Introduction
Diabetes mellitus (DM) is an increasingly prevalent
worldwide disease that is challenging human health and is currently
of primary concern. As one of the major risk factors for
cardiovascular diseases, type 2 diabetes contributes greatly to the
occurrence of disabilities in later life and to mortality (1). Over 50% of mortality events resulting
from type 2 diabetes are attributable to cardiovascular diseases
(2), including stroke and
myocardial infarction. Microvascular endothelial apoptosis is
important in the development of the initial vessel lesions of
vascular complications in DM (3).
Dipeptidyl peptidase-4 (DPP-4) inhibitors are of a
class of oral hypoglycemic agents, which reduce blood glucose
levels with a low risk of hypoglycemia and weight gain. DPP-4 is an
enzyme expressed on blood vessels, myocardium and myeloid cells and
is responsible for the inactivation of endogenous glucoregulatory
peptides, termed incretins (4).
Glucagon-like peptide 1 (GLP-1) and gastric inhibitory peptide are
two well-studied incretins. GLP-1 prolongs gastric emptying,
reduces appetite, inhibits glucagon release and stimulates insulin
in a glucose-dependent manner. GLP-1 receptor (GLP-1R) agonists
have been used in the treatment of patients with type 2 diabetes
(5). Sitagliptin was the first
clinically used DPP-4 inhibitor and was approved by the US Food and
Drug Administration for the treatment of type 2 diabetes in 2006
(6). Recent studies in
apolipoprotein E-deficient mice revealed that sitagliptin improved
endothelial dysfunction, enhanced endothelial nitric oxide synthase
(eNOS) phosphorylation (7) and
reduced the atherosclerotic plaque area (8), suggesting that DPP-4 inhibitors may
have further potential therapeutic effects beyond the
incretin-dependent hypoglycemic action. DPP-4 inhibitors have been
demonstrated to exhibit cardiovascular protective functions,
however their effect on endothelial apoptosis and the underlying
mechanism in diabetes remains to be fully elucidated.
Adenosine monophosphate-activated protein kinase
(AMPK) is a cellular energy and stress sensor (9). In diabetes, AMPK has been observed to
be dephosphorylated and inactive (10). A previous study revealed that AMPK
activation significantly prevents the oxidative stress-induced
apoptosis of human umbilical vein endothelial cells (HUVECs)
(11). Various studies have
additionally demonstrated that AMPK prevents apoptosis via
inhibiting reactive oxygen species (ROS) generation by mitochondria
(12) and nicotinamide adenine
dinucleotide phosphate [NAD(P)H] oxidase (13). The molecular mechanism regarding
how the DPP-4 inhibitor regulates endothelial homeostasis and the
associated functional role of AMPK remains to be elucidated. The
present study aimed to verify the mechanism by which the DPP-4
inhibitor sitagliptin protects against high glucose (HG)-induced
vascular endothelial cell apoptosis and examine if AMPK is involved
in this regulatory process.
Materials and methods
Reagents
Sitagliptin (phosphate) was provided by Cayman
Chemical Company (Ann Arbor, MI, USA) and
5-aminoimidazole-4-carboxamide riboside (AICAR) was purchased from
Beyotime Institute of Biotechnology (Haimen, China). Compound C was
obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Monoclonal rabbit anti-phospho-AMPKα antibody (catalog no. 2535p)
and anti-AMPK antibody (catalog no. 2603p) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The following
antibodies were also used: Monoclonal mouse anti-β-actin antibody
(catalog no. sc-47778) and horseradish peroxidase-conjugated goat
anti-rabbit/mouse secondary antibody (catalog no. sc-2004/sc-2005)
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
HUVECs were isolated by collagenase digestion from
fresh cord umbilical veins, as previously described (14). The flesh cord umbilical veins were
obtained from normal cesarean section surgery. This was approved by
Air Force General Hospital ethics committee with informed written
consent. HUVECs between passages 3 and 6 were cultured in
endothelial cell medium (ScienCell Research Laboratories, Inc.,
Carlsbad, CA, USA) containing basal medium, supplemented with 5%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 1% endothelial cell growth supplement with antibiotics
(100 U/ml penicillin G and 100 µg/ml streptomycin sulfate). This
was conducted in a humidified atmosphere containing 5% CO2, at
37°C.
Western blotting
To determine the effect of sitagliptin on AMPK
activation, the HUVECs were treated with 1 µM sitagliptin for 0.5,
1, 2 and 4 h or 100 µM AMPK activator AICAR, for 0.5 h. To detect
the inhibitory effect of the AMPK inhibitor compound C on
sitagliptin-induced AMPKα phosphorylation, HUVECs were incubated
with 1 µM sitagliptin, 10 µM compound C or 1 µM sitagliptin plus 10
µM compound C for 2 h. The cytoplasmic protein of cells was
extracted with ice-cold hypotonic lysis buffer [50 mM Tris-HCl, pH
7.5, 15 mM EGTA, 0.1% (vol/vol) Triton X-100, 100 mM NaCl and
complete protease inhibitor cocktail] as previously described
(15). Cell lysates were first
snap frozen in liquid nitrogen and then centrifuged at 12,000 × g
at 4°C for 20 min, for collection of the supernatant. Protein
concentration was measured using the BCA method. Equal amounts of
protein (10 µg per sample) were separated on 10% sodium dodecyl
sulfate-polyacrylamide gels electrophoresis and blotted onto
polyvinylidene difluoride membranes. Following incubation with no
fat milk at 25°C for 20 min, the membranes were reacted with
anti-phospho-AMPKα antibody (1:1,000) and anti-AMPKα antibody
(1:1,000) at 4°C overnight, then reacted with appropriate
horseradish peroxidase-conjugated secondary antibodies (1:3,000)
for 2 h at 25°C. Proteins were visualized with an enhanced
chemiluminescence kit, as previously described (16). Densitometry analysis was performed
for three independent experiments using the Image J Gel Analysis
tool (National Institutes of Health, Bethesda, MD, USA).
Measurement of endothelial
apoptosis
HUVECs (1×105) were incubated with HG (33
mM) in the presence of 1 µM sitagliptin, 100 µM AICAR or 1 µM
sitagliptin plus 10 µM AMPK inhibitor compound C for 48 h.
Induction of apoptosis in the treated groups was assessed by
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
double staining detection kit (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China), according to the manufacturer's protocol. Briefly,
cells were incubated with 33 mM D-glucose in the presence of the
aforementioned agents for 48 h and gently digested with 1 ml 0.25%
trypsin (Thermo Fisher Scientific, Inc.) for 2 min. The trypsinized
cells were washed once with endothelial cell medium containing 5%
fetal bovine serum prior to collection by centrifugation at 1,000 ×
g and room temperature for 20 min. Cells were resuspended in 500 µl
of 1X binding buffer, followed by incubation with 5 µl of Annexin
V-FITC and 5 µl of PI (50 µg/ml) for 10 min in the dark. Binding
buffer, Annexin V-FITC and PI are components of the detection kit.
All procedures subsequent to cell incubation were performed at room
temperature. Stained cells were monitored by flow cytometry (BD
FACSCalibur; BD Biosciences, San Jose, CA, USA) and analyzed with
BD FACSDiva™ software (version 6.0; BD Biosciences).
Measurement of ROS generation
A ROS-specific fluorescent probe,
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular
Probes; Thermo Fisher Scientific, Inc.) was used for the
measurement of cytosolic ROS production. HUVECs were incubated with
33 mM D-glucose in the presence of 1 µM sitagliptin, 100 µM AICAR
or 1 µM sitagliptin plus 10 µM compound C for 48 h, then cells were
stained with 10 µM H2DCFDA fluorescent probe in serum-free
endothelial cell medium at 37°C for 30 min. The labeled cells were
then washed twice with serum-free endothelial cell medium and the
levels of ROS were immediately analyzed by flow cytometry (BD
FACSCalibur; BD Biosciences).
Mitochondrial membrane potential (ΔΨm)
assay
To measure ΔΨm, HUVECs were treated with 1 µM
sitagliptin, 100 µM AICAR or 1 µM sitagliptin plus 10 µM compound C
prior to exposure to 33 mM D-glucose for 48 h. Following
incubation, cells were collected and stained with 2 µM ΔΨm-specific
fluorescent dye JC-1 (Molecular Probes; Thermo Fisher Scientific,
Inc.) at 37°C in an atmosphere containing 5% CO2, for 20 min. Flow
cytometry (BD FACSCalibur; BD Biosciences) was used to detect ΔΨm
for each treatment group. JC-1 accumulates in mitochondria in a
potential-dependent manner. In normal mitochondria with high ΔΨm,
JC-1 aggregates with red fluorescence. In apoptotic cells with
injured mitochondria membrane, JC-1 alters to monomers, and emits
green fluorescence. ΔΨm is determined by red/green fluorescence
intensity ratio.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. One-way analysis of variance was used to determine
differences among the mean values of treatments. SPSS software,
version 20.0 (IBM SPSS, Armonk, NY, USA) was used for the
statistical data analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sitagliptin prevents HG-induced
endothelial apoptosis
The present study examined the effect of sitagliptin
on HUVECs incubated with HG. Cell apoptosis of the pretreated
groups was measured by Annexin V-FITC/PI double staining and
monitored by flow cytometry (Fig.
1A). It was observed that HG significantly increased cell
apoptosis, and this HG-induced endothelial cell apoptosis was
prevented by sitagliptin or the AMPK activator, AICAR. However,
compound C, an AMPK inhibitor, reversed the inhibition of apoptosis
by sitagliptin (Fig. 1B). This
therefore indicated that AMPK is important in the regulatory action
of sitagliptin.
 | Figure 1.Sitagliptin prevents HG-induced
apoptosis in vascular endothelial cells. (A) The apoptosis rate of
endothelial cells in the five groups was assessed by Annexin
V-FITC/PI staining and monitored with flow cytometry. The lower
right quadrant: Annexin V-FITC-positive/PI-negative cells,
representing early apoptotic cells. The upper right quadrant:
Annexin V-FITC-positive/PI-positive cells, indicating late
apoptotic cells. The values represent the percentage of the total
cells in the two quadrants. (B) Quantification of apoptotic cell
rate compared with control group. HG-induced cell apoptosis was
prevented by sitagliptin or AMPK activator, AICAR. Compound C, an
AMPK inhibitor reversed inhibition of cell apoptosis by
sitagliptin. Data are expressed as the mean ± standard error of the
mean from three independent experiments. **P<0.01 vs. HG group,
#P<0.05. Sita, sitagliptin; CC, compound C; HG, high glucose;
AICAR, 5-aminoimidazole-4-carboxamide riboside; FITC, fluorescein
isothiocyanate; PI, propidium iodide. |
Sitagliptin activates AMPKα
phosphorylation in vascular endothelial cells
As AMPK was observed to be involved in
sitagliptin-mediated prevention of endothelial cell apoptosis, the
present study aimed to determine the effect of sitagliptin on AMPK
activity. HUVECs were incubated with 1 µM sitagliptin at different
times ranging from 0.5–4 h. Phosphorylation of AMPKα (p-AMPKα) was
determined by western blotting (Fig.
2A). Sitagliptin stimulated AMPKα (Thr172)
phosphorylation from 2 h, and this phosphorylation activity
prevailed until 4 h. AICAR enhanced AMPK phosphorylation in
endothelial cells in a similar manner to sitagliptin, following
incubation with the cells for 0.5 h (Fig. 2B). The effect of compound C on
sitagliptin-induced AMPKα phosphorylation was additionally examined
(Fig. 2C). As presented in
(Fig. 2D), sitagliptin-stimulated
AMPKα activation was significantly inhibited by compound C. These
findings suggested that sitagliptin induces AMPKα
phosphorylation.
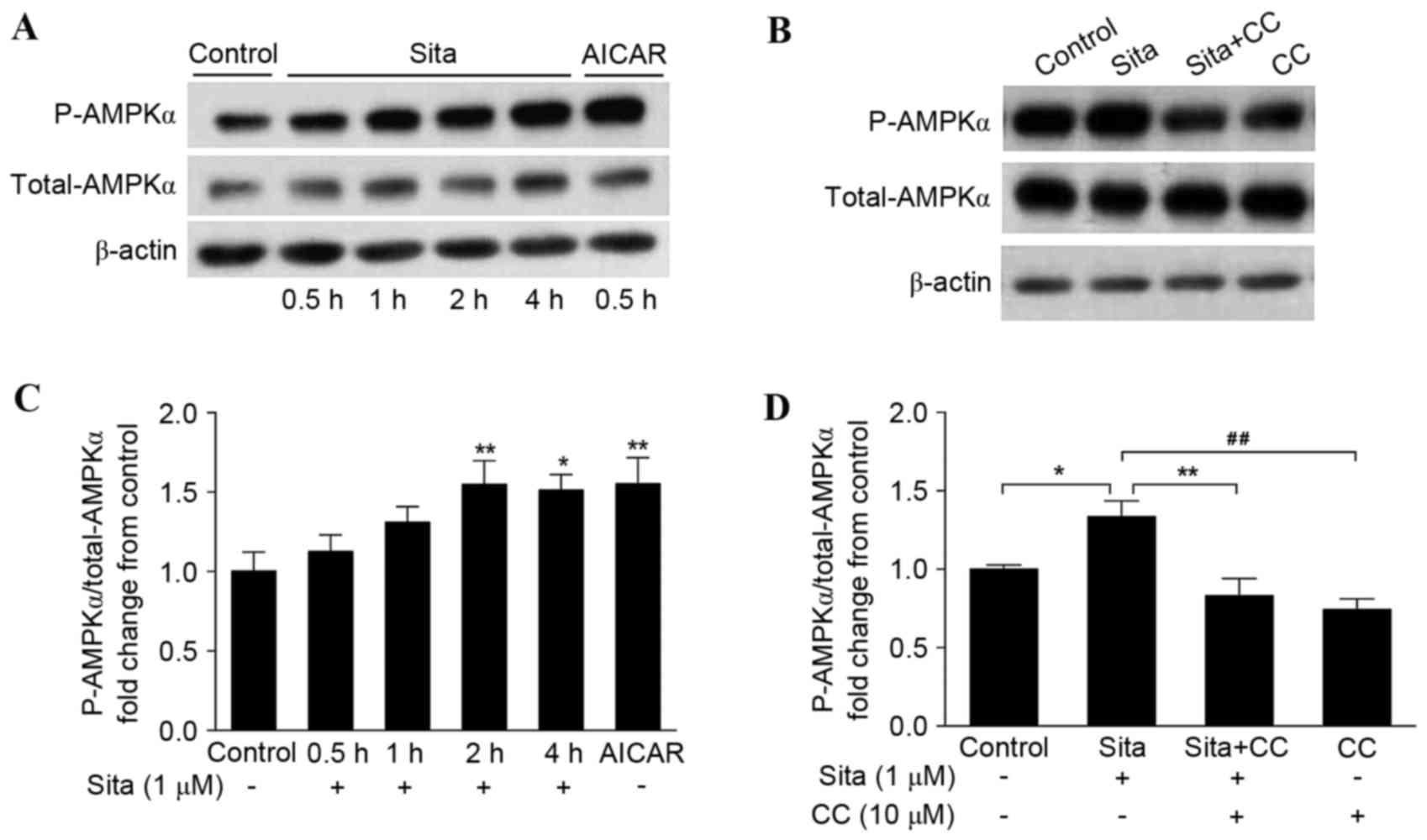 | Figure 2.Sitagliptin activates AMPKα
phosphorylation. (A) Phosphorylation of AMPKα was determined by
western blotting. AMPKα phosphorylation was enhanced from 2–4 h.
AICAR additionally activated AMPKα phosphorylation. (B) Compound C
inhibited sitagliptin-induced AMPKα activation. The results were
quantified and expressed as AMPKα phosphorylation normalized to
total AMPKα following (C) sitagliptin plus AICAR treatment
(*P<0.05, **P<0.01 vs. control), and (D) sitagliptin plus
compound C, in bar graphs. Data are presented as the mean ±
standard error of the mean of three independent experiments
(*P<0.05, **P<0.01, ##P<0.01). Sita,
sitagliptin; CC, compound C; AICAR, 5-aminoimidazole-4-carboxamide
riboside; AMPK, adenosine monophosphate-activated protein kinase;
P, phosphorylated. |
Sitagliptin decreases high
glucose-induced ROS generation
In vascular endothelial cells, the hyperglycemia
load increases generation of ROS (17), which subsequently contributes to
cell apoptosis. To observe the effect of sitagliptin pretreatment
on HG-induced cytosolic ROS generation, cytosolic ROS levels were
detected via flow cytometry (Fig.
3A). It was observed that high glucose significantly increased
ROS production, however this was suppressed with pretreatment with
1 µM sitagliptin. In addition, AICAR effectively inhibited
generation of ROS, whereas compound C diminished the inhibitory
effect of sitagliptin (Fig. 3B).
These data suggested that sitagliptin inhibits cytosolic ROS via
AMPK activation. The effect of sitagliptin on ROS-mediated
mitochondrial dysfunction, under conditions of hyperglycemia were
then examined.
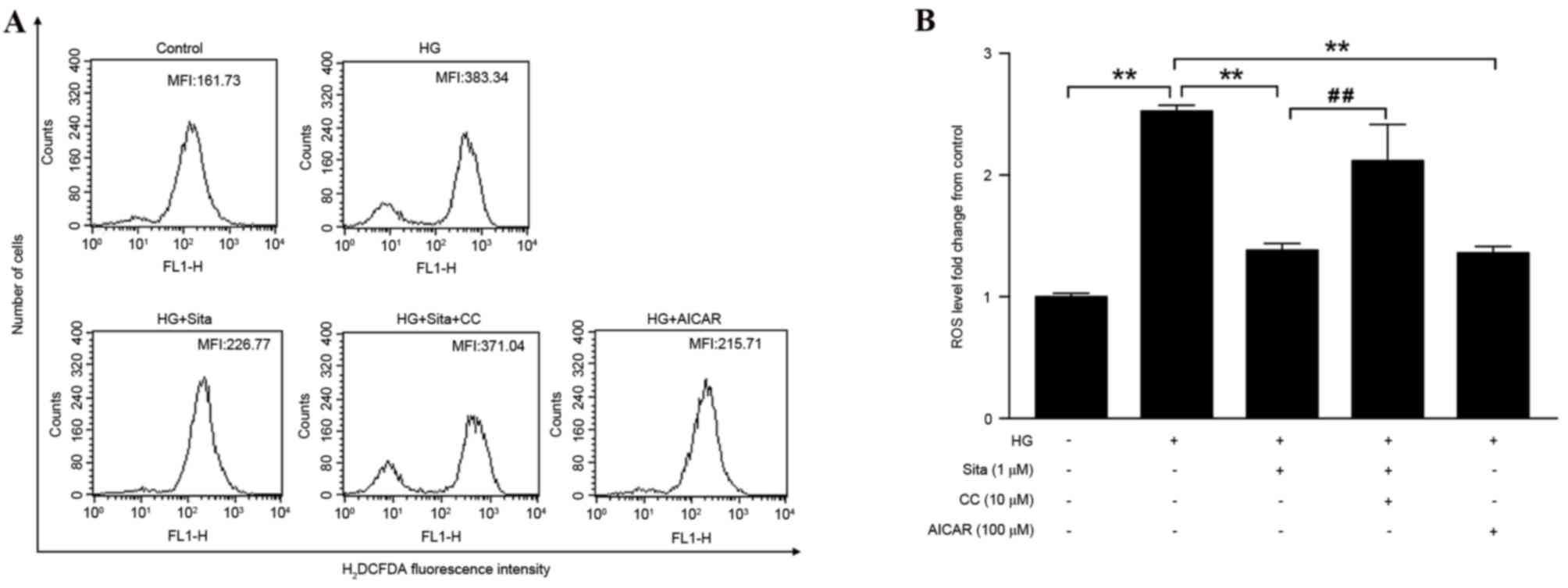 | Figure 3.Sitagliptin decreases HG-induced
cytosolic ROS generation. (A) The MFI of the cells was monitored
using flow cytometry. (B) ROS level was quantified as MFI of each
group compared with control group. Sitagliptin decreased HG-induced
ROS generation, which was blocked by compound C. AICAR similarly
inhibited hyperglycemia-induced intracellular ROS. Data are
presented as the mean ± standard error of the mean of three
independent experiments. **P<0.01, ##P<0.01. Sita,
sitagliptin; CC, compound C; HG, high glucose; ROS, reactive oxygen
species; AICAR, 5-aminoimidazole-4-carboxamide riboside; MFI, mean
fluorescence intensity; H2DCFDA, 2′,7′-dichlorodihydrofluorescein
diacetate. |
Sitagliptin restores the loss of
ΔΨm
ROS-mediated ΔΨm collapse was previously
demonstrated to initiate mitochondrial-dependent apoptosis in DM
(18). The present study proceeded
to characterize HG-induced ΔΨm alterations and examine if
sitagliptin protects against ΔΨm collapse. JC-1 staining detection
by flow cytometry was performed. Mitochondrial depolarization is
determined by a decrease in aggregate/monomer fluorescence ratio
(Fig. 4A). In a similar manner to
that exhibited by AICAR, 1 µM sitagliptin restored HG-induced ΔΨm
collapse, and this effect was blocked by compound C (Fig. 4B). These results suggested that
AMPK is important in the regulatory actions of sitagliptin in
HG-induced endothelial apoptosis.
Discussion
The present study demonstrated that the DPP-4
inhibitor, sitagliptin, functions as a regulator of endothelial
cell apoptosis. In HUVECs, sitagliptin effectively prevented
HG-induced apoptosis. The underlying mechanisms may involve
inhibition of ROS and the downstream ΔΨm collapsing pathway,
induced via AMPKα activation, as summarized in Fig. 5. Overall, the present study has
reported novel findings, suggesting DPP-4 inhibitor-mediated AMPK
activation as a therapeutic target for vascular endothelial
apoptosis.
Diabetes is a major risk factor for cardiovascular
disease. The risk of the development of cardiovascular
complications in diabetes suggests a need for further therapeutic
treatments, which may modulate disease-specific mechanisms,
including endothelial apoptosis. DPP-4 inhibitors, including
sitagliptin, alogliptin and vildagliptin are safe, well-tolerated
hypoglycemic agents that have exhibited beneficial therapeutic
effects in diabetes. Previous studies have demonstrated that DPP-4
inhibitors have substantial implications in the cardiovascular
system. Alogliptin relaxes reconstructed aortic segments (19), and incubation of HUVECs with
vildagliptin has been demonstrated to result in phosphorylation of
eNOS and serine/threonine kinase 1, increasing nitric oxide
synthesis (20). Various animal
model studies have demonstrated beneficial effects of DPP-4
inhibitors in improving blood pressure and endothelial dysfunction
(7,21). The present study revealed the novel
mechanism of DPP-4 inhibitor-mediated apoptosis prevention. The
results demonstrated that sitagliptin protected against HG-induced
apoptosis in HUVECs, an effect additionally exhibited by the AMPK
activator AICAR. Compound C, an AMPK inhibitor, diminished the
inhibitory effect of the sitagliptin pretreatment. The potential
link between sitagliptin and AMPK was then determined. The data
demonstrated that AMPKα phosphorylation was activated by
sitagliptin and compound C inhibited sitagliptin-induced AMPKα
activation. Therefore, it was demonstrated that AMPK activation is
important in sitagliptin-mediated protection against HG-induced
vascular endothelial apoptosis.
AMPK is composed of catalytic α-subunit and
regulatory β- and γ-subunits (9).
The activation of AMPK occurs via α-subunit phosphorylation at
Thr172 (22). AMPK is
an important regulator of metabolic homeostasis, and is considered
a therapeutic target for the prevention of diabetic complications
(23). Various reports have
demonstrated that the AMPK signaling pathway exhibits a protective
effect against endothelial dysfunction (24) and prevents apoptosis of HUVECs
(11) consistent with the findings
of the present study. A previous study reported that AMPKα acts as
a physiological suppressor of NAD(P)H oxidase and ROS generation in
endothelial cells (12), whereas a
further study conversely indicated that AMPK is activated by ROS
(25). Hyperglycemia-induced
intracellular ROS production and associated downstream
mitochondrial fission, stimulated ΔΨm collapse, which resulted in
mitochondrial-dependent apoptosis (26). The present study observed a
significant increase in cytosolic ROS generation and ΔΨm collapse
upon incubation with HG in HUVECs. The ROS production and ΔΨm
collapse were suppressed by pretreatment with sitagliptin or AICAR.
Compound C reversed the effect of sitagliptin. Therefore, there may
be a negative feedback loop between AMPK and ROS, in which ROS
generation potentiates AMPK activation, resulting in a further
inhibition in intracellular ROS production.
Sitagliptin was demonstrated to prevent endothelial
apoptosis via AMPKα activation, therefore it is necessary to
elucidate the mechanism by which sitagliptin activates AMPKα. The
Ca2+/calmodulin-activated protein kinase kinases
(CaMKK), particularly CaMKKβ (27)
and the liver kinase B1 (LKB1)-STRAD-MO25 complex (28) are major upstream kinases of AMPK in
mammals, and the upstream kinase LKB1 is important for the
activation of AMPK by AICAR (29).
Sitagliptin mimicked the preventive effect of AICAR on HG-induced
ROS production, ΔΨm collapse, and endothelial cell apoptosis,
indicating that sitagliptin-mediated AMPK HG-induced AMPKα
activation may involve LKB1. It was additionally reported that
sitagliptin treatment improved endothelial function in vivo
via sequential activation of the LKB1/AMPKα/eNOS axis (30).
In conclusion, the results of the present study
indicated that the DPP-4 inhibitor sitagliptin effectively
prevented HG-induced cytosolic ROS generation, ΔΨm collapse and
apoptosis via activation of AMPKα in endothelial cells. These
results suggested sitagliptin may act as a potential novel
therapeutic agent to treat vascular complications in diabetes.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant no. 81070209). The
authors would like to thank Ms. Guohua Ma (Department of
Cardiology, Beijing Tian Tan Hospital, Beijing, China) for
providing HUVECs, Dr Zhipeng Wang (Institute of Cardiovascular
Science, Peking University, Beijing, China) for helpful discussions
and Dr Yahan Liu (Institute of Cardiovascular Science, Peking
University) for technical assistance.
Glossary
Abbreviations
Abbreviations:
|
DPP-4
|
dipeptidyl peptidase-4
|
|
ROS
|
reactive oxygen species
|
|
DM
|
diabetes mellitus
|
|
HG
|
high glucose
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
References
|
1
|
Mazzone T, Chait A and Plutzky J:
Cardiovascular disease risk in type 2 diabetes mellitus: Insights
from mechanistic studies. Lancet. 371:1800–1809. 2008. View Article : Google Scholar :
|
|
2
|
Zhong J, Maiseyeu A, Davis SN and
Rajagopalan S: DPP4 in cardiometabolic disease: Recent insights
from the laboratory and clinical trials of DPP4 inhibition. Circ
Res. 116:1491–1504. 2015. View Article : Google Scholar :
|
|
3
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar
|
|
4
|
Drucker DJ and Nauck MA: The incretin
system: Glucagon-like peptide-1 receptor agonists and dipeptidyl
peptidase-4 inhibitors in type 2 diabetes. Lancet. 368:1696–1705.
2006. View Article : Google Scholar
|
|
5
|
Ussher JR and Drucker DJ: Cardiovascular
biology of the incretin system. Endocr Rev. 33:187–215. 2012.
View Article : Google Scholar :
|
|
6
|
Drucker D, Easley C and Kirkpatrick P:
Sitagliptin. Nat Rev Drug Discov. 6:109–110. 2007. View Article : Google Scholar
|
|
7
|
Matsubara J, Sugiyama S, Sugamura K,
Nakamura T, Fujiwara Y, Akiyama E, Kurokawa H, Nozaki T, Ohba K,
Konishi M, et al: A dipeptidyl peptidase-4 inhibitor,
des-fluoro-sitagliptin, improves endothelial function and reduces
atherosclerotic lesion formation in apolipoprotein E-deficient
mice. J Am Coll Cardiol. 59:265–276. 2012. View Article : Google Scholar
|
|
8
|
Zeng Y, Li C, Guan M, Zheng Z, Li J, Xu W,
Wang L, He F and Xue Y: The DPP-4 inhibitor sitagliptin attenuates
the progress of atherosclerosis in apolipoprotein-E-knockout mice
via AMPK- and MAPK-dependent mechanisms. Cardiovasc Diabetol.
13:322014. View Article : Google Scholar :
|
|
9
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View
Article : Google Scholar
|
|
10
|
Eid AA, Ford BM, Block K, Kasinath BS,
Gorin Y, Ghosh-Choudhury G, Barnes JL and Abboud HE: AMP-activated
protein kinase (AMPK) negatively regulates Nox4-dependent
activation of p53 and epithelial cell apoptosis in diabetes. J Biol
Chem. 285:37503–37512. 2010. View Article : Google Scholar :
|
|
11
|
Ido Y, Carling D and Ruderman N:
Hyperglycemia-induced apoptosis in human umbilical vein endothelial
cells: Inhibition by the AMP-activated protein kinase activation.
Diabetes. 51:159–167. 2002. View Article : Google Scholar
|
|
12
|
Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu
C, Viollet B, Yan D and Zou MH: AMPKalpha2 deletion causes aberrant
expression and activation of NAD(P)H oxidase and consequent
endothelial dysfunction in vivo: Role of 26S proteasomes. Circ Res.
106:1117–1128. 2010. View Article : Google Scholar :
|
|
13
|
Colombo SL and Moncada S: AMPKalpha1
regulates the antioxidant status of vascular endothelial cells.
Biochem J. 421:163–169. 2009. View Article : Google Scholar
|
|
14
|
Pan B, Yu B, Ren H, Willard B, Pan L, Zu
L, Shen X, Ma Y, Li X, Niu C, et al: High-density lipoprotein
nitration and chlorination catalyzed by myeloperoxidase impair its
effect of promoting endothelial repair. Free Radic Biol Med.
60:272–281. 2013. View Article : Google Scholar
|
|
15
|
Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin
L, Liu X and Wang N: Tumor-derived microRNA-494 promotes
angiogenesis in non-small cell lung cancer. Angiogenesis.
18:373–382. 2015. View Article : Google Scholar
|
|
16
|
Yin R, Fang L, Li Y, Xue P, Li Y, Guan Y,
Chang Y, Chen C and Wang N: Pro-inflammatory Macrophages suppress
PPARγ activity in Adipocytes via S-nitrosylation. Free Radic Biol
Med. 89:895–905. 2015. View Article : Google Scholar
|
|
17
|
Paneni F, Mocharla P, Akhmedov A,
Costantino S, Osto E, Volpe M, Lüscher TF and Cosentino F: Gene
silencing of the mitochondrial adaptor p66(Shc) suppresses vascular
hyperglycemic memory in diabetes. Circ Res. 111:278–289. 2012.
View Article : Google Scholar
|
|
18
|
Yee C, Yang W and Hekimi S: The intrinsic
apoptosis pathway mediates the pro-longevity response to
mitochondrial ROS in C. Elegans. Cell. 157:897–909. 2014.
View Article : Google Scholar :
|
|
19
|
Shah Z, Pineda C, Kampfrath T, Maiseyeu A,
Ying Z, Racoma I, Deiuliis J, Xu X, Sun Q, Moffatt-Bruce S, et al:
Acute DPP-4 inhibition modulates vascular tone through GLP-1
independent pathways. Vascul Pharmacol. 55:2–9. 2011. View Article : Google Scholar :
|
|
20
|
Ishii M, Shibata R, Kondo K, Kambara T,
Shimizu Y, Tanigawa T, Bando YK, Nishimura M, Ouchi N and Murohara
T: Vildagliptin stimulates endothelial cell network formation and
ischemia-induced revascularization via an endothelial nitric-oxide
synthase-dependent mechanism. J Biol Chem. 289:27235–27245. 2014.
View Article : Google Scholar :
|
|
21
|
Aroor AR, Sowers JR, Bender SB, Nistala R,
Garro M, Mugerfeld I, Hayden MR, Johnson MS, Salam M,
Whaley-Connell A and Demarco VG: Dipeptidylpeptidase inhibition is
associated with improvement in blood pressure and diastolic
function in insulin-resistant male Zucker obese rats.
Endocrinology. 154:2501–2513. 2013. View Article : Google Scholar :
|
|
22
|
Oakhill JS, Steel R, Chen ZP, Scott JW,
Ling N, Tam S and Kemp BE: AMPK is a direct adenylate
charge-regulated protein kinase. Science. 332:1433–1435. 2011.
View Article : Google Scholar
|
|
23
|
Viollet B, Lantier L, Devin-Leclerc J,
Hebrard S, Amouyal C, Mounier R, Foretz M and Andreelli F:
Targeting the AMPK pathway for the treatment of Type 2 diabetes.
Front Biosci (Landmark Ed). 14:3380–3400. 2009. View Article : Google Scholar :
|
|
24
|
Xu Q and Si LY: Protective effects of
AMP-activated protein kinase in the cardiovascular system. J Cell
Mol Med. 14:2604–2613. 2010. View Article : Google Scholar :
|
|
25
|
Hawley SA, Ross FA, Chevtzoff C, Green KA,
Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM and
Hardie DG: Use of cells expressing gamma subunit variants to
identify diverse mechanisms of AMPK activation. Cell Metab.
11:554–565. 2010. View Article : Google Scholar :
|
|
26
|
Bhatt MP, Lim YC, Kim YM and Ha KS:
C-peptide activates AMPKα and prevents ROS-mediated mitochondrial
fission and endothelial apoptosis in diabetes. Diabetes.
62:3851–3862. 2013. View Article : Google Scholar :
|
|
27
|
Hawley SA, Pan DA, Mustard KJ, Ross L,
Bain J, Edelman AM, Frenguelli BG and Hardie DG:
Calmodulin-dependent protein kinase kinase-beta is an alternative
upstream kinase for AMP-activated protein kinase. Cell Metab.
2:9–19. 2005. View Article : Google Scholar
|
|
28
|
Shaw RJ, Kosmatka M, Bardeesy N, Hurley
RL, Witters LA, DePinho RA and Cantley LC: The tumor suppressor
LKB1 kinase directly activates AMP-activated kinase and regulates
apoptosis in response to energy stress. In: Proc Natl Acad Sci USA.
101. pp. 3329–3335. 2004; View Article : Google Scholar :
|
|
29
|
Fisslthaler B and Fleming I: Activation
and signaling by the AMP-activated protein kinase in endothelial
cells. Circ Res. 105:114–127. 2009. View Article : Google Scholar
|
|
30
|
Liu L, Liu J, Wong WT, Tian XY, Lau CW,
Wang YX, Xu G, Pu Y, Zhu Z, Xu A, et al: Dipeptidyl peptidase 4
inhibitor sitagliptin protects endothelial function in hypertension
through a glucagon-like peptide 1-dependent mechanism.
Hypertension. 60:833–841. 2012. View Article : Google Scholar
|



















