Introduction
Lung cancer remains a daunting health problem with
more than 1.1 million mortalities worldwide attributed to lung
cancer annually (1). Although
there is growing evidence that the immune system has the potential
to protect against malignancies, the precise mechanisms of immune
modulation in lung cancer patients remain elusive, particularly
that exerted by the innate immune system, which is considered to be
the primary response against tumor growth and development. Previous
studies (2,3) have indicated that the immune system
may be involved in the protection and promotion of
carcinogenesis.
Innate lymphoid cells (ILCs) are a heterogeneous
group of cell types that serve a role in innate immune responses
and which have received a great deal of attention. ILCs are
categorized into three distinct populations based on the surface
markers they express, the transcription factors that regulate their
development and function and the effective cytokines they can
produce (4,5). Group 2 ILCs mainly produce
interleukin (IL)-13 and IL-5, and express the transcription factors
retinoic acid receptor related orphan receptor α (RORα) or GATA
binding protein 3 (GATA3; 4,6,7). These cells respond to IL-25 and
IL-33 in vitro due to IL-17 receptor (R) B or ST2 expression
on their membrane (8,9).
Myeloid-derived suppressor cells (MDSCs) are defined
as a heterogeneous population of activated immature myeloid cells
characterized by a morphological mixture of granulocytic and
monocytic cells. However, they lack the expression of cell-surface
markers that are specific to the fully differentiated monocytes,
macrophages, or dendritic cells. In mice, granulocytic MDSCs
possess a CD11b+Ly6G+Ly6Clow
phenotype, whereas MDSCs with monocytic morphology are
CD11b+Ly6G−Ly6Chigh; the two
phenotypes exhibit different functions in cancer and autoimmune
diseases. By contrast, the human MDSCs are traditionally defined as
CD14−CD11b+CD33+CD15+cells
or cells that express the CD33 marker but lack the expression of
markers of mature myeloid and lymphoid cells and the major
histocompatibility complex class-II molecule human leukocyte
antigen-antigen D related (10).
The identification and isolation of human MDSC subsets have been
challenging due to the heterogeneous characteristics of these
immature cells and accumulating data suggest a significant
diversity in the MDSC subsets identified in different human cancers
(11). The frequency of each MDSCs
subset appears to be influenced by the type of cancer (12). MDSCs are suspected of serving a
crucial role in local and systemic tumor development, providing a
microenvironment in which tumor cells can proliferate, expand,
acquire new mutations and escape host immunosurveillance (13). Elevated numbers of MDSCs in many
cancer patients have been demonstrated, including patients with
lung cancer (14–16), and their inhibitory role in
adaptive immunity via their suppression of T cell activation is
well established (17–20). However, the influence of MDSCs on
Th1/Th2 balance is little known in lung cancer. In previous studies
of the authors (21,22), it was identified that there were
markedly enhanced ILC2s and a predominant Th2 phenotype in patients
with gastric cancer. However, it remains controversial whether
these ILC2s serve a role in the progression of lung cancer. The
present study was designed to investigate the changes in the
frequency of ILC2s and the expression levels of ILC2s-associated
factors in patients with lung cancer, and to analyze the
association between circulating ILC2s, or associated factors, and
Th1/Th2 immune imbalance, with the aim of understanding the
significance of ILC2s in lung cancer.
Materials and methods
Patients and healthy controls
A total of 36 patients (26 male, 10 female), newly
diagnosed with lung cancer based on the guideline of International
Union Against Cancer Tumor Node Metastasis (23) were included in the current study.
These patients ranged between 43 and 70 years of age (mean 52.35
years) and 30 healthy subjects, age- and sex-matched to the
patients, were studied as the controls. None of the patients had
been treated preoperatively or had a history of autoimmune disease,
and none of the healthy controls had a prior history of cancer or
any chronic inflammation. The present study was approved by the
Ethical Committee of the Affiliated People's Hospital of Jiangsu
University (Zhenjiang, China), and written informed consent was
obtained from all individuals.
Cell preparation
Peripheral blood samples were collected from lung
cancer patients and healthy controls. Plasma samples were frozen at
−80°C immediately following centrifugation at 500 × g and at 4°C
until use. Peripheral blood mononuclear cells (PBMCs) were obtained
by Ficoll-Hypaque centrifugation at 500 × g and at 4°C. The cell
suspensions were divided into two equal aliquots, one was
immediately used for experiments, and then 1 ml TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added to the
other aliquot, which was stored at −80°C for extracting total
RNA.
Flow cytometric quantification of
ILC2s and MDSCs
ILC2s population was defined as lineage (CD2, CD3,
CD14, CD16, CD19, CD56 and CD235a) and
lineage−−/inducible T-cell costimulator
(ICOS)+/IL-17RB+; MDSCs population was
defined as lineage−/HLA-DR−/CD33+.
For flow cytometric quantification of ILC2s: PBMCs were stained
with a fluorescently labeled antibody mix (FITC-conjugated
anti-human CD2, CD3, CD14, CD16, CD19, CD56 and CD235a; 22-7778-72;
1:50; eBioscience, Inc.; Thermo Fisher Scientific, Inc.), with
Allophycocyanin- (APC) -conjugated anti-human ICOS (FAB6975A;
1:200; R&D Systems, Inc., Minneapolis, MN, USA) and
PerCP-conjugated anti-human IL-17RB (FAB1207C; 1:200; R&D
Systems, Inc.). For flow cytometric quantification of MDSCs: PBMCs
were stained with antibody mix (FITC-conjugated anti-human CD2,
CD3, CD14, CD16, CD19, CD56 and CD235a; 22-7778-72; 1:50;
eBioscience, Inc.; Thermo Fisher Scientific, Inc.) with
PE-conjugated anti-human HLA-DR (FAB4869P; 1:200; R&D Systems,
Inc.) and PE conjugated anti-human CD33 (15–0339; 1:100;
eBioscience Inc.; Thermo Fisher Scientific, Inc.); incubated at
room temperature for 30 min. Following incubation, the samples were
washed with phosphate buffered saline (PBS) and the pellets were
resuspended in 250 µl PBS. The isotype control antibody was used in
all cases. The labeled cells were analyzed with an Accuri C6 flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using FlowJo
software version 7.6 (Tree Star, Inc., Ashland, OR, USA).
RNA extraction, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from individual PBMCs and
500 ng RNA was reverse transcribed using PrimeScript® RT
reagent kit Perfect Real Time (Takara, Bio, Inc., Ostu, Japan),
according to the manufacturer's protocols. For RT-qPCR, reverse
transcribed cDNA (1 µl) was amplified by RT-PCR using the SYBR
Green Premix EX Taq kit (Takara Bio, Inc.). The reaction was
performed in a 10 µl solution containing 1 µl DNA template, a
forward primer (10 µM), a reverse primer (10 µM), 2X SYBR Premix EX
Taq II (1X, final concentration) and distilled water. Then the
reaction was carried out as follows: 95°C 5 min then 40 cycles of
denaturation at 95°C for 10 sec, annealing at 72°C for 30 sec,
extension at 72°C for 30 sec and a final extension at 72°C for 5
sec. Each sample was analyzed in duplicate with the CFXA96 Cycler
(Thermo Fisher Scientific, Inc.) and the expression data for each
target gene was then normalized relative to β-actin. All primer
sequences are presented in Table
I.
 | Table I.Primers used in real-time polymerase
chain reaction. |
Table I.
Primers used in real-time polymerase
chain reaction.
| Gene | Sequence (5′-3′) | Length (bp) |
|---|
| IL-33 | Forward:
ATCCCAACAGAAGGCCAAAG | 198 |
|
| Reverse:
CCAAAGGCAAAGCACTCCAC |
|
| RORα | Forward:
CTGACGAGGACAGGAGTAGG | 204 |
|
| Reverse:
GTGCGCAGACAGAGCTATTC |
|
| GATA3 | Forward:
TTGTGGTGGTCTGACAGTTC | 294 |
|
| Reverse:
AGTACAGCTCCGGACTCTTC |
|
| T-bet | Forward:
CGGGAGAACTTTGAGTCCAT | 115 |
|
| Reverse:
ACTGGTTGGGTAGGAGAGGAG |
|
| IL-13 | Forward:
GGCTGAGGTCTAAGCTAAGG | 370 |
|
| Reverse:
GACAGCTGGCATGTACTGTG |
|
| IL-5 | Forward:
ACTCTCCAGTGTGCCTATTC | 102 |
|
| Reverse:
CTGCTGATAGCCAATGAGAC |
|
| IL-4 | Forward:
GACATCTTTGCTGCCTCCA | 99 |
|
| Reverse:
TACTCTGGTTGGCTTCCTTCA |
|
| Arg1 | Forward:
CAAGAAGAACGGAAGAATCAGC | 149 |
|
| Reverse:
TTGTGGTTGTCAGTGGAGTGTT |
|
| iNOS | Forward:
CTTTCCAAGACACACTTCACCA | 236 |
|
| Reverse:
TATCTCCTTTGTTACCGCTTCC |
|
ELISA
The protein expression levels of IL-33, IL-13 and
IL-5 in plasma were measured by an ELISA kit following the
manufacturer's protocols (IL-33; BMS2048; eBioscience Inc.; Thermo
Fisher Scientific, Inc.; IL-13; BMS231TNST; eBioscience Inc.;
Thermo Fisher Scientific, Inc.; and IL-5; BMS278TNSTCE;
eBioscience, Inc.; Thermo Fisher Scientific, Inc.). Hemolyzed and
lipoidaemia samples were excluded. All samples were run in batches
to minimize inter-assay variability and in triplicate, and the mean
absorbance was calculated from the standard curve.
Statistical analysis
Statistical comparisons between groups were
performed using the Student's unpaired or paired t-test. The
correlation between two continuous variables was analyzed by the
Spearman test. Calculations were performed using GraphPad Prism,
software version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
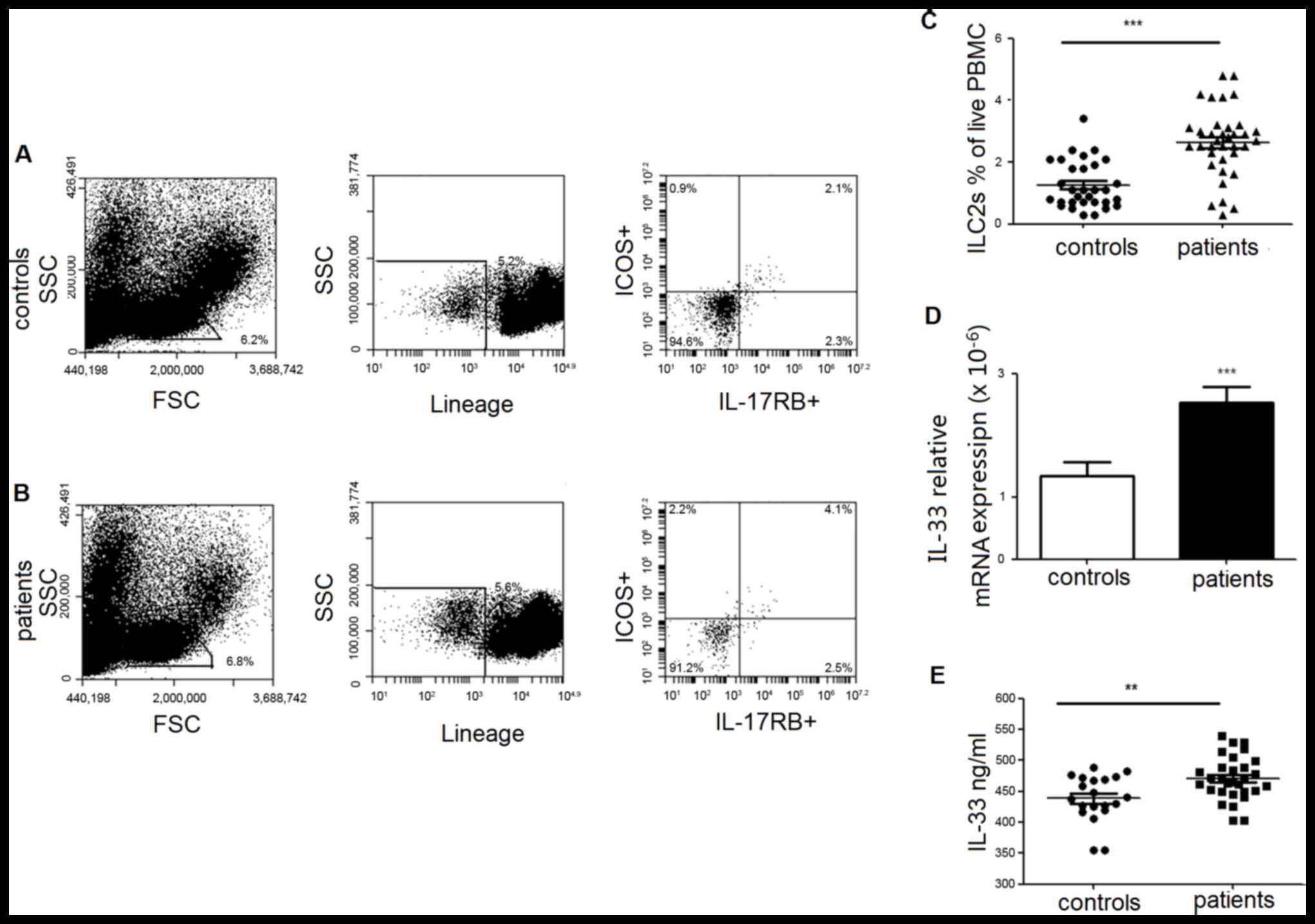
Elevated ILC2s in PBMCs are
accompanied by an increased level of IL-33 in PBMCs and plasma in
lung cancer patients
ILC2s, defined as
lineage−/ICOS+/IL-17RB+ cells,
were determined by multicolor flow cytometry and calculated as the
percentage of PBMCs. As demonstrated in Fig. 1, the frequency of individual ILC2s
was significantly increased in patients compared with healthy
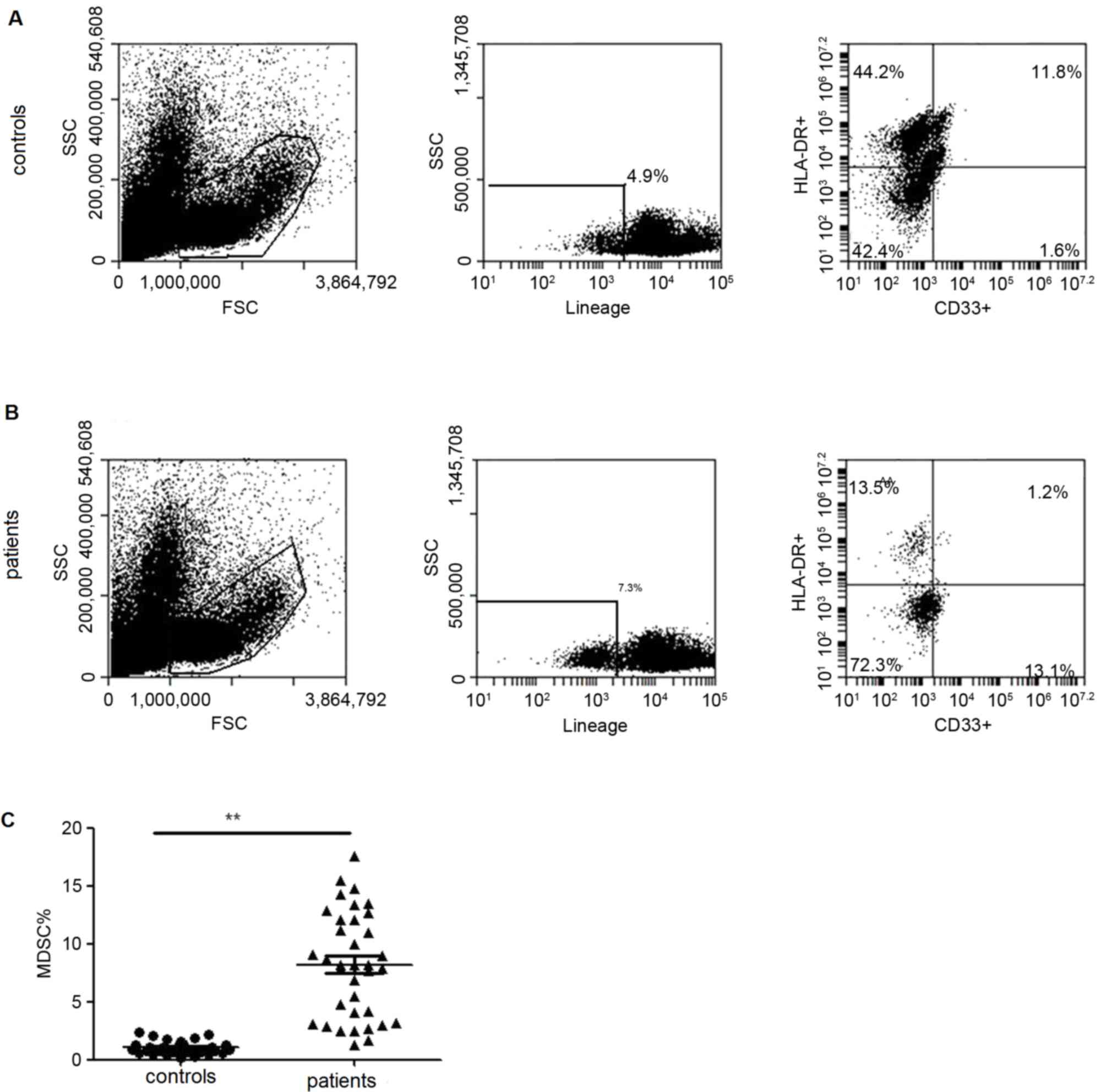
controls. MDSCs from PBMCs of lung cancer patients were also
analyzed and demonstrated a significantly elevated frequency
(Fig. 2).
The plasma concentration of IL-33, an important
factor with the potential to induce the dominant differentiation of
human ILC2s, was also measured. The results demonstrated that IL-33
expression was clearly increased in mRNA (Fig. 1D) and protein level (Fig. 1E) in patients with lung cancer
compared with healthy controls.
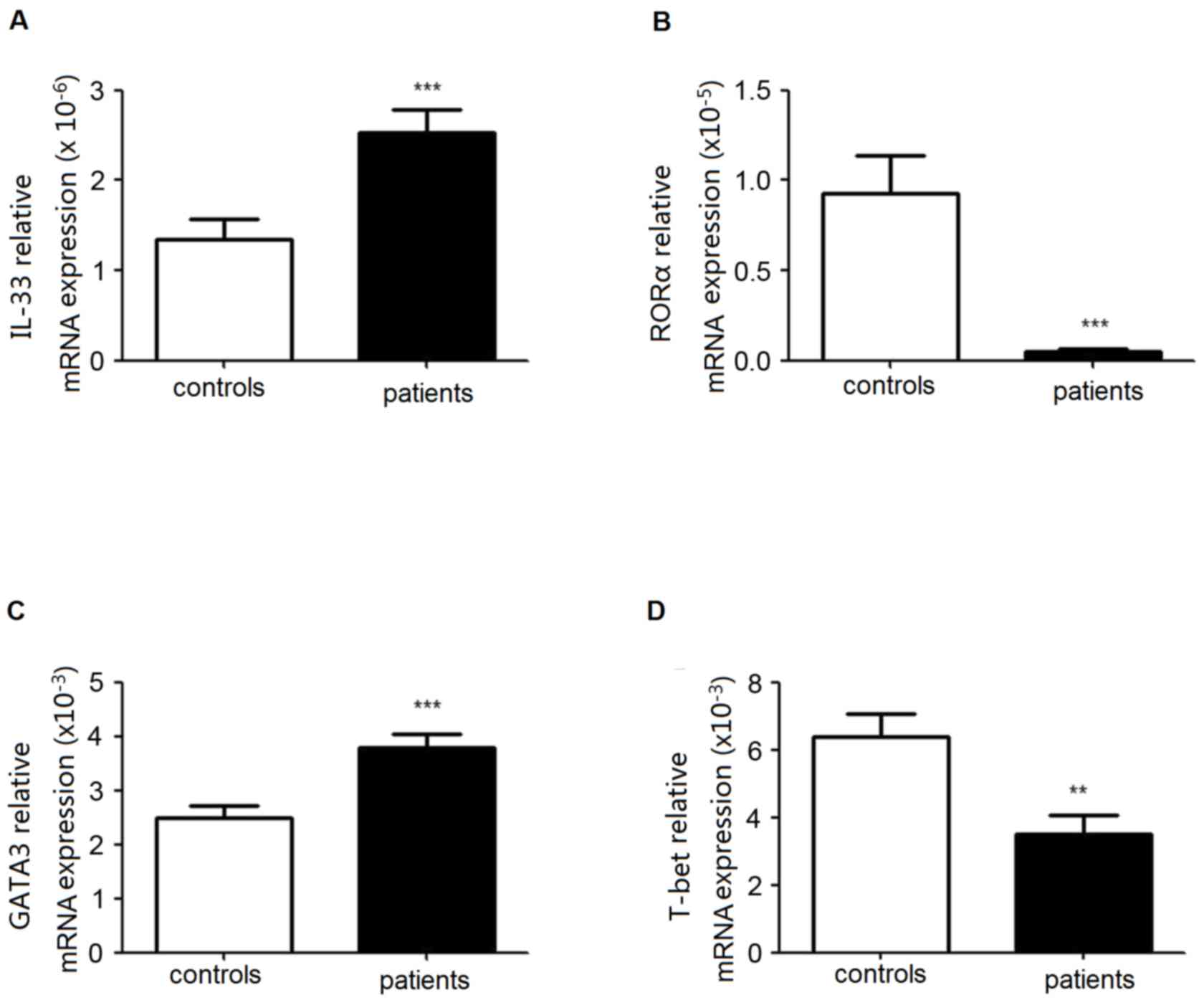
Increased expression levels of RORα
and GATA3 in PBMCs from patients with lung cancer
The transcription factors RORα and/or GATA3 are
essential for the development and function of human ILC2s. GATA3
and T-bet are involved in Th1/Th2 balance. To analyze the level of
ILC2s and Th1/Th2 equilibrium in patients with lung cancer, the
expression levels of RORα, GATA3 and T-bet in PBMCs were
identified. As presented in Fig.
3, there was an increased mRNA expression of GATA3 in patients,
although RORα mRNA decreased. Simultaneously, the expression level
of T-bet was markedly decreased.
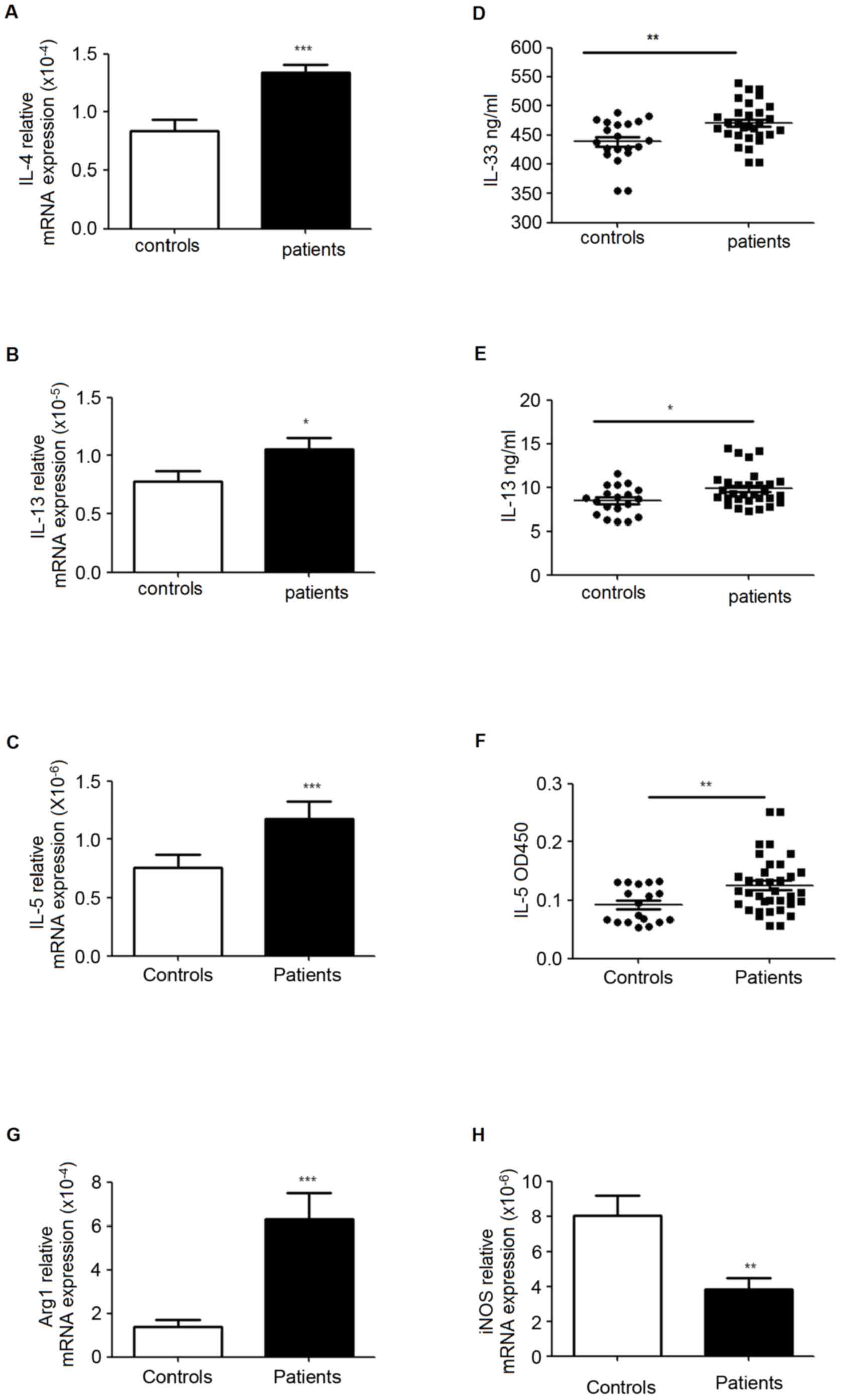
Enhanced ILC2s, MDSCs and Th2 related
cytokines in PBMC from patients with lung cancer
RT-qPCR was used to analyze the mRNA levels of IL-4,
IL-5, IL-13, autophagy related 1 (Arg1) and inducible nitric oxide
(iNOS) in PBMCs, and ELISA was performed to evaluate the levels of
these signature cytokines in plasma. The data indicated that IL-5
and IL-13 were significantly increased in mRNA and protein
expression levels in patients. The results also demonstrated
increased mRNA levels of Arg1 and IL-4, while the level of iNOS was
decreased (Fig. 4).
The correlation between the expression
levels of Th2 cytokines and ILC2s or MDSCs
IL-33 serves an important role in inducing ILC2s,
while IL-5 and IL-13 are the signature cytokines produced by ILC2s.
Correlation analysis demonstrated that the expression of IL-33 was
associated with RORa, IL-5 or IL-13 expression levels (Fig. 5A-C). In order to understand the
association between ILC2s and Th2s, the connection between ILC2s
associated factors (IL-33, IL-5, IL-13 or RORa) and Th2s related
molecules (GATA3 or IL-4) were analyzed; the results demonstrated
that there was a positive correlation between them in patients with
lung cancer (Fig. 5D-G). In
addition, the mRNA expression levels of Arg1 and iNOS (the
representative cytokines of MDSCs) were affected significantly and
positively by predominant Th2signature cytokines in patients with
lung cancer (Fig. 5H-K).
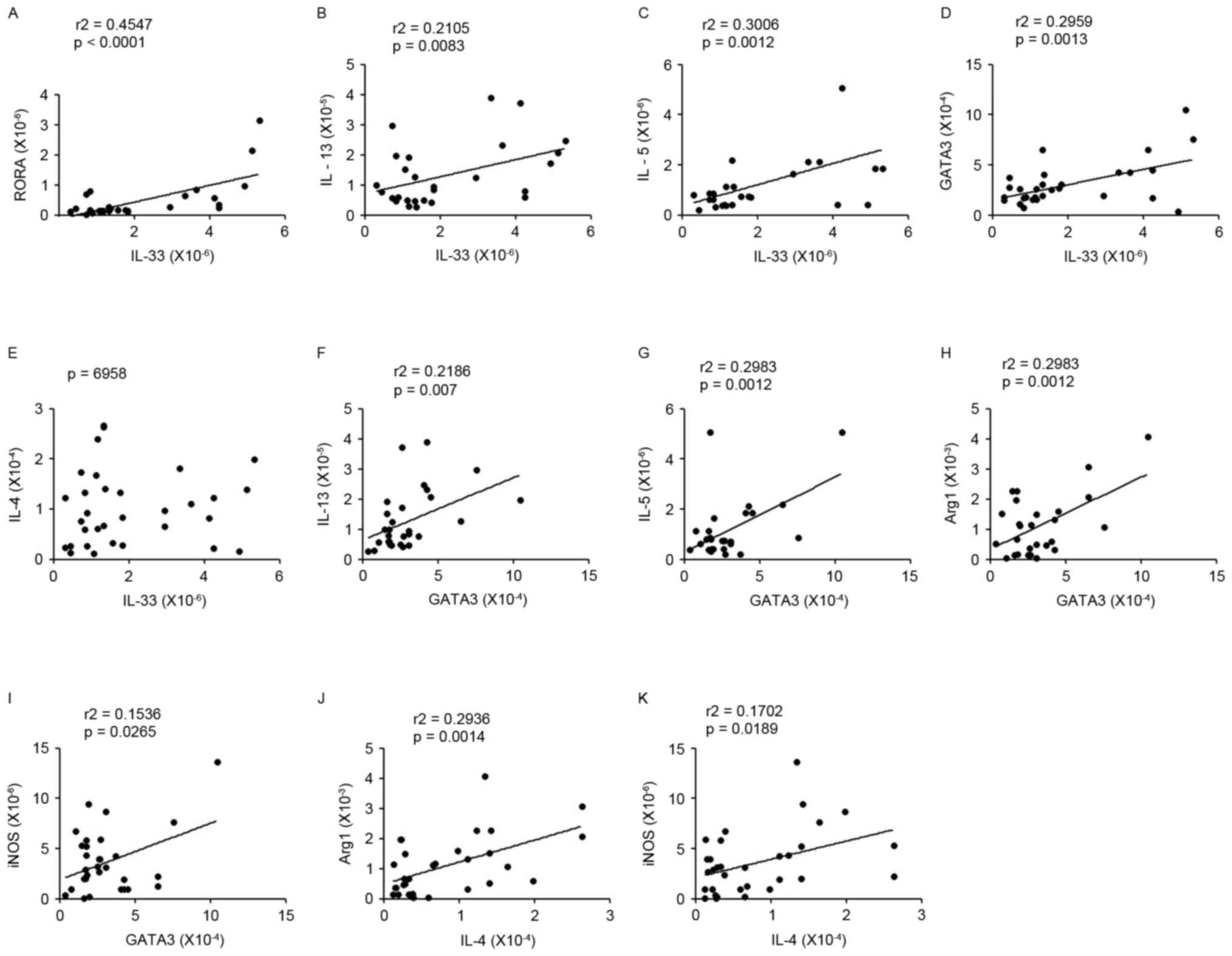 | Figure 5.Correlations between Th2 cytokines and
ILC2s or MDSCs. (A-C) The correlation between IL-33 and RORα, IL-5
or IL-13 expression levels. (D-G) The correlation between ILC2s
associated factors (IL-33, IL-5, IL-13 or RORα) and Th2s related
molecules (GATA3 or IL-4); a significantly positive correlation was
observed between them in patients with lung cancer. (H-K) There
were also positive correlations between GATA3/IL-4 and Arg1/iNOS
mRNA expression. The data were analyzed by the Spearman test. Th2,
type 2 T helper cell; ILC2s, group 2 innate lymphoid cells; MDSCs,
myeloid-derived suppressor cells; IL, interleukin; RORa, retinoic
acid receptor related orphan receptor α; GATA3, GATA binding
protein 3; Arg1, autophagy related 1; iNOS, inducible nitric
oxide. |
Discussion
Human immunity is an elegant, powerful and complex
system that fights invading pathogens and transformed cells,
including cancer. The system is divided into the major components
of innate and adaptive immunity. In past years, the regulation of
cancer by adaptive immunity has received a great deal of attention,
although little has been focused on innate immunity. The ILC2s,
which is a subset of the newly identified innate lymphoid cell
family, are essential in the initiation and coordination of
efficient Th2 cell-mediated immunity by producing IL-13 and IL-5
when stimulated by IL-25 or IL-33 (24). Under the tumor induced
immunosuppressive environment, T helper cells acquire Th2, while
MDCS are hypothesized to inhibit effector immune responses. It has
become clear that IL-4 and IL-13 are also important factors, which
can promote the expansion of these MDSCs or induce their activation
(10), and it has been widely
accepted that the ILC2s were the early source of IL-13 (25). Therefore, it was hypothesized that
these two heterogeneous groups may coexist in the tumor immune
microenvironment. In the present study, the frequencies of ILC2s
and MDSCs in the peripheral blood in patients with lung cancer was
evaluated. It was confirmed that the circulating ILC2s
(lineage−/ICOS+/IL-17RB+) and
MDSCs (lineage−/HLA-DR−/CD33+)
were significantly increased in patients with lung cancer. In
addition, IL-5 and IL-13 were significantly increased in mRNA and
protein expression levels in patients. It was hypothesized that the
IL-13 or IL-5 produced by ILC2s may cause the expansion of
circulating MDSCs or induce their activation. It was noteworthy
that the expression of RORα, as a transcription factor of ILC2s,
was clearly decreased in lung cancer patients in the present study.
This phenomenon may be due to increased circulating MDSCs executing
their inhibitory function in ILC2s by certain yet to be elucidated
mechanisms, including suppression of T cell activation (16–19).
A previous study (26) indicated
that Th2s are also important for ILC2s function, and identified
that numbers of ILC2s are not maintained following several days
without T cells.
IL-33 and IL-25 are also potent activators of ILC2s,
however, recent studies have paid attention on the more forcible
function of IL-33 on ILC2s. Mjösberg et al (8) reported that human fetal gut and blood
CRTH2+ ILC2 lines responded to IL-25, although the
response to IL-33 was more pronounced. In addition, an
influenza-induced airway hyper-reactivity model confirmed the
hypothesis that IL-33 is more potent in the induction of ILC2s
(27–29). Consistent with these previous
studies, the data from the present study demonstrated that lung
cancer patients exhibited a marked increase of IL-33 in mRNA and
protein levels. The mRNA level of ILC2s-required transcription
factor, GATA3, was also demonstrated to be significantly increased,
while the mRNA expression of transcription factor RORα was reduced
compared with healthy controls. This discrepancy may due to the
diversity of the tumor microenvironment, or the faster degradation
rate of mRNA compared with protein (30). It has been reported (31) that GATA3 has the potential to
control the function of ILC2s through multiple mechanisms.
Considering the Th1/Th2 balance in this type of cancer, the mRNA
level of T-bet, the Th1 cells-required transcription factor, was
then analyzed in the present study and the results demonstrated
that the mRNA level of T-bet was decreased, indicating that there
was a predominant Th2 phenotype in patients with lung cancer.
IL-13 is one of the main products of ILC2s, and
Gabitass et al (32)
reported that the elevated MDSCs in pancreatic and gastric cancer
is an independent prognostic factor associated with the elevation
of IL-13. Thus, the functional products of MDSCs were analyzed; the
mRNA level of Arg1 was elevated, and the level of iNOS was reduced.
This phenomenon was similar to one study (33) where in vitro cultured MDSCs
with IL-13 exhibited clearly increased Arg1, although no
significantly increased iNOS. Indeed, monocytic MDSCs appear to fit
in the concept of classically activated (or M1) vs. alternatively
activated (or M2) mononuclear phagocytes, induced by Th1 vs. Th2
cytokines, respectively. Monocytic MDSCs are also distinguished by
the production of enzymes involved in L-arginine metabolism (high
iNOS/NO for M1; high Arg1 for M2) (34). IL-4 and IL-13 are crucial for
inducing the M2-MDSCs, which can produce high levels of Arg1.
Studies (35–37) demonstrate that Th2s and ILC2s
exhibit a close association with MDSCs. In view of this, the
correlations between the products of Th2s and ILC2s or MDSCs were
analyzed, and it was identified that there were positive
correlations between Th2 cytokines and ILC2s or MDSCs associated
factors. These results require confirmation using an in vivo
model.
In conclusion, the present study demonstrated
significant increases in circulating ILC2s and MDSCs in lung cancer
patients whose peripheral circulation had an elevated expression
level of IL-33. The positive correlations between Th2 cytokines and
ILC2s or MDSCs associated factors indicated that enhanced ILC2s and
MDSCs were accompanied by a predominant Th2 phenotype, which may
lead to new immunotherapeutic approaches for lung cancer based on
the associated metabolites and cytokines.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31270947,
31170849 and 81370084), the Natural Science Foundation of Jiangsu
Province (grant no. BK2011472) and the Postdoctoral Foundation of
China (grant nos. 2014T70490 and 2013T60508).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Danelli L, Frossi B and Pucillo CE: Mast
cell/MDSC a liaison immunosuppressive for tumor microenvironment.
Oncoimmunology. 4:e10012322015. View Article : Google Scholar :
|
|
3
|
Basith S, Manavalan B, Yoo TH, Kim SG and
Choi S: Roles of Toll-like receptors in cancer: A double-edged
sword for defense and offense. Arch Pharmacal Res. 35:1297–1316.
2012. View Article : Google Scholar
|
|
4
|
Hoyler T, Klose CS, Souabni A,
Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M
and Diefenbach A: The transcription factor GATA-3 controls cell
fate and maintenance of type 2 innate lymphoid cells. Immunity.
37:634–648. 2012. View Article : Google Scholar :
|
|
5
|
Roediger B and Weninger W: Group 2 innate
lymphoid cells in the regulation of immune responses. Adv Immunol.
125:111–154. 2015. View Article : Google Scholar
|
|
6
|
Halim TY, MacLaren A, Romanish MT, Gold
MJ, McNagny KM and Takei F: Retinoic-acid-receptor-related orphan
nuclear receptor alpha is required for natural helper cell
development and allergic inflammation. Immunity. 37:463–474. 2012.
View Article : Google Scholar
|
|
7
|
Wong SH, Walker JA, Jolin HE, Drynan LF,
Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al:
Transcription factor RORα is critical for nuocyte development. Nat
Immunol. 13:229–236. 2012. View
Article : Google Scholar :
|
|
8
|
Mjösberg JM, Trifari S, Crellin NK, Peters
CP, Van Drunen CM, Piet B, Fokkens WJ, Cupedo T and Spits H: Human
IL-25- and IL-33-responsive type 2 innate lymphoid cells are
defined by expression of CRTH2 and CD161. Nat Immunol.
12:1055–1062. 2011. View
Article : Google Scholar
|
|
9
|
Monticelli LA, Sonnenberg GF, Abt MC,
Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ,
Yang CY, Sathaliyawala T, et al: Innate lymphoid cells promote
lung-tissue homeostasis following infection with influenza virus.
Nat Immunol. 12:1045–1054. 2011. View
Article : Google Scholar :
|
|
10
|
Trikha P and Carson WE III: Signaling
pathways involved in MDSC regulation. Biochim Biophys Acta.
1846:55–65. 2014.
|
|
11
|
Mantovani A: The growing diversity and
spectrum of action of myeloid-derived suppressor cells. Eur J
Immunol. 40:3317–3320. 2010. View Article : Google Scholar
|
|
12
|
Khaled YS, Ammori BJ and Elkord EJ:
Increased levels of granulocytic myeloid-derived suppressor cells
in peripheral blood and tumour tissue of pancreatic cancer
patients. Immunol Res. 2014:8798972014.
|
|
13
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar :
|
|
14
|
Feng PH, Lee KY, Chang YL, Chan YF, Kuo
LW, Lin TY, Chung FT, Kuo CS, Yu CT, Lin SM, et al:
CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and
their clinical relevance in non-small cell lung cancer. Am J Respir
Crit Care Med. 186:1025–1036. 2012. View Article : Google Scholar :
|
|
15
|
Heuvers ME, Muskens F, Bezemer K, Lambers
M, Dingemans AM, Groen HJ, Smit EF, Hoogsteden HC, Hegmans JP and
Aerts JG: Arginase-1 mRNA expression correlates with
myeloid-derived suppressor cell levels in peripheral blood of NSCLC
patients. Lung Cancer. 81:468–474. 2013. View Article : Google Scholar
|
|
16
|
Ortiz ML, Lu L, Ramachandran I and
Gabrilovich DI: Myeloid- derived suppressor cells in the
development of lung cancer. Cancer Immunol Res. 2:50–58. 2014.
View Article : Google Scholar
|
|
17
|
Gabrilovich D: Mechanisms and functional
significance of tumor-induced dendritic-cell defects. Nat Rev
Immunol. 4:941–952. 2004. View
Article : Google Scholar
|
|
18
|
Bronte V, Serafini P, Apolloni E and
Zanovello P: Tumor-induced immune dysfunctions caused by myeloid
suppressor cells. J Immunother. 24:431–446. 2001. View Article : Google Scholar
|
|
19
|
Sinha P, Clements VK and Ostrand-Rosenberg
S: Reduction of myeloid-derived suppressor cells and induction of
M1 macrophages facilitate the rejection of established metastatic
disease. J Immunol. 174:636–645. 2005. View Article : Google Scholar
|
|
20
|
Kusmartsev SA, Li Y and Chen SH: Gr-1+
myeloid cells derived from tumor-bearing mice inhibit primary T
cell activation induced through CD3/CD28 costimulation. J Immunol.
165:779–785. 2000. View Article : Google Scholar
|
|
21
|
Bie Q, Zhang P, Su Z, Zheng D, Ying X, Wu
Y, Yang H, Chen D, Wang S and Xu H: Polarization of ILC2s in
peripheral blood might contribute to immunosuppressive
microenvironment in patients with gastric cancer. J Immunol Res.
2014:9231352014. View Article : Google Scholar :
|
|
22
|
Xu Y, Gao J, Su Z, Dai X, Li Y, Liu Y,
Chen J, Tong J, Zhang Y, Wu C, et al: Down regulation of Hlx
closely related to the decreased expressions of T-bet and Runx3 in
patients with gastric cancer may be associated with a pathological
event leading to the imbalance of Th1/Th2. Clin Dev Immunol.
2012:9498212012. View Article : Google Scholar :
|
|
23
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed: Communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar
|
|
24
|
Halim TY, Steer CA, Mathä L, Gold MJ,
Martinez-Gonzalez I, McNagny KM, McKenzie AN and Takei F: Group 2
innate lymphoid cells are critical for the initiation of adaptive T
helper 2 cell-mediated allergic lung inflammation. Immunity.
40:425–435. 2014. View Article : Google Scholar :
|
|
25
|
Matthew T, Melissa H, Shinji T, Dawn C,
Kasia G, Kelli B, Marc Q, Martin L, Tina V, Kelli B, et al:
Respiratory syncytial virus infection activates IL-13-producing
group 2 innate lyphoid cells through thymic stromal lymphopoietin.
J Allergy Clin Immunol. 138:814–824.e11. 2016. View Article : Google Scholar :
|
|
26
|
Neill DR, Wong SH, Bellosi A, Flynn RJ,
Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al:
Nuocytes represent a new innate effector leukocyte that mediates
type-2 immunity. Nature. 464:1367–1370. 2010. View Article : Google Scholar :
|
|
27
|
Chang YJ, Kim HY, Albacker LA, Baumgarth
N, McKenzie AN, Smith DE, Dekruyff RH and Umetsu DT: Innate
lymphoid cells mediate influenza-induced airway hyperreactivity
independently of adaptive immunity. Nat Immunol. 12:631–638. 2011.
View Article : Google Scholar :
|
|
28
|
Shaw JL, Fakhri S, Citardi MJ, Porter PC,
Corry DB, Kheradmand F, Liu YJ and Luong A: IL-33-responsive innate
lymphoid cells are an important source of IL-13 in chronic
rhinosi-nusitis with nasal polyps. Am J Respir Crit Care Med.
188:432–439. 2013. View Article : Google Scholar
|
|
29
|
Spits H, Artis D, Colonna M, Diefenbach A,
Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius
RE, et al: Innate lymphoid cells-a proposal for uniform
nomenclature. Nat Rev Immunol. 13:145–149. 2013. View Article : Google Scholar
|
|
30
|
Reppert S, Boross I, Koslowski M, Türeci
Ö, Koch S, Lehr HA and Finotto S: A role for T-bet-mediated tumour
immune surveillance in anti-IL-17A treatment of lung cancer. Nat
Commun. 2:6002011. View Article : Google Scholar
|
|
31
|
Wan YY: GATA3: A master of many trades in
immune regulation. Cell. 35:233–242. 2014.
|
|
32
|
Gabitass RF, Annels NE, Stocken DD, Pandha
HA and Middleton GW: Elevated myeloid-derived suppressor cells in
pancreatic, esophageal and gastric cancer are an independent
prognostic factor and are associated with significant elevation of
the Th2 cytokine interleukin-13. Cancer Immonol Immunother.
60:1419–1430. 2011. View Article : Google Scholar
|
|
33
|
Highfill SL, Rodriguez PC, Zhou Q, Goetz
CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody
JS, Munn DH, et al: Bone marrow myeloid-derived suppressor cells
(MDSCs) inhibit graft-versus-host disease (GVHD) via an
arginase-1-dependent mechanism that is upregulated by
interleukin-13. Blood. 116:5738–5747. 2010. View Article : Google Scholar :
|
|
34
|
Van Ginderachter JA, Beschin A, De
Baetselier P and Raes G: Myeloid-derived suppressor cells in
parasitic infections. Eur J Immunol. 40:2976–2985. 2010. View Article : Google Scholar
|
|
35
|
Wu Y, Yan Y, Su Z, Bie Q, Wu J, Wang S, Yu
Y, Ding H, Lu P and Xu H: Enhanced circulating ILC2s accompany by
upregulated MDSCs in patients with asthma. Int J Clin Exp Pathol.
8:3568–3579. 2015.
|
|
36
|
Wolterink RG Klein, Serafini N, Van
Nimwegen M, Vosshenrich CA, de Bruijn MJ, Pereira D Fonseca,
Fernandes H Veiga, Hendriks RW and Di Santo JP: Essential,
dose-dependent role for the transcription factor Gata3 in the
development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc
Natl Acad Sci USA. 110:pp. 10240–10245. 2013; View Article : Google Scholar :
|
|
37
|
Bando JK, Nussbaum JC, Liang HE and
Locksley RM: Type 2 innate lymphoid cells constitutively express
arginase-I in the naive and inlamed lung. J Leukoc Biol.
94:877–884. 2013. View Article : Google Scholar :
|