Introduction
The brain uses glucose as a primary energy substrate
under physiological conditions, however, the brain is also capable
of oxidizing other intermediate metabolites, such as lactate
(1,2). The reduced glucose and oxygen levels
induced by ischemia causes a rapid reduction in ATP supplies by
increasing the rate of anaerobic glycolysis, which leads to lactate
accumulation, rather than initiating the respiratory chain reaction
by the tricarboxylic acid cycle (3,4). In
the brain, lactate is catalyzed by several monocarboxylate
transporters (MCTs), which rapidly transport lactate across the
plasma membrane (5). Among MCTs,
MCT1, MCT2 and MCT4 isoforms have been previously detected in the
brain (6). MCT1 has been
predominantly observed in the endothelial cells of microvessels and
also in glial cells (7–9). In contrast, MCT2 and MCT4 isoforms
have been observed in neurons and astrocytes (9–14).
Several studies have reported cell-specific changes in the
expression of MCTs occurring in different rat models of ischemia
(14–17). In a previous study of the authors,
MCT4 was indicated to be expressed in the pyramidal cells of the
gerbil hippocampus following the induction of transient forebrain
ischemia. In addition, the authors indicated that MCT4 may be one
of the factors that serves a key role in ischemic preconditioning
(13). However, there are few
other studies investigating the changes of hippocampal MCTs
isoforms following the induction of transient forebrain ischemia.
To improve the understanding of the role of MCT4 in transient
forebrain ischemia, the present study investigated the temporal and
spatial changes of MCT4 in the hippocampal CA1 region of the
Mongolian gerbil at several time points following ischemia.
Materials and methods
Experimental animals
A total of 85 male Mongolian gerbils (3-months-old;
50–60 g) were purchased from Japan SLC, Inc. (Shizuoka, Japan).
They were housed under standard conditions with adequate
temperature (22°C) and humidity (60%) control, a 12 h light/12 h
dark cycle and had free access to food and water. The handling and
care of the animals conformed to the guidelines established to
comply with current international laws and policies [NIH Guide for
the Care and Use of Laboratory Animals, NIH Publication No. 85–23,
1985, revised 1996 (18)], and
were approved by the Institutional Animal Care and Use Committee of
Seoul National University (Seoul, Korea). All of the experiments
were conducted with an effort to minimize the number of animals
used and the suffering caused by the procedures employed in the
present study.
Induction of transient forebrain
ischemia
The animals were anesthetized with a mixture of 2.5%
isoflurane (Hana Pharmaceutical Co., Ltd., Seoul, South Korea) in
33% oxygen and 67% nitrous oxide. Bilateral common carotid arteries
were isolated and occluded using non-traumatic aneurysm clips. The
complete interruption of blood flow was confirmed by observing the
central artery in retinae using an ophthalmoscope (HEINE
K180®; Heine Optotechnik, Herrsching, Germany).
Following 5 min of occlusion, the aneurysm clips were removed from
the common carotid arteries. Blockade of blood flow by occlusion of
the common carotid arteries was not performed in the animals of the
sham-operated group. The body (rectal) temperature under
free-regulating or normothermic (37±0.5°C) conditions was monitored
with a rectal temperature probe (TR-100; Fine Science Tools GmbH,
Foster City, CA, USA) and maintained using a thermometric blanket
prior to, during and following surgery until the animals completely
recovered from anesthesia. Thereafter, animals were kept on the
thermal incubator to maintain the body temperature of animals until
the animals were euthanized.
Immunohistochemistry
For histology, sham- and ischemia-operated animals
(n=5 per group) were anesthetized with 1 g/kg urethane
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at various time
points following ischemia/reperfusion and perfused transcardially
with 0.1 M PBS (pH 7.4), which was followed by 4% paraformaldehyde
in 0.1 M phosphate-buffered saline (pH 7.4). The brains were
removed and post-fixed in 4% paraformaldehyde for 12 h at 25°C. The
brain tissues were cryoprotected by infiltration with 30% sucrose
overnight at 4°C. Brain sections (30 µm) in coronal plane were
serially cut using a cryostat (Leica Microsystems GmbH, Wetzlar,
Germany). The sections were collected into 6-well plates containing
PBS for further processes.
To ensure that the immunohistochemical data were
comparable between groups, the sections were carefully processed
under the same conditions. The tissue sections were selected
between 1.4 mm and 2.0 mm posterior to the bregma in reference to a
gerbil brain atlas (19) for each
animal. A total of 5 sections per group, 90 µm apart from each
other, were sequentially treated with 0.3% hydrogen peroxide in PBS
for 30 min and 10% normal goat serum (Vector Laboratories, Inc.,
Burlingame, CA, USA) in 0.05 M PBS for 30 min at 25°C. They were
subsequently incubated with rabbit anti-MCT4 antibodies (1:200;
catalog no. sc-50329; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) overnight at 25°C, followed by treatment with biotinylated
goat anti-rabbit IgG for 2 h at 25°C (1:200; catalog no. BA-1000;
Vector Laboratories, Inc.) and a streptavidin-peroxidase complex
(catalog no. PK-6100; Vector Laboratories, Inc.). Sections were
subsequently visualized by reaction with 3,3′-diaminobenzidine
tetrachloride (Sigma-Aldrich; Merck KGaA) in 0.1 M Tris-HCl buffer
(pH 7.2) and mounted on gelatin-coated slides. Sections were
mounted in Canada Balsam (Kanto Chemical Co., Inc., Tokyo, Japan)
following dehydration.
Analysis of the regions of interest in the stratum
oriens, stratum pyramidale and stratum radiatum of the hippocampal
CA1 region was performed using an image analysis system and ImageJ
software version 1.50 (National Institutes of Health, Bethesda, MD,
USA). Images were calibrated into an array of 512×512 pixels
corresponding to a tissue area of 140×140 µm (x40 magnification).
Each pixel resolution was 256 gray levels. The intensity of MCT4
immunoreactivity was evaluated by relative optical density (ROD),
which was obtained following transformation of the mean gray level
using the following formula: ROD=log (256/mean gray level). ROD of
background was determined in unlabeled portions and this value was
subtracted for correction, yielding high ROD values in the presence
of preserved structures and low values following structural loss
using ImageJ software version 1.50 (National Institutes of Health).
A ratio of the ROD was calibrated as percentage compared with the
control.
Double immunofluorescence
To confirm the co-localization of MCT4 and glial
fibrillary acidic protein (GFAP) in the brain, sections taken 7
days following ischemia were processed under the same conditions as
the immunohistochemistry analysis prior to addition of the primary
antibody. Double immunofluorescence staining for rabbit anti-MCT4
(1:50) and mouse anti-GFAP (1:500; catalog no. AB5804; EMD
Millipore, Billerica, MA, USA) was performed. The sections were
incubated in the mixture of antisera overnight at 25°C. Following
three washes for 10 min each with PBS, they were subsequently
incubated in a mixture of Cy3-conjugated donkey anti-rabbit IgG
(1:600; catalog no. 711-165-152; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) and FITC-conjugated donkey
anti-mouse IgG (1:600; catalog no. 715-095-151; Jackson
ImmunoResearch Laboratories, Inc.) for 2 h at 25°C. The
immunoreactions were observed under a confocal microscope (LSM 510
META NLO; Zeiss GmbH, Jena, Germany).
Western blot analysis
To confirm the changes in the expression of MCT4 in
the hippocampus, animals in sham- and ischemia-operated animals
(n=5) were sacrificed and western blot analysis was performed.
Following sacrifice and removal of tissues, the tissues were cut
into 500 µm thick sections on a vibratome (Leica Microsystems GmbH)
and the hippocampus was cut out using a surgical blade. The
hippocampal tissues were homogenized in 50 mM PBS (pH 7.4)
containing 0.1 mM EGTA (pH 8.0), 0.2% Nonidet P-40, 10 mM EDTA (pH
8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM
sodium fluoride, 150 mM sodium chloride, 2 mM sodium orthovanadate,
1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol (DTT).
Following centrifugation for 5 min at 16,000 × g at 4°C, the
protein concentration was determined in the supernatants using a
Micro BCA protein assay kit with bovine serum albumin as the
standard (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Aliquots containing 20 µg of total protein were boiled in
loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS,
0.3% bromophenol blue and 30% glycerol. Each aliquot was
subsequently loaded onto a 12% polyacrylamide gel. Following
electrophoresis, the gels were transferred to nitrocellulose
membranes (Pall Life Sciences, Port Washington, NY, USA). To reduce
background staining, the membranes were incubated with 5% non-fat
dry milk in PBS containing 0.1% Tween-20 for 45 min at 25°C, which
was followed by incubation with peroxidase-conjugated rabbit
anti-MCT4 (1:500; catalog no. sc-50329, Santa Cruz Biotechnology,
Inc.) and rabbit anti- β actin (1:2,000; catalog no. ab8227; Abcam,
Cambridge, UK) overnight at 4°C and an ECL chemiluminescent kit
(Pierce; Thermo Fisher Scientific, Inc.). The blot was
densitometrically scanned for the quantification of ROD of each
band using Scion Image software version 4.0.3 (Scion Corporation.,
Frederick, MD). Expression was normalized against β-actin.
Statistical analysis
Data are presented as the mean + standard error of
the mean. The differences among means were analyzed by one-way
analysis of variance followed by Bonferroni's post-hoc correction
to compare the time-dependent changes in MCT4 immunoreactivity.
Analysis was performed using GraphPad Prism 5.01 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes of MCT4 immunoreactivity in
the hippocampal CA1 region
Microscopy images are presented in Fig. 1 and quantification of
immunoreactivity are presented in Fig.
2. MCT4 immunoreactivity was observed in the stratum pyramidale
of the CA1 region of the sham-operated group (Fig. 1A). At 30 min and 3 h after
ischemia, reduced MCT4 immunoreactivity was observed in the stratum
pyramidale compared with the immunoreactivity observed in the
sham-operated group (Figs. 1B and
C and 2). Between 6 and 24 h
after ischemia, MCT4 immunoreactivity levels increased
time-dependently in the CA1 region, however, the difference in the
MCT4 immunoreactivity was not statistically different between sham
and these groups (Figs. 1D-F and
2). MCT4 immunoreactivity was
significantly increased 2 days following ischemia in the stratum
pyramidale compared with the sham-operated group (Figs. 1G and 2). At 3 days following the treatment,
MCT4 immunoreactivity decreased significantly in the stratum
pyramidale compared with at 2 days following ischemia and was
barely detectable in this region (Figs. 1H and 2). At days 4 and 5 following ischemia,
MCT4 immunoreactivity increased in the strata oriens and radiatum
compared with sham-operated animals (Fig. 1I and J). However, few MCT4
immunoreactive structures were detected in the stratum pyramidale
(Fig. 1I and J). At 7 and 10 days
following ischemia, MCT4 immunoreactivity was observed in the
strata oriens, radiatum and pyramidale (Fig. 1K and L). In these groups, MCT4
immunoreactivity levels significantly increased compared with the
group at 5 days following ischemia (P<0.05; Fig. 2). Numerous MCT4 immunoreactive
structures were colocalized with activated (exhibiting a
hypertrophied cytoplasm) GFAP immunoreactive astrocytes in the CA1
region of the hippocampus (Fig.
3).
 | Figure 1.Immunohistochemical staining for MCT4
in the hippocampal CA1 region of sham-operated and operated groups.
(A) Sham-operated group. MCT4 staining in ischemia-operated group
at (B) 30 min, (C) 3 h, (D) 6 h, (E) 12 h, (F) 24 h, (G) 2 days,
(H) 3 days, (I) 4 days, (J) 5 days, (K) 7 days and (L) 10 days
following ischemia. MCT4 immunoreactivity is observed in the SP of
the sham-operated group. MCT4 exhibits strong immunoreactivity in
the SP 2 days following ischemia. However, MCT4 immunoreactivity is
decreased in the same region 3 days following ischemia. Thereafter,
MCT4 immunoreactivity is observed in the SO, SR and SP. Scale
bar=50 µm. MCT4, monocarboxylate transporter 4; h, hour; d, days;
SO, stratum oriens; SP, stratum pyramidale; SR, stratum
radiatum. |
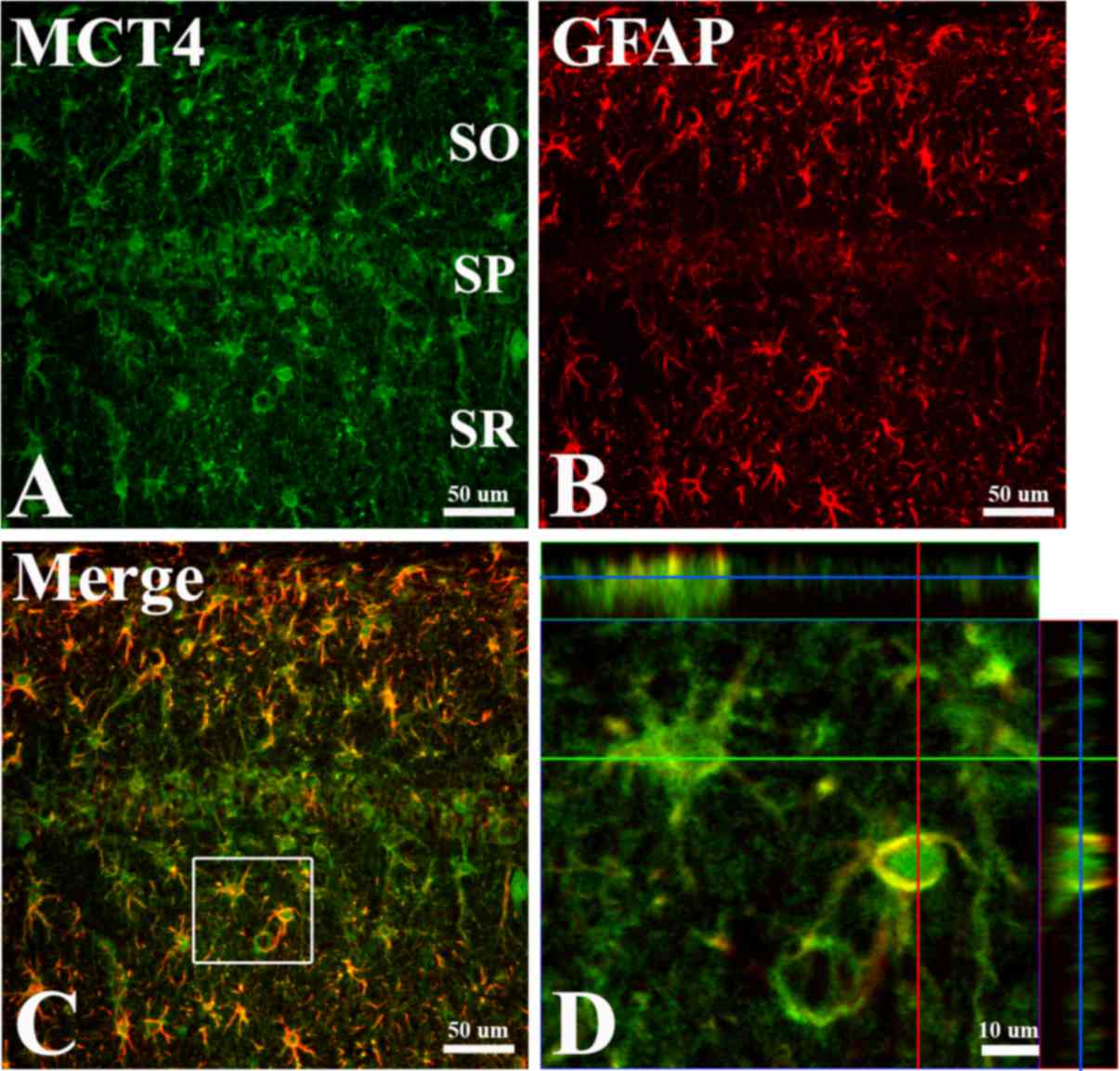 | Figure 3.Double immunofluorescence staining for
(A) MCT4, (B) GFAP, (C) merged images and (D) magnified image with
orthogonal configuration in the hippocampal CA1 region 7 days
following ischemia. Much of the MCT4 immunoreactivity is also
detected in the GFAP immunoreactive astrocytes in the CA1 region.
Green line, × axis; red line, y axis; blue line, position of the
central panel image in the z stack. (A-C) Scale bar=50 µm and (D)
scale bar=10 µm. MCT4, monocarboxylate transporter; GFAP, glial
fibrillary acidic protein SO, stratum oriens; SP, stratum
pyramidale; SR, stratum radiatum. |
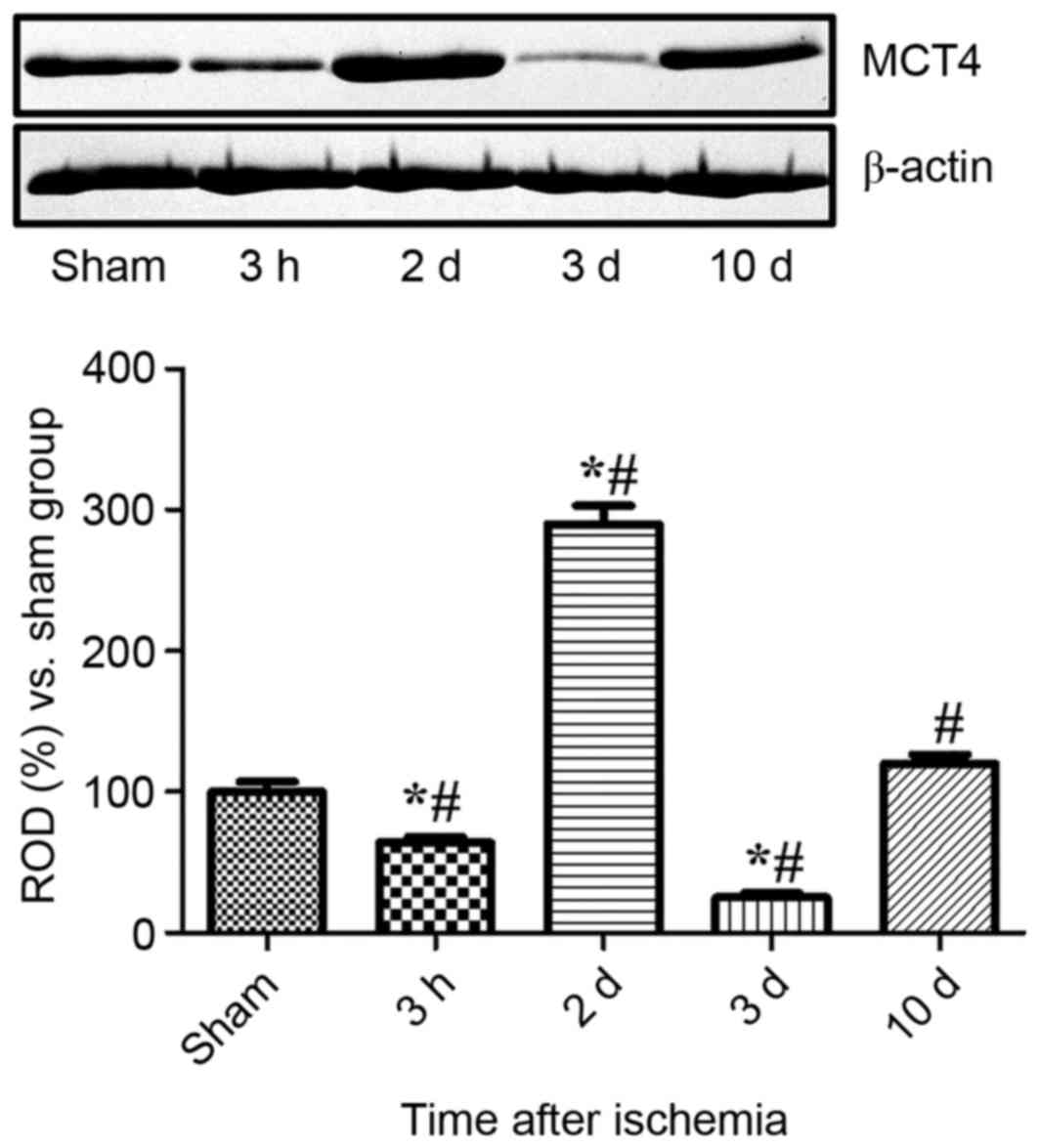
Changes of MCT4 protein levels in the
hippocampal CA1 region
MCT4 protein levels decreased significantly in
hippocampal CA1 homogenates 3 h following ischemia compared with
CA1 homogenates sampled from the sham-operated group (P<0.05).
The protein levels of MCT4 exhibited a significant 2.9-fold
increase 2 days following ischemia compared with the sham-operated
group, and a 4.5-fold increase at 2 days compared with the group at
3 h following ischemia. Only weak MCT4 bands were detected 3 days
after ischemia and MCT4 levels were 25% of the sham-operated group
levels. At 10 days after ischemia, MCT4 levels were marginally
higher in the experimental group compared the sham-operated group
(Fig. 4).
Discussion
Hypoxia-induced glycolysis promotes the synthesis of
lactate in the brain. Under this pathological condition, lactate
concentration in the extracellular space may undergo a 10-fold
increase relative to a control brain (20–22).
It has previously been suggested that lactate itself may be
considered an important energy substrate, and that it may have an
essential role in cellular metabolism (3) and memory formation (23,24).
Uptake of lactate by MCTs is operated by several isoforms of MCTs.
In particular, MCT4 is expressed in the hippocampal CA1 pyramidal
neurons of the Mongolian gerbil (13). The present study investigated the
spatial and temporal changes of MCT4 expression in the hippocampal
CA1 region. In the sham-operated group, MCT4 immunoreactivity was
observed in the pyramidal cells of the hippocampal CA1 region. This
result is consistent with findings previously reported by
colleagues of the authors (13).
In addition, MCT4 has been demonstrated as expressed in the neurons
of ob/ob obese mice, high-fat diet fed mice and also
db/db diabetic mice (11). However, MCT4 immunoreactivity is
observed in the astrocytes of control rats and mice (9–12).
This observation may be associated with characteristic factors in
plasma lipid response to dietary carbohydrate and fatty acids
previously reported in Mongolian gerbils; Mongolian gerbils have
been reported to be more sensitive to fatty acids in the presence
and absence of dietary cholesterol (25–27).
The present study observed the fluctuation of MCT4
levels and immunoreactivity following the induction of transient
forebrain ischemia. MCT4 levels were significantly increased 2 days
after ischemia in the CA1 pyramidal cells and significantly
decreased in this region 3 days after ischemia, compared with the
sham-operated group. The sharp decrease in MCT4 levels observed in
the hippocampal CA1 region may be associated with the
ischemia-induced neuronal damage that has been previously detected
in the hippocampal CA1 region. Inhibition of lactate transport
induced by blocking of MCT has been reported to exacerbate neuronal
damage in different rat models of cerebral ischemia (28,29),
while ischemic preconditioning significantly increased MCT4
immunoreactivity in the pyramidal cells of the CA1 region (13). Following the ischemia-induced
decrease in MCT4 levels, MCT4 immunoreactivity was increased in the
astrocytes of the CA1 region. The initial decrease in MCT4
expression may be associated with the increased use of MCT4 aimed
at reducing the ischemia-induced increase in lactate levels. The
subsequent rise in MCT4 levels may be interpreted as a compensatory
response to the ischemic damage. The elevated levels of MCT4 may be
due to an increase in the synthesis of MCT4 in the pyramidal cells
and an upregulation of MCT4 activity in astrocytes caused by
ischemia-induced neuronal death. The results are supported by
previous studies that demonstrated that MCT4 immunoreactivity is
increased 1 h post-ischemia and is no longer detected 24 h after
the treatment, which suggests neuronal death in the brain by middle
cerebral artery occlusion (14).
At 5 days following ischemia, MCT4 immunoreactivity is
significantly increased within and around the infarct zone of
permanent focal ischemia in rats (16).
In conclusion, the results of the present study
indicated that MCT4 is expressed in the pyramidal cells of the
hippocampal CA1 region of the Mongolian gerbil, and the location
and immunoreactivity of MCT4 undergo time-dependent changes that
aim to remove or process the lactate in the CA1 region. Acute
reduction in MCT4 may be associated with neuronal damage in the
hippocampal CA1 region. The present study demonstrated that
time-dependent expressional changes in MCT4 after transient
ischemia may be involved in lactate metabolism after ischemic
damage in the hippocampus.
Acknowledgements
The authors would like to thank to Dr Seung-Hae Kwon
of the Korean Basic Science Institute Chuncheon Center (Daejeon,
Korea) for technical assistance with the confocal image analyses.
This work was supported by the Basic Science Research Program
through the National Research Foundation of Korea, funded by the
Ministry of Education (grant no. NRF-2013R1A1A2059364). In
addition, the present study was supported by the Research Institute
for Veterinary Science of Seoul National University.
References
|
1
|
Boumezbeur F, Petersen KF, Cline GW, Mason
GF, Behar KL, Shulman GI and Rothman DL: The contribution of blood
lactate to brain energy metabolism in humans measured by dynamic
13C nuclear magnetic resonance spectroscopy. J Neurosci.
30:13983–13991. 2010. View Article : Google Scholar :
|
|
2
|
Wyss MT, Jolivet R, Buck A, Magistretti PJ
and Weber B: In vivo evidence for lactate as a neuronal energy
source. J Neurosci. 31:7477–7485. 2011. View Article : Google Scholar
|
|
3
|
Schurr A: Lactate: The ultimate cerebral
oxidative energy substrate? J Cereb Blood Flow Metab. 26:142–152.
2006. View Article : Google Scholar
|
|
4
|
Brooks GA: Lactate: Link between
glycolytic and oxidative metabolism. Sports Med. 37:341–343. 2007.
View Article : Google Scholar
|
|
5
|
Halestrap AP and Price NT: The
proton-linked monocarboxylate transporter (MCT) family: Structure,
function and regulation. Biochem J 343 Pt. 2:281–299. 1999.
View Article : Google Scholar
|
|
6
|
Pierre K and Pellerin L: Monocarboxylate
transporters in the central nervous system: Distribution,
regulation and function. J Neurochem. 94:1–14. 2005. View Article : Google Scholar
|
|
7
|
Gerhart DZ, Enerson BE, Zhdankina OY,
Leino RL and Drewes LR: Expression of monocarboxylate transporter
MCT1 by brain endothelium and glia in adult and suckling rats. Am J
Physiol. 273:E207–E213. 1997.
|
|
8
|
Hanu R, McKenna M, O'Neill A, Resneck WG
and Bloch RJ: Monocarboxylic acid transporters, MCT1 and MCT2, in
cortical astrocytes in vitro and in vivo. Am J Physiol Cell
Physiol. 278:C921–C930. 2000.
|
|
9
|
Pierre K, Pellerin L, Debernardi R,
Riederer BM and Magistretti PJ: Cell-specific localization of
monocarboxylate transporters, MCT1 and MCT2, in the adult mouse
brain revealed by double immunohistochemical labeling and confocal
microscopy. Neuroscience. 100:617–627. 2000. View Article : Google Scholar
|
|
10
|
Rafiki A, Boulland JL, Halestrap AP,
Ottersen OP and Bergersen L: Highly differential expression of the
monocarboxylate transporters MCT2 and MCT4 in the developing rat
brain. Neuroscience. 122:677–688. 2003. View Article : Google Scholar
|
|
11
|
Pierre K, Parent A, Jayet PY, Halestrap
AP, Scherrer U and Pellerin L: Enhanced expression of three
monocarboxylate transporter isoforms in the brain of obese mice. J
Physiol. 583:469–486. 2007. View Article : Google Scholar :
|
|
12
|
Gao C, Wang C, Liu B, Wu H, Yang Q, Jin J,
Li H, Dong S, Gao G and Zhang H: Intermittent hypoxia
preconditioning-induced epileptic tolerance by upregulation of
monocarboxylate transporter 4 expression in rat hippocampal
astrocytes. Neurochem Res. 39:2160–2169. 2014. View Article : Google Scholar
|
|
13
|
Hong S, Ahn JY, Cho GS, Kim IH, Cho JH,
Ahn JH, Park JH, Won MH, Chen BH, Shin BN, et al: Monocarboxylate
transporter 4 plays a significant role in the neuroprotective
mechanism of ischemic preconditioning in transient cerebral
ischemia. Neural Regen Res. 10:1604–1611. 2015. View Article : Google Scholar :
|
|
14
|
Rosafio K, Castillo X, Hirt L and Pellerin
L: Cell-specific modulation of monocarboxylate transporter
expression contributes to the metabolic reprograming taking place
following cerebral ischemia. Neuroscience. 317:108–120. 2016.
View Article : Google Scholar
|
|
15
|
Tseng MT, Chan SA and Schurr A:
Ischemia-induced changes in monocarboxylate transporter 1 reactive
cells in rat hippocampus. Neurol Res. 25:83–86. 2003. View Article : Google Scholar
|
|
16
|
Zhang F, Vannucci SJ, Philp NJ and Simpson
IA: Monocarboxylate transporter expression in the spontaneous
hypertensive rat: Effect of stroke. J Neurosci Res. 79:139–145.
2005. View Article : Google Scholar
|
|
17
|
Moreira TJ, Pierre K, Maekawa F, Repond C,
Cebere A, Liljequist S and Pellerin L: Enhanced cerebral expression
of MCT1 and MCT2 in a rat ischemia model occurs in activated
microglial cells. J Cereb Blood Flow Metab. 29:1273–1283. 2009.
View Article : Google Scholar
|
|
18
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. 7th. National Academy Press;
Washington, DC: 1996
|
|
19
|
Loskota WA, Lomax P and Verity MA: A
stereotaxic atlas of the Mongolian Gerbil Brain (Meriones
unguiculatus). Ann Arbor Science Publishers Inc.; Ann Arbor: pp.
70–79. 1974
|
|
20
|
Rehncrona S, Rosén I and Siesjö BK: Brain
lactic acidosis and ischemic cell damage: 1. Biochemistry and
neurophysiology. J Cereb Blood Flow Metab. 1:297–311. 1981.
View Article : Google Scholar
|
|
21
|
Folbergrová J, Memezawa H, Smith ML and
Siesjö BK: Focal and perifocal changes in tissue energy state
during middle cerebral artery occlusion in normo- and hyperglycemic
rats. J Cereb Blood Flow Metab. 12:25–33. 1992. View Article : Google Scholar
|
|
22
|
Wagner KR, Kleinholz M, de Courten-Myers
GM and Myers RE: Hyperglycemic versus normoglycemic stroke:
Topography of brain metabolites, intracellular pH, and infarct
size. J Cereb Blood Flow Metab. 12:213–222. 1992. View Article : Google Scholar
|
|
23
|
Bouzier-Sore AK, Voisin P, Canioni P,
Magistretti PJ and Pellerin L: Lactate is a preferential oxidative
energy substrate over glucose for neurons in culture. J Cereb Blood
Flow Metab. 23:1298–1306. 2003. View Article : Google Scholar
|
|
24
|
Newman LA, Korol DL and Gold PE: Lactate
produced by glycogenolysis in astrocytes regulates memory
processing. PLoS One. 6:e284272011. View Article : Google Scholar :
|
|
25
|
Mercer NJ and Holub BJ: Response of free
and esterified plasma cholesterol levels in the Mongolian gerbil to
the fatty acid composition of dietary lipid. Lipids. 14:1009–1014.
1979. View Article : Google Scholar
|
|
26
|
Andersen DB and Holub BJ: Effects of
dietary cholesterol level and type of dietary carbohydrate on
hepatic and plasma sterols in the gerbil. Can J Physiol Pharmacol.
60:885–892. 1982. View
Article : Google Scholar
|
|
27
|
Dictenberg JB, Pronczuk A and Hayes KC:
Hyperlipidemic effects of trans fatty acids are accentuated by
dietary cholesterol in gerbils. J Nutr Biochem. 6:353–361. 1995.
View Article : Google Scholar
|
|
28
|
Schurr A, Payne RS, Miller JJ, Tseng MT
and Rigor BM: Blockade of lactate transport exacerbates delayed
neuronal damage in a rat model of cerebral ischemia. Brain Res.
895:268–272. 2001. View Article : Google Scholar
|
|
29
|
Wang Y, Guo SZ, Bonen A, Li RC,
Kheirandish-Gozal L, Zhang SX, Brittian KR and Gozal D:
Monocarboxylate transporter 2 and stroke severity in a rodent model
of sleep apnea. J Neurosci. 31:10241–10248. 2011. View Article : Google Scholar :
|