Introduction
Polymers may be naturally-occurring, including
collagen and polysaccharides, or synthesized, including polylactide
(PLA), polyethylene and poly lactic-co-glycolic acid (PLGA)
(1–7). Polymers have been extensively studied
and used for biomedical and pharmaceutical applications (1,8). As
polymer synthesis may be conducted under controlled conditions, and
the properties of polymers, including hydrophilicity, degradability
and biocompatibility, may be tailored for specific applications,
numerous studies have focused on the development of novel synthetic
polymers for tissue-engineering (polymers as scaffolds for tissue
regeneration) and controlled drug release (polymers as drug
carriers) (3,4,9).
Additionally, synthetic polymers are frequently less expensive as
they may be synthesized in large quantities and exhibit long shelf
lives (4).
Aliphatic polyesters, including PLA, polyglycolide
(PGA) and PLGA, are among the most commonly used synthetic polymers
in medicine, due to their excellent biocompatibility and
biodegradability (10). PLA is
used for manufacturing sutures and bone screws; PLA, PGA and
particularly PLGA are being investigated for drug delivery
applications (7,11,12).
Polypropylene carbonate (PPC), a relatively new aliphatic
polyester, is synthesized via the copolymerization of
CO2 and propylene oxide (13,14).
It is known that PPC possesses limited mechanical strength, and is
therefore not suited to serve as a scaffold to support tissue
regeneration (10), although it
may be suitable for drug delivery; however, this requires further
investigation.
Electrospinning has been used for the fabrication of
polymer fibers (15–17). Electrospinning uses an electric
field, wherein a polymer solution is charged and driven by the
electric potential. When the charged jet of polymer solution
travels across an air gap, the solvent evaporates while the polymer
fibers, with diameters ranging between nanometers and micrometres,
are generated. In the present study, the applicability of the PPC
polymer as a drug carrier for sirolimus, also known as rapamycin,
was investigated. Sirolimus is a cell growth-inhibiting agent used
to treat vascular restenosis. Electrospinning was employed to
synthesize PPC polymer containing sirolimus. The properties of the
drug-containing PPC polymer were investigated, drug loading and
entrapment efficiency were determined, and in vitro
sirolimus release was assessed. In addition, the effect of the
PPC-sirolimus polymer on cell growth was studied in
vitro.
Materials and methods
Materials
PPC (MW 100,000; purity >99%) was purchased from
Inner Mongolia Mengxi High Tech Group Co., Ltd. (Ordos, China).
Sirolimus (rapamycin) was obtained from Fujian Kerui Pharmaceutical
Co., Ltd. (Fuzhou, China). All chemicals were from Jiete'ao
Biotechnology Co., Ltd. (Beijing, China). MTT was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Eagle's minimum
essential medium (EMEM), fetal bovine serum (FBS), penicillin and
streptomycin were obtained from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Preparation of PPC polymer loaded with
sirolimus
Various ratios of PPC and sirolimus were used to
produce PPC-sirolimus polymers and cell growth experiments,
performed by MTT assay as previously described (18), demonstrated that the polymer made
with 900 mg PPC and 100 mg sirolimus resulted in the greatest cell
inhibitory effect; therefore, this ratio was used in the present
study. The polymer solution was as follows: 1,000 mg PPC alone (PPC
polymer) or 900 mg PPC plus 100 mg sirolimus (PPC-sirolimus
polymer) was dissolved in 10 ml acetonitrile following stirring for
6 h. Electrospinning was performed using the electrospinning
apparatus (Model ET2535; Beijing Ucalery Technology and Development
Co., Ltd., Beijing, China). The polymer solution was transferred
into a syringe attached with a blunt tipped needle. The anode of
the power supply was clamped to the needle tip and the cathode was
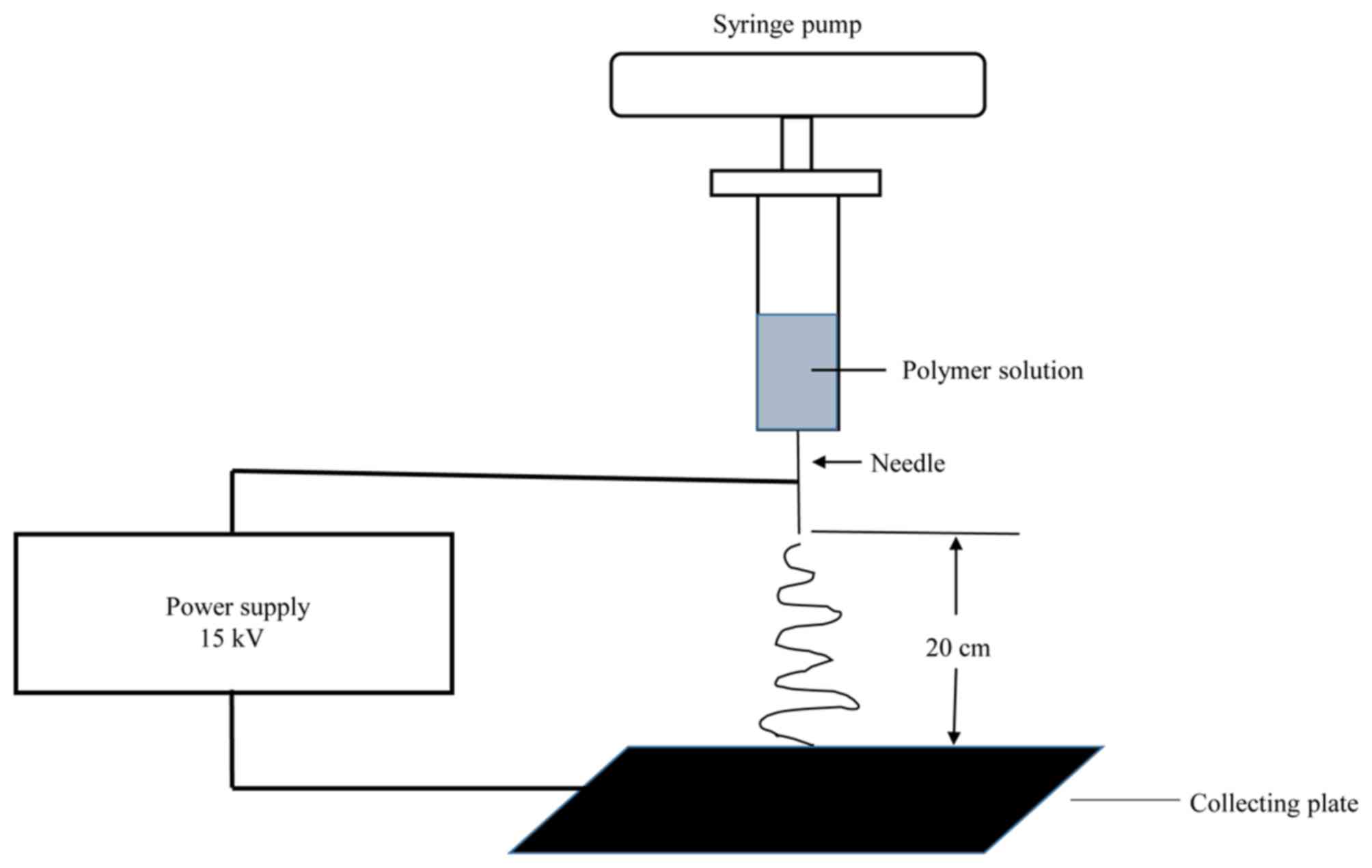
connected to the grounded collector plate (Fig. 1). Electrospinning was performed
according to the established protocol (Institute of Polymer
Science, Tsinghua University, Beijing, China) (19).
Scanning electron microscopy
(SEM)
The polymers were coated with silver using the
vacuum evaporator (HUS 5GB; Hitachi Ltd., Tokyo, Japan) following
an established protocol at the Imaging Center, Shandong University
(Jinan, China) (20). The silver
coated polymers were analyzed using a scanning electron microscope
(H-8010; Hitachi, Ltd., Japan) operated at 15 kV, and images were
captured.
Differential scanning calorimetry
(DSC)
The thermal behavior of the PPC-sirolimus polymers
was assessed by DSC analysis using the DSC822e differential
scanning calorimeter (Mettler Toledo, Greifensee, Switzerland). A
total of 4 mg sample (pure sirolimus or PPC-sirolimus polymer) was
weighed in a 40 µl aluminum pan and placed in the sample chamber of
the calorimeter, and an empty pan was used as the reference. A flow
of nitrogen gas was maintained over the sample to eliminate air
oxidation of the sample at high temperatures, and to create a
reproducible and dry atmosphere. DSC analysis was performed using
the STARe software version 8.10 (Mettler Toledo) with the
temperature range 20–300°C and the temperature increase being set
at a rate of 10°C/min. The endothermic or exothermic peak was
automatically recorded.
In vitro PPC-sirolimus polymer
degradation assessment
A PPC-sirolimus polymer of 1×1 cm was placed in a
5-ml test tube and soaked in PBS (pH 7.2). The tube was gently
shaken (60 rpm) at 37°C for 4 weeks. Subsequently, the structure of
the polymer was examined using SEM.
Determination of drug loading and
entrapment efficiency
The efficiency of drug loading and entrapment was
determined as follows: 1,000 mg PPC-sirolimus polymer was dissolved
in dichloromethane by stirring for 2 h followed by addition of 0.1
M hydrochloric acid. Following mixing by vortex, lower
dichloromethane and upper hydrochloric acid layers were formed. The
hydrochloric acid layer was collected, centrifuged at 1,000 × g for
5 min at room temperature and the supernatant was collected. The
precipitate was mixed with 0.1 M hydrochloric acid containing 0.1%
EDTA. When the precipitate was completely dissolved, the solution
was added to the previously collected hydrochloric acid
supernatant. The sirolimus in the combined solution was measured by
high pressure liquid chromatography as described previously
(21). The sirolimus loading
efficiency (%) was calculated using the following formula: (Total
sirolimus amount in the combined solution/polymer weight) × 100.
Drug entrapment efficiency was calculated using the following
formula: [Total sirolimus amount in the combined solution/total
sirolimus amount used for polymer preparation (100 mg)] × 100. A
total of three PPC-sirolimus polymer preparations were used to
determine the drug loading and entrapment efficiency.
In vitro measurement of sirolimus
release from polymers
A total of 5 mg PPC-sirolimus polymer was placed in
14 separate 5-ml test tubes. The polymer was immersed in 4 ml
PBS/tube and gently shaken (60 rpm) at 37°C. At day 2, 4, 6, 8, 10,
12, 14, 16, 18, 20, 22, 24, 26 and 28 following shaking, the
polymer was removed and the sirolimus contained in the polymer was
isolated and measured as described in the determination of drug
loading and entrapment efficiency section. At each time point, the
amount of released sirolimus was calculated using the following
formula: Total sirolimus in the original 5 mg polymer-total
sirolimus in the soaked polymer. The release percentage was
calculated using the following formula: (Amount of released
sirolimus/total sirolimus in the original 5 mg polymer) × 100.
Inhibition of cell growth by
PPC-sirolimus polymers
An MTT assay was performed to examine the anti-cell
proliferative effect of the PPC-sirolimus polymer. Rat aortic
adventitial fibroblast cells (RAAFCs) were isolated as described
previously (22,23). A total of 4 Wistar rats (2 males
and 2 females, ~ 7 weeks old, weighing 180–220 g) were used for
RAAFC isolation. The animals were purchased from Shandong
Experimental Animal Center (Jinan, China), housed in the Shandong
University (Jinan, China) Animal Room with temperature at 20±2°C,
and fed with standard chow. The use of animals was approved by the
Research Ethics Committee of Shandong University in compliance with
the Guidelines for the Care and Use of Laboratory Animals issued by
the Ministry of Science and Technology, China. Cells were cultured
in EMEM with 10% FBS, 50 IU/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. For the MTT assay, 5×103 cells suspended
in 100 µl culture medium were seeded into each well of a 96-well
plate, into which had previously been added 2 mg of PPC or
PPC-sirolimus polymer (each sample in triplicate). Cells seeded in
wells with no polymer were taken as the control. Following
culturing for 1 and 3 days, the MTT assay was performed as follows:
10 µl MTT solution was added into each well and incubated at 37°C
for 4 h, followed by the addition of 50 µl dimethyl sufoxide/well.
Following gentle shaking for 10 min, absorbance at a wavelength of
570 nm was measured using a 96-well plate reader (Model 680,
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The relative cell
viability (%) of each sample was calculated using the following
formula: (Average sample absorbance/average control absorbance) ×
100.
Statistical analysis
Cell growth data are expressed as the mean ±
standard error of the mean. The SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Statistical
significance was determined using the Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Electrospinning and polymer
characterization
Electrospinning conditions were optimized and used
as follows: The voltage was set at 15 kV; the distance from the
needle tip to the collector was 20 cm, and the flow rate of the
spinning solution was controlled at 0.2 ml/h by a syringe pump
(TJ-3A/W0109-1B; Longer Precision Pump Co., Ltd., Baoding, China)
(Fig. 1). SEM analysis
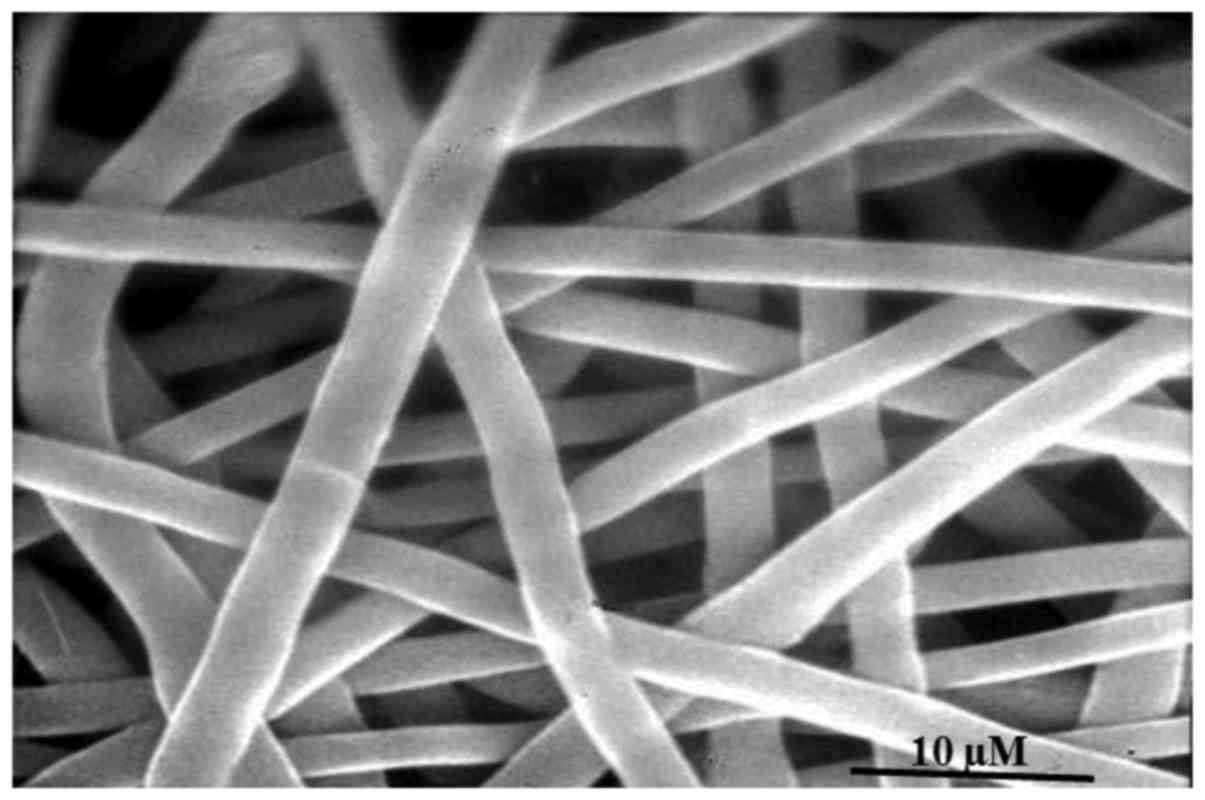
demonstrated that the polymer exhibited a regular
three-dimensional, grid-intertwined, net-like structure with a
smooth surface, and the diameter was ~3 µm (Fig. 2). DSC analysis demonstrated that
the crystalline pure sirolimus produced an endothermic peak at
~230°C, the melting point of sirolimus (Fig. 3A). By contrast, the PPC-sirolimus
polymer exhibited a glass transition temperature of ~40°C for PPC
(Fig. 3B). The in vitro
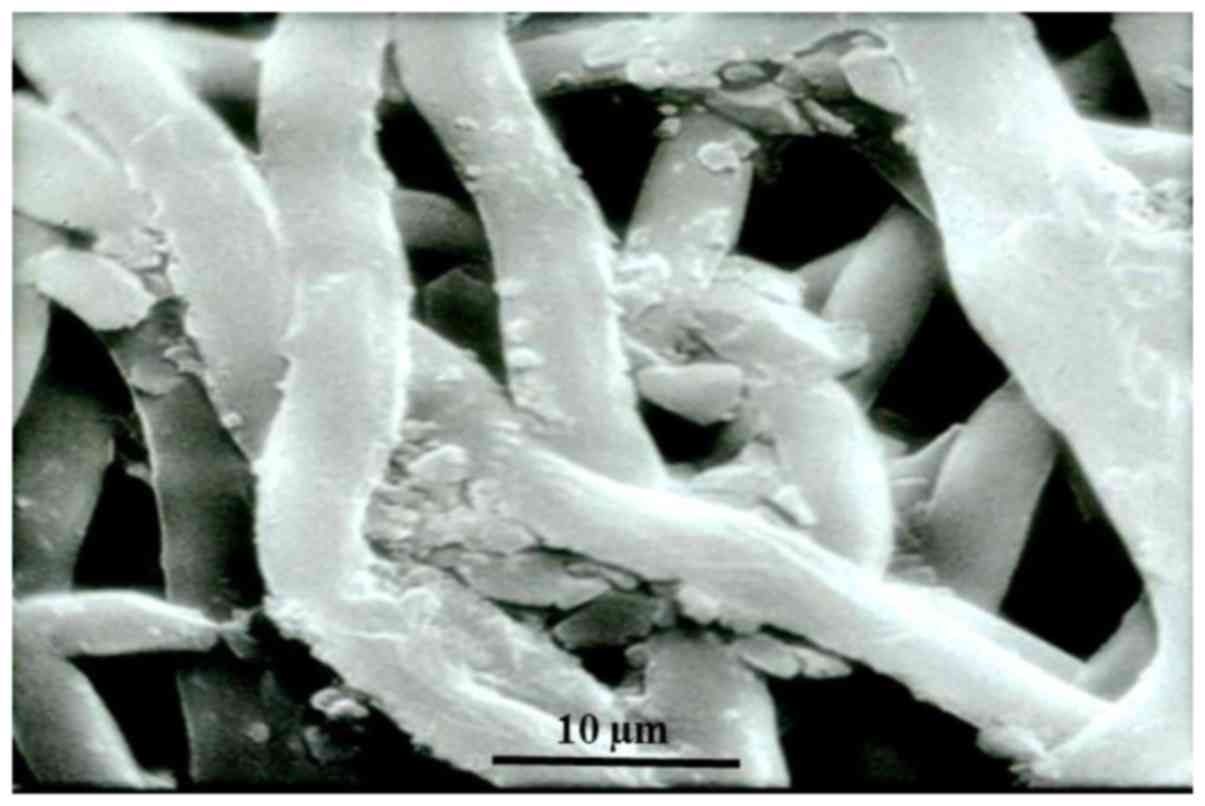
degradation experiment demonstrated that, following soaking in PBS
for 4 weeks, the polymer swelled and the regular three-dimensional
grid-intertwined structure broke down and fragmented (Fig. 4).
Sirolimus loading, entrapment
efficiency and release from polymer
Sirolimus loading and entrapment efficiency were
10.3±3.2 and 95.1±10.6%, respectively. The release of sirolimus was
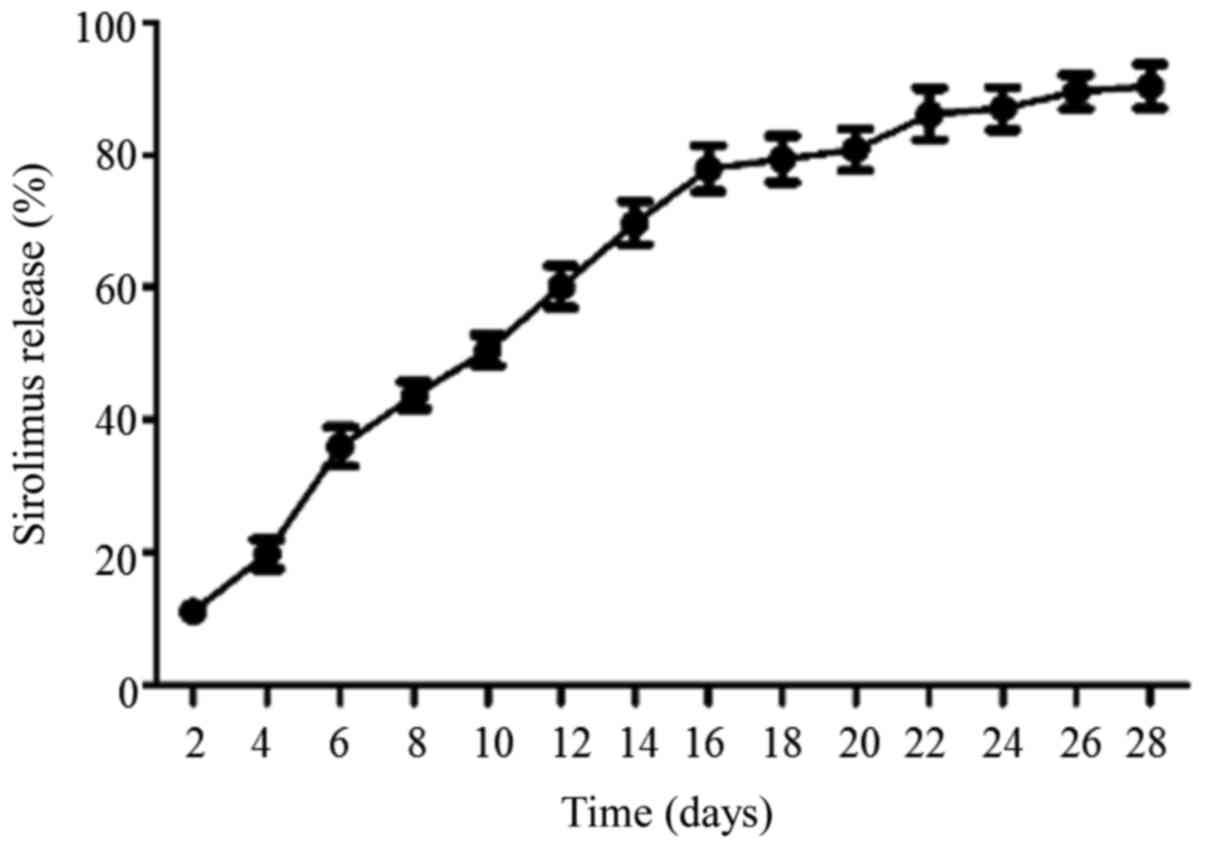
measured for 28 days. As presented in Fig. 5, sirolimus was gradually released
from the PPC-sirolimus polymer in a linear-like fashion,
particularly for the first 3 weeks. A total of ~90% of the
sirolimus entrapped in the polymer was released after 28 days.
Inhibition of RAAFC growth by the
PPC-sirolimus polymer
An MTT assay was performed in order to evaluate the
effect of the PPC-sirolimus polymer on RAAFC growth. The viability
of control cells (without the addition of any polymer) was
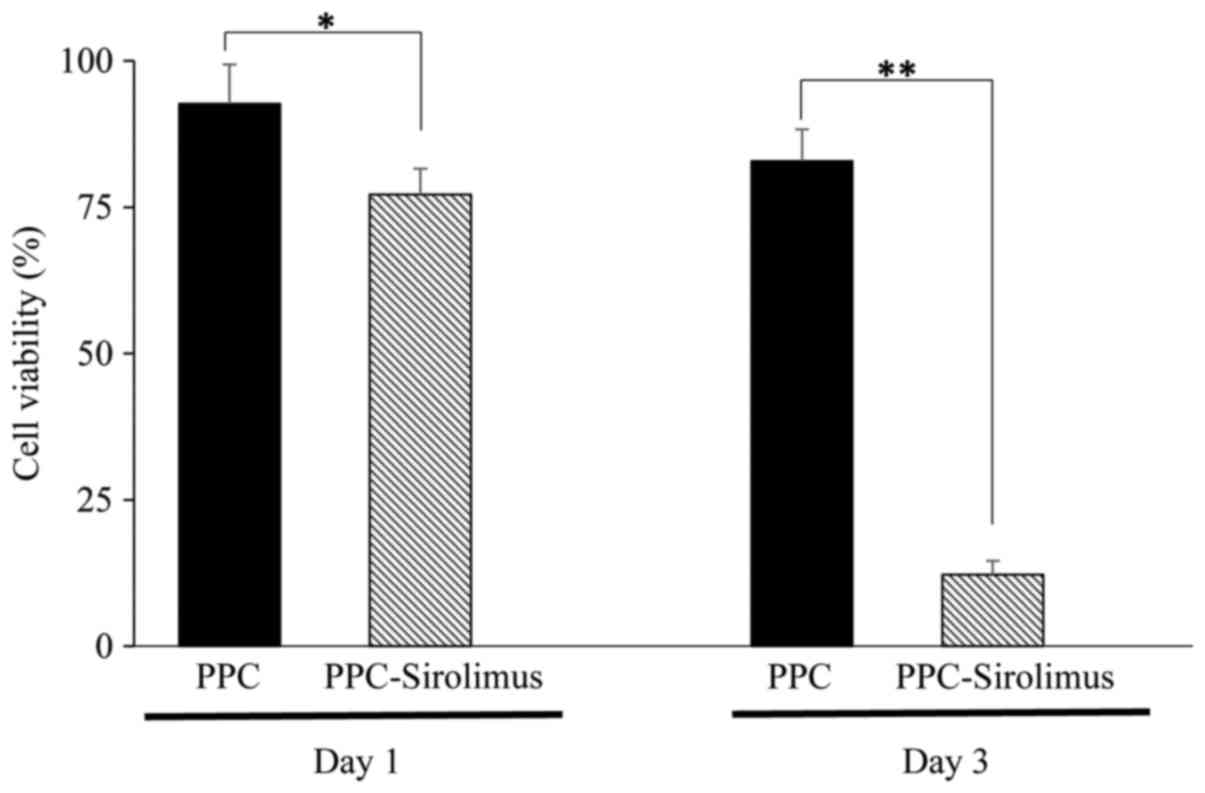
arbitrarily set at 100%. As presented in Fig. 6, following culture for 1 day, the
viability of cells treated with PPC and PPC-sirolimus was 92.7±6.7
and 77.2±4.4%, respectively. At day 3, the viability of cells
treated with PPC and PPC-sirolimus was 82.9±5.4 and 12.2±2.4%,
respectively. Statistical analysis demonstrated that the
PPC-sirolimus polymer significantly inhibited the growth of RAAFCs
at the two time points, while the PPC polymer did not (Fig. 6).
Discussion
Subsequent to the first investigations into
polymer-controlled drug release in cancer therapy (8,24,25),
the field has rapidly expanded and applied to the treatment of
other diseases (18,26,27).
Aliphatic polyesters, particularly PLGA, have been extensively
explored for local drug delivery. However, one disadvantage of
traditional aliphatic polyesters is that they generate acidic
products following degradation in vivo, which may cause
aseptic inflammation and tissue necrosis (10,28–30).
By contrast, PCC, a novel aliphatic polyester, is distinct in that
it primarily produces H2O and CO2 following
degradation, eliminating the side effects caused by PLA, PGA and
PLGA (14). Additionally, PPC
exhibits an increased bioadhesive capacity compared with PLA, PGA
and PLGA (21), enabling PPC-drug
conjugates to be retained in the target area and exert prolonged
therapeutic effects.
Using electrospinning technology, PPC fibers
containing sirolimus were generated in the present study. As
examined by SEM, the PPC-sirolimus polymer formed a uniform
three-dimensional and grid-intertwined net-like structure. The
structure formed in electrospun fibers is hypothesized to exhibit a
large surface area which is ideal for drug release (31). DSC analysis demonstrated that the
endothermic peak for sirolimus was not present in PPC-sirolimus
polymer, indicating that sirolimus may exist in the polymer in an
amorphous state. Notably, DSC sensitivity may be affected by the
low concentration of the drug in the polymer fibers. It is thought
that the amorphous state enhances the release of the drug from the
polymers (32). In a previous
study, PLGA was used to load sirolimus, and the entrapment and drug
loading efficiencies were reported to be ~80 and ~5%, respectively
(21), slightly lower compared
with what was observed with PPC in the present study.
It is desirable that entrapped therapeutic agents in
a polymer are able to be released for a period of time, thus
maintaining a sustained action and decreasing the number of
administration procedures required. The results of the present
study demonstrate that sirolimus encapsulated in a PPC polymer was
released in a linear-like manner, particularly for the first 3
weeks. At week 4, ~90% of the sirolimus had been released. Zou
et al (21) demonstrated
the application of PLGA with carbopol, a compound which enhances
PLGA bioadhesion, for local sirolimus delivery; it was observed in
an in vitro experiment that sirolimus was able to be
released for 4 weeks. Kang et al (33) observed that the release of
doxorubicin from polyorganophosphazene hydrogels lasted 20–30 days,
which is consistent with the results of the present study. In
vitro experiments in the present study demonstrated that PPC
did not affect the growth of RAAFCs. However, the PPC-sirolimus
polymer significantly reduced cell viability, warranting further
in vivo investigation of the effects of PPC-sirolimus.
In conclusion, compared with PLGA, the most widely
studied aliphatic polyester for drug delivery, PPC exhibits a
similar drug release curve and slightly increased loading and
entrapment efficiency with respect to sirolimus encapsulation. As
PPC exhibits increased bioadhesion and primarily produces
H2O and CO2 following degradation in
vivo, causing minimal side effects, PPC may be a promising
alternative polymer to PLGA for controlled drug delivery.
Acknowledgements
The present study was supported by the Independent
Innovation Foundation of Shandong University (grant no. 2012TS171)
and the Research Award Fund for Outstanding Young Scientists of
Shandong Province (grant no. 2006BS03014).
References
|
1
|
Lendlein A: Polymers in biomedicine.
Macromol Biosci. 10:993–997. 2010. View Article : Google Scholar
|
|
2
|
Chattopadhyay S and Raines RT: Review
collagen-based biomaterials for wound healing. Biopolymers.
101:821–833. 2014. View Article : Google Scholar :
|
|
3
|
Williams CK: Synthesis of functionalized
biodegradable polyesters. Chem Soc Rev. 36:1573–1580. 2007.
View Article : Google Scholar
|
|
4
|
Gunatillake P, Mayadunne R and Adhikari R:
Recent developments in biodegradable synthetic polymers. Biotechnol
Annu Rev. 12:301–347. 2006. View Article : Google Scholar
|
|
5
|
Fambri L, Pegoretti A, Fenner R, Incardona
SD and Migliaresi C: Biodegradable fibres of poly(L-lactic acid)
produced by melt spinning. Polymer. 38:79–85. 1997. View Article : Google Scholar
|
|
6
|
Peyton SR, Raub CB, Keschrumrus VP and
Putnam AJ: The use of poly(ethylene glycol) hydrogels to
investigate the impact of ECM chemistry and mechanics on smooth
muscle cells. Biomaterials. 27:4881–4893. 2006. View Article : Google Scholar
|
|
7
|
Kapoor DN, Bhatia A, Kaur R, Sharma R,
Kaur G and Dhawan S: PLGA: A unique polymer for drug delivery. Ther
Deliv. 6:41–58. 2015. View Article : Google Scholar
|
|
8
|
Langer RS and Peppas NA: Present and
future applications of biomaterials in controlled drug delivery
systems. Biomaterials. 2:201–214. 1981. View Article : Google Scholar
|
|
9
|
Middleton JC and Tipton AJ: Synthetic
biodegradable polymers as orthopedic devices. Biomaterials.
21:2335–2346. 2000. View Article : Google Scholar
|
|
10
|
Seyednejad H, Ghassemi AH, Van Nostrum CF,
Vermonden T and Hennink WE: Functional aliphatic polyesters for
biomedical and pharmaceutical applications. J Control Release.
152:168–176. 2011. View Article : Google Scholar
|
|
11
|
Cameron DJ and Shaver MP: Aliphatic
polyester polymer stars: Synthesis, properties and applications in
biomedicine and nanotechnology. Chem Soc Rev. 40:1761–1776. 2011.
View Article : Google Scholar
|
|
12
|
Jain R, Shah NH, Malick AW and Rhodes CT:
Controlled drug delivery by biodegradable poly(ester) devices:
Different preparative approaches. Drug Dev Ind Pharm. 24:703–727.
1998. View Article : Google Scholar
|
|
13
|
Li XH, Meng YZ, Chen GQ and Li RKY:
Thermal properties and rheological behavior of biodegradable
aliphatic polycarbonate derived from carbon dioxide and propylene
oxide. J Appl Polym Sci. 94:711–716. 2004. View Article : Google Scholar
|
|
14
|
Zhong X and Dehghani F: Solvent free
synthesis of organometallic catalysts for the copolymerisation of
carbon dioxide and propylene oxide. Appl Catal B. 98:101–111. 2010.
View Article : Google Scholar
|
|
15
|
Li WJ, Laurencin CT, Caterson EJ, Tuan RS
and Ko FK: Electrospun nanofibrous structure: A novel scaffold for
tissue engineering. J Biomed Mater Res. 60:613–621. 2002.
View Article : Google Scholar
|
|
16
|
Ji Y, Ghosh K, Shu XZ, Li B, Sokolov JC,
Prestwich GD, Clark RA and Rafailovich MH: Electrospun three
dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials.
27:3782–3792. 2006. View Article : Google Scholar
|
|
17
|
Tipduangta P, Belton P, Fábián L, Wang LY,
Tang H, Eddleston M and Qi S: Electrospun polymer blend nanofibers
for tunable drug delivery: The role of transformative phase
separation on controlling the release rate. Mol Pharm. 13:25–39.
2016. View Article : Google Scholar
|
|
18
|
Zheng L, Chen J, Ma Z, Liu W, Yang F, Yang
Z, Wang K, Wang X, He D, Li L and Zeng J: Capsaicin enhances
anti-proliferation efficacy of pirarubicin via activating TRPV1 and
inhibiting PCNA nuclear translocation in 5637 cells. Mol Med Rep.
13:881–887. 2016.
|
|
19
|
Zeng J, Chen X, Xu X, Liang Q, Bian X,
Yang L and Jing X: Ultrafine fibers electrospun from biodegradable
polymers. J Appl Polym Sci. 89:1085–1092. 2003. View Article : Google Scholar
|
|
20
|
Ni S, Xia T, Li X, Zhu X, Qi H, Huang S
and Wang J: Sustained delivery of chondroitinase ABC by
poly(propylene carbonate)-chitosan micron fibers promotes axon
regeneration and functional recovery after spinal cord hemisection.
Brain Res. 1624:469–478. 2015. View Article : Google Scholar
|
|
21
|
Zou W, Cao G, Xi Y and Zhang N: New
approach for local delivery of rapamycin by bioadhesive
PLGA-carbopol nanoparticles. Drug Deliv. 16:15–23. 2009. View Article : Google Scholar
|
|
22
|
Gu M and Brecher P: Nitric oxide-induced
increase in p21(Sdi1/Cip1/Waf1) expression during the cell cycle in
aortic adventitial fibroblasts. Arterioscler Thromb Vasc Biol.
20:27–34. 2000. View Article : Google Scholar
|
|
23
|
Tsuruda T, Kato J, Cao YN, Hatakeyama K,
Masuyama H, Imamura T, Kitamura K, Asada Y and Eto T:
Adrenomedullin induces matrix metalloproteinase-2 activity in rat
aortic adventitial fibroblasts. Biochem Biophys Res Commun.
325:80–84. 2004. View Article : Google Scholar
|
|
24
|
Duncan R: Polymer conjugates as anticancer
nanomedicines. Nat Rev Cancer. 6:688–701. 2006. View Article : Google Scholar
|
|
25
|
Davis ME, Chen Z and Shin DM: Nanoparticle
therapeutics: An emerging treatment modality for cancer. Nat Rev
Drug Discov. 7:771–782. 2008. View
Article : Google Scholar
|
|
26
|
Liechty WB, Kryscio DR, Slaughter BV and
Peppas NA: Polymers for drug delivery systems. Annu Rev Chem Biomol
Eng. 1:149–173. 2010. View Article : Google Scholar :
|
|
27
|
Merkle HP: Drug delivery's quest for
polymers: Where are the frontiers? Eur J Pharm Biopharm.
97:293–303. 2015. View Article : Google Scholar
|
|
28
|
Fu K, Pack DW, Klibanov AM and Langer R:
Visual evidence of acidic environment within degrading
poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res.
17:100–106. 2000. View Article : Google Scholar
|
|
29
|
Danmark S, Finne-Wistrand A, Schander K,
Hakkarainen M, Arvidson K, Mustafa K and Albertsson AC: In vitro
and in vivo degradation profile of aliphatic polyesters subjected
to electron beam sterilization. Acta Biomater. 7:2035–2046. 2011.
View Article : Google Scholar
|
|
30
|
Ding AG and Schwendeman S: Acidic
microclimate pH distribution in PLGA microspheres monitored by
confocal laser scanning microscopy. Pharm Res. 25:2041–2052. 2008.
View Article : Google Scholar :
|
|
31
|
Zamani M, Prabhakaran MP and Ramakrishna
S: Advances in drug delivery via electrospun and electrosprayed
nanomaterials. Int J Nanomedicine. 8:2997–3017. 2013.
|
|
32
|
Zahedi P and Lee PI: Solid molecular
dispersions of poorly water-soluble drugs in poly(2-hydroxyethyl
methacrylate) hydrogels. Eur J Pharm Biopharm. 65:320–328. 2007.
View Article : Google Scholar
|
|
33
|
Kang GD, Cheon SH and Song SC: Controlled
release of doxorubicin from thermosensitive poly(organophosphazene)
hydrogels. Int J Pharm. 319:29–36. 2006. View Article : Google Scholar
|