Introduction
Cyclooxygenases (COXs) are major inflammatory
mediators that catalyze the production of thromboxane and
prostaglandins from arachidonic acid. COXs consist of COX-1 and
COX-2, and they possess 65% amino acid sequence homology and
virtually identical catalytic sites (1). Among these, COX-2 has attracted the
majority of attention, as it is induced by inflammation. COX-2 is
constitutively expressed in specific organs, including the brain,
thymus, gut and kidneys (2), and
constitutive expression of COX-2 in the brain is considered to
serve a major role in synaptic plasticity, with prostaglandins
generated by COX-2 modulating local cerebral blood flow and
learning (3–6). Previous studies have demonstrated
that treadmill exercise significantly increases neurogenesis and
COX-2 immunoreactivity in the rat hippocampus (7,8). In
addition, the pharmacological or genetic inhibition of COX-2
significantly reduces constitutive COX-2 immunoreactivity, as well
as the number of differentiated neuroblasts in the hippocampus
(7,9).
As life expectancy continues to increase, ~20–30% of
the population are likely to be >65 years of age by the year
2030 in the USA (10–12). Therefore, it is important to
understand the alterations in physiological parameters and synaptic
plasticity that occur during normal healthy aging, not just in
neurodegenerative conditions. A number of previous studies have
demonstrated that synaptic plasticity significantly decreases with
increasing age in healthy animals (13–15).
Accumulating evidence has demonstrated that COX-2 is
significantly upregulated in the brain of patients with Alzheimer's
disease (16,17). The administration of non-steroidal
anti-inflammatory drugs reduces the risk of Alzheimer's disease
(18–20). However, there are a limited number
of studies investigating constitutive COX-2 immunoreactivity and
protein levels in the hippocampus during aging of healthy
individuals. Therefore, the aim of the present study was to
investigate age-associated alterations in COX-2 immunoreactivity
and protein levels in the hippocampus in naive healthy mice.
Materials and methods
Experimental animals
A total of 50 male mice [postnatal month (PM)1;
14–17 g, PM3; 25–28 g, PM6; 28–32 g, PM12; 31–36 g, PM24; 30–34 g]
were purchased from Japan SLC Inc. (Shizuoka, Japan). They were
housed under standard conditions with adequate temperature (22°C)
and humidity (60%) control, 12-h light/12-h dark cycles and access
to food and water ad libitum. The handling and care of the
animals conformed to the guidelines established to comply with
current international laws and policies (National Institutes of
Health Guide for the Care and Use of Laboratory Animals, NIH
Publication No. 85-23, 1985, revised 1996) and were approved by the
Institutional Animal Care and Use Committee of Seoul National
University (Seoul, Republic of Korea). Animals were equally divided
into the following 5 groups (n=10 in each group): PM1, PM3, PM6,
PM12 and PM24 groups. All experiments were conducted in an effort
to minimize the number of animals used and the suffering caused by
the procedures employed.
Blood sampling
The blood from mice in each group was used for
immunohistochemical and western blot analyses, and was retrieved
from the retro-orbital sinus for collection in blood collection
tubes containing 3.8% sodium citrate. Total blood cell counts were
measured using the HEMAVET® 950 (Drew Scientific Inc.,
Miami Lakes, FL, USA) within 5 h after collection. The analyzer
required a volume of 20 µl of whole blood for a successful
measurement; therefore, 50–60 µl of blood was collected. Blood
sample was maintained at 25°C for at least 5 min prior to
measurement in order to stabilize the cells.
Tissue processing
For histological analysis, the animals (n=5 in each
group) at PM1, 3, 6, 12 and 24 were anesthetized with
intraperitoneal injection of 1.5 g/kg urethane (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and the blood was retrieved from
the retro-orbital sinus and collected in tubes containing 3.8%
sodium citrate. The animals were perfused transcardially with 0.1 M
phosphate-buffered saline (PBS, pH 7.4) followed by 4%
paraformaldehyde in 0.1 M PBS (pH 7.4). The brains were removed and
postfixed in the same fixative at 25°C for 12 h prior to undergoing
cryoprotection via overnight storage in 30% sucrose. Serial coronal
brain sections (30-µm in thickness) were generated using a cryostat
(Leica Microsystems GmbH, Wetzlar, Germany) and transferred to
6-well plates containing PBS for further processing.
Immunohistochemistry
In order to ensure that the immunohistochemical data
were comparable between groups, sections were carefully processed
under parallel conditions. Tissue sections located at a distance of
90 µm from each other were selected from an area between 1.46 and
2.46 mm posterior to the bregma, as defined by a mouse atlas
(21). A total of 10 samples of
tissue sections located at a distance of 90 µm from each other were
sequentially incubated with 0.3% hydrogen peroxide in PBS for 30
min and 10% normal goat serum (S-1000, Vector Laboratories, Inc.,
Burlingame, CA, USA) in 0.05 M PBS for 30 min at 25°C. Sections
were then incubated with a rabbit anti-COX-2 antibody (dilution,
1:200; cat. no. 160126; Cayman Chemical Company, Ann Arbor, MI,
USA) overnight at room temperature. Sections were then incubated
with biotinylated goat anti-rabbit IgG secondary antibody
(dilution, 1:200; cat. no. BA-1000; Vector Laboratories, Inc.) for
2 h at 25°C, followed by a streptavidin-peroxidase complex
(dilution, 1:200; cat. no. SA-5004; Vector Laboratories, Inc.) for
1 h at room temperature. Immunostaining was visualized by reaction
with 3,3′-diaminobenzidine (1 mg/ml in 0.1 M Tris-HCl buffer; pH
7.2). Sections were dehydrated and mounted on gelatin-coated slides
in Canada balsam (Kanto Chemical, Co., Inc. Tokyo, Japan).
Analysis of the hippocampal CA2/3 region and dentate
gyrus was performed using an image analysis system and ImageJ
software (version 1.50; National Institutes of Health, Bethesda,
MD, USA). Digital images of the mid-point of each region were
captured using an Olympus BX51 light microscope (Olympus
Corporation, Tokyo, Japan) equipped with a digital camera (DP72;
Olympus Corporation) connected to a computer monitor. Images were
calibrated into an array of 512×512 pixels corresponding to a
tissue area of 1,200×900 µm (primary magnification, ×100). Each
pixel resolution was 256 gray levels and the intensity of COX-2
immunoreactivity was evaluated by the relative optical density
(ROD), which was obtained following transformation of the mean gray
level using the following formula: ROD=log10 (256/mean
gray level). The ROD of background staining was determined using
the unlabeled portions of the sections using Photoshop CC 2015
software (Adobe Systems Inc., San Jose, CA, USA), and this value
was subtracted to correct for nonspecific staining using ImageJ
software (version, 1.50; National Institutes of Health). Data are
expressed as a percentage of the PM1 group values (set to
100%).
Western blot analysis
To quantify alterations in COX-2 expression levels
in the hippocampus, animals were euthanized with intraperitoneal
injection using 1.5 g/kg urethane (Sigma-Aldrich; Merck KGaA) at
PM1, 3, 6, 12 and 24 (n=5 from each group) and their brains were
removed. Tissues were dissected for use in western blot analysis.
Briefly, brain tissue sections (500-µm in thickness) were produced
using a vibratome (Leica Microsystems GmbH) and the hippocampal
region was dissected out using a surgical blade. Hippocampal
tissues were homogenized in 50 mM PBS (pH 7.4), containing 0.1 mM
ethylene glycol-bis (2-aminoethylether)-N,N,N', N'-tetraacetic acid
(pH 8.0), 0.2% Nonidet P-40, 10 mM ethylenediaminetetraacetic acid
(pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50
mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM
phenylmethylsulfonyl fluoride and 1 mM dithiothreitol (DTT).
Following centrifugation for 5 min at 16,000 × g at 4°C, the
protein levels in the supernatants were determined using a Micro
BCA Protein assay kit (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocols.
Aliquots containing 20 µg total protein were denatured by boiling
in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6%
sodium dodecyl sulfate, 0.3% bromophenol blue and 30% glycerol.
Each aliquot was loaded onto a 12% polyacrylamide gel. Following
electrophoresis, proteins were transferred to nitrocellulose
membranes (Pall Corporation, Port Washington, NY, USA), which were
then blocked in 5% non-fat dry milk in PBS containing 0.1% Tween 20
for 45 min at 25°C. Membranes were subsequently incubated with a
rabbit anti-COX-2 antibody (dilution, 1:500; cat. no. 160126;
Cayman Chemical Company) overnight at 4°C. Detection was performed
using peroxidase-conjugated anti-rabbit IgG (dilution 1:200; cat.
no. PI-1000; Vector Laboratories, Inc.) for 2 h at 25°C and an
enhanced luminol-based chemiluminescent kit (Pierce; Thermo Fisher
Scientific, Inc.) for 1 min. The blots were scanned, and
densitometry analysis was performed using Scion Image software
(version 4.0.3; Scion Corporation, Walkersville, MD, USA). Blots
were stripped and reprobed with an antibody against β-actin
(dilution 1:2,000; cat. no. ab8227; Abcam, Cambridge, UK) overnight
at 4°C as an internal loading control. Data were normalized to the
β-actin level in each lane.
Statistical analysis
Data are presented as the mean ± standard error.
Differences among the means of each group were analyzed by one-way
analysis of variance followed by a Bonferroni's post hoc test using
GraphPad Prism software (version 5.01; GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Age-associated alterations in white
blood cell counts
The total white blood cell count began to decrease
from PM12, and was significantly decreased in the PM24 group when
compared with the PM1 group (Table
I). Similarly, the lymphocyte count was significantly decreased
in the PM24 group when compared with that of the PM1, PM3, PM6 and
PM12 groups. By contrast, monocyte, neutrophil, eosinophil and
basophil counts demonstrated a tendency to increase in the PM24
group, however, this did not reach statistical significance when
compared with the other groups (Table
I).
 | Table I.Age-associated alterations in white
blood cell counts in C57BL/6 mice. |
Table I.
Age-associated alterations in white
blood cell counts in C57BL/6 mice.
| Cell count
(cells/µ1) | PM1 | PM3 | PM6 | PM12 | PM24 |
|---|
| White blood
cells | 3,282±296 | 3,101±263 | 3,158±285 | 2,869±259 |
2,471±301a |
| Lymphocytes | 2,820±275 | 2,571±284 | 2,683±280 | 2,410±273 |
1,629±225a–d |
| Monocytes | 52.3±7.4 | 46.9±6.9 | 58.4±8.2 | 62.1±8.5 |
99.7±19.4 |
| Neutrophils | 385.6±48.2 | 401.3±41.7 | 418.7±49.8 | 488.1±63.8 |
720.8±115.2 |
| Eosinophils |
13.6±1.79 |
14.5±2.02 |
12.7±1.75 |
13.3±2.08 |
14.2±2.38 |
| Basophils |
3.1±1.3 |
3.7±1.1 |
3.5±1.6 |
4.3±1.3 |
5.6±1.5 |
COX-2 immunoreactivity
In the PM1 group, COX-2 immunoreactivity was
identified in particular granule cells of the dentate gyrus in
addition to the pyramidal cells of the hippocampal CA2/3 region
(Fig. 1A). In the PM3 to PM12
groups, the pattern of COX-2 immunoreactivity distribution was
similar to that observed in the PM1 group. However, COX-2
immunoreactivity in the dentate gyrus was significantly decreased
in the PM3 group when compared with the PM1 group (Fig. 1). COX-2 immunoreactivity was
increased in the dentate gyrus of the PM6 group when compared with
the PM3 group; however, COX-2 immunoreactivity was lower in the
dentate gyrus of the PM6 group when compared with the PM1 group
(Fig. 1). In the PM12 group, COX-2
immunoreactivity in the dentate gyrus was similar to that of the
PM6 group (Fig. 1). However, COX-2
immunoreactivity in the CA2/3 region was not significantly altered
in the PM1 to PM12 group. In the PM24 group, COX-2 immunoreactivity
was significantly decreased in the dentate gyrus and the
hippocampal CA2/3 region when compared to the other groups
(Fig. 1). In this group, weak
COX-2 immunoreactivity was identified in the hippocampal CA1
region.
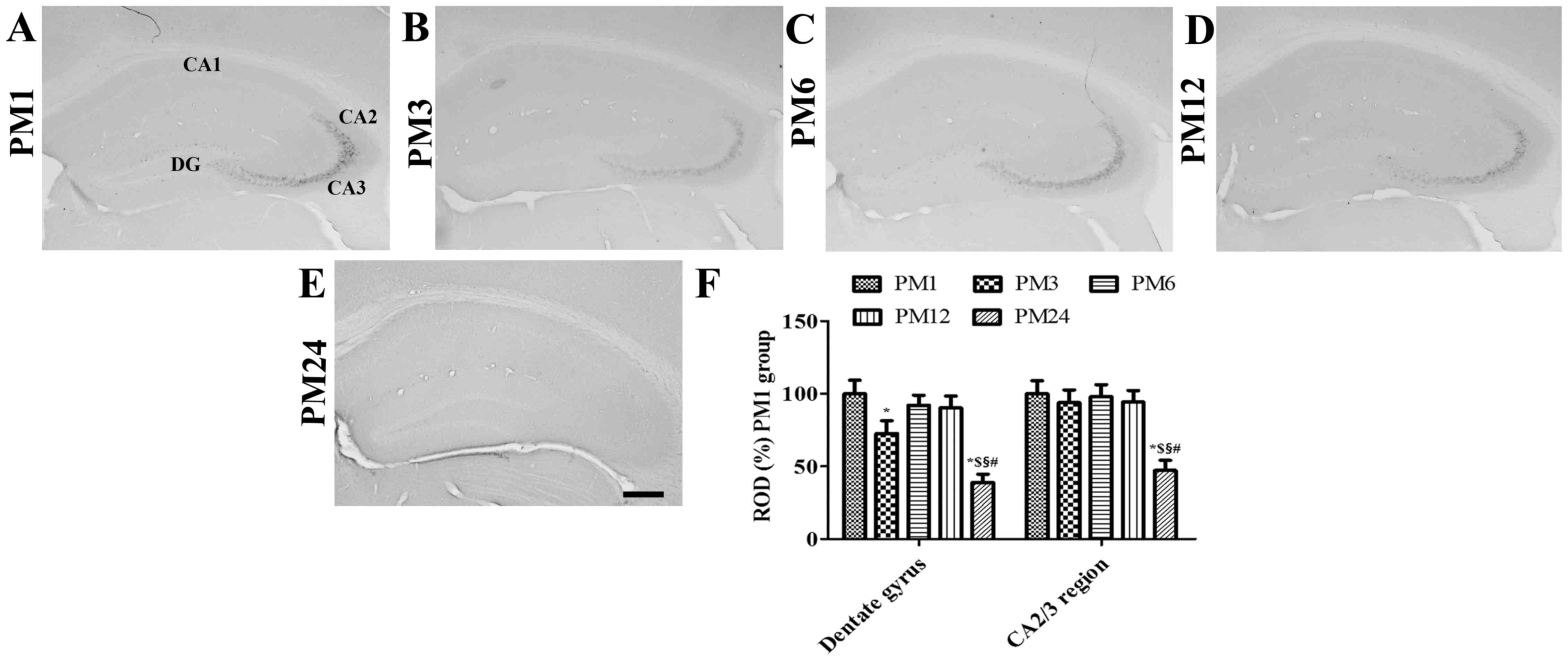 | Figure 1.Immunohistochemical staining for COX-2
in the mouse hippocampus in the (A) PM1, (B) PM3, (C) PM6, (D) PM12
and (E) PM24 groups (scale bar, 200 µm). COX-2 immunoreactivity was
constitutively observed in the granule cell layer of the DG and the
SP of the hippocampal CA2/3 region. Of note, COX-2 immunoreactivity
was significantly decreased in these regions in the PM24 group. (F)
ROD values for each section are expressed as a percentage of the
COX-2 immunoreactivity detected in the dentate gyrus and
hippocampal CA2/3 region of mice in the PM1 group (n=5/group;
*P<0.05 vs. PM1 group; $P<0.05 vs. PM3 group;
§P<0.05 vs. PM6 group; #P<0.05 vs. PM12
group). Data are presented as the mean ± standard error. COX-2,
cyclooxygenase-2; ROD, relative optical density; PM, postnatal
month; DG, dentate gyrus; SP, stratum pyramidale. |
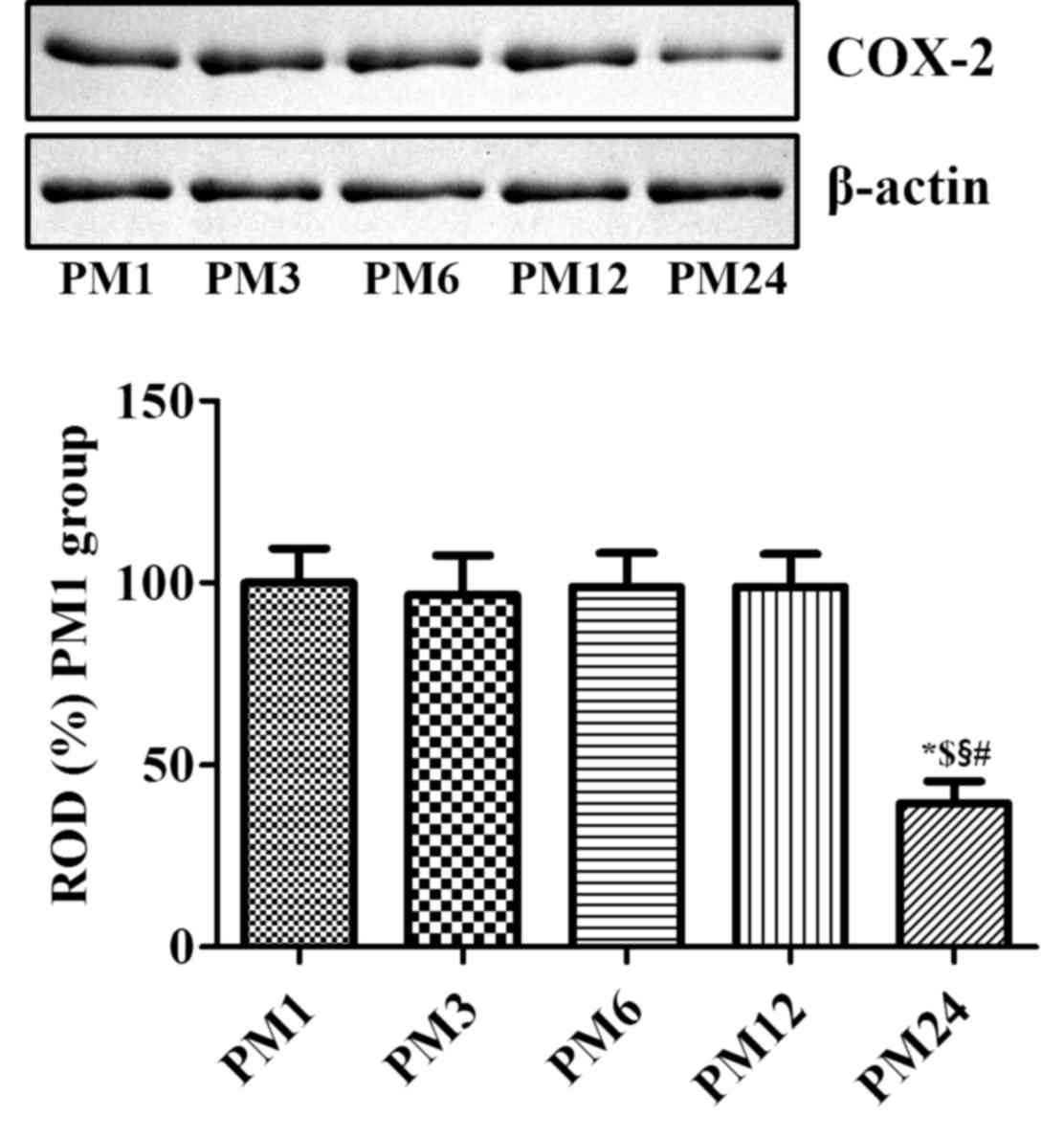
COX-2 protein levels
Western blot analysis of brain tissue sections from
mice in the PM1, PM3, PM6, PM12 and PM24 groups demonstrated
results similar to the immunohistochemical analysis of COX-2
expression in the hippocampus. COX-2 protein levels were
significantly decreased in the hippocampal homogenates of the PM24
group when compared with the other groups (Fig. 2).
Discussion
COX-2 is an inducible inflammatory mediator, which
is constitutively expressed in the brain, kidney, gut and thymus
(2). In particular, the
constitutive expression of COX-2 is enriched in the hippocampus and
cortex (3). In the brain, basal
expression of COX-2 is regulated by N-methyl-D-aspartate
receptor-dependent synaptic activity. In addition, induction of
long-term potentiation by high-frequency stimulation increases
COX-2 expression (22).
Previous studies have demonstrated that constitutive
COX-2 expression is closely associated with neuroblast
differentiation in the dentate gyrus (7,9). The
present study observed age-associated alterations in constitutive
COX-2 expression in the hippocampus. COX-2 immunoreactivity was
identified in the granule cells of the dentate gyrus, and the
pyramidal cells of the hippocampal CA2/3 region. This result is
consistent with previous studies involving mice and rats (7,9). In
humans, significant neuronal COX-2 immunoreactivity has been
identified in the CA3 region, subiculum, entorhinal cortex and
transentorhinal cortex (17). The
present study demonstrated that COX-2 immunoreactivity was
significantly decreased in the dentate gyrus and hippocampal CA2/3
region of PM24 mice when compared with younger mice. This result
was supported by those of a previous study, which demonstrated that
COX protein levels are reduced in the aged (18 months of age)
cortex of male and female rats when compared with sex-matched young
(3 months of age) rats (23). In
addition, COX-2 mRNA levels were observed to be significantly
decreased at PM30, and not at PM24, in hippocampal homogenates,
with a 2-fold increase in brain thromboxane B2 levels in
PM24 and PM30 groups (24).
However, in the female rhesus monkey, COX-2 protein levels in the
hippocampus were maintained at a constant level with increasing
age, while COX-2 protein levels were significantly decreased in the
frontal pole of middle-aged (8–11 years of age) rhesus monkeys when
compared with younger (2–5 years of age) monkeys (25).
A previous study revealed a significant increase in
COX-2 immunoreactivity in the gerbil hippocampal CA1 region in PM18
and PM24 groups when compared with PM1, PM3, PM6 or PM12 groups
(26). However, this increase in
COX-2 immunoreactivity was not observed in additional brain
regions. These results contrast those of the present study, and may
be associated with the inflammatory status of the animals. The
present study confirmed that mice were not in a state of
inflammation using white blood cell analysis. All white blood cell
parameters were within the normal range in the PM24 group, although
the lymphocyte counts were at baseline levels (27,28).
The parameters were similar to those identified in a previous study
(29), which demonstrated that
total white blood cell and lymphocyte counts decrease with age,
while neutrophil, monocyte and eosinophil counts increase in mice.
However, in humans, lymphocyte counts are significantly decreased
in the first two decades and remain constant for the following
three decades, and demonstrate a more prominent decrease thereafter
(30).
In the present study, the observed decrease in COX-2
immunoreactivity in the hippocampus of aged mice may have been
associated with decreased synaptic plasticity. Previous studies
have identified that adult neurogenesis decreases with age
(31–34). In addition, it was demonstrated
that treadmill exercise increased neural plasticity and COX-2
expression in the dentate gyrus (7). Furthermore, the concentration of
arachidonic acid in the cell membrane was significantly decreased
in the hippocampus of aged (22 months of age) rats compared with
that of younger adults (4 months of age) (35). Treatment with arachidonic acid
ameliorated the age-associated impairments of long-term
potentiation (35).
In conclusion, constitutive COX-2 expression was
identified in the granule cells of the dentate gyrus and pyramidal
cells of the hippocampal CA2/3 region in PM1, PM3, PM6, PM12 and
PM24 mice. COX-2 expression was significantly reduced in these
regions in PM24 mice in the absence of any significant increases in
the white blood cell count, when compared with younger mice. These
results suggest that a reduction in constitutive COX-2 expression
may be correlated with an age-associated decrease in synaptic
plasticity in the hippocampus.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education (grant no.
NRF-2013R1A1A2059364). In addition, the current study was supported
by the Research Institute for Veterinary Science of Seoul National
University.
References
|
1
|
Radi ZA: Comparative pathophysiology and
toxicology of cyclooxygenase. John Wiley & Sons; Hoboken: pp.
15–19. 2012
|
|
2
|
Kirkby NS, Zaiss AK, Urquhart P, Jiao J,
Austin PJ, Al-Yamani M, Lundberg MH, MacKenzie LS, Warner TD,
Nicolaou A, et al: LC-MS/MS confirms that COX-1 drives vascular
prostacyclin whilst gene expression pattern reveals non-vascular
sites of COX-2 expression. PLoS One. 8:e695242013. View Article : Google Scholar :
|
|
3
|
Yamagata K, Andreasson KI, Kaufmann WE,
Barnes CA and Worley PF: Expression of a mitogen-inducible
cyclooxygenase in brain neurons: Regulation by synaptic activity
and glucocorticoids. Neuron. 11:371–386. 1993. View Article : Google Scholar
|
|
4
|
Li DY, Hardy P, Abran D, Martinez-Bermudez
AK, Guerguerian AM, Bhattacharya M, Almazan G, Menezes R, Peri KG,
Varma DR and Chemtob S: Key role for cyclooxygenase-2 in PGE2 and
PGF2alpha receptor regulation and cerebral blood flow of the
newborn. Am J Physiol. 273:R1283–R1290. 1997.
|
|
5
|
Hewett SJ, Bell SC and Hewett JA:
Contributions of cyclooxygenase-2 to neuroplasticity and
neuropathology of the central nervous system. Pharmacol Ther.
112:335–357. 2006. View Article : Google Scholar
|
|
6
|
Lacroix A, Toussay X, Anenberg E, Lecrux
C, Ferreirós N, Karagiannis A, Plaisier F, Chausson P, Jarlier F,
Burgess SA, et al: COX-2-derived prostaglandin E2 produced by
pyramidal neurons contributes to neurovascular coupling in the
rodent cerebral cortex. J Neurosci. 35:11791–11810. 2015.
View Article : Google Scholar
|
|
7
|
Hwang IK, Yi SS, Yoo KY, Park OK, Yan B,
Kim IY, Kim YN, Song W, Moon SM, Won MH, et al: Effects of
treadmill exercise on cyclooxygenase-2 in the hippocampus in type 2
diabetic rats: correlation with the neuroblasts. Brain Res.
1341:84–92. 2010. View Article : Google Scholar
|
|
8
|
Hwang IK, Yi SS, Song W, Won MH, Yoon YS
and Seong JK: Effects of age and treadmill exercise in chronic
diabetic stages on neuroblast differentiation in a rat model of
type 2 diabetes. Brain Res. 1341:63–71. 2010. View Article : Google Scholar
|
|
9
|
Nam SM, Kim JW, Yoo DY, Choi JH, Kim W,
Jung HY, Won MH, Hwang IK, Seong JK and Yoon YS: Comparison of
pharmacological and genetic inhibition of cyclooxygenase-2: Effects
on adult neurogenesis in the hippocampal dentate gyrus. J Vet Sci.
16:245–251. 2015. View Article : Google Scholar :
|
|
10
|
Vincent GK and Velkoff VA: The next four
decades: The older population in the United States: 2010 to
2050United States Department of Commerce, C.B. (edition).
Washington, DC: 2010
|
|
11
|
Wimo A, Jönsson L, Bond J, Prince M and
Winblad B: Alzheimer Disease International: The worldwide economic
impact of dementia 2010. Alzheimers Dement. 9:1–11.e3. 2013.
View Article : Google Scholar
|
|
12
|
Alzheimer's Association, . 2014
Alzheimer's disease facts and figures. Alzheimers Dement.
10:e47–e92. 2014. View Article : Google Scholar
|
|
13
|
Drapeau E, Mayo W, Aurousseau C, Le Moal
M, Piazza PV and Abrous DN: Spatial memory performances of aged
rats in the water maze predict levels of hippocampal neurogenesis.
Proc Natl Acad Sci USA. 100:pp. 14385–14390. 2003; View Article : Google Scholar :
|
|
14
|
Marlatt MW, Philippens I, Manders E, Czéh
B, Joels M, Krugers H and Lucassen PJ: Distinct structural
plasticity in the hippocampus and amygdala of the middle-aged
common marmoset (Callithrix jacchus). Exp Neurol. 230:291–301.
2011. View Article : Google Scholar
|
|
15
|
Puzzo D, Bizzoca A, Loreto C, Guida CA,
Gulisano W, Frasca G, Bellomo M, Castorina S, Gennarini G and
Palmeri A: Role of F3/contactin expression profile in synaptic
plasticity and memory in aged mice. Neurobiol Aging. 36:1702–1715.
2015. View Article : Google Scholar
|
|
16
|
Yokota O, Terada S, Ishihara T, Nakashima
H, Kugo A, Ujike H, Tsuchiya K, Ikeda K, Saito Y, Murayama S, et
al: Neuronal expression of cyclooxygenase-2, a pro-inflammatory
protein, in the hippocampus of patients with schizophrenia. Prog
Neuropsychopharmacol Biol Psychiatry. 28:715–721. 2004. View Article : Google Scholar
|
|
17
|
Fujimi K, Noda K, Sasaki K, Wakisaka Y,
Tanizaki Y, Iida M, Kiyohara Y, Kanba S and Iwaki T: Altered
expression of COX-2 in subdivisions of the hippocampus during aging
and in Alzheimer's disease: The Hisayama Study. Dement Geriatr Cogn
Disord. 23:423–431. 2007. View Article : Google Scholar
|
|
18
|
McGeer PL, Schulzer M and McGeer EG:
Arthritis and anti-inflammatory agents as possible protective
factors for Alzheimer's disease: A review of 17 epidemiologic
studies. Neurology. 47:425–432. 1996. View Article : Google Scholar
|
|
19
|
Miguel-Álvarez M, Santos-Lozano A,
Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Garatachea N and
Lucia A: Non-steroidal anti-inflammatory drugs as a treatment for
Alzheimer's disease: A systematic review and meta-analysis of
treatment effect. Drugs Aging. 32:139–147. 2015. View Article : Google Scholar
|
|
20
|
Malkki H: Alzheimer disease: NSAIDs
protect neurons and preserve memory in a mouse model of AD. Nat Rev
Neurol. 12:370–371. 2016. View Article : Google Scholar
|
|
21
|
Franklin KBJ and Paxinos G: The mouse
brain in stereotaxic coordinates. 3rd. Academic Press; San Diego:
1997
|
|
22
|
Bliss TV and Collingridge GL: A synaptic
model of memory: Long-term potentiation in the hippocampus. Nature.
361:31–39. 1993. View
Article : Google Scholar
|
|
23
|
Sanguino E, Roglans N, Alegret M, Sánchez
RM, Vázquez-Carrera M and Laguna JC: Prevention of age-related
changes in rat cortex transcription factor activator protein-1 by
hypolipidemic drugs. Biochem Pharmacol. 68:1411–1421. 2004.
View Article : Google Scholar
|
|
24
|
Aïd S and Bosetti F: Gene expression of
cyclooxygenase-1 and Ca(2+)-independent phospholipase A(2) is
altered in rat hippocampus during normal aging. Brain Res Bull.
73:108–113. 2007. View Article : Google Scholar :
|
|
25
|
Weerasinghe GR, Coon SL, Bhattacharjee AK,
Harry GJ and Bosetti F: Regional protein levels of cytosolic
phospholipase A2 and cyclooxygenase-2 in Rhesus monkey brain as a
function of age. Brain Res Bull. 69:614–621. 2006. View Article : Google Scholar :
|
|
26
|
Lee CH, Yoo KY, Choi JH, Park OK, Hwang
IK, Kang IJ and Won MH: Cyclooxygenase-2 immunoreactivity and
protein level in the gerbil hippocampus during normal aging.
Neurochem Res. 35:99–106. 2010. View Article : Google Scholar
|
|
27
|
Quinby FL: Clinical chemistry of the
laboratory mouseFox JG: The mouse in biomedical research. 2nd. 3.
Elsevier, Academic Press; Amsterdam: pp. 171–216. 2007
|
|
28
|
Suckow MA, Danneman P and Brayton PC: The
laboratory mouse. (The laboratory animal pocket reference series).
CRC Press; Boca Raton: 2001
|
|
29
|
Hemmeryckx B, Emmerechts J, Bovill EG,
Hoylaerts MF and Lijnen HR: Effect of ageing on the murine venous
circulation. Histochem Cell Biol. 137:537–546. 2012. View Article : Google Scholar
|
|
30
|
MacKinney AA Jr: Effect of aging on the
peripheral blood lymphocyte count. J Gerontol. 33:213–216. 1978.
View Article : Google Scholar
|
|
31
|
Kuhn HG, Dickinson-Anson H and Gage FH:
Neurogenesis in the dentate gyrus of the adult rat: Age-related
decrease of neuronal progenitor proliferation. J Neurosci.
16:2027–2033. 1996.
|
|
32
|
Hwang IK, Yoo KY, Li H, Choi JH, Kwon YG,
Ahn Y, Lee IS and Won MH: Differences in doublecortin
immunoreactivity and protein levels in the hippocampal dentate
gyrus between adult and aged dogs. Neurochem Res. 32:1604–1609.
2007. View Article : Google Scholar
|
|
33
|
Hwang IK, Yoo KY, Yi SS, Kwon YG, Ahn YK,
Seong JK, Lee IS, Yoon YS and Won MH: Age-related differentiation
in newly generated DCX immunoreactive neurons in the subgranular
zone of the gerbil dentate gyrus. Neurochem Res. 33:867–872. 2008.
View Article : Google Scholar
|
|
34
|
Seib DR and Martin-Villalba A:
Neurogenesis in the normal ageing hippocampus: A mini-review.
Gerontology. 61:327–335. 2015. View Article : Google Scholar
|
|
35
|
McGahon B, Clements MP and Lynch MA: The
ability of aged rats to sustain long-term potentiation is restored
when the age-related decrease in membrane arachidonic acid
concentration is reversed. Neuroscience. 81:9–16. 1997. View Article : Google Scholar
|