; Lin, lineage
antibody; ICOS, inducible T cell costimulator; ST2, interleukin-33
receptor. |
GITRL treatment increases the number
of lung ILC2s
To determine the effect of GITRL on AHR, sensitized
mice were treated intravenously with 20 µg GITRL or control protein
prior to the first OVA challenge, and lung ILC2s were subsequently
analyzed by flow cytometry. Compared with the controls, GITRL
administration induced a significant increase in the frequency of
GITR+ cells (Fig. 6B) and ILC2s
(Fig. 6C) in lung tissue.
Discussion
The present study, to the best of our knowledge,
demonstrates for the first time that GITR- and GITRL-positive cells
were simultaneously increased in the lung tissue of asthmatic mice,
which are associated with ILC2 activation and contribute to immune
imbalance, and participate in pathological mechanisms involved in
asthma. Previous studies (28,29)
have demonstrated that GITR is primarily expressed by naive and
activated T cells. In addition, CD4+ CD25+forkhead box P3+ Treg
cells have been demonstrated to express high levels of GITR. GITR
exerts a differential effect on Th cell proliferation and cytokine
release by fully differentiated Th1 and Th2 cells in vitro;
GITR increases Th2 and cytokine production; however, it does not
have this effect on Th1. A similar effect on Th2 effector functions
was observed in vivo in a mouse model of asthma; increased
AHR, serum IgE response and cytokine production by Th2 cells was
observed (11). Activation of
CD4+CD25+ Tregs by the agonistic anti-GITR monoclonal antibody
decreased Treg numbers (30), and
reversed the suppressive activity of Treg. Previous research has
demonstrated that GITRL has a wider range of tissue and cell
distribution; it is not only expressed on macrophages, dendritic
cells and B cells, but is additionally expressed on endothelial
cells and certain stromal cells (31,32).
It has become clear that the functional interaction of GITRL with
GITR drives T-cell activation via either co-stimulation of CD4+
effector T cells or inhibition of the suppressive function of
Tregs.
It has been previously demonstrated that the
inhibition of Treg function was due to the upregulation of
GITR/GITRL expression (32).
Notably, the present study observed that GITR was additionally
expressed on ILC2s, and the expression level was significantly
increased in asthmatic mice, which indicates that GITRL may have a
wider range of tissue and cell distribution than previously
observed. ILC2s have an important role in acute allergic lung
inflammation, including asthma and allergic diarrhoea. Given their
distribution and function, lung ILC2s are hypothesized to
coordinate epithelial responses to the external environment; the
activity of ILC2s is additionally associated with interactions with
many other immune cells. Enhanced GITR expression on ILC2s was
observed in the lung tissue from asthmatic mice in the present
study, which led to consideration of the potential outcome of
GITR-GITRL interaction. On the one hand, the function of Tregs
maybe inhibited by increased GITRL in lung tissue; however, it may
additionally enhance the activity of ILC2s. Allergy is a major
health problem associated with the industrialized world and
overproduction of Th2-type cytokines, which include certain ILs
(IL-4, 5 and 9) and IgE, are involved in the type 2 immune response
that is responsible for the majority of allergen-induced
inflammation at mucosal surfaces (33). There is currently limited
understanding of the underlying mechanisms that may be responsible
for allergen-induced initiation of Th0 to Th2 differentiation
during the sensitization phase. However, it is accepted that the
cytokine environment has a role in dictating the differentiation of
Th0 cells into different Th cell populations. Specifically, IL-4
and the transcription factor GATA binding protein 3are considered
essential for Th2 cell differentiation and the production of type-2
cytokines. However, the initial source of IL-4 is not established
as multiple cell populations, including natural killer T cells and
dendritic cells amongst others, may contribute. Furthermore, Th2
cell differentiation has been induced in vitro without the
presence of exogenous IL-4, which indicates that a Th2 cell
differentiation signaling pathway that is independent of IL-4-may
exist. Halim et al (34)
indicated that ILC2s induce Th2 cell differentiation, which was
deduced based on increased type 2 cytokine production, increased
IgE titers and the presence of eosinophilic lung inflammation,
which was partially confirmed by our previous studies (35). The earliest studies on asthma
pathology demonstrated that CD4+ T lymphocytes were present in
asthma biopsies. To identify the ILC2 activity in lung tissue from
asthmatic mice, the present study detected the mRNA expression
levels of ILC2-associated transcription factors and cytokines. As
expected, RORα, ICSO, IL-5 and IL-13 were significantly increased
in asthmatic mice compared with control mice, which was associated
with upregulated expression of GITR/GITRL, and a positive
correlation was observed between GITR/GITRL expression and the
expression of ILC2-associated factors. The administration of
recombinant mGITRL protein induced an increase in the numbers of
lung ILC2s. The results indicated that GITR-GITRL signaling may
contribute to ILC2 polarization and its cytokine production in a
direct or indirect way. The mechanism behind this requires further
investigation.
In conclusion, to the best of our knowledge, the
results of the present study demonstrated for the first time that
in asthmatic mice, the number of ILC2s and GITR+/GITRL+ cells were
simultaneously increased, and increased GITR expression on ILC2s
was observed. There was a significant, positive correlation between
GITR-GITRL expression levels and the expression of ILC2-associated
molecules. Furthermore, GITRL treatment increased the number of
ILC2s and/or GITR+ cells in lung tissue. These results indicated
that the activity of ILC2s maybe enhanced by the interaction of
GITRL and GITR, which may subsequently contribute to the
pathogenesis of asthma. These findings present potential
therapeutic targets for the treatment of asthma.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 31270947, 31170849
and 81370084) and the Postdoctoral Foundation of China (grant nos.
2014T70490 and 2013T60508).
References
|
1
|
Neill DR and McKenzie AN: Nuocytes and
beyond: New insights into helminth expulsion. Trends Parasitol.
27:214–221. 2011. View Article : Google Scholar
|
|
2
|
Neill DR, Wong SH, Bellosi A, Flynn RJ,
Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al:
Nuocytes represent a new innate effector leukocyte that mediates
type-2 immunity. Nature. 464:1367–1370. 2010. View Article : Google Scholar :
|
|
3
|
Maazi H, Patel N, Sankaranarayanan I,
Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH and Akbari O:
ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid
cell function, homeostasis, and induction of airway
hyperreactivity. Immunity. 42:538–551. 2015. View Article : Google Scholar :
|
|
4
|
Wong SH, Walker JA, Jolin HE, Drynan LF,
Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al:
Transcription factor RORα is critical for nuocyte development. Nat
Immunol. 13:229–236. 2012. View
Article : Google Scholar :
|
|
5
|
Kearley J, Barker JE, Robinson DS and
Lloyd CM: Resolution of airway inflammation and hyperreactivity
after in vivo transfer of CD4+CD25+ regulatory T cells is
interleukin 10 dependent. J Exp Med. 202:1539–1547. 2005.
View Article : Google Scholar :
|
|
6
|
Jaffar Z, Sivakuru T and Roberts K:
CD4+CD25+ T cells regulate airway eosinophilic inflammation by
modulating the Th2 cell phenotype. J Immunol. 172:3842–3849. 2004.
View Article : Google Scholar
|
|
7
|
Lewkowich IP, Herman NS, Schleifer KW,
Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Köhl J, Belkaid
Y and Wills-Karp M: CD4+CD25+ T cells protect against
experimentally induced asthma and alter pulmonary dendritic cell
phenotype and function. J Exp Med. 202:1549–1561. 2005. View Article : Google Scholar :
|
|
8
|
Placke T, Kopp HG and Salih HR:
Glucocorticoid-induced TNFR-related (GITR) protein and its ligand
in antitumor immunity: Functional role and therapeutic modulation.
Clin Dev Immunol. 2010:2390832010. View Article : Google Scholar :
|
|
9
|
Joetham A, Ohnishi H, Okamoto M, Takeda K,
Schedel M, Domenico J, Dakhama A and Gelfand EW: Loss of T
regulatory cell suppression following signaling through
glucocorticoid-induced tumor necrosis receptor (GITR) is dependent
on c-Jun N-terminal kinase activation. J Biol Chem.
287:17100–17108. 2012. View Article : Google Scholar :
|
|
10
|
Lommatzsch M, Julius P, Kuepper M, Garn H,
Bratke K, Irmscher S, Luttmann W, Renz H, Braun A and Virchow JC:
The course of allergen-induced leukocyte infiltration in human and
experimental asthma. J Allergy Clin Immunol. 118:91–97. 2006.
View Article : Google Scholar
|
|
11
|
Motta AC, Vissers JL, Gras R, VanEsch BC,
Van Oosterhout AJ and Nawijn MC: GITR signaling potentiates airway
hyperresponsiveness by enhancing Th2 cell activity in a mouse model
of asthma. Respir Res. 10:932009. View Article : Google Scholar :
|
|
12
|
Shimizu J, Yamazaki S, Takahashi T, Ishida
Y and Sakaguchi S: Stimulation of CD25(+)CD4(+) regulatory T cells
through GITR breaks immunological self-tolerance. Nat Immunol.
3:135–142. 2002. View
Article : Google Scholar
|
|
13
|
McHugh RS, Whitters MJ, Piccirillo CA,
Young DA, Shevach EM, Collins M and Byrne MC: CD4(+)CD25(+)
immunoregulatory T cells: Gene expression analysis reveals a
functional role for the glucocorticoid-induced TNF receptor.
Immunity. 16:311–323. 2002. View Article : Google Scholar
|
|
14
|
Tone M, Tone Y, Adams E, Yates SF, Frewin
MR, Cobbold SP and Waldmann H: Mouse glucocorticoid-induced tumor
necrosis factor receptor ligand is costimulatory for T cells. Proc
Natl Acad Sci USA. 100:pp. 15059–15064. 2003; View Article : Google Scholar :
|
|
15
|
Yu KY, Kim HS, Song SY, Min SS, Jeong JJ
and Youn BS: Identification of a ligand for glucocorticoid-induced
tumor necrosis factor receptor constitutively expressed in
dendritic cells. Biochem Biophys Res Commun. 310:433–438. 2003.
View Article : Google Scholar
|
|
16
|
Kim JD, Choi BK, Bae JS, Lee UH, Han IS,
Lee HW, Youn BS, Vinay DS and Kwon BS: Cloning and characterization
of GITR ligand. Genes Immun. 4:564–569. 2003. View Article : Google Scholar
|
|
17
|
Ronchetti S, Ricci E, Petrillo MG, Cari L,
Migliorati G, Nocentini G and Riccardi C: Glucocorticoid-induced
tumour necrosis factor receptor-related protein: A key marker of
functional regulatory T cells. J Immunol Res. 2015:1715202015.
View Article : Google Scholar :
|
|
18
|
Kanamaru F, Youngnak P, Hashiguchi M,
Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I and Azuma M:
Costimulation via glucocorticoid-induced TNF receptor in both
conventional and CD25+ regulatory CD4+ T cells. J Immunol.
172:7306–7314. 2004. View Article : Google Scholar
|
|
19
|
Kohm AP, Williams JS and Miller SD:
Cutting edge: Ligation of the glucocorticoid-induced TNF receptor
enhances autoreactive CD4+ T cell activation and experimental
autoimmune encephalomyelitis. J Immunol. 172:4686–4690. 2004.
View Article : Google Scholar
|
|
20
|
Stephens GL, McHugh RS, Whitters MJ, Young
DA, Luxenberg D, Carreno BM, Collins M and Shevach EM: Engagement
of glucocorticoid-induced TNFR family-related receptor on effector
T cells by its ligand mediates resistance to suppression by
CD4+CD25+ T cells. J Immunol. 173:5008–5020. 2004. View Article : Google Scholar
|
|
21
|
Zhan Y, Funda DP, Every AL, Fundova P,
Purton JF, Liddicoat DR, Cole TJ, Godfrey DI, Brady JL, Mannering
SI, et al: TCR-mediated activation promotes GITR upregulation in T
cells and resistance to glucocorticoid-induced death. Int Immunol.
16:1315–1321. 2004. View Article : Google Scholar
|
|
22
|
Wang SY, Yang M, Xu XP, Qiu GF, Ma J, Wang
SJ, Huang XX and Xu HX: Intranasal delivery of T-bet modulates the
profile of helper T cell immune responses in experimental asthma. J
Investig Allergol Clin Immunol. 18:357–365. 2008.
|
|
23
|
He Z, Shotorbani SS, Jiao Z, Su Z, Tong J,
Liu Y, Shen P, Ma J, Gao J, Wang T, et al: HMGB1 promotes the
differentiation of Th17 via up-regulating TLR2 and IL-23 of CD14+
monocytes from patients with rheumatoid arthritis. Scand J Immunol.
76:483–490. 2012. View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Zhengjun H, Shengjun W, Yanping Z, Junfeng
B, Jia T, Jie M, Jun C, Changgui S and Huaxi X: Construction of
eukaryotic expression plasmid containing mGITRL gene and its
expression in Lewis cell. J Jiangsu Univ (Medicine Edition).
18:107–110. 2008.
|
|
26
|
Shengjun W, Bin M, Jia T, Huaxi H and
Shengli Y: Cloning and sequence analysis of mouse
glucocorticoid-induced tumor necrosis factor receptor ligand gene.
J Jiangsu Univ (Medicine Edition). 14:97–99. 2004.
|
|
27
|
Martinez-Gonzalez I, Steer CA and Takei F:
Lung ILC2s link innate and adaptive responses in allergic
inflammation. Trends Immunol. 36:189–195. 2015. View Article : Google Scholar
|
|
28
|
Wenzel SE: Asthma: Defining of the
persistent adult phenotypes. Lancet. 368:804–813. 2006. View Article : Google Scholar
|
|
29
|
Ronchetti S, Zollo O, Bruscoli S, Agostini
M, Bianchini R, Nocentini G, Ayroldi E and Riccardi C: GITR, a
member of the TNF receptor superfamily, is costimulatory to mouse T
lymphocyte subpopulations. Eur J Immunol. 34:613–622. 2004.
View Article : Google Scholar
|
|
30
|
Wang S, Shi Y, Yang M, Ma J, Tian J, Chen
J, Mao C, Jiao Z, Ko KH, Baidoo SE, et al: Glucocorticoid-induced
tumor necrosis factor receptor family-related protein exacerbates
collagen-induced arthritis by enhancing the expansion of Th17
cells. Am J Pathol. 180:1059–1067. 2012. View Article : Google Scholar
|
|
31
|
Olsen PC, Kitoko JZ, Ferreira TP,
de-Azevedo CT, Arantes AC and Martins ΜA: Glucocorticoids decrease
Treg cell numbers in lungs of allergic mice. Eur J Pharmacol.
747:52–58. 2015. View Article : Google Scholar
|
|
32
|
Nocentini G and Riccardi C: GITR: A
modulator of immuneresponse and inflammation. Adv Exp Med Biol.
647:156–173. 2009. View Article : Google Scholar
|
|
33
|
Oczypok EA, Milutinovic PS, Alcorn JF,
Khare A, Crum LT, Manni ML, Epperly MW, Pawluk AM, Ray A and Oury
TD: Pulmonary receptor for advanced glycation end-products promotes
asthma pathogenesis through IL-33 and accumulation of group 2
innate lymphoid cells. J Allergy Clin Immunol. 136:747–756. 2015.
View Article : Google Scholar :
|
|
34
|
Halim TY, Steer CA, Mathä L, Gold MJ,
Martinez-Gonzalez I, McNagny KM, McKenzie AN and Takei F: Group 2
innate lymphoid cells are critical for the initiation of adaptive T
helper 2 cell-mediated allergic lung inflammation. Immunity.
40:425–435. 2014. View Article : Google Scholar :
|
|
35
|
Wu Y, Yan Y, Su Z, Bie Q, Wu J, Wang S, Yu
Y, Ding H, Lu P and Xu H: Enhanced circulating ILC2s accompany by
upregulated MDSCs in patients with asthma. Int J Clin Exp Pathol.
8:3568–3579. 2015.
|