Introduction
Aspergillus fumigatus (A. fumigatus)
is one of the most important opportunistic pathogens that form
airborne spores (conidia) in our environment (1). Immunocompromised individuals may
develop invasive aspergillosis (IA), which demonstrates high
morbidity and mortality rates (2).
Invasive pulmonary aspergillosis (IPA) is the most common form of
IA, and affects immunocompromised hosts including those with
malignancies, advanced human immunodeficiency virus infection,
patients that have undergone hematopoietic stem cell
transplantation and those receiving immunosuppressive treatments
(3). Due to the increasing number
of immunocompromised patients, the number of cases of IPA is
increasing substantially (3).
Thus, an improved understanding of the host defense against A.
fumigatus is urgently required.
Alveolar macrophages (AMs) serve a critical role in
the host's innate defense against A. fumigatus. AMs express
several different pattern recognition receptors (PRRs) that mediate
recognition of the invading pathogens via pathogen-associated
molecular patterns (4). The A.
fumigatus cell wall is mainly composed of chitin, α-glucans,
β-(1,3)(1,4)-glucans and galactomannans, which are
recognized by specific PRRs, including C-type lectin receptors
(CTLs) and toll-like receptors (TLRs) (4). Dendritic cell-associated C-type
lectin receptor (Dectin-1) is one of the most important CTLs that
recognize the major fungal cell wall component, β-1,3-glucan.
Dectin-1−/− mice are more susceptible to A.
fumigatus and produce fewer pro-inflammatory cytokines
(5). Additionally, the presence of
a Dectin-1 polymorphism increases the risk of IA in patients with
allogeneic stem cell transplantation (6). In a recent study (3), overexpression of Dectin-1 in MH-S
murine AM cells promoted cytokine release and killing ability
during A. fumigatus stimulation. Among the TLRs, TLR-2 and
TLR-4 have been extensively studied, and are key recognition
components for host defense against A. fumigatus via the
common TLR adaptor protein, myeloid differentiation primary
response gene 88 (MyD88) (7). AMs
from TLR-2-deficient mice produce fewer inflammatory cytokines in
response to A. fumigatus, including tumor necrosis factor
(TNF)-α, chemokine (C-X-C motif) ligand 2 and macrophage
inflammatory protein-2 (8,9). TLR-4−/− mice and TLR-4
polymorphisms in donor cells from hematopoietic stem cell
transplant recipients demonstrate an increased susceptibility to
A. fumigatus (10,11). Therefore, Dectin-1-dependent and
MyD88-dependent pathways are critical for the host defense against
A. fumigatus in mice and humans.
Human transcription factor PU.1 (encoded by the
SPI1 gene) is a member of the E26-transformation-specific
(Ets) family, which is important for macrophage development and the
transcriptional control of inducible genes in mature macrophages
(12). In mice and humans, PU.1
binds to the gene promoter and enhances the transcription of
Dectin-1 (13,14), TLR-2 (15,16)
and TLR-4 (15,17,18).
Based on the evidence that PU.1 may be critical for the regulation
of these three receptors in macrophages, the authors of the present
study hypothesized that alterations in the expression of PU.1 may
affect the innate defense against A. fumigatus. In the
present study, phorbol-12-myristate-13-acetate (PMA)-induced THP-1
derived macrophages were used as a model of AMs. The aim was to
investigate whether alterations in the expression of PU.1 affects
innate immune functions in AMs during A. fumigatus
stimulation, including cytokine release, phagocytosis and the
killing ability.
Materials and methods
THP-1 derived macrophages and
preparation of conidia
The human monocyte cell line, THP-1 (American Type
Culture Collection, Manassas, VA, USA) was cultured in RPMI 1640
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
antibiotic-antimycotic solution (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. To prepare THP-1-derived
macrophages, THP-1 cells were incubated with PMA (cat. no. P1585;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a concentration
of 100 ng/ml for 48 h. Following incubation, adherent macrophages
were maintained in complete medium at 37°C with 5%
CO2.
A. fumigatus A1 was kindly provided by the
Microbiological Laboratory of Jinling Hospital (Nanjing, China).
A. fumigatus was cultured on Sabouraud dextrose agar (Thermo
Fisher Scientific, Inc.) containing penicillin (100 U/ml) and
streptomycin (100 µg/ml; Gibco; Thermo Fisher Scientific, Inc.) for
5 days at 37°C.
Conidia were washed from agar plates using 5 ml
sterile phosphate-buffered saline (PBS) supplemented with 0.025%
Tween-20. The suspension was filtered through nylon filters with
25-µm pores to separate the conidia from the contaminating
mycelium. Resting conidia were diluted in PBS and counted using a
hemocytometer. To prepare fluorescein isothiocyanate (FITC)-labeled
conidia, a total of 5×108/ml conidia were suspended in
0.1 M carbonate buffer (pH 9.0) prior to incubation with FITC (cat.
no. F-4274; Sigma-Aldrich; Merck KGaA) at a final concentration of
0.16 mg/ml overnight at 4°C. The suspension was then diluted and
washed twice with PBS. FITC-labeled conidia were diluted in PBS to
the desired concentration (4×108/ml) and stored at 4°C
in the dark (3,19).
PU.1 gene overexpression
The PU.1 recombinant adenovirus vector used in the
study was provided by Shanghai R&S Biotechnology Co., Ltd.
(Shanghai, China) and the final titer was 8×1011 IU/ml.
THP-1 derived macrophages were seeded in six-well plates (Corning
Incorporated, Corning, NY, USA) at a density of 8×105
cells/well. Cells were divided into the following three groups:
Mock untransduced group; an empty-vector transduction group (Ad
group), where cells were transduced at a multiplicity of infection
(MOI) of 1,500; and a PU.1 recombinant adenovirus vector-transduced
group (Ad-PU.1), where were transduced at an MOI of 1,500.
Following co-culture in 1 ml of RPMI 1640 medium for 12 h, the
adenovirus-containing medium was removed and replaced with complete
RPMI 1640 medium. Total RNA and protein were extracted at 48 h
post-transduction and stored at −80°C until required.
PU.1 gene silencing
Small interfering RNA (siRNA) targeting PU.1 and
negative control siRNA were obtained from Shanghai GenePharma Co.,
Ltd. (Shanghai, China). The sequence for the PU.1 siRNA was as
follows: Forward, 5′-UAUAGAUCCGUGUCAUAGGGCACCA-3′, and reverse,
5′-UGGUGCCCUAUGACACGGAUCUAUA-3′. THP-1-derived macrophages were
seeded in six-well plates (Corning Incorporated) at a density of
8×105 cells/well. For each transfection, 160 pmol PU.1
siRNA (siPU.1 group) or negative control siRNA (siNC group) was
mixed with 8 µl Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) and 500 µl Opti-MEM (Invitrogen; Thermo
Fisher Scientific, Inc.), and incubated at room temperature for 20
min. The mixture was then added to each well containing the cells
and the plate was incubated for 8 h at 37°C. Cells that were
untransfected were used as a mock transfection group. Following
incubation, 1.5 ml complete RPMI 1640 medium was added to each
well. Total RNA and protein were extracted at 48 h
post-transfection and stored at −80°C until required.
Stimulation of cells with resting
conidia
Following overexpression or silencing of PU.1,
THP-1-derived macrophages were stimulated with A. fumigatus
resting conidia (MOI=1, if not indicated otherwise) for 0, 4, 8 and
12 h, respectively. Total RNA and culture supernatants were then
isolated at these indicated time points. Samples were stored at
−80°C until required.
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay
lysis buffer containing 25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1%
NP-40 and 1% sodium deoxycholate with 1% protease inhibitor
cocktail (Sigma-Aldrich; Merck KGaA). The protein concentration was
determined using a bicinchoninic acid assay protein assay kit
(Beyotime Institute of Biotechnology, Hangzhou, China) according to
the manufacturer's protocol. Protein lysates (10 µg) were separated
using 10% SDS-polyacrylamide gels (cat. no. P0012A; Beyotime
Institute of Biotechnology) and transferred to polyvinylidene
difluoride membranes (GE Healthcare Life Sciences, Chalfont, UK).
Membranes were then blocked with 5% skimmed milk (Sigma-Aldrich;
Merck KGaA) for 2 h at room temperature. The membranes were
subsequently hybridized with primary rabbit anti-human polyclonal
antibodies against PU.1 (cat. no. 2258; Cell Signaling Technology,
Inc., Danvers, MA, US), Dectin-1 (cat. no. 9051; Cell Signaling
Technology, Inc.), TLR-2 (cat. no. 2229S; Cell Signaling
Technology, Inc.) and TLR-4 (cat. no. sc-10741; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 1:1,000 dilution overnight
at 4°C. Membranes were also incubated with primary rabbit
anti-human polyclonal antibodies against β-tubulin (cat. no. 2146S;
Cell Signaling Technology, Inc.) as a loading control for PU.1, and
β-actin (cat. no. 12620; Cell Signaling Technology, Inc.) for
Dectin-1, TLR-2 and TLR-4, at a 1:1,000 dilution overnight at 4°C.
Following incubation with the relevant horseradish
peroxidase-conjugated polyclonal goat anti-rabbit secondary
antibody (cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) at
1:5,000 dilution at room temperature for 2 h, an enhanced
chemiluminescence detection kit (Beyotime Institute of
Biotechnology) was used for detection of immunoreactive proteins.
Images of protein bands were acquired using the G:BOX Chemi XR5
system (Syngene, Frederick, MD, USA) (20). All antibodies were diluted in
Tris-buffered saline with 0.1% Tween-20.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. The concentration and purity of extracted
RNA were (260/280 ratio) was determined using a
NanoDrop™ 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). cDNA was synthesized from total RNA (1 µg) using
PrimeScript RT Master Mix (cat. no. RR036A; Takara Biotechnology
Co., Ltd., Dalian, China). The sequences of human-specific primers
were synthesized by GenScript (Nanjing, China) and are listed in
Table I. qPCR was performed using
an Applied Biosystems Prism 7500 PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using SYBR® Premix Ex
Taq™ (cat. no. RR420A; Takara Biotechnology Co., Ltd.).
PCR was performed as follows: Denaturation at 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
Relative mRNA expression levels were normalized to GAPDH, and
calculated using the 2−∆∆Cq method (21).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| PU.1 |
CGTGCACAGCGAGTTCGA |
GCTCTGGAGCTCCGTGAAGT |
| Dectin-1 |
GCAATACCAGGATAGCTGTTG |
CCAAGCATAGGATTCCCAA |
| TLR-2 |
CCTCTCGGTGTCGGAATGT |
CATCCCGCTCACTGTAAGAAA |
| TLR-4 |
TGGATACGTTTCCTTATAAG |
GAAATGGAGGCACCCCTTC |
| IL-1β |
TGATGGCTTATTACAGTGGCAATG |
GTAGTGGTGGTCGGAGATTCG |
| IL-10 |
ACCTGCCTAACATGCTTCGAG |
CTGGGTCTTGGTTCTCAGCTT |
| TNF-α |
TGCTTGTTCCTCAGCCTCTT |
CAGAGGGCTGATTAGAGAGAGGT |
| GAPDH |
ACAGTCAGCCGCATCTTCTT |
ACGACCAAATCCGTTGACTC |
Enzyme-linked immunosorbent assay
(ELISA)
TNF-α and interleukin (IL)-10 levels in the cell
culture supernatants were determined using commercially available
Quantikine ELISA kits (cat. nos. DTA00C and D1000B, respectively;
R&D Systems, Minneapolis, MN, USA), strictly according to the
manufacturer's protocols. Absorbance was recorded at 450 nm in a
microplate reader (Thermomax; Molecular Devices, LLC, Sunnyvale,
CA, USA).
Confocal laser-scanning
microscopy
THP-1-derived macrophages were seeded on 22×22 mm
coverslips in six-well plates (Corning Incorporated) at a density
of 8×105 cells/well. Following transfection with PU.1
siRNA or transduction with Ad-PU.1 for 48 h, cells were co-cultured
with 4×106 FITC-labeled resting conidia (MOI=5) in 1 ml
complete RPMI 1640 medium at 37°C and 5% CO2 for 4 h.
Cells were then washed vigorously three times with PBS, and fixed
in 4% paraformaldehyde for 10 min at room temperature. The cells
were gently washed three times with PBS and incubated with
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
(DiI; cat. no. C1036; dilution, 1:500; Beyotime Institute of
Biotechnology) and DAPI (cat. no. C1002; dilution, 1:1,000;
Beyotime Institute of Biotechnology) to stain the membrane and
nucleus, respectively. Finally, the coverslips were removed, gently
washed three times with PBS and mounted onto glass slides using
SlowFade Gold antifade reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). A confocal laser-scanning microscope
(Fluoview® FV10i; Olympus Corporation, Tokyo, Japan)
with a ×63 objective oil immersion lens was used to obtain all
images. FITC-labeled resting conidia, cell membranes and nuclei
were observed by the fluorescence intensities of FITC (green;
excitation, 495 nm; emission, 525 nm), DiI (red; excitation, 549
nm; emission range collected, 565 nm) and DAPI (blue; excitation,
364 nm; emission, 454 nm).
Killing experiments
THP-1-derived macrophages (8×105
cells/well) were exposed to resting conidia (MOI=0.5) in six-well
plates for 2 h. Killing experiments were performed as previously
described (3). Briefly,
non-adherent cells and non-phagocytosed conidia were removed by
washing the cells with pre-warmed PBS, and fresh medium was added
(0 h time point). The cells were subsequently incubated for 4 or 8
h. At 0, 4 and 8 h time points, the plates were frozen at −80°C,
and immediately thawed at 37°C to lyse the cells and harvest the
conidia. Serial dilutions were performed in sterile medium, and
solutions were used to inoculate Sabouraud dextrose agar (Thermo
Fisher Scientific, Inc.). Colonies were counted following
incubation for 24 h at 37°C. The percentage of killed conidia was
calculated by dividing the difference between the number of conidia
recovered at 0 and 4 or 8 h by the total number of conidia
recovered at 0 h, and then multiplying by 100 (22).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA) and the SPSS statistical analysis software program (version,
19.0; IBM SPSS, Armonk, NY, USA). All experiments were performed in
triplicate. Data are presented as the mean ± standard deviation.
One-way analysis of variance followed by a Bonferroni post hoc test
was used to compare differences among three groups when equal
variances were assumed, and a Dunnett's T3 multiple comparisons
test was used when equal variances were not assumed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Overexpression and silencing of PU.1
in THP-1-derived macrophages
A recombinant adenoviral vector carrying the PU.1
gene SPI1 (Ad-PU.1) was provided by Shanghai R&S
Biotechnology Co., Ltd. and used to transduce THP-1 derived
macrophages. RT-qPCR analysis demonstrated that PU.1 mRNA increased
by 80.4-fold in the Ad-PU.1 group when compared with the Mock group
(P<0.05; Fig. 1A). Western blot
analysis confirmed the results of the RT-qPCR analysis (Fig. 1B). In addition, RT-qPCR analysis
demonstrated that PU.1 mRNA levels were reduced to 19.0% of the
Mock group following transfection with siPU.1 (P<0.05; Fig. 1C). Consistent with these
observations, western blotting revealed that PU.1 protein levels
were markedly decreased in the siPU.1-transfected group when
compared with the mock group (Fig.
1D). The results demonstrated that PU.1 was successfully
overexpressed and silenced in THP-1-derived macrophages.
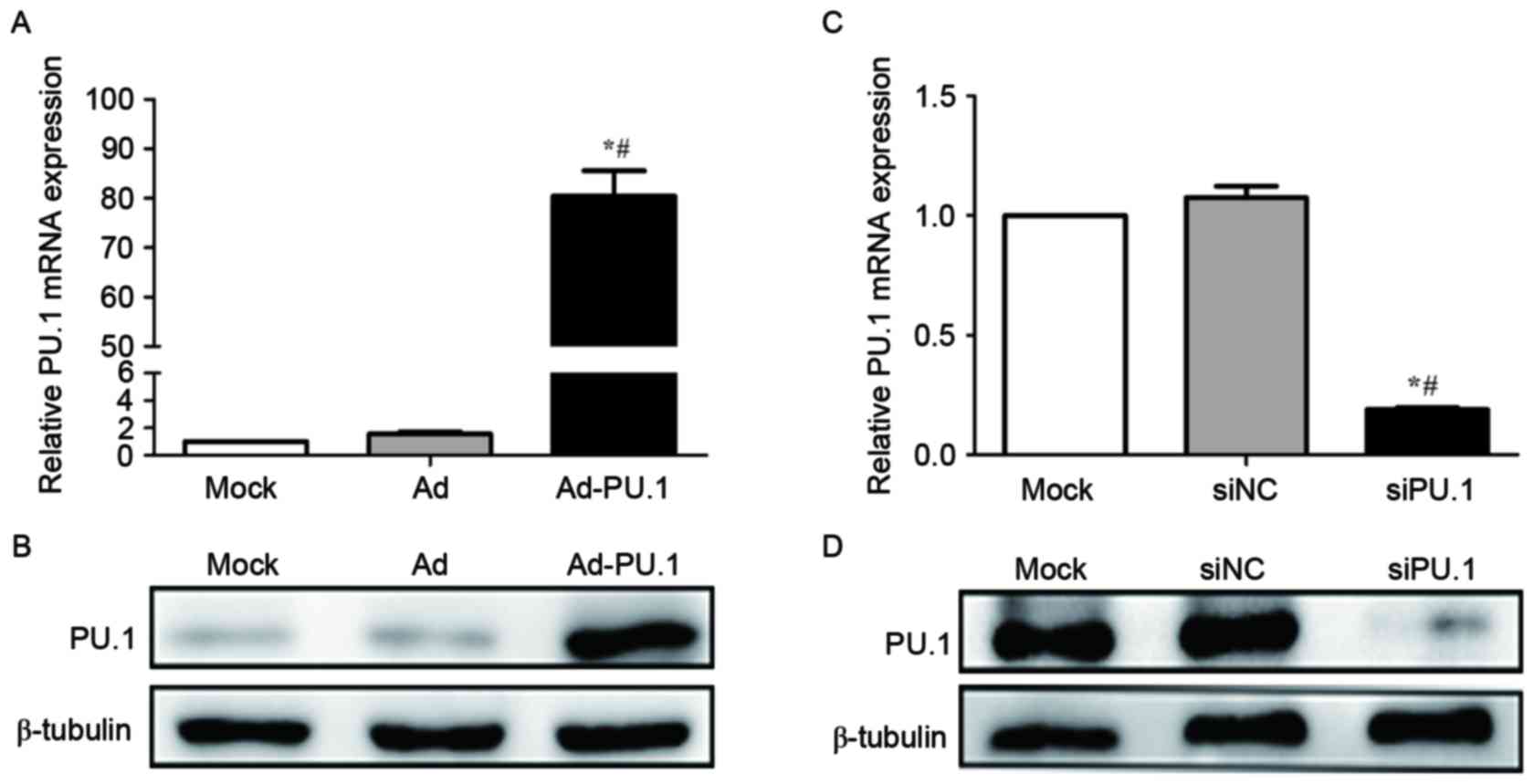 | Figure 1.Relative PU.1 (A) mRNA and (B)
protein expression levels were measured by RT-qPCR and western blot
analyses, respectively, in mock, Ad and Ad-PU.1-transduced THP-1
cells (*P<0.05 vs. Mock group; #P<0.05 vs. Ad
group). Relative PU.1 (C) mRNA and (D) protein expression levels
were measured by RT-qPCR and western blotting analyses,
respectively, in mock, siNC and siPU.1-transfected THP-1 cells
(*P<0.05 vs. Mock group; #P<0.05 vs. siNC group).
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; Ad-PU.1, recombinant adenoviral-PU.1 vector; siPU.1,
small-interfering RNA targeting PU.1; mock,
untransfected/transduced cells; Ad, adenovirus-transduced cells;
siNC, negative control siRNA. |
Expression of Dectin-1, TLR-2 and
TLR-4 following overexpression and silencing of PU.1
As PU.1 is essential for the transcriptional
regulation of Dectin-1, TLR-2 and TLR-4 (13–18),
the mRNA and protein expression levels of these genes in
THP-1-derived macrophages following transduction with Ad-PU.1 and
transfection with siPU.1 were analyzed. When compared with the mock
group, Dectin-1, TLR-2 and TLR-4 mRNA expression increased by 8.31,
2.56 and 4.28-fold, respectively (Fig.
2A). Similarly, western blot analysis revealed that the protein
expression levels of these three receptors increased when compared
with the mock group (Fig. 2B).
Conversely, the siPU.1 group exhibited a significant reduction in
Dectin-1, TLR-2 and TLR-4 mRNA expression levels by 4.64, 4.04 and
4.33-fold, respectively, when compared with the mock controls
(Fig. 2C). Consistent with these
results, the protein expression levels of these factors were
decreased in the siPU.1 group when compared with the mock group
(Fig. 2D). The results indicate
that PU.1 overexpression led to upregulation of Dectin-1, TLR-2 and
TLR-4, and silencing of PU.1 led to downregulation of Dectin-1,
TLR-2 and TLR-4.
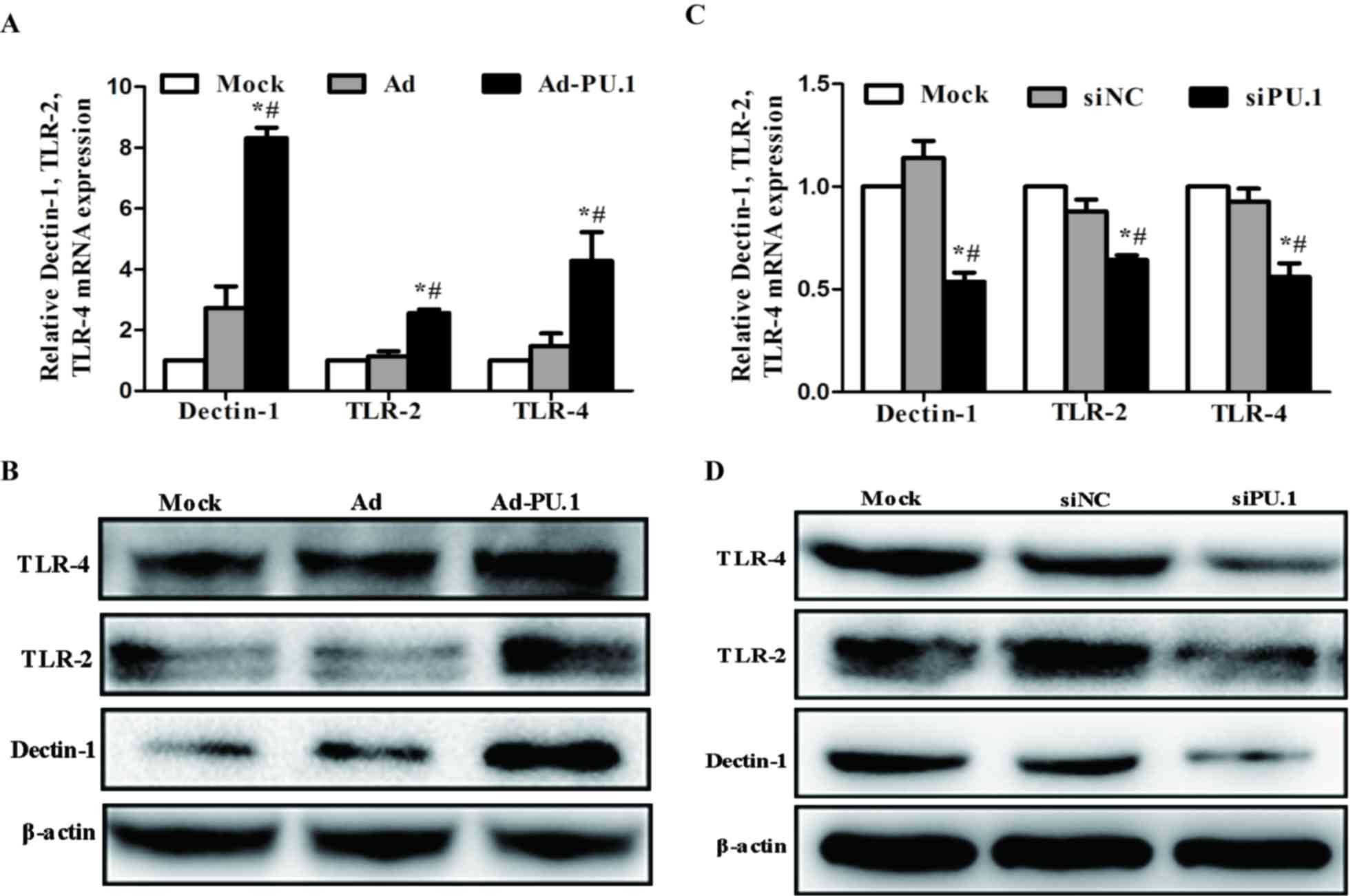 | Figure 2.Relative levels of Dectin-1, TLR-2
and TLR-4 (A) mRNA and (B) protein expression in THP-1 cells at 48
h following transduction with AdPU.1, as determined by RT-qPCR and
western blot analyses, respectively (*P<0.05 vs. Mock group;
#P<0.05 vs. Ad group). Relative levels of Dectin-1,
TLR-2 and TLR-4 (C) mRNA and (D) protein expression in THP-1 cells
at 48 h following transfection with siPU.1, as determined by
RT-qPCR and western blot analyses, respectively (*P<0.05 vs.
Mock group; #P<0.05 vs. siNC group). Dectin-1,
dendritic cell-associated C-type lectin receptor-1; TLR, toll-like
receptor; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; Ad-PU.1, recombinant adenoviral-PU.1 vector;
siPU.1, small-interfering RNA targeting PU.1; mock,
untransfected/transduced cells; Ad, adenovirus-transduced cells;
siNC, negative control siRNA. |
Cytokine release, phagocytosis and the
killing ability of THP-1-derived macrophages following
overexpression of PU.1
In order to investigate whether overexpression of
PU.1 promotes immune defense against A. fumigatus, the
cytokine release, phagocytosis and killing ability of THP-1-derived
macrophages was examined. Cells were stimulated for 0, 4, 8 and 12
h with resting conidia (MOI=1) at 48 h following transduction with
Ad-PU.1. The mRNA levels of TNF-α and IL-1β increased in a
time-dependent manner in the Ad-PU.1 group, whereas, IL-10
exhibited no significant increase in expression (Fig. 3A-C). The protein expression
patterns of TNF-α and IL-10 in cell culture supernatants were
consistent with the observed mRNA expression patterns (Fig. 3D and E).
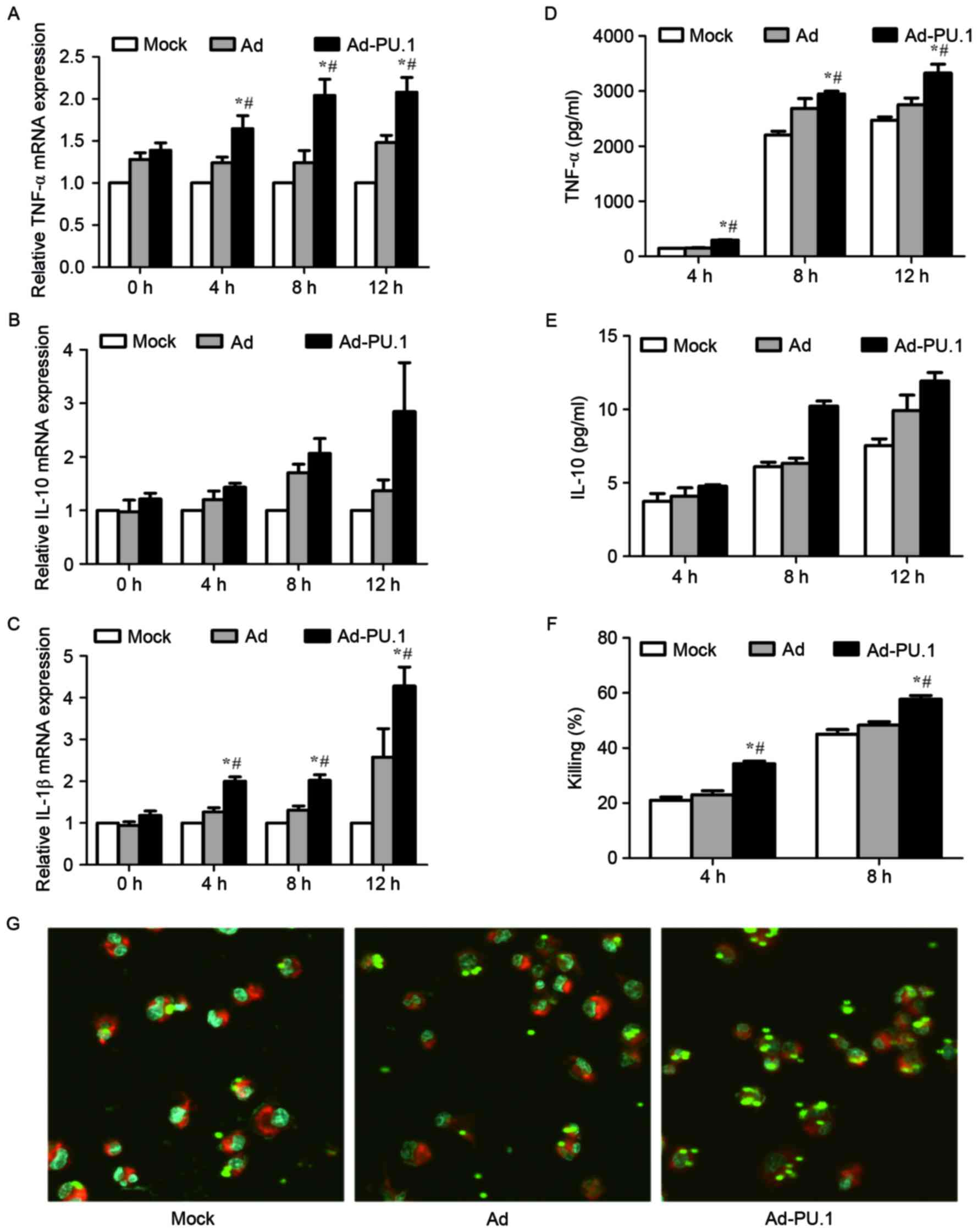 | Figure 3.mRNA levels of (A) TNF-α, (B) IL-10
and (C) IL-1β in mock, Ad, or Ad-PU.1-transduced THP-1 cells at 0,
4, 8 and 12 h of exposure to resting conidia, as determined by
reverse transcription-quantitative polymerase chain reaction
analysis. The protein levels of (D) TNF-α and (E) IL-10 were
assayed by enzyme-linked immunosorbent assay analysis. (F) Killing
ability was assessed by serial dilution of cells following
co-incubation with resting conidia, and quantification of the
number of colony forming units. (G) Confocal laser-scanning
microscopy was used to observe phagocytosis after 4 h of incubation
with resting conidia. Magnification, ×63 (objective oil immersion
lens). *P<0.05 vs. Mock group; #P<0.05 vs. Ad
group. TNF, tumor necrosis factor; IL, interleukin; Mock,
untransduced cells; Ad, adenovirus-transduced cells; Ad-PU.1,
recombinant adenoviral-PU.1 vector. |
AMs constitute the first line of a host's immune
defense, and facilitate the initiation of the innate immune
response to A. fumigatus infections, including phagocytosis
and killing (23,24). Therefore, the phagocytosis and
killing ability of THP-1-derived macrophages following
overexpression of PU.1 was examined. Cells were stimulated with
resting conidia (MOI=0.5) for 4 and 8 h, respectively. The killing
rate in the Ad-PU.1 group was significantly higher when compared
with the control groups (Fig. 3F).
For the analysis of phagocytosis, cells were stimulated with
FITC-labeled resting conidia (MOI=5) for 4 h. The confocal
laser-scanning microscopy results shown in Fig. 3G, demonstrated that phagocytosis
was enhanced following overexpression of PU.1. The results
therefore suggest that overexpression of PU.1 promoted cytokine
release, and enhanced phagocytosis and the killing ability of
THP-1-derived macrophages during A. fumigatus conidia
stimulation.
Cytokine release, phagocytosis and the
killing ability of THP-1-derived macrophages following silencing of
PU.1
As overexpression of PU.1 appears to enhance the
immune defense against A. fumigatus, the authors of the
present study investigated whether silencing of PU.1 expression was
associated with decreased cytokine release, phagocytosis and a
reduced killing ability of THP-1-derived macrophages. Cells were
stimulated for 0, 4, 8 and 12 h with resting conidia (MOI=1) at 48
h following transfection with PU.1 siRNA. The mRNA levels of TNF-α
and IL-1β decreased, while IL-10 levels increased significantly in
the siPU-1 group when compared with the mock group (Fig. 4A-C). Similar results were observed
for the protein levels of TNF-α and IL-10 in culture supernatants
(Fig. 4D and E). To determine the
killing ability, cells were stimulated with resting conidia
(MOI=0.5) for 4 and 8 h. As shown in Fig. 4F, the killing rate in siPU.1 group
was significantly lower compared with the control groups at 4 h;
however, no significant difference at 8 h was observed (Fig. 4F). Following the stimulation of
cells with FITC-labeled resting conidia (MOI=5) for 4 h, a decrease
in phagocytosis was observed in the siPU.1 group when compared with
the controls (Fig. 4G). These
results demonstrate that silencing of PU.1 decreased cytokine
release, phagocytosis and the killing ability of THP-1-derived
macrophages during A. fumigatus conidia stimulation.
 | Figure 4.mRNA levels of (A) TNF-α, (B) IL-10
and (C) IL-1β in mock, siNC, or siPU.1-transfected THP-1 cells at
0, 4, 8 and 12 h of exposure to resting conidia, as determined by
reverse transcription-quantitative polymerase chain reaction
analysis. The protein levels of (D) TNF-α and (E) IL-10 were
assayed by enzyme-linked immunosorbent assay analysis. (F) Killing
ability was assessed by serial dilution of cells following
co-incubation with resting conidia, and quantification of the
number of colony forming units. (G) Confocal laser-scanning
microscopy was used to observe phagocytosis after 4 h of incubation
with resting conidia. Magnification, ×63 (objective oil immersion
lens). *P<0.05 vs. Mock group; #P<0.05 vs. siNC
group. TNF, tumor necrosis factor; IL, interleukin; Mock,
untransfected cells; siNC, negative control small-interfering RNA;
siPU.1, small-interfering RNA targeting PU.1. |
Discussion
A. fumigatus resting conidia are inhaled
frequently and, if not effectively cleared by the innate immune
system, the conidia germinate and form invasive hyphae, which
activate innate and adaptive immune responses (25). The innate immune response is
mediated by phagocytic cells, primarily macrophages, which prevent
the growth of inhaled conidia and hyphae. Recognition of conidia by
different PRRs expressed on the membrane of AMs leads to the
phagocytosis and killing of inhaled conidia, and induces the
expression of inflammatory cytokines and chemokines (23).
PU.1 is an important transcription factor that
belongs to the Ets family, and is a critical regulator of cellular
communication in the immune system (26). AMs are the only mature tissue
macrophages that express PU.1 (27). PU.1 regulates several genes that
serve critical roles in cellular functions, including phagocytosis,
TLR signaling pathways, CTLs and surfactant clearance (14,15,28).
Overexpression of PU.1 using a retrovirus vector has been reported
in two important studies (15,27).
One such study demonstrated that retrovirus-mediated expression of
PU.1 in AMs from GM−/− mice rescued the marked reduction
in TLR-2 and TLR-4 mRNA expression (15). The other study demonstrated that
overexpression of PU.1 in bone marrow-derived dendritic cells
induced the expression of cluster of differentiation (CD) 80 and
CD86, suggesting that PU.1 may be a potential target for the
treatment of immune-associated diseases (29). Similar to the results of previous
studies (14,16,17),
the present study observed decreased expression levels of TLR-2,
TLR-4 and Dectin-1 following silencing of PU.1 expression. However,
an adenoviral vector containing the PU.1 gene was successfully
constructed in the present study, and the results revealed that
overexpression of PU.1 leads to the increase of Dectin-1, TLR-2 and
TLR-4 expression at the transcriptional and translational levels.
Although its precise regulatory mechanism has not yet been fully
elucidated, the results from gene overexpression and silencing
experiments have demonstrated that PU.1 may be an essential
regulator of Dectin-1, TLR-2 and TLR-4 expression in THP-1-derived
macrophages.
Following inhalation, A. fumigatus conidia
undergo a progression from conidial swelling to hyphal growth.
During this process, conidia cell wall components are exposed and
are detected by various PRRs on macrophages, particularly CTLs and
TLRs (30). Dectin-1, TLR-2 and
TLR-4 are the major PRRs on phagocytic cells that recognize fungal
components (31). In human and
murine macrophages, several studies have demonstrated that
Dectin-1, TLR-2 and TLR-4 are involved in the secretion of
pro-inflammatory cytokines in response to live and dead A.
fumigatus (3,20,32–34).
TNF-α and IL-1β are major pro-inflammatory cytokines, and IL-10 is
a key anti-inflammatory cytokine. Notably, TNF-α enhances the host
response to A. fumigatus during the early and later stages
of infection (4). In the present
study, THP-1-derived macrophages were stimulated with A.
fumigatus resting conidia following overexpression or silencing
of PU.1 expression. TNF-α and IL-1β levels increased significantly
and in a time-dependent manner in THP-1 cells following
overexpression of PU.1 and exposure to A. fumigatus, whereas
IL-10 exhibited no significant increase. By contrast, TNF-α and
IL-1β levels decreased significantly following PU.1 silencing. The
present study demonstrated that overexpression of PU.1 increased
Dectin-1, TLR-2 and TLR-4 expression, which led to increased
cytokine levels during A. fumigatus stimulation. As
expected, silencing of PU.1 led to a decrease in the levels of
these receptors and cytokines.
Dectin-1, TLR-2 and TLR-4 facilitate the recognition
of several morphological forms of A. fumigatus, including
swollen conidia, germinating conidia and hyphae (not resting
conidia) (4). A. fumigatus
conidia begin to swell at ~3 h following inhalation, and swollen
conidia are prerequisites for efficient phagocytosis and killing
(24,35). Therefore, the ability of
THP-1-derived macrophages to phagocytose resting conidia following
4 h of co-incubation was examined using confocal laser-scanning
microscopy in the present study. The results demonstrated that
phagocytosis was markedly enhanced in the Ad-PU.1 group when
compared with the control groups. By contrast, a reduction in
phagocytosis was observed in the siPU.1 group when compared with
the control group. Killing is commonly induced following conidia
phagocytosis (36). The killing
ability of THP-1-derived macrophages following overexpression or
silencing of PU.1 expression was examined further, and the results
demonstrated that the killing ability of the THP-1-derived
macrophages was significantly higher in the Ad-PU.1 group when
compared with the control groups. In the siPU.1 group, the killing
ability following conidia stimulation was lower following PU.1
silencing when compared with the control groups.
Previous studies have demonstrated that PU.1 induced
the expression of a number of genes that have critical roles in the
immune response to several infectious pathogens, including
Mycobacterium tuberculosis, pneumocystis, Epstein-Barr virus
and Ehrlichia chaffeensis (14,37–39).
In a previous in vivo study (40), decreased inflammatory cytokine
release and survival benefits were observed in mice with
macrophages that lacked functional PU.1, which were challenged with
lipopolysaccharide, a component of the outer membrane of
gram-negative bacteria. Thus, PU.1 serves a protective mechanistic
role in response to endotoxemia. To the best of our knowledge, the
present study was the first to demonstrate that PU.1 serves a role
in A. fumigatus infection. The results demonstrated that
PU.1 may function to induce pro-inflammatory cytokines, such as
TNF-α and IL-1β, and the anti-inflammatory cytokine IL-10. Of
particular note, excessive inflammation may negatively affect the
host; therefore, efficient protection against fungal infection
requires a fine-tuned balance between pro-inflammatory cytokines
and anti-inflammatory cytokines.
In conclusion, the present study constructed a
recombinant adenoviral vector containing the PU.1 gene and
generated an AM model that overexpressed and silenced PU.1
expression. The results demonstrated that Dectin-1, TLR-2 and TLR-4
expression were upregulated and downregulated following
overexpression and silencing of PU.1, respectively. It was
confirmed that overexpression of PU.1 promoted the release of
pro-inflammatory cytokines, and enhanced the phagocytosis and
killing ability of THP-1-derived macrophages during A.
fumigatus stimulation. Indeed, silencing of PU.1 led to a
reduction in the release of pro-inflammatory cytokines,
phagocytosis, and the killing ability of THP-1-derived macrophages.
Therefore, PU.1 may be an essential factor for defense against
A. fumigatus infection, and overexpression of PU.1 may be a
novel method for the prophylaxis or treatment of IPA.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81270064,
81200063 and 81200004).
References
|
1
|
Sugui JA, Kwon-Chung KJ, Juvvadi PR, Latge
JP and Steinbach WJ: Aspergillus fumigatus and Related Species.
Cold Spring Harb Perspect Med. 5:a197862015. View Article : Google Scholar
|
|
2
|
Cramer RA, Rivera A and Hohl TM: Immune
responses against Aspergillus fumigatus: What have we learned? Curr
Opin Infect Dis. 24:315–322. 2011. View Article : Google Scholar :
|
|
3
|
Xia D, Sun WK, Tan MM, Ding Y, Liu ZC, Li
P, Qian Q, Su X and Shi Y: An adenoviral vector encoding
full-length dectin-1 promotes aspergillus-induced innate immune
response in macrophages. Lung. 193:549–557. 2015. View Article : Google Scholar
|
|
4
|
Margalit A and Kavanagh K: The innate
immune response to Aspergillus fumigatus at the alveolar surface.
Fems Microbiol Rev. 39:670–687. 2015. View Article : Google Scholar
|
|
5
|
Werner JL, Metz AE, Horn D, Schoeb TR,
Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD and Steele C:
Requisite role for the dectin-1 beta-glucan receptor in pulmonary
defense against Aspergillus fumigatus. J Immunol. 182:4938–4946.
2009. View Article : Google Scholar :
|
|
6
|
Cunha C, Di Ianni M, Bozza S, Giovannini
G, Zagarella S, Zelante T, D'Angelo C, Pierini A, Pitzurra L,
Falzetti F, et al: Dectin-1 Y238X polymorphism associates with
susceptibility to invasive aspergillosis in hematopoietic
transplantation through impairment of both recipient- and
donor-dependent mechanisms of antifungal immunity. Blood.
116:5394–5402. 2010. View Article : Google Scholar
|
|
7
|
Hohl TM and Feldmesser M: Aspergillus
fumigatus: Principles of pathogenesis and host defense. Eukaryot
Cell. 6:1953–1963. 2007. View Article : Google Scholar :
|
|
8
|
Mehrad B, Strieter RM and Standiford TJ:
Role of TNF-alpha in pulmonary host defense in murine invasive
aspergillosis. J Immunol. 162:1633–1640. 1999.
|
|
9
|
Meier A, Kirschning CJ, Nikolaus T, Wagner
H, Heesemann J and Ebel F: Toll-like receptor (TLR) 2 and TLR4 are
essential for aspergillus-induced activation of murine macrophages.
Cell Microbiol. 5:561–570. 2003. View Article : Google Scholar
|
|
10
|
Bellocchio S, Montagnoli C, Bozza S,
Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM
and Romani L: The contribution of the toll-like/IL-1 Receptor
superfamily to innate and adaptive immunity to fungal pathogens in
vivo. J Immunol. 172:3059–3069. 2004. View Article : Google Scholar
|
|
11
|
Bochud PY, Chien JW, Marr KA, Leisenring
WM, Upton A, Janer M, Rodrigues SD, Li S, Hansen JA, Zhao LP, et
al: Toll-like receptor 4 polymorphisms and aspergillosis in
stem-cell transplantation. N Engl J Med. 359:1766–1777. 2008.
View Article : Google Scholar :
|
|
12
|
Ghisletti S, Barozzi I, Mietton F,
Polletti S, De Santa F, Venturini E, Gregory L, Lonie L, Chew A,
Wei CL, et al: Identification and characterization of enhancers
controlling the inflammatory gene expression program in
macrophages. Immunity. 32:317–328. 2010. View Article : Google Scholar
|
|
13
|
Serezani CH, Kane S, Collins L,
Morato-Marques M, Osterholzer JJ and Peters-Golden M: Macrophage
dectin-1 expression is controlled by leukotriene B4 Via a
GM-CSF/PU.1 axis. J Immunol. 189:906–915. 2012. View Article : Google Scholar :
|
|
14
|
Zhang C, Wang SH, Liao CP, Shao S, Lasbury
ME, Durant PJ and Lee CH: Downregulation of PU.1 leads to decreased
expression of dectin-1 in alveolar macrophages during pneumocystis
pneumonia. Infect Immun. 78:1058–1065. 2010. View Article : Google Scholar :
|
|
15
|
Shibata Y, Berclaz PY, Chroneos ZC,
Yoshida M, Whitsett JA and Trapnell BC: GM-CSF regulates alveolar
macrophage differentiation and innate immunity in the lung through
PU.1. Immunity. 15:557–567. 2001. View Article : Google Scholar
|
|
16
|
Haehnel V, Schwarzfischer L, Fenton MJ and
Rehli M: Transcriptional regulation of the human toll-like receptor
2 gene in monocytes and macrophages. J Immunol. 168:5629–5637.
2002. View Article : Google Scholar
|
|
17
|
Park SY, Lee SW, Baek SH, Lee CW, Lee WS,
Rhim BY, Hong KW and Kim CD: Suppression of PU.1-linked TLR4
expression by cilostazol with decrease of cytokine production in
macrophages from patients with rheumatoid arthritis. Br J
Pharmacol. 168:1401–1411. 2013. View Article : Google Scholar :
|
|
18
|
Moon HG, Yang J, Zheng Y and Jin Y:
MiR-15a/16 regulates macrophage phagocytosis after bacterial
infection. J Immunol. 193:4558–4567. 2014. View Article : Google Scholar :
|
|
19
|
Persat F, Noirey N, Diana J, Gariazzo MJ,
Schmitt D, Picot S and Vincent C: Binding of live conidia of
Aspergillus fumigatus activates in vitro-generated human langerhans
cells via a lectin of galactomannan specificity. Clin Exp Immunol.
133:370–377. 2003. View Article : Google Scholar :
|
|
20
|
Sun WK, Lu X, Li X, Sun QY, Su X, Song Y,
Sun HM and Shi Y: Dectin-1 is inducible and plays a crucial role in
Aspergillus-induced innate immune responses in human bronchial
epithelial cells. Eur J Clin Microbiol Infect Dis. 31:2755–2764.
2012. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Sun H, Xu XY, Tian XL, Shao HT, Wu XD,
Wang Q, Su X and Shi Y: Activation of NF-κB and respiratory burst
following Aspergillus fumigatus stimulation of macrophages.
Immunobiology. 219:25–36. 2014. View Article : Google Scholar
|
|
23
|
Heinekamp T, Schmidt H, Lapp K, Pähtz V,
Shopova I, Köster-Eiserfunke N, Krüger T, Kniemeyer O and Brakhage
AA: Interference of Aspergillus fumigatus with the immune response.
Semin Immunopathol. 37:141–152. 2015. View Article : Google Scholar
|
|
24
|
Ibrahim-Granet O, Philippe B, Boleti H,
Boisvieux-Ulrich E, Grenet D, Stern M and Latgé JP: Phagocytosis
and intracellular fate of Aspergillus fumigatus conidia in alveolar
macrophages. Infect Immun. 71:891–903. 2003. View Article : Google Scholar :
|
|
25
|
Dagenais TR and Keller NP: Pathogenesis of
Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol
Rev. 22:447–465. 2009. View Article : Google Scholar :
|
|
26
|
Turkistany SA and DeKoter RP: The
transcription factor PU.1 is a critical regulator of cellular
communication in the immune system. Arch Immunol Ther Exp (Warsz).
59:431–440. 2011. View Article : Google Scholar
|
|
27
|
Nakata K, Kanazawa H and Watanabe M: Why
does the autoantibody against granulocyte-macrophage
colony-stimulating factor cause lesions only in the lung?
Respirology. 11(Suppl): S65–S69. 2006. View Article : Google Scholar
|
|
28
|
Trapnell BC and Whitsett JA: Gm-CSF
regulates pulmonary surfactant homeostasis and alveolar
macrophage-mediated innate host defense. Annu Rev Physiol.
64:775–802. 2002. View Article : Google Scholar
|
|
29
|
Kanada S, Nishiyama C, Nakano N, Suzuki R,
Maeda K, Hara M, Kitamura N, Ogawa H and Okumura K: Critical role
of transcription factor PU.1 in the expression of CD80 and CD86 on
dendritic cells. Blood. 117:2211–2222. 2011. View Article : Google Scholar
|
|
30
|
Becker KL, Ifrim DC, Quintin J, Netea MG
and van de Veerdonk FL: Antifungal innate immunity: Recognition and
inflammatory networks. Semin Immunopathol. 37:107–116. 2015.
View Article : Google Scholar
|
|
31
|
Bonfim CV, Mamoni RL and Blotta MH: TLR-2,
TLR-4 and Dectin-1 expression in human monocytes and neutrophils
stimulated by paracoccidioides brasiliensis. Med Mycol. 47:722–733.
2009. View Article : Google Scholar
|
|
32
|
Braedel S, Radsak M, Einsele H, Latgé JP,
Michan A, Loeffler J, Haddad Z, Grigoleit U, Schild H and Hebart H:
Aspergillus fumigatus antigens activate innate immune cells via
toll-like receptors 2 and 4. Br J Haematol. 125:392–399. 2004.
View Article : Google Scholar
|
|
33
|
Mambula SS, Sau K, Henneke P, Golenbock DT
and Levitz SM: Toll-like receptor (TLR) signaling in response to
Aspergillus fumigatus. J Biol Chem. 277:39320–39326. 2002.
View Article : Google Scholar
|
|
34
|
Meier A, Kirschning CJ, Nikolaus T, Wagner
H, Heesemann J and Ebel F: Toll-like receptor (TLR) 2 and TLR4 are
essential for Aspergillus-induced activation of murine macrophages.
Cell Microbiol. 5:561–570. 2003. View Article : Google Scholar
|
|
35
|
Marr KA, Balajee SA, Hawn TR, Ozinsky A,
Pham U, Akira S, Aderem A and Liles WC: Differential role of MyD88
in macrophage-mediated responses to opportunistic fungal pathogens.
Infect Immun. 71:5280–5286. 2003. View Article : Google Scholar :
|
|
36
|
Philippe B, Ibrahim-Granet O, Prévost MC,
Gougerot-Pocidalo MA, Sanchez PM, Van der Meeren A and Latgé JP:
Killing of Aspergillus fumigatus by alveolar macrophages is
mediated by reactive oxidant intermediates. Infect Immun.
71:3034–3042. 2003. View Article : Google Scholar :
|
|
37
|
Zhang G, Zhou B, Li S, Yue J, Yang H, Wen
Y, Zhan S, Wang W, Liao M, Zhang M, et al: Allele-specific
induction of IL-1β expression by C/EBPβ and PU.1 contributes to
increased tuberculosis susceptibility. Plos Pathog.
10:e10044262014. View Article : Google Scholar :
|
|
38
|
Lin JH, Lin JY, Chou YC, Chen MR, Yeh TH,
Lin CW, Lin SJ and Tsai CH: Epstein-barr virus LMP2A suppresses MHC
class II expression by regulating the b-cell transcription factors
E47 and PU.1. Blood. 125:2228–2238. 2015. View Article : Google Scholar
|
|
39
|
Lin M and Rikihisa Y: Ehrlichia
chaffeensis downregulates surface toll-like receptors 2/4, CD14 and
transcription factors PU.1 and inhibits lipopolysaccharide
activation of NF-kappa BERK 1/2 and P38 MAPK in host monocytes.
Cell Microbiol. 6:175–186. 2004. View Article : Google Scholar
|
|
40
|
Karpurapu M, Wang X, Deng J, Park H, Xiao
L, Sadikot RT, Frey RS, Maus UA, Park GY, Scott EW and Christman
JW: Functional PU.1 in macrophages has a pivotal role in NF-κB
activation and neutrophilic lung inflammation during endotoxemia.
Blood. 118:5255–5266. 2011. View Article : Google Scholar :
|


















