Introduction
Paraplegia caused by spinal cord ischemia is a
debilitating complication following endovascular intervention for
thoracoabdominal aortic aneurysm (1). Interruption of the aorta underneath
the renal artery depletes glucose and oxygen supply to the spinal
cord and causes neuronal damage in the spinal cord by various
mechanisms including glutamate toxicity and increased oxidative
stress-induced reactive oxygen species (ROS). Various
neuroprotective approaches have been proposed to reduce the
neuronal death of the spinal cord, including surgical, physical and
chemical methods (2–4).
Small ubiquitin-like modifier (SUMO) is a reversible
protein modifier. Numerous key proteins involved in ischemia have
been demonstrated to be SUMOylated (5–10).
SUMOs consist of three isoforms, and SUMO-1 shares ~50% homology
with SUMO-2 and −3, with some overlap in the target proteins.
SUMO-1 and SUMO-2/3 are widely expressed in the adult brain
(11,12). In the ischemic brain, the SUMO
conjugation is dramatically increased in the penumbral area
following transient focal cerebral ischemia (6,13).
In addition, elevated SUMO conjugation has demonstrated
neuroprotection against ischemic damage (12,14).
However, most studies have focused on the alterations in and
effects of SUMO-2/3 levels or conjugation following transient
forebrain (6,13,14)
or spinal cord ischemia (9).
The brain has a unique structure called the
blood-brain barrier, which is the first defense system against
toxins in the blood; however, this structure makes it difficult for
drugs to access the brain. Previously, cell-penetrating peptides
have drawn great attention regarding intracellular access in drug
delivery within neuroscience fields (15–17).
In previous studies, trans-activator of transcription (Tat)-fusion
proteins have been successfully delivered into the brain and spinal
cord following administration (18,19).
Therefore, the present study investigated the
effects of SUMO-1 on neuronal damage in the ventral horn of the
spinal cord using rabbits, as the blood vessels of rabbits are
supplied segmentally and there is a lack of collateral circulation
(20,21).
Materials and methods
Experimental animals
Male New Zealand white rabbits (n=48, weight,
1.2–1.5 kg) were obtained from the Experimental Animal Center of
Cheonan Yonam College (Cheonan, South Korea). They were housed
under standard conditions at 22°C and 60% humidity in a 12-h
light/dark cycle and with free access to food and water. The
handling and care of the animals was in accordance with the NIH
Guide for the Care and Use of Laboratory Animals (NIH publication
no. 85-23, 1985, revised 1996) (22). Ethical approval was obtained from
the Institutional Animal Care and Use Committee of Seoul National
University (Seoul, South Korea; approval no. SNU-141021-2).
Tat-SUMO-1 fusion protein expression
and purification
Preparation of the Tat expression vector has been
described in a previous study (23). Human SUMO-1 was amplified by
polymerase chain reaction (PCR) using the following primer
sequences: Forward, 5′-CTCGAGATGTCTGACCAGGAGGCA-3′ (containing an
XhoI restriction site) and reverse,
5′-GGATCCCTAAACTGTTGAATGACCCC-3′ (containing a BamHI
restriction site). The resulting PCR products were ligated into the
TA vector and cut with XhoI and BamHI. Fragments were
subsequently ligated into the Tat expression vector to generate
Tat-SUMO-1. Control SUMO-1 was manufactured without the Tat
peptide. Recombinant Tat-SUMO-1 plasmid was transformed into E.
coli BL21 (DE3) and cultured in 0.5 mM
isopropyl-β-D-thio-galactoside (Duchefa Biochemie, Haarlem,
Netherlands) at 18°C for >24 h. Harvested cells were lysed by
sonication and the Tat-SUMO-1 protein was purified using
Ni2+-nitrilotriacetic acid sepharose affinity column and
PD-10 column (Qiagen, Inc., Valencia, CA, USA) chromatography to
generate a Tat-SUMO-1 protein. Various concentration (0–1,000 mg)
of bovine serum albumin (Thermo Fisher Scientific, Waltham, MA,
USA) was used as a standard and protein concentration was measured
by Bradford assay (24).
Experimental groups and drug
treatment
To evaluate the effects of Tat-SUMO-1 against
ischemic damage, rabbits were divided into the following groups:
Sham-operated, vehicle (glycerol)-treated ischemia-operated and
Tat-SUMO-1-treated ischemia-operated groups, at 24 h and 72 h
following sham operation or ischemia/reperfusion. Tat-SUMO-1 (1
mg/kg) or an equal volume of glycerol was administered to rabbits
immediately following reperfusion by intraperitoneal injection.
Arterial blood gases [partial pressure (Pa)O2 and
PaCO2], pH and glucose levels were measured using a GEM
Premier 3,000 gas analyzer (Instrumentation Laboratory, Milan,
Italy) 10 min after occlusion of the abdominal aorta and 10 min
after reperfusion. Additionally, mean arterial pressure (MAP) was
recorded from the caudal artery by physiography (Gould Instrument
Systems, Cleveland, OH, USA) and Ponemah acquisition software
version 3.0 (Gould Instrument Systems).
Induction of transient spinal cord
ischemia
Rabbits were anesthetized with 2.5% isoflurane
(Baxter, Deerfield, IL, USA) in 67% N2O and 33%
O2. To spare mesenteric flow to the gut, the abdomen was
incised at the ventral midline and the abdominal aorta was isolated
beneath the left renal artery, following which it was occluded
using an aneurysm clip. Spinal cord ischemia was subsequently
induced as described by Kiyoshima et al (25). The aneurysm clip was removed after
15 min of occlusion, and reperfusion was observed from the
abdominal aorta. This produced delayed paraplegia within several h
of ischemia (26). Free-regulating
or normothermic (38.7±0.3°C) conditions and a thermometric blanket
were used to maintain body temperature, which was monitored with a
rectal temperature probe (TR-100; Fine Science Tools, Foster City,
CA, USA) throughout the surgical procedure. Once the anesthesia had
worn off, rabbits were placed in a thermal incubator (Mirae Medical
Industry, Seoul, South Korea) prior to sacrifice. Sham-operated
animals underwent the same procedure with the exception of
occlusion of the abdominal aorta.
Neurological assessment
To assess neurological function, modified Tarlov
criteria were used (27) as
follows: 0) no voluntary hind-limb function; i) only perceptible
joint movement; ii) active movement but unable to stand; iii) able
to stand but unable to walk; or iv) complete normal hind-limb motor
function (28,29). Assessments were conducted by two
blinded observers for each experiment. Evaluation of control,
vehicle-treated, and 1 mg/kg Tat-SUMO-1-treated groups (n=8/group)
were conducted under identical conditions. Neurological function
was examined 24, 48, and 72 h after reperfusion as it begins to
deteriorate 12–24 h after reperfusion, progressing to complete
delayed-onset paraplegia by 48 h (30,31).
Cresyl violet staining in the spinal
cord
For histological analysis, animals (n=4/group) were
anesthetized with 2 g/kg urethane (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 24 and 72 h after ischemia/reperfusion and
perfused transcardially with 0.1 M phosphate-buffered saline (pH
7.4), followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (pH
7.4). The spinal cords were removed and the 5–6th lumbar segments
(L5-L6) of the spinal cord were fixed in the
same fixative for 4 h. The spinal cord tissues were cryoprotected
by incubation with 30% sucrose overnight, and 30-µm-thick spinal
cord sections were serially cut in the coronal plane using a
cryostat (Leica Microsystems GmbH, Wetzlar, Germany). The sections
were stained with cresyl violet acetate as previously described
(19).
Counting of cresyl violet-positive neurons at the
center of the ventral horn was performed using an analysis system
equipped with a computer-based CCD camera (OPTIMAS software version
6.5; CyberMetrics® Corporation, Phoenix, AZ, USA;
magnification, ×100). The image was converted to a gray-scale image
and cresyl violet-positive neurons were automatically selected
according to the intensity of cresyl violet staining. Cell counts
were averaged from 25 sections at 300-µm intervals from each
rabbit. Values are reported as a percentage of that obtained from
the control groups.
Tissue malondialdehyde (MDA)
analyses
The effects of Tat-SUMO-1 on lipid peroxidation in
the control, vehicle-, and Tat-SUMO-treated groups (n=4/group) were
assessed by measuring MDA formation using a 2-thiobarbituric acid
reactive substances assay kit (Cayman Chemical Company, Ann Arbor,
MI, USA) 24 h after ischemia/reperfusion. The MDA concentration was
determined from a standard curve and expressed in nmol/g of wet
tissue. All experiments were conducted in triplicate.
Tissue Cu, Zn-superoxide dismutase
(SOD1) analyses
The enzyme activity of SOD1 was measured using
superoxide dismutase assay kits (Cayman Chemical Company) to assess
the effects of Tat-SUMO-1 in the control, and vehicle- and
Tat-SUMO-1-treated groups (n=4/group). Readings were obtained at
550 nm using a Beckman DU-640B spectrophotometer (Beckman Coulter,
Inc., Brea, CA, USA). Final SOD1 activity levels were expressed as
units of enzymatic activity/mg protein sample (U/mg protein). One
unit of SOD was defined as the amount of enzyme necessary to
achieve 50% dismutation of the superoxide radical. All assays
experiments were conducted in triplicate.
Statistical analysis
Multiple comparisons were analyzed by one-way or
two-way analysis of variance followed by Bonferroni's post hoc test
using GraphPad Prism software verson 5.01 (GraphPad Software, Inc.,
La Jolla, CA, USA). Data are expressed as the mean ± standard
error. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of Tat-SUMO-1 on physiological
parameters prior to, during and following ischemia
In the vehicle-treated and Tat-SUMO-1 treated
groups, blood gases (PaCO2 and PaO2), pH and
glucose levels were not significantly changed 10 min before
induction of ischemia or after reperfusion (Table I). The Tat-SUMO-1 and
vehicle-treated groups demonstrated similar patterns of MAP; levels
significantly decreased 10 min after occlusion (during ischemia)
and were restored to baseline levels 10 min after reperfusion
(Table I).
 | Table I.Physiological parameters prior to,
during and following ischemia. |
Table I.
Physiological parameters prior to,
during and following ischemia.
| Group | pH | Distal MAP
(mmHg) | PaCO2
(mmHg) | PaO2
(mmHg) | Glu (mM) |
|---|
| Pre-ischemia |
|
|
|
|
|
|
Control | 7.40±0.03 | 85.1±8.9 | 37.2±3.1 | 102.4±8.3 | 6.5±1.1 |
|
Vehicle | 7.41±0.02 | 83.7±9.3 | 37.0±3.4 | 104.1±9.5 | 6.3±1.1 |
|
Tat-SUMO-1 | 7.42±0.03 | 84.3±9.0 | 36.8±2.9 | 105.3±9.9 | 6.7±1.2 |
| Ischemia 10
min |
|
|
|
|
|
|
Control | 7.38±0.04 | 84.4±9.5 | 37.1±3.4 |
103.5±11.6 | 6.7±1.2 |
|
Vehicle | 7.37±0.04 |
8.9±2.5a | 37.6±3.3 | 100.5±9.4 | 7.2±1.8 |
|
Tat-SUMO-1 | 7.39±0.03 |
9.3±1.9a | 36.9±4.0 | 105.2±9.9 | 7.0±1.6 |
| Reperfusion 10
min |
|
|
|
|
|
|
Control | 7.42±0.05 | 86.3±9.9 | 36.1±3.2 | 101.3±8.9 | 6.7±1.0 |
|
Vehicle | 7.40±0.04 |
85.3±12.1 | 36.6±3.7 |
106.3±10.5 | 7.0±1.9 |
|
Tat-SUMO-1 | 7.39±0.04 | 84.7±9.5 | 37.0±3.5 |
105.9±11.4 | 7.1±1.4 |
Effect of Tat-SUMO-1 on Tarlov
criteria following spinal cord ischemia
In the control group, all animals exhibited
completely normal function 72 h after the sham operation, and had a
neurological score of 4.00. In the vehicle-treated group, numerous
animals had no voluntary or barely perceptible joint movement 24,
48 and 72 h after ischemia/reperfusion, and had a mean neurological
score of 0.625. In the Tat-SUMO-1-treated group, animals could
stand; however, could not walk, and had an average neurological
score of 2.750 at 24 h after ischemia/reperfusion. These animals
did not stand or move voluntarily, and had an average neurological
score of 1.125 at 72 h after ischemia/reperfusion (Fig. 1).
Effect of Tat-SUMO-1 on neuronal
protection following spinal cord ischemia
In the control group, cresyl violet-positive neurons
were abundantly detected in the ventral horn of the spinal cord at
24 h (Fig. 2A) and 72 h (Fig. 2B) after the sham operation;
however, no significant differences were observed between the two
groups. In the vehicle-treated group, cresyl violet-positive neuron
counts were decreased in the ventral horn of the spinal cord at 24
h (Fig. 2C) and 72 h (Fig. 2D) after ischemia/reperfusion (28.5
and 12.4% of the control group, respectively, at 24 h). In the
Tat-SOD1-treated groups, cresyl violet-positive neurons were
abundantly detected in the ventral horn of the spinal cord 24 h
after ischemia/reperfusion (Fig.
2E). However, they were markedly reduced in number 72 h after
ischemia/reperfusion (Fig 2F). The
proportion of cresyl violet-positive neurons 24 h and 72 h after
ischemia/reperfusion was 67.5% and 17.2% of the control group,
respectively, 24 h after the sham operation (Fig. 2G).
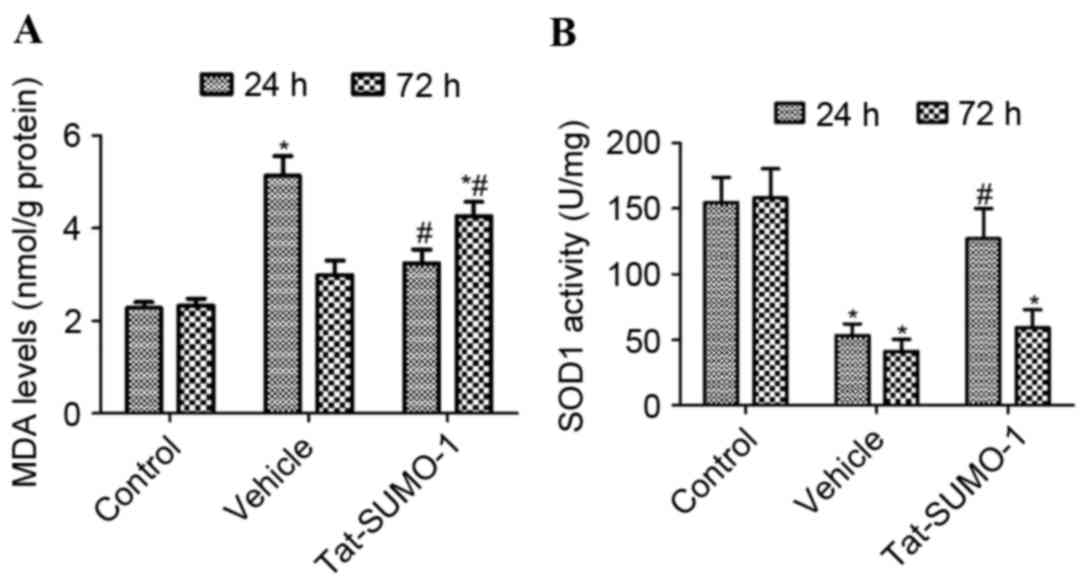
Effect of Tat-SUMO-1 on lipid
peroxidation following spinal cord ischemia
In the control group, MDA levels in
L5-L6 spinal cord homogenates were 2.278 and
2.325 nmol/g protein at 24 and 72 h after the sham operation,
respectively. In the vehicle-treated group, MDA levels were
significantly increased compared with the control group to 5.134
nmol/g protein 24 h after ischemia/reperfusion (P<0.05), and
were 2.994 nmol/g protein after 72 h. In the Tat-SUMO-1-treated
group, MDA levels in L5-L6 spinal cord
homogenates decreased significantly to 3.244 nmol/g 24 h after
ischemia/reperfusion, compared with the vehicle-treated group
(P<0.05). However, compared with the control and vehicle-treated
groups, the MDA levels in L5-L6 spinal cord
homogenates increased to 4.264 nmol/g after 72 h in the
Tat-SUMO-1-treated group (P<0.05; Fig. 3A).
Effect of Tat-SUMO-1 on SOD1 activity
following spinal cord ischemia
In the control groups, SOD1 activity in
L5-L6 spinal cord homogenates was 154.7 and
158.2 U/mg at 24 and 72 h, respectively, after the sham operation,
whereas in the vehicle-treated group, SOD1 activity was
significantly decreased compare with the control to 53.4 and 41.2
U/mg after 24 and 72 h, respectively (P<0.05). In the
Tat-SUMO-1-treated group, SOD1 activity was significantly increased
to 127.3 U/mg after 24 h, compared with the vehicle-treated group
(P<0.05). However, SOD1 activity in the Tat-SUMO-1-treated group
was significantly decreased to 59.3 U/mg at 72 h, compared with
Tat-SUMO-1-treated group at 24 h (Fig.
3B).
Discussion
SUMOylation may be part of an endogenous
neuroprotective response to cellular stress, particularly in brain
ischemia (7,8,12).
There have been conflicting reports regarding of SUMOylation by
SUMO-1 and SUMO-2/3 following ischemic damage. Shao et al
(32) demonstrated that under
hypoxia, SUMO-1 protein and mRNA expression levels were markedly
increased in the brain and heart (32). Cimarosti et al (5) revealed that SUMO-1 and SUMO-2/3
levels were markedly increased after 6 and 24 h in the striatal
infarct area. However, there have been reports that SUMO-1
conjugation is not altered in the ischemic hippocampus (5) or spinal cord (9). Under normal conditions, there is a
large pool of free SUMO-2 and −3, and limited SUMO-1 (33). The present study examined the
effects of Tat-SUMO-1 following ischemic damage in the rabbit
spinal cord. The administration of Tat-SUMO-1 did not have any
effect on blood biochemical parameters including pH, blood glucose,
and the arterial blood gases PaCO2 and PaO2,
prior to or following transient spinal cord ischemia. Significant
decreases in MAP levels in the caudal artery were observed in
vehicle- and Tat-SUMO-1-treated groups 10 min after ischemia, which
thereafter returned to normal levels in the two groups. This result
suggested that the transient spinal cord ischemia is completely
induced in the two groups and that Tat-SUMO-1 has no effect on
blood gases or pressure prior to or following
ischemia/reperfusion.
The present study observed alterations in hind limb
behaviors on the basis of the Tarlov criteria (27). Administration of Tat-SUMO-1
significantly ameliorated the ischemia-induced reduction in Tarlov
score 24 h after ischemia/reperfusion. However, the Tarlov score
was significantly decreased 72 h after ischemia/reperfusion,
similar to in the vehicle-treated group. Morphological evidence
based on cresyl violet histochemistry supported the behavioral
results; positively-stained neurons were abundantly detected in the
ventral horn of the spinal cord 24 h after ischemia/reperfusion;
however, the number significantly decreased after 72 h. This result
suggested that Tat-SUMO-1 does not protect against neuronal damage
following spinal cord ischemia, although it transiently delays it.
There have been reports that overexpression of SUMO-1 significantly
reduces damage from oxygen-glucose deprivation (34) and rescues cardiac function in a
mouse and swine model of heart failure (35,36).
In addition, knockdown of SUMO-1 by small interfering- or short
hairpin RNA increased the susceptibility of SHSY5Y human
neuroblastoma cells and cultured cortical neurons (34) and accelerated the deterioration of
cardiac function (35,36). The discrepancies in the effects of
SUMO-1 between the present and other studies may be associated with
the delivery methods of SUMO-1. The present study directly
delivered SUMO-1 by using the Tat-SUMO-1 fusion protein; however,
others administered SUMO-1 via genetic engineering with viral
vectors. These results suggested that transient treatment with
SUMO-1 protein did not induce neuroprotection against ischemic
damage in the spinal cord; however, delayed neuronal damage.
The mechanisms underlying this result were not fully
elucidated in this study. The present study focused on lipid
peroxidation and SOD1 because SUMOylation is known to be increased
following exposure to various stressors, including strong oxidative
stress (37). The present study
demonstrated that lipid peroxidation was significantly increased 24
h after ischemia/reperfusion, whereas SOD1 activity was decreased.
This result was supported by previous studies, which demonstrated
that spinal cord ischemia increases lipid peroxidation and the
subsequent inactivation and consumption of SOD1 in the spinal cord
(38–40). A previous study reported that at
H2O2 concentrations of 1 mM and below,
SUMO-1, −2, and −3 conjugates were almost completely lost (37). In the present study, administration
of Tat-SUMO-1 ameliorated the increases in lipid peroxidation and
depletion of SOD1 levels 24 h after ischemia/reperfusion in the
spinal cord. This result was supported by a previous study, which
revealed that SUMO-1 conjugation resulted in a decrease in
intracellular ROS generation (41). In addition, inhibition of
endogenous SUMOylation increased ROS production from Nox5 human
embryonic kidney cells, human vascular smooth muscle cells and
neutrophils (42). Additionally,
administration of antioxidants was previously demonstrated to
reduce cisplatin-induced SUMOylation (43). However, these effects in the
present study were not maintained at 72 h after
ischemia/reperfusion. At this time point, lipid peroxidation and
SOD1 levels were demonstrated to be similar to those in the
vehicle-treated group. This result suggested that administration of
SUMO-1 may improve neuronal damage transiently by regulation of
antioxidant or lipid peroxidation during early ischemia. SUMO-1 may
facilitate neuroprotection against ischemic damage, in combination
with antioxidants, calcium modulators, or other therapeutic
compounds in the spinal cord. It has been reported that
pET-glutathione transferase-SUMO-metallothionein fusion proteins
improved memory impairments and increased lipid peroxidation in a
chemically-induced ageing model (44).
In conclusion, the present study demonstrated that
Tat-SUMO-1 administration delays neuronal death from ischemic
damage in the spinal cord by regulating oxidative stress. These
results suggested that Tat-SUMO-1 treatment, in combination with
antioxidants, calcium modulators or other compounds, may provide a
therapeutic approach to reduce neuronal damage.
Acknowledgements
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
NRF-2014R1A1A2056492) and a Priority Research Centers Program grant
from the National Research Foundation (grant no. NRF-2009-0093812)
funded by the Ministry of Science, ICT & Future Planning in the
Republic of Korea.
References
|
1
|
Schepens MA, Heijmen RH, Ranschaert W,
Sonker U and Morshuis WJ: Thoracoabdominal aortic aneurysm repair:
Results of conventional open surgery. Eur J Vasc Endovasc Surg.
37:640–645. 2009. View Article : Google Scholar
|
|
2
|
Cambria RP, Davison JK, Carter C, Brewster
DC, Chang Y, Clark KA and Atamian S: Epidural cooling for spinal
cord protection during thoracoabdominal aneurysm repair: A
five-year experience. J Vasc Surg. 31:1093–1102. 2000. View Article : Google Scholar
|
|
3
|
Coselli JS, LeMaire SA, Köksoy C,
Schmittling ZC and Curling PE: Cerebrospinal fluid drainage reduces
paraplegia after thoracoabdominal aortic aneurysm repair: Results
of a randomized clinical trial. J Vasc Surg. 35:631–639. 2002.
View Article : Google Scholar
|
|
4
|
Safi HJ, Miller CC III, Huynh TT, Estrera
AL, Porat EE, Winnerkvist AN, Allen BS, Hassoun HT and Moore FA:
Distal aortic perfusion and cerebrospinal fluid drainage for
thoracoabdominal and descending thoracic aortic repair: Ten years
of organ protection. Ann Surg. 238:372–381. 2003.
|
|
5
|
Cimarosti H, Lindberg C, Bomholt SF, Rønn
LC and Henley JM: Increased protein SUMOylation following focal
cerebral ischemia. Neuropharmacology. 54:280–289. 2008. View Article : Google Scholar
|
|
6
|
Yang W, Sheng H, Warner DS and Paschen W:
Transient focal cerebral ischemia induces a dramatic activation of
small ubiquitin-like modifier conjugation. J Cereb Blood Flow
Metab. 28:892–896. 2008. View Article : Google Scholar
|
|
7
|
Lee YJ and Hallenbeck JM: SUMO and
ischemic tolerance. Neuromolecular Med. 15:771–781. 2013.
View Article : Google Scholar
|
|
8
|
Silveirinha V, Stephens GJ and Cimarosti
H: Molecular targets underlying SUMO-mediated neuroprotection in
brain ischemia. J Neurochem. 127:580–591. 2013. View Article : Google Scholar
|
|
9
|
Wang Z, Wang R, Sheng H, Sheng SP, Paschen
W and Yang W: Transient ischemia induces massive nuclear
accumulation of SUMO2/3-conjugated proteins in spinal cord neurons.
Spinal Cord. 51:139–143. 2013. View Article : Google Scholar
|
|
10
|
Yang W and Paschen W: SUMO proteomics to
decipher the SUMO-modified proteome regulated by various diseases.
Proteomics. 15:1181–1191. 2015. View Article : Google Scholar
|
|
11
|
Zhang FP, Mikkonen L, Toppari J, Palvimo
JJ, Thesleff I and Jänne OA: Sumo-1 function is dispensable in
normal mouse development. Mol Cell Biol. 28:5381–5390. 2008.
View Article : Google Scholar :
|
|
12
|
Lee YJ, Mou Y, Maric D, Klimanis D, Auh S
and Hallenbeck JM: Elevated global SUMOylation in Ubc9 transgenic
mice protects their brains against focal cerebral ischemic damage.
PLoS One. 6:e258522011. View Article : Google Scholar :
|
|
13
|
Hochrainer K, Jackman K, Anrather J and
Iadecola C: Reperfusion rather than ischemia drives the formation
of ubiquitin aggregates after middle cerebral artery occlusion.
Stroke. 43:2229–2235. 2012. View Article : Google Scholar :
|
|
14
|
Cimarosti H, Ashikaga E, Jaafari N,
Dearden L, Rubin P, Wilkinson KA and Henley JM: Enhanced
SUMOylation and SENP-1 protein levels following oxygen and glucose
deprivation in neurones. J Cereb Blood Flow Metab. 32:17–22. 2012.
View Article : Google Scholar
|
|
15
|
Kilic E, Kilic U and Hermann DM: TAT
fusion proteins against ischemic stroke: Current status and future
perspectives. Front Biosci. 11:1716–1721. 2006. View Article : Google Scholar
|
|
16
|
Fonseca SB, Pereira MP and Kelley SO:
Recent advances in the use of cell-penetrating peptides for medical
and biological applications. Adv Drug Deliv Rev. 61:953–964. 2009.
View Article : Google Scholar
|
|
17
|
Stalmans S, Bracke N, Wynendaele E,
Gevaert B, Peremans K, Burvenich C, Polis I and De Spiegeleer B:
Cell-penetrating peptides selectively cross the blood-brain barrier
in vivo. PLoS One. 10:e01396522015. View Article : Google Scholar :
|
|
18
|
Eum WS, Kim DW, Hwang IK, Yoo KY, Kang TC,
Jang SH, Choi HS, Choi SH, Kim YH, Kim SY, et al: In vivo protein
transduction: Biologically active intact pep-1-superoxide dismutase
fusion protein efficiently protects against ischemic insult. Free
Radic Biol Med. 37:1656–1669. 2004. View Article : Google Scholar
|
|
19
|
Kim W, Kim DW, Jeong HJ, Yoo DY, Jung HY,
Nam SM, Kim JH, Choi JH, Won MH, Yoon YS, et al: Tat-DJ-1 protects
neurons from ischemic damage in the ventral horn of rabbit spinal
cord via increasing antioxidant levels. Neurochem Res. 39:187–193.
2014. View Article : Google Scholar
|
|
20
|
DeGirolami U and Zivin JA: Neuropathology
of experimental spinal cord ischemia in the rabbit. J Neuropathol
Exp Neurol. 41:129–149. 1982. View Article : Google Scholar
|
|
21
|
Weir CJ, Zivin JA and Lyden PD:
Inter-relationships between spinal cord blood flow, neuronal death
and neurological function in rabbit spinal cord ischemia. Brain
Res. 946:43–51. 2002. View Article : Google Scholar
|
|
22
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. 7th. National Academy Press;
Washington DC: 1996
|
|
23
|
Kwon HY, Eum WS, Jang HW, Kang JH, Ryu J,
Lee B Ryong, Jin LH, Park J and Choi SY: Transduction of Cu,
Zn-superoxide dismutase mediated by an HIV-1 Tat protein basic
domain into mammalian cells. FEBS Lett. 485:163–167. 2000.
View Article : Google Scholar
|
|
24
|
Bradford MA: A rapid and sensitive method
for the quantification of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar
|
|
25
|
Kiyoshima T, Fukuda S, Matsumoto M, Iida
Y, Oka S, Nakakimura K and Sakabe T: Lack of evidence for apoptosis
as a cause of delayed onset paraplegia after spinal cord ischemia
in rabbits. Anesth Analg. 96:839–846. 2003.
|
|
26
|
Murakami H, Tsukube T, Kawanishi Y and
Okita Y: Transcranial myogenic motor-evoked potentials after
transient spinal cord ischemia predicts neurologic outcome in
rabbits. J Vasc Surg. 39:207–213. 2004. View Article : Google Scholar
|
|
27
|
Tarlov IM: Spinal cord compression;
mechanism of paralysis and treatment. Thomas CC: Springfield,
Illinois: pp. 1471957
|
|
28
|
Jacobs TP, Kempski O, McKinley D, Dutka
AJ, Hallenbeck JM and Feuerstein G: Blood flow and vascular
permeability during motor dysfunction in a rabbit model of spinal
cord ischemia. Stroke. 23:367–373. 1992. View Article : Google Scholar
|
|
29
|
Huang Y, Xie K, Li J, Xu N, Gong G, Wang
G, Yu Y, Dong H and Xiong L: Beneficial effects of hydrogen gas
against spinal cord ischemia-reperfusion injury in rabbits. Brain
Res. 1378:125–136. 2011. View Article : Google Scholar
|
|
30
|
Moore WM Jr and Hollier LH: The influence
of severity of spinal cord ischemia in the etiology of
delayed-onset paraplegia. Ann Surg. 213:427–432. 1991. View Article : Google Scholar :
|
|
31
|
Wisselink W, Patetsios P, Panetta TF,
Ramirez JA, Rodino W, Kirwin JD and Zikria BA: Medium molecular
weight pentastarch reduces reperfusion injury by decreasing
capillary leak in an animal model of spinal cord ischemia. J Vasc
Surg. 27:109–116. 1998. View Article : Google Scholar
|
|
32
|
Shao R, Zhang FP, Tian F, Friberg P
Anders, Wang X, Sjöland H and Billig H: Increase of SUMO-1
expression in response to hypoxia: Direct interaction with
HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett.
569:293–300. 2004. View Article : Google Scholar
|
|
33
|
Saitoh H and Hinchey J: Functional
heterogeneity of small ubiquitin-related protein modifiers SUMO-1
versus SUMO-2/3. J Biol Chem. 275:6252–6258. 2000. View Article : Google Scholar
|
|
34
|
Lee YJ, Castri P, Bembry J, Maric D, Auh S
and Hallenbeck JM: SUMOylation participates in induction of
ischemic tolerance. J Neurochem. 109:257–267. 2009. View Article : Google Scholar :
|
|
35
|
Kho C, Lee A, Jeong D, Oh JG, Chaanine AH,
Kizana E, Park WJ and Hajjar RJ: SUMO1-dependent modulation of
SERCA2a in heart failure. Nature. 477:601–605. 2011. View Article : Google Scholar :
|
|
36
|
Tilemann L, Lee A, Ishikawa K, Aguero J,
Rapti K, Santos-Gallego C, Kohlbrenner E, Fish KM, Kho C and Hajjar
RJ: SUMO-1 gene transfer improves cardiac function in a
large-animal model of heart failure. Sci Transl Med.
5:211ra1592013. View Article : Google Scholar
|
|
37
|
Bossis G and Melchior F: Regulation of
SUMOylation by reversible oxidation of SUMO conjugating enzymes.
Mol Cell. 21:349–357. 2006. View Article : Google Scholar
|
|
38
|
Kim W, Kim DW, Yoo DY, Chung JY, Hwang IK,
Won MH, Choi SY, Jeon SW, Jeong JH, Hwang HS and Moon SM:
Neuroprotective effects of PEP-1-Cu, Zn-SOD against ischemic
neuronal damage in the rabbit spinal cord. Neurochem Res.
37:307–313. 2012. View Article : Google Scholar
|
|
39
|
Gürer B, Kertmen H, Kasim E, Yilmaz ER,
Kanat BH, Sargon MF, Arikok AT, Ergüder BI and Sekerci Z:
Neuroprotective effects of testosterone on ischemia/reperfusion
injury of the rabbit spinal cord. Injury. 46:240–248. 2015.
View Article : Google Scholar
|
|
40
|
Jung HY, Kim DW, Yim HS, Yoo DY, Kim JW,
Won MH, Yoon YS, Choi SY and Hwang IK: Heme oxygenase-1 protects
neurons from ischemic damage by upregulating expression of Cu,
Zn-superoxide dismutase, catalase, and brain-derived neurotrophic
factor in the rabbit spinal cord. Neurochem Res. 41:869–879. 2016.
View Article : Google Scholar
|
|
41
|
Kim HJ, Yun J, Lee J, Hong H, Jeong J, Kim
E, Bae YS and Lee KJ: SUMO1 attenuates stress-induced ROS
generation by inhibiting NADPH oxidase 2. Biochem Biophys Res
Commun. 410:555–562. 2011. View Article : Google Scholar
|
|
42
|
Pandey D, Chen F, Patel A, Wang CY,
Dimitropoulou C, Patel VS, Rudic RD, Stepp DW and Fulton DJ: SUMO1
negatively regulates reactive oxygen species production from NADPH
oxidases. Arterioscler Thromb Vasc Biol. 31:1634–1642. 2011.
View Article : Google Scholar :
|
|
43
|
Guo C, Wei Q, Su Y and Dong Z: SUMOylation
occurs in acute kidney injury and plays a cytoprotective role.
Biochim Biophys Acta. 1852:482–489. 2015. View Article : Google Scholar
|
|
44
|
Huang Y, Su Z, Li Y, Zhang Q, Cui L, Su Y,
Ding C, Zhang M, Feng C, Tan Y, et al: Expression and purification
of glutathione transferase-small ubiquitin-related
modifier-metallothionein fusion protein and its neuronal and
hepatic protection against D-galactose-induced oxidative damage in
mouse model. J Pharmacol Exp Ther. 329:469–478. 2009. View Article : Google Scholar
|