Introduction
In traditional Chinese medicine, the pathogenesis of
hypertension is mainly attributed to liver, kidney, heart and
spleen dysfunction, that cause an imbalance in the body (1). Traditional Chinese treatments focus
on the etiology and pathology of hypertension, and aim, via
restoring the function of the immune system and other organs, to
eradicate the underlying causes of hypertension. Traditional
Chinese formulas do not produce serious adverse events, such as
those associated with antihypertensive agents used in western
medicine (2). Traditional Chinese
treatments aim to repair the damages induced by increased blood
pressure, via facilitating blood circulation, alleviating blood
stasis, regulating blood lipid contents and restoring the blood
pressure regulatory mechanisms (3).
Gardenia jasminoides Ellis is an edible plant
that has also been used in traditional Chinese medicine (4). In traditional Chinese medicine,
Gardenia jasminoides has been used for its liver- and
gallbladder-protecting properties, in the treatment of contusions
to stop bleeding and reduce swelling, as well as in the treatment
of diabetes (5). Previous studies
on the chemical composition of Gardenia jasminoides have
reported that iridoid glycosides, diterpenoids and organic acid
esters are among its main ingredients (5,6).
More than 20 different types of iridoid glycosides have been
identified in Gardenia jasminoides extracts, including
genipin 1-gentiobioside and gardenoside. Among Gardenia
jasminoides diterpenoids, crocin 1 and crocin 2 are the most
abundant. Genipin 1-gentiobioside, gardenoside, crocin 1 and crocin
2 have been suggested to mediate the biological actions of
Gardenia jasminoides (7).
L-NG-nitroarginine (L-NNA) is an
inhibitor of nitric oxide synthase (NOS), which can increase blood
pressure by inhibiting NO production. L-NNA is often used in
experimental hypertension models, as it can inhibit the
endothelium-dependent relaxation of blood vessels (8,9).
When used in the induction of experimental hypertension, L-NNA
potently inhibits NO production in vascular endothelium, whereas it
also promotes vascular smooth muscle cell proliferation, and thus
changes vascular structure, impairs endothelial function and
increases arterial blood pressure (10). The present study employed an
L-NNA-induced mouse model of hypertension to investigate the
effects of Gardenia jasminoides on hypertension. The
molecular mechanisms underlying the actions of Gardenia
jasminoides were also examined, via the evaluation of serum
levels of key proteins and the expression of related genes.
Furthermore, the chemical composition of Gardenia
jasminoides was analyzed in order to identify the main
components responsible for its effects.
Materials and methods
Preparation of Gardenia jasminoides
extracts
Gardenia jasminoides plants of the Jiangjin
County variety (CJGJ) were purchased in Chongqing local markets
(Jiangjin, China); Gardenia jasminoides plants of the
Lichuan City variety (HLGJ) were purchased in Lichuan local markets
(Hubei, China). The plants were authenticated using an ultraviolet
spectrophotometer, ultraviolet absorption spectrum and thin layer
chromatography by Dr Long Song (Department of Pharmacognosy,
Shanghai University of Traditional Chinese Medicine, Shanghai,
China). Gardenia jasminoides was stored at −80°C and
freeze-dried to produce a powder. A 20-fold volume of 70% methanol
was added to the powdered sample for the sonic extraction of
Gardenia jasminoides components (power, 250 W; frequency, 40
kHz). Methanol extracts were evaporated using a rotary evaporator
(N-1100; Eywla, Tokyo, Japan).
Liquid chromatography
Standards for genipin 1-gentiobioside, gardenoside,
crocin 1 and crocin 2 were prepared in 20 ml volumetric flasks.
Methanol was used to obtain stock solutions with concentrations of
1.03, 1.07, 0.103 and 1.02 mg/ml. Standards were prepared by 5-fold
dilution of the stock solutions. A Waters® ACQUITY
UPLC® BEH C18 chromatography column (2.1×50 mm, 1.7 µm)
was used (Waters China, Ltd., Shatin, Hong Kong). Acetonitrile (A)
and 0.2% phosphoric acid solution (B) were used as the mobile phase
during gradient elution (8–20% A during 0–3 min, 20–35% A during
3–8 min). The detection wavelength was 238 nm during 0–5 min and
440 nm during 5–8 min. The flow rate was 0.3 ml/min and the column
temperature was 30°C. Sample volumes were 2 µl.
Animal experiment
A total of 40 male ICR mice (age, 7 weeks; body
weight, 20–25 g) were purchased from the Experimental Animal Center
of Chongqing Medical University (Chongqing, China). ICR mice were
randomly assigned to the following four groups (n=10 mice/group):
Normal group, control group, CJGJ group, HLGJ group. Mice in the
control, CJGJ and HLGJ groups were treated with 700 mg/kg L-NNA (2
ml) daily (Shanghai Golden Time Biological Technology Co., Ltd.,
Shanghai, China) via gavage for 20 days. Mice in the normal group
were treated with normal saline (2 ml) via gavage for 20 days.
Following L-NNA treatment for 7 days, mice in the CJGJ and HLGJ
groups were treated with 500 mg/kg CJGJ and HLGJ via gavage. On day
20, 45 min after L-NNA the systolic (SBP), diastolic (DBP) and mean
blood pressure (MBP) were measured using the Tail-cuff method (MRBP
non-invasive blood pressure meter, Shanghai Yuyan Instruments Co.,
Ltd., Shanghai, China). Subsequently, mice were sacrificed via
CO2 inhalation and the heart, liver, kidney and stomach
were collected. Blood and heart whole blood vessel samples were
also obtained. The present study was approved by the Animal Ethics
Committee of Chongqing Medical University.
Metabolite levels
NO (cat. no A012) and malondialdehyde (MDA; cat. no
A003-1) levels in heart, liver, kidney and stomach tissue samples
were determined using detection kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). Serum NO (cat. no A012),
MDA (cat. no A003-1), endothelin (ET)-1 (cat. no H093) and
calcitonin gene-related peptide (CGRP; cat. no H217) levels were
determined using the appropriate detection kits (Nanjing Jiancheng
Bioengineering Institute). Serum vascular endothelial growth factor
(VEGF; cat. no 111202; Beijing BLKW Biotechnology Co., Ltd.,
Beijing, China) and E-selectin (cat. no ml002207; Shanghai
Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) levels were
assessed using an ELISA kit.
Reverse transcription-polymerase chain
reaction (RT-qPCR)
Total RNA was extracted from myocardial and vascular
endothelial tissue samples using RNAzol reagent, (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA. The concentration
of the extracted RNA was adjusted to 1 µg/µl. Total RNA (2 µl) was
reverse transcribed into cDNA using 1 µl each of oligo
(dT)18, RNase, deoxyribonucleotide triphosphate and
M-MLV reverse transcriptase (Roche Diagnostics, Basel, Switzerland)
in 5X buffer (10 µl). RT was performed with incubation at 37°C for
120 min, 99°C for 4 min and 4°C for 3 min. The primers used for PCR
amplification of heme oxygenase (HO)-1, adrenomedullin (ADM),
receptor activity modifying protein (RAMP) 2, interleukin (IL)-1β,
tumor necrosis factor (TNF)-α, B-cell lymphoma-2 (Bcl-2),
Bcl-2-associated X protein (Bax), caspase-3, caspase-8, caspase-9,
monocyte chemoattractant protein (MCP)-1, nuclear factor-κB
(NF-κB), matrix metalloproteinase (MMP)-2, MMP-9, neuronal (n)NOS,
endothelial (e)NOS and inducible (i)NOS are presented in Table I. GAPDH was used as an internal
control. cDNA (2 µl) was mixed with 1 µl of each 10 µM primer and
16 µl of DNase-free water in a PCR premix tube
(AccuPower® PCR PreMix; Bioneer Corporation, Daejeon,
Korea) and PCR was performed in an automatic thermocycler (Bioneer
Corporation) for 40 cycles of 94°C for 5 min, 58°C for 30 sec and
72°C for 90 sec, followed by a 10 min cycle at 95°C. Then these PCR
products were resolved by 1.2% agarose gel electrophoresis using 1%
ethidium bromide. Gene expression was semi-quantitatively analyzed
using ImageJ software version 1.44 (National Institutes of Health,
Bethesda, MD, USA) and normalized to GAPDH, as previously described
(11). The following formula was
used to calculate the fold-ratio: Target gene expression/GAPDH ×
control numerical value (control fold ratio=1).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence |
|---|
| HO-1 | F:
5′-GGAACTTTCAGAAGGGCCAG-3′ |
|
| R:
5′-GTCCTTGGTGTCATGGGTCA-3′ |
| ADM | F:
5′-GCTGGTTTCCGTCGCCCTGATGT-3′ |
|
| R:
5′-CGTTGTCCTTGTCCTTATCTGTG-3′ |
| RAMP2 | F:
5′-GGACGGTGAAGAACTATGAG-3′ |
|
| R:
5′-ATCATGGCCAGGAGTACATC-3′ |
| IL-1β | F:
5′-CTCCATGAGCTTTGTACAAGG-3′ |
|
| R:
5′-TGCTGATGTACCAGTTGGGG-3′ |
| TNF-α | F:
5′-CTCCCTCCAGAAAAGACACCAT-3′ |
|
| R:
5′-ATCACCCCGAAGTTCAGTAGACAG-3′ |
| nNOS | F:
5′-GAATACCAGCCTGATCCATGGAA-3′ |
|
| R:
5′-TCCTCCAGGAGGGTG' TCCACCG CATG-3 |
| eNOS | F:
5′-GGAGAGGCTGCATGACATTG-3′ |
|
| R:
5′-GGTAGAGCCATAGTGGAATGAC-3′ |
| iNOS | F:
5′-AGAGAGATCGGGTTCACA-3′ |
|
| R:
5′-CACAGAACTGAGGGTACA-3′ |
| Bax | F:
5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ |
|
| R:
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-2 | F:
5′-CTCGTCGCTACCGTCGTGACTTGG-3′ |
|
| R:
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Caspase-3 | F:
5′-CAAACTTTTTCAGAGGGGATCG-3′ |
|
| R:
5′-GCATACTGTTTCAGCATGGCA-3′ |
| Caspase-8 | F:
5′-CCCCACCCTCACTTTGCT-3′ |
|
| R:
5′-GGAGGACCAGGCTCACTTA-3′ |
| Caspase-9 | F:
5′-GGCCCTTCCTCGCTTCATCTC-3′ |
|
| R:
5′-GGTCCTTGGGCCTTCCTGGTAT-3′ |
| MCP-1 | F:
5′-CACGTCGTAGCAAACCACCAA-3′ |
|
| R:
5′-GTTGGTTGTCTTTGAGATCCAT-3′ |
| NF-κB | F:
5′-CACTTATGGACAACT'ATGAGGTCTC TGG-3 |
|
| R:
5′-CTGTCTTGTGGACAACGCAGTGGAAT' TTTAGG-3 |
| MMP-2 | F:
5′-CTTCTTCAAGGACCGGTTCA-3′ |
|
| R:
5′-GCTGGCTGAGTACCAGTA-3′ |
| MMP-9 | F:
5′-TGGGCTACGTGACCTATGAC-3′ |
|
| R:
5′-GCCCAGCCCACCTCCACTCC-3′ |
| GAPDH | F:
5′-CGGAGTCAACGGATTTGGTC-3′ |
|
| R:
5′-AGCCTTCTCCATGGTCGTGA-3′ |
Statistical analysis
The statistical significance of the difference
between groups was assessed by one-way analysis of variance
followed by a post hoc Duncan's test for multiple comparisons. Data
are expressed as the mean ± standard deviation of three independent
experiments. The analysis was performed using SAS statistical
software version 9.1 (SAS Institute Inc., Cary, NC, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Gardenia jasminoides chemical
constituents
Genipin 1-gentiobioside, gardenoside, crocin 1 and
crocin 2 standards were dissolved in methanol. Genipin
1-gentiobioside standard solutions were adjusted to 1.0300, 0.5150,
0.2575, 0.1288, 0.0644, 0.0322 mg/ml; gardenoside standard
solutions were adjusted to 1.0700, 0.5350, 0.2675, 0.1338, 0.0669,
0.0334 mg/ml; crocin 1 standard solutions were adjusted to 0.2575,
0.1288, 0.0644, 0.0322, 0.0161 and 0.0080 mg/ml; and crocin 2
standard solutions were adjusted to 0.0638, 0.0319, 0.0159, 0.0080,
0.0040, 0.0020 mg/ml. The standards were assessed using liquid
chromatography and standard curves were determined. The following
equations were used: Genipin 1-gentiobioside,
y=5×106x+17,485; gardenoside,
y=1×107x+96,992; crocin 1,
y=4×107x+10,1764; and crocin 2,
y=4×107x-784.4. Subsequently, genipin
1-gentiobioside, gardenoside, crocin 1 and crocin 2 contents of the
two Gardenia jasminoides varieties were chromatographically
determined according to the standard curves (Fig. 1). The chemical compositions of CJGJ
and HLGJ are presented in Table
II.
 | Table II.Analysis of the chemical composition
of Gardenia jasminoides using liquid chromatography. |
Table II.
Analysis of the chemical composition
of Gardenia jasminoides using liquid chromatography.
| Sample | Genipin
1-gentiobioside (%) | Gardenoside
(%) | Crocin 1 (%) | Crocin 2 (%) |
|---|
| CJGJ | 0.59 | 2.74 | 0.38 | 0.06 |
| HLGJ | 0.33 | 2.91 | 0.39 | 0.04 |
Blood pressure
Mice in the control group exhibited the highest SBP,
DBP and MBP following L-NNA treatment, whereas normal mice
exhibited the lowest blood pressure measurements (Table III). Treatment with CJGJ and HLGJ
appeared to significantly reduce SBP, DBP and MBP in L-NNA-treated
mice compared with L-NNA-treated control mice (P<0.05). The
effect of HLGJ on blood pressure was significantly greater compared
to the effects of CJGJ (Table
III).
 | Table III.Effects of treatment with Gardenia
jasminoides on SBP, DBP and MBP in mice. |
Table III.
Effects of treatment with Gardenia
jasminoides on SBP, DBP and MBP in mice.
| Group | SBP (mmHg) | DBP (mmHg) | MBP (mmHg) |
|---|
| Normal |
101.7±3.2d |
82.0±2.1d |
70.2±1.7d |
| Control |
137.6±4.2a |
108.7±3.0a |
91.5±2.2a |
| CJGJ |
115.2±2.8b |
94.1±2.2b |
82.3±1.8b |
| HLGJ |
107.7±2.4c |
87.0±1.6c |
78.1±1.4c |
Serum and tissue NO levels
Normal mice had the highest serum and heart, liver,
kidney and stomach tissue NO levels, whereas control mice exhibited
the lowest NO contents (Table
IV). L-NNA-treated mice exhibited significantly increased serum
and tissue NO contents following HLGJ and CJGJ treatment compared
with control mice (P<0.05). NO contents in HLGJ-treated mice
were closer to the NO contents of normal mice compared with in
CJGJ-treated mice (Table IV).
 | Table IV.Nitric oxide contents in mouse serum,
heart, liver, kidney and stomach tissue samples. |
Table IV.
Nitric oxide contents in mouse serum,
heart, liver, kidney and stomach tissue samples.
| Group | Serum
(µmol/gprot) | Heart
(µmol/gprot) | Liver
(µmol/gprot) | Kidney
(µmol/gprot) | Stomach
(µmol/gprot) |
|---|
| Normal |
65.71±4.08a |
8.08±0.71a |
2.68±0.15a |
7.67±0.75a |
9.42±0.41a |
| Control |
32.10±2.78d |
3.11±0.42d |
0.50±0.05d |
4.03±0.39d |
5.39±0.26d |
| CJGJ |
44.63±2.77c |
5.42±0.41c |
1.39±0.24c |
5.01±0.44c |
6.71±0.52c |
| HLGJ |
52.61±2.82b |
6.39±0.32b |
2.11±0.18b |
6.18±0.46b |
8.10±0.43b |
Serum and tissue MDA levels
Following the induction of hypertension by L-NNA,
control mice demonstrated the highest MDA serum, heart, liver,
kidney and stomach tissue contents (Table V). Treatment with CJGJ and HLGJ
significantly reduced MDA serum and tissue levels in L-NNA-treated
mice (P<0.05). MDA contents in HLGJ-treated mice were lower and
closer to MDA contents of normotensive mice compared with in
CJGJ-treated mice (Table V).
 | Table V.Malondialdehyde contents in mouse
serum, heart, liver, kidney and stomach tissue samples. |
Table V.
Malondialdehyde contents in mouse
serum, heart, liver, kidney and stomach tissue samples.
| Group | Serum
(µmol/gprot) | Heart
(µmol/gprot) | Liver
(µmol/gprot) | Kidney
(µmol/gprot) | Stomach
(µmol/gprot) |
|---|
| Normal |
4.35±0.28d |
2.12±0.20d |
0.42±0.07d |
1.28±0.08d |
1.35±0.12d |
| Control |
11.78±0.88a |
4.63±0.28a |
1.08±0.06a |
2.97±0.19a |
3.87±0.11a |
| CJGJ |
8.36±0.42b |
3.41±0.22b |
0.79±0.05b |
2.09±0.16b |
2.59±0.17b |
| HLGJ |
6.97±0.27c |
2.81±0.19c |
0.65±0.04c |
1.72±0.18c |
1.92±0.15c |
Serum ET-1, CGRP, VEGF and E-selectin
levels
L-NNA-induced hypertensive mice (control) exhibited
the highest ET-1, VEGF and E-selectin serum levels and the lowest
CGRP serum levels among the groups (Table VI). Treatment with CJGJ and HLGJ
significantly reduced ET-1, VEGF and E-selectin serum levels, and
increased CGRP serum levels compared with L-NNA-treated control
mice. HLGJ-treated mice demonstrated lower ET-1, VEGF and
E-selectin and higher CGRP levels compared with CJGJ-treated mice,
and these levels were closer to those reported in normal mice
(Table VI).
 | Table VI.Serum levels of ET-1, CGRP and VEGF
in mice. |
Table VI.
Serum levels of ET-1, CGRP and VEGF
in mice.
| Group | ET-1 (pg/ml) | CGRP (pg/ml) | VEGF (pg/ml) | E-selectin
(ng/ml) |
|---|
| Normal |
73.87±3.82d |
180.68±5.03a |
119.75±4.98d |
445.63±17.42d |
| Control |
126.54±6.74a |
64.78±3.25d |
292.63±7.85a |
735.95±28.43a |
| CJGJ |
95.62±3.55b | 116.97±4.58
c |
208.71±6.31b |
608.34±20.64b |
| HLGJ |
83.47±3.08c | 135.20±4.03
b |
177.98±7.81c |
541.36±16.50c |
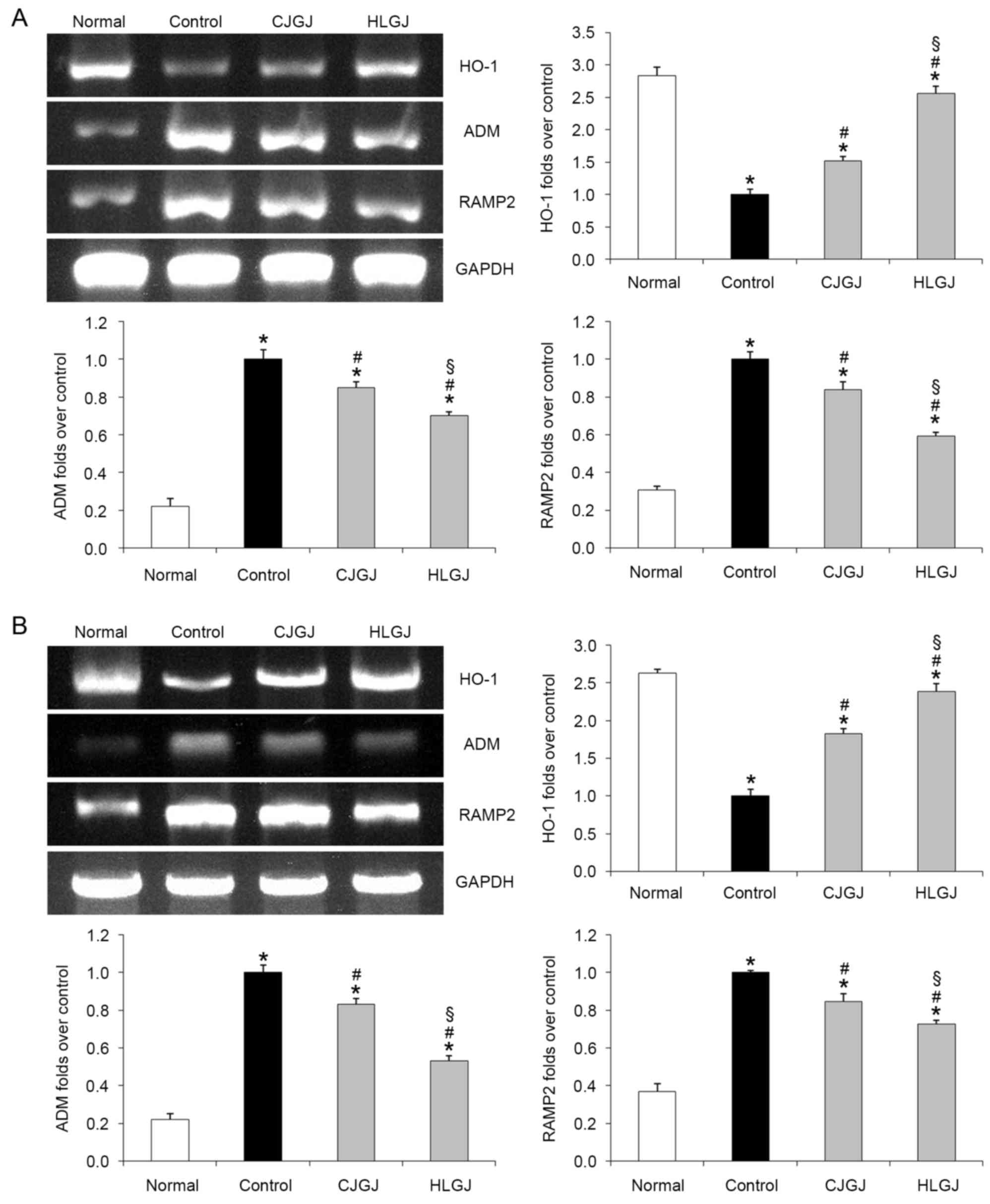
HO-1, ADM and RAMP2 mRNA expression
levels
HO-1 mRNA expression levels were the highest in
myocardial and blood vessel tissue samples of normal mice (2.84-
and 2.63-folds over control, respectively). Conversely, ADM and
RAMP2 mRNA expression levels in normal mice were the lowest (ADM,
0.22- and 0.22-folds over control; RAMP2, 0.31- and 0.37-folds over
control, in myocardial and endothelial samples, respectively).
HJGJ-treated hypertensive mice exhibited reduced ADM and RAMP2, and
increased HO-1 myocardial (ADM, 0.70-; RAMP2, 0.59-; and HO-1,
2.56-folds over control) and endothelial (ADM, 0.53-; RAMP2, 0.73-;
and HO-1, 2.39-folds over control) mRNA expression levels compared
with CJGJ-treated mice (ADM, 0.85- and 0.83-folds over control;
RAMP2, 0.84- and 0.85-folds over control; HO-1, 1.51- and
1.83-folds over control, in myocardial and endothelial samples,
respectively). HO-1, ADM and RAMP-2 mRNA expression levels in
HJGJ-treated mice were closer to the levels reported in normal mice
compared with in CJGJ-treated mice (Fig. 2).
IL-1β and TNF-α mRNA expression
levels
Blood vessel samples isolated from control mice
exhibited the highest IL-1β and TNF-α mRNA expression levels
(Fig. 3). IL-1β and TNF-α mRNA
expression levels were decreased in L-NNA-induced hypertensive mice
following treatment with CJGJ (0.85- and 0.80-folds over control,
respectively) and HLGJ (0.64- and 0.57-folds over control,
respectively). IL-1β and TNF-α mRNA expression levels in
HJGJ-treated mice were closer to the levels reported in normal mice
(0.38- and 0.27-folds over control, respectively) compared with in
CJGJ-treated mice (Fig. 3).
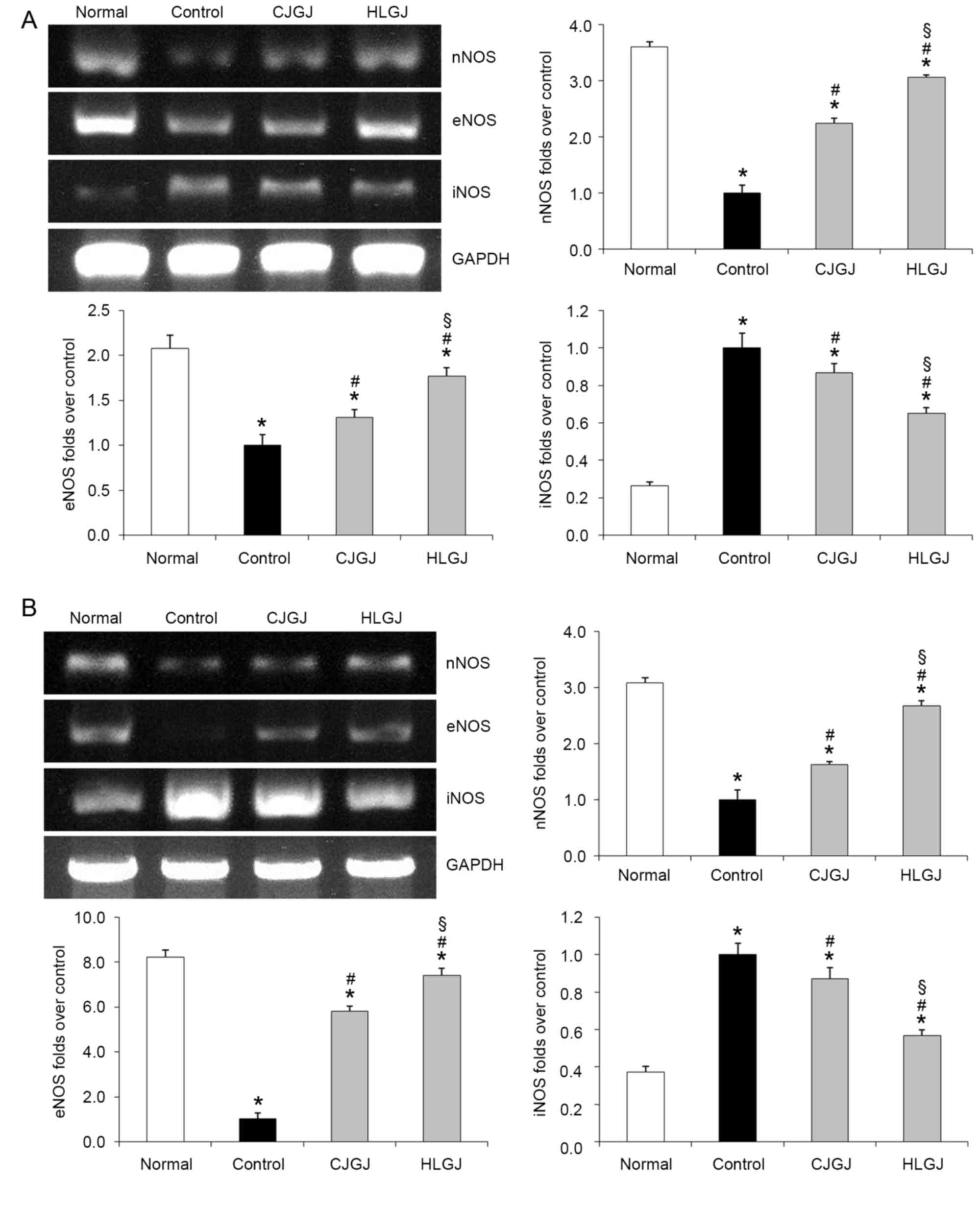
nNOS, eNOS and iNOS mRNA expression
levels
Normal, and CJGJ- and HLGJ-treated mice demonstrated
increased myocardial nNOS mRNA expression levels compared with
control hypertensive mice (3.61-, 2.23- and 3.06-folds over
control, respectively; Fig. 4A).
In addition, nNOS mRNA expression levels in blood vessel samples
isolated from normal (3.09-folds over control), CJGJ-(1.62-folds
over control) and HLGJ-treated (2.68-folds over control) mice were
increased compared with in control mice (Fig. 4B). Myocardial and endothelial eNOS
expression levels in normal (2.07- and 8.22-folds over control,
respectively), CJGJ-(1.31- and 5.79-folds over control,
respectively) and HLGJ-treated (1.77- and 7.41-folds over control,
respectively) mice were significantly upregulated compared with in
control hypertensive mice. Conversely, myocardial and endothelial
iNOS expression levels were significantly lower in normal (0.26-
and 0.37-folds over control, respectively), CJGJ-(0.87- and
0.87-folds over control, respectively) and HLGJ-treated (0.65- and
0.57-folds over control, respectively) mice compared with in
control mice (Fig. 4).
Bax and Bcl-2 mRNA expression
levels
Treatment with CJGJ and HLGJ was demonstrated to
significantly upregulate Bax and downregulate Bcl-2 myocardial mRNA
expression levels compared with control hypertensive mice (Fig. 5). Bax mRNA expression levels
appeared to be higher in tissue samples isolated from HLGJ-treated
mice (3.02-folds over control) compared with from CJGJ-treated mice
(1.38-folds over control). Conversely, Bcl-2 mRNA expression levels
appeared to be lower in HLGJ-treated tissue (0.23-folds over
control) compared with in CJGJ-treated tissue (0.39-folds over
control).
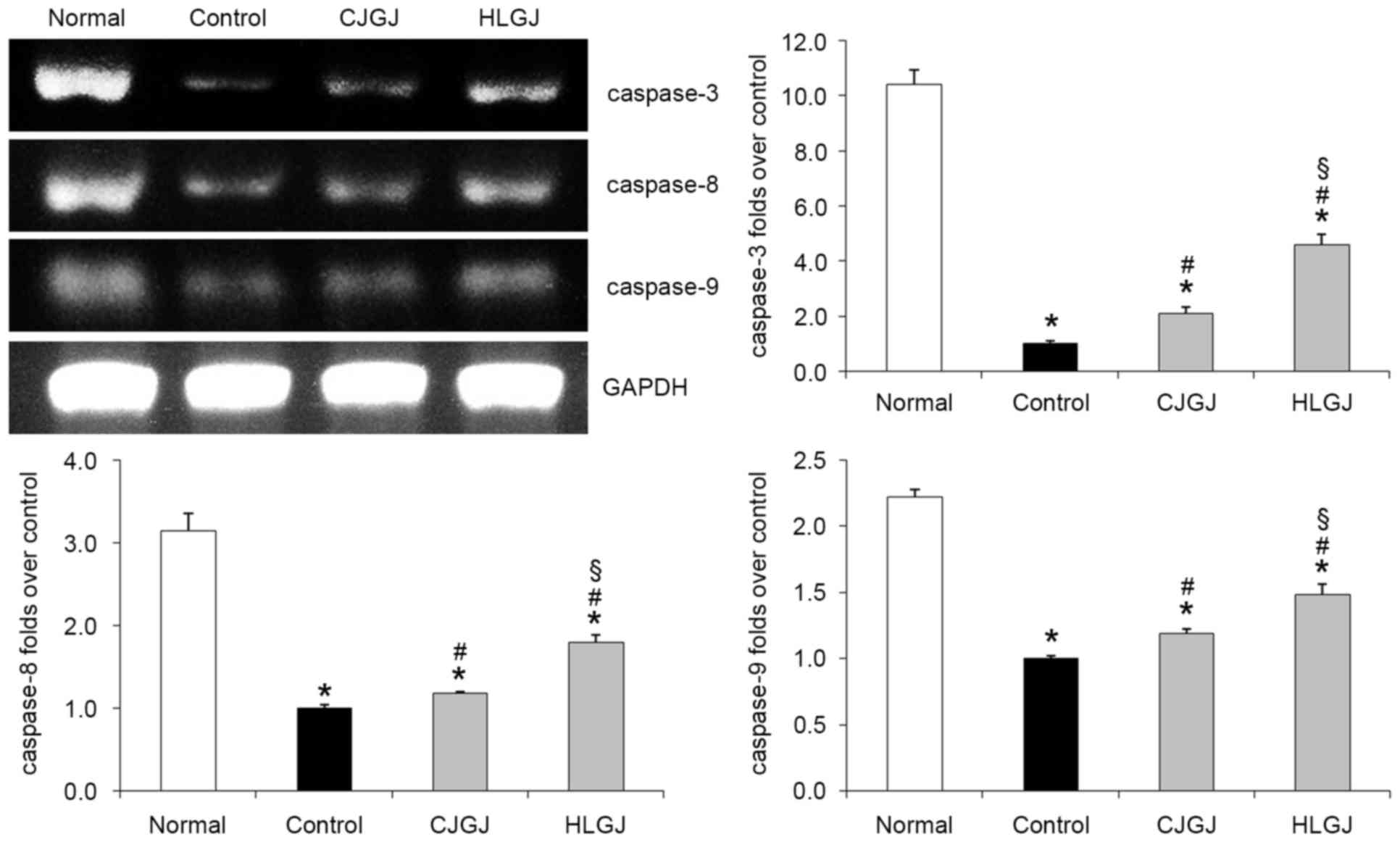
Caspase-3, caspase-8 and caspase-9
mRNA expression levels
Caspase-3, caspase-8 and caspase-9 mRNA expression
levels in myocardial tissue of control hypertensive mice were the
lowest (10.42-, 3.14- and 2.22-folds over control, respectively).
HLGJ-treated hypertensive mice exhibited significantly increased
caspase-3, caspase-8 and caspase-9 mRNA expression levels (4.58-,
1.79- and 1.48-folds over control, respectively) compared with mice
in the control and CJGJ-treated groups (Fig. 6).
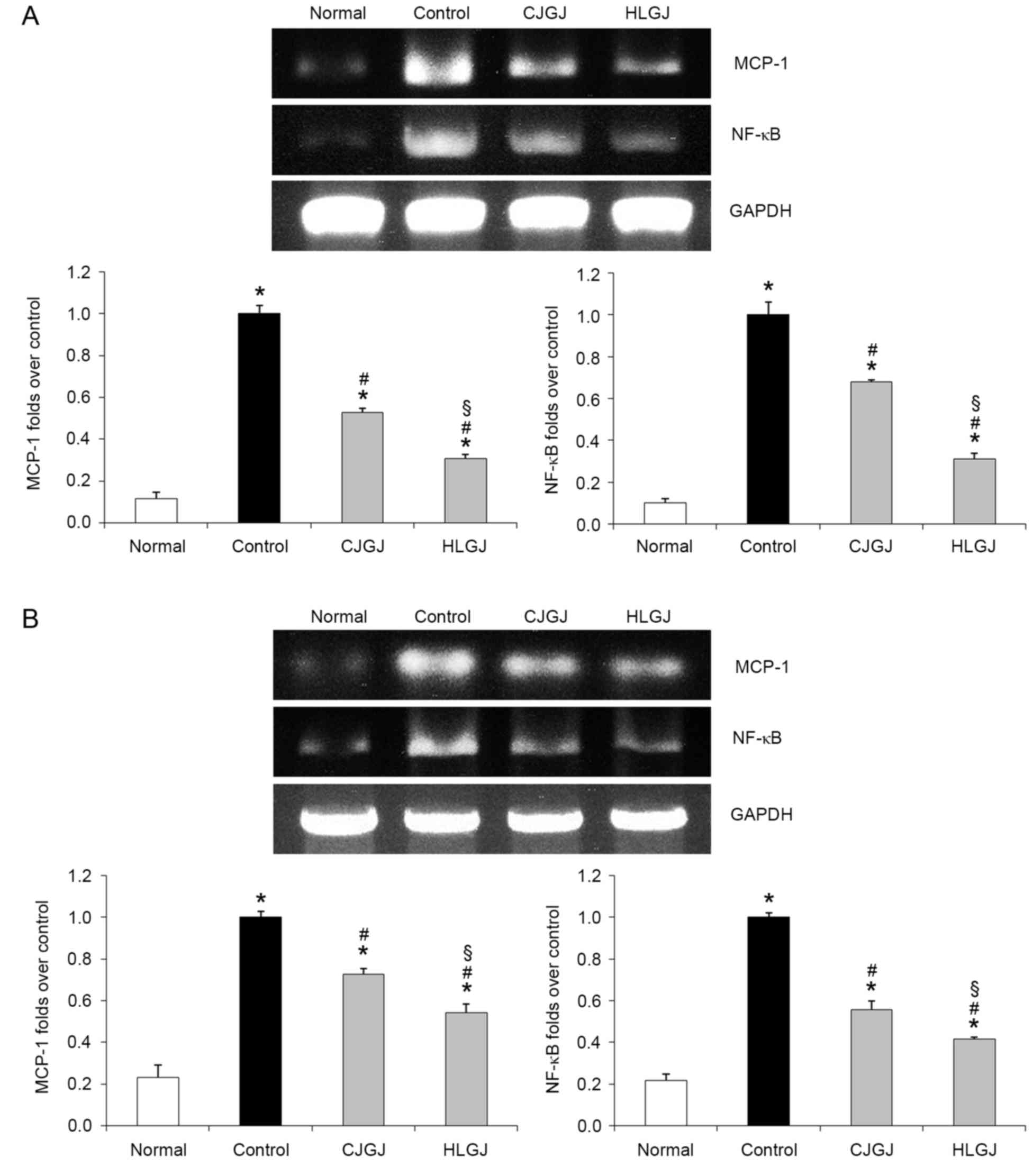
MCP-1 and NF-κB mRNA expression
levels
Myocardial and endothelial MCP-1 and NF-κB mRNA
expression levels were the lowest in normotensive (MCP-1, 0.11- and
0.23-folds over control; NF-κB, 0.10- and 0.22-folds over control,
respectively) and the highest in control hypertensive mice
(Fig. 7). Treatment with HLGJ
significantly downregulated MCP-1 (0.31- and 0.54-folds over
control, respectively) and NF-κB (0.31- and 0.41-folds over
control, respectively) mRNA expression levels compared with CJGJ
treatment (MCP-1, 0.53- and 0.72-folds over control; NF-κB, 0.68-
and 0.56-folds over control, respectively) in heart and blood
vessel tissue samples.
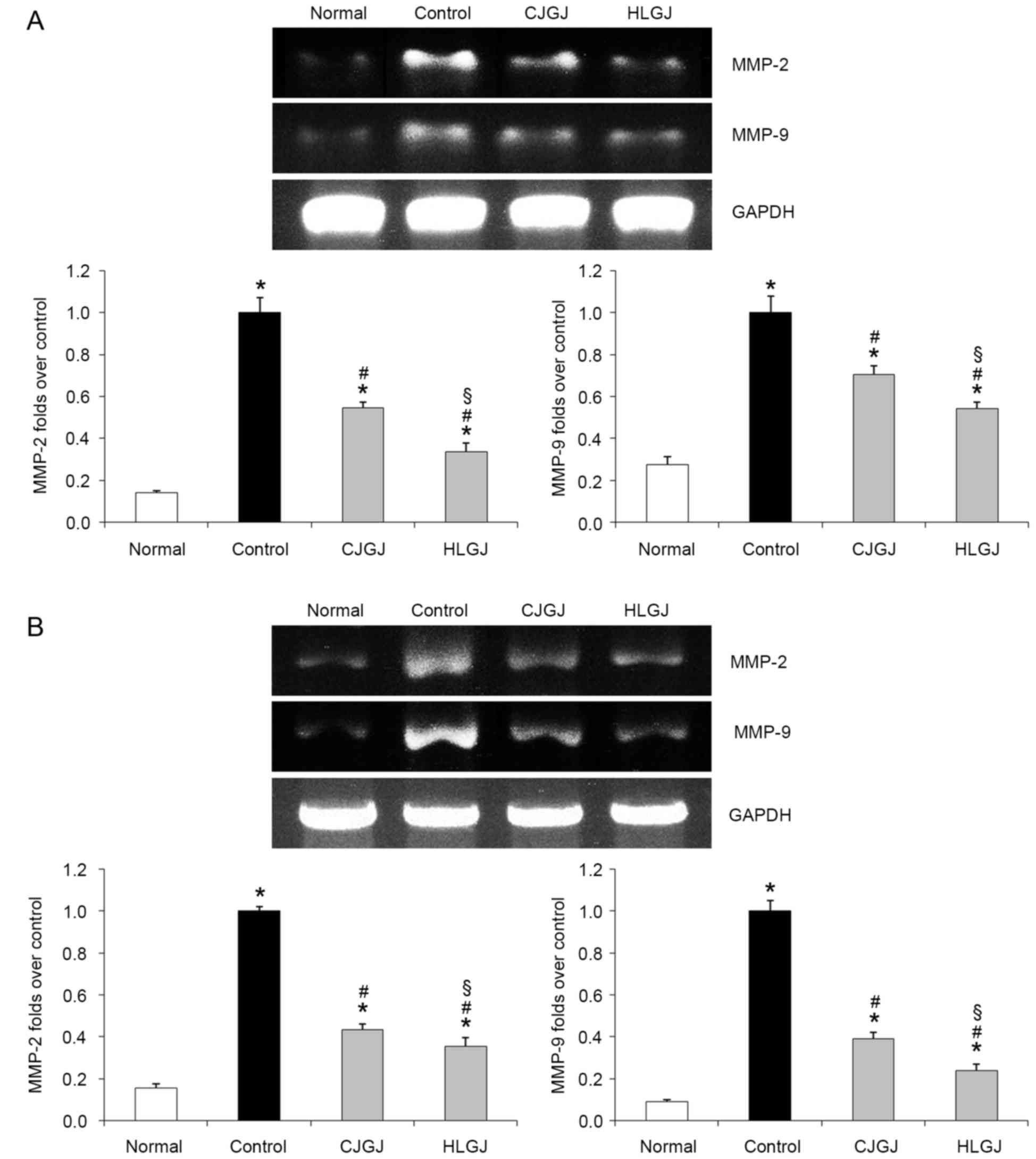
MMP-2 and MMP-9 mRNA expression
levels
Control hypertensive mice demonstrated the highest
MMP-2 and MMP-9 mRNA expression levels in myocardial and blood
vessel samples. Treatment with CJGJ and HLGJ significantly
downregulated the expression of MMP-2 and MMP-9 (Fig. 8). MMP-2 and MMP-9 mRNA expression
levels in myocardial (0.34- and 0.54-folds over control,
respectively) and blood vessel (0.27- and 0.09-folds over control,
respectively) tissue samples isolated from HLGJ-treated mice were
significantly reduced compared with in CJGJ-treated tissue
samples.
Discussion
ET-1 has been identified as one of the most potent
and long-lasting vasoconstrictor polypeptides, and it has also been
reported to promote cellular proliferation, which may result in
vascular smooth muscle hypertrophy (12). Previous studies have reported the
potential of the ET-1 gene as a candidate gene to predict the risk
of hypertension, whereas ET-1 polymorphisms have been associated
with various hypertension risk factors, such as left ventricular
hypertrophy and fibrosis, and renal insufficiency (13–15).
Therefore, plasma ET-1 levels may be used as an indicator of the
severity of hypertension and of hypertension-associated damage of
related organs (14). Furthermore,
ET-1 has previously been reported to promote the synthesis and
release of NO, which has vasodilatory actions (16), whereas their interaction maintains
the vascular tension under physiological conditions. NOS is the
enzyme responsible for catalyzing the biosynthesis of NO (17). In hypertensive vascular
endothelium, the activity of NOS is decreased, due to the
insufficient response of the vasculature to endothelium-dependent
dilators (17). Three NOS
isoforms, nNOS, eNOS and iNOS, are expressed in different cell and
tissue types and under different conditions (18). Under physiological conditions, iNOS
is not expressed in endothelial cells, whereas eNOS and nNOS
constantly synthesize small amounts of NO to maintain the vascular
tone (19,20). However, under pathological
conditions, endotoxins and various cytokines can induce the
expression of iNOS in macrophages and leukocytes, leading to
increased NO production, which can exert potent vasodilatory
effects (21). NO synthesized by
eNOS has been reported to cause smooth muscle relaxation, inhibit
vascular smooth muscle cellular proliferation, inhibit platelet
aggregation, and maintain normal vascular tension and arterial
blood pressure (22). However,
previous studies have suggested that under pathological conditions,
such as hypertension and atherosclerosis, eNOS-produced NO may be
insufficient to maintain normal vascular tension, resulting in iNOS
activation and increased NO production, in an effort to maintain
physiological blood pressure levels (22,23).
E-selectin is a cell adhesion molecule specifically
expressed on cytokine-activated endothelial cells; therefore,
soluble E-selectin in peripheral blood can be used as a specific
marker of endothelial cell activation (24). Serum levels of soluble E-selectin
have been reported to be significantly increased in hypertensive
patients, thus suggesting that vascular endothelial cells may be
activated by high blood pressure (25). In addition, E-selectin has been
reported to mediate leukocyte adhesion, which may damage vascular
endothelial cells and cause their degeneration, necrosis and
apoptosis, thus leading to atherosclerotic lesion formation, as
well as lumen deformation and stenosis (26).
CGRP has been identified as one of the most potent
vasodilatory neuropeptides. It has previously been reported that
CGRP plasma levels in patients with essential hypertension were
significantly lower compared with in healthy subjects, thus
suggesting a role for CGRP in the pathogenesis of hypertension
(27). CGRP has been reported to
promote NOS activity and NO production in vascular smooth muscle
cells, and NO may participate in its vasodilatory mechanism.
Inadequate plasma CGRP secretion in patients with essential
hypertension may lead to reduced NOS activity and NO levels, thus
resulting in increased blood pressure (28). ADM has been identified in plasma
and several tissues, whereas vascular endothelial cells have been
reported as the main source of plasma ADM. ADM is a member of the
CGRP superfamily and shares high sequence homology with CGRP, thus
suggesting that ADM may exert its actions via binding to CGRP
receptors in endothelial cells. In addition, ADM may participate in
the regulation of vasoactive substance levels, which serve critical
roles in the maintenance of vascular tension (29). Furthermore, ADM has been
demonstrated to inhibit the release of ET-1 and angiotensin (Ang),
and to suppress their vasoconstrictive actions (30). Sumimoto et al (31) reported that plasma ADM levels in
hypertensive patients were markedly increased compared with in
healthy subjects. It has previously been revealed that following
myocardial infarction and heart failure in mice, ADM and RAMP2 mRNA
expression levels were increased in ischemic, as well as in
non-infarcted myocardial tissue (32). Under pathological conditions, such
as shock, congestive heart failure and obstructive nephropathy, ADM
and RAMP2 expression in cardiovascular tissue may be altered as a
result of compensatory mechanisms aiming to protect cardiovascular
function (33). In accordance with
a previous study (34), the
present results demonstrated that ADM and RAMP2 mRNA expression
levels were increased in myocardial and blood vessel tissue
isolated from hypertensive mice compared with in normal mice.
Following hypertension-induced cerebral tissue
damage, immune cells, astrocytes, endothelial cells and neurons
have been reported to express TNF-α and other pro-inflammatory
cytokines, such as IL-6 (35).
IL-6 has been reported to induce fibrinogen activation and blood
clot formation, leading to fibroblast hyperplasia and collagen
deposition in inflamed vasculature. In addition, IL-6 is able to
promote the activation and aggregation of platelets, thus leading
to endothelial cell damage (36).
Ang II has also been revealed to stimulate vascular smooth muscle
cells to increase the synthesis and release of IL-6, andIL-6
upregulates Ang II receptors. Furthermore, during the pathogenesis
of essential hypertension, Ang II has been demonstrated to increase
peripheral resistance via smooth muscle contraction, and thus
increase blood pressure (37).
VEGF is a glycosylated secretory peptide factor, and
the important pro-angiogenic protein, which selectively stimulates
mitosis and proliferation of endothelial cells, promotes
angiogenesis and increases vascular permeability (38). Hypertensive patients have been
reported to exhibit increased serum VEGF levels compared with
healthy subjects. The reduced blood flow leading to partial
ischemia hypoxia and increased cytokine levels in tissues of
hypertensive patients may underlie the increased serum levels of
VEGF observed in these patients (39).
Oxidative stress has been associated with
hypertension and reactive oxygen species have been suggested to be
implicated in its pathogenesis (40). It has previously been demonstrated
that damage induced by free radicals participates in the
development of hypertension (41).
MDA is a product of unsaturated fatty acid decomposition, which can
be used as a biomarker to reflect the production of oxygen free
radicals. Research has demonstrated that patients with hypertension
and ischemic stroke may exhibit higher MDA compared with controls
(42).
The HO enzymatic system has been reported to exert
cytoprotective effects against oxidative damage. HO is the key
enzyme involved in heme degradation, and catalyzes the catabolism
of heme to produce the antioxidant molecule bilirubin, the
vasodilatory gas carbon monoxide and Fe2+ (43). Under physiological or pathological
conditions, the induction of HO-1 activity has been demonstrated to
protect against oxidative damage; HO-1-associated cytoprotection
has been attributed to the antioxidant properties of bilirubin
(44). In addition, HO-1 has been
reported to exert protective effects on cardiovascular function,
as, through its antioxidant action, it protects vascular
endothelial cells and cardiomyocytes from oxidative damage
(45). HO-1 also directly inhibits
cardiomyocyte hypertrophy and reduces the left/right ventricle
weight ratio. Furthermore, HO-1 was previously demonstrated to
exert anti-hypertensive effects, which were associated with
improved endothelial function (46).
Previous studies have reported that crocin prevented
endothelial cell injury, and thus counteracted the development of
hypertension and atherosclerosis, as well as cerebral edema, spinal
cord injury, papilloma and arthritis; crocin-1 appeared to exert
stronger effects (47,48). Genipin 1-gentiobioside has been
reported to exhibit protective properties against heart failure
(49). Furthermore, it has
previously been reported that gardenoside protected vascular
endothelial cells against oxidative damage and prevented the
development of hypertension (50).
Bax and Bcl-2 are apoptosis-associated genes.
Previous studies have demonstrated that in hypertensive mice with
left ventricular hypertrophy, myocardial cell apoptosis was
enhanced, and the expression of Bax was upregulated, whereas the
expression of Bcl-2 was downregulated (51–53).
Therefore, to successfully treat hypertension, myocardial cell
apoptosis, myocardial hypertrophy and the development of heart
failure should be addressed (54).
The cardiovascular actions of Ang II have been reported to be
primarily mediated by type 1 Ang II receptors. Ang II receptor
activation stimulated the expression of Bax, inhibited the
expression of Bcl-2 and increased the phosphorylation of target
proteins, which eventually led to caspase activation and the
induction of apoptosis (55). Ang
II induced myocardial cell apoptosis and increased blood pressure,
and caspase-3 reduced the effects of Ang II and reduced blood
pressure (56).
The development of hypertension and
hypertension-induced organ damage, as well as the progression of
atherosclerosis, are associated with vascular inflammation.
Hypertensive patients exhibit increased levels of pro-inflammatory
cytokines, including TNF-α, IL-1β and MCP-1, compared with
normotensive subjects. The inhibition of pro-inflammatory factors
has been demonstrated to reduce MCP-1 expression and inhibit the
activation of NF-κB (57).
Inhibiting Ang II led to a series of effects on inflammation,
including reduced levels of macrophages and T lymphocytes, which
subsequently led to reduced blood pressure (58).
MMPs are a family of Zn2+-containing
proteases, that are responsible for the degradation of
extracellular matrix components; therefore, they are critical in
tissue remodeling (59). High
blood pressure has been reported to cause left ventricular
remodeling, which has been identified as a risk factor for heart
failure and other cardiovascular events (60). Previous studies using mouse models
of hypertension and heart failure have demonstrated that the
activity of MMP-2 and MMP-9 was significantly enhanced in
myocardial tissue. These results suggested that MMP-2 and MMP-9 may
serve important roles in ventricular remodeling processes induced
by hypertension (61,62).
The present study demonstrated that crocin-1 and
crocin-2 contents were similar in CJGJ and HLGJ Gardenia
jasminoides varieties. CJGJ appeared to be enriched in genipin
1-gentiobioside compared with HLGJ. Conversely, HLGJ exhibited
higher gardenoside contents compared with CJGJ. Notably, HLGJ
appeared to exert more potent anti-hypertensive effects compared
with CJGJ in the L-NNA-induced mouse model of hypertension.
Therefore, it may be hypothesized that the higher gardenoside
contents of HLGJ are critical for its anti-hypertensive effects.
These results were similar to previous research where gardenoside
was demonstrated to reduce blood pressure (50). In addition, treatment of
L-NNA-induced hypertensive mice with Gardenia jasminoides
increased their NO, CGRP, HO-1, nNOS, eNOS, Bax, caspase-3,
caspase-8 and caspase-9 levels, and reduced their MDA, ET-1, VEGF,
E-selectin, ADM, RAMP2, IL-1β, TNF-α, iNOS, Bcl-2, MCP-1, NF-κB,
MMP-2 and MMP-9 levels. Previous studies demonstrated that changes
in the expression of these genes were associated with changes in
blood pressure (63–65), which was confirmed by the results
of the present study. These results may demonstrate the mechanism
of action of Gardenia jasminoides, and may guide the
development of treatments based on the structure of Gardenia
jasminoides. Further experiments involving chemical structure
analysis and application to clinical experiments are required prior
to the development and application of Gardenia
jasminoides-based treatments.
Acknowledgements
The present study was supported by the Science and
Technology Innovation Action Plan of Shanghai (grant no.
14495800400), the Basic Research Project of Chongqing Frontier and
Application (grant no. cstc2014jcyjA1466), the Construction Program
of Chongqing Engineering Research Center (grant no.
cstc2015yfpt_gcjsyjzx0027) and the Program for Innovation Team
Building at Institutions of Higher Education in Chongqing (grant
no. CXTDX201601040).
References
|
1
|
Gu WL, Shi ZX, Yu YX, Wu YW, Lu BW and Hui
KK: Distribution characteristics of syndrome types in essential
hypertension. Zhong Xi Yi Jie He Xue Bao. 8:842–847. 2010.(In
Chinese). View Article : Google Scholar
|
|
2
|
Xu HY and Wang YQ: Investigation and
application for the therapy of hypertension with traditional
Chinese medicine. China Prac Med. 3:189–192. 2008.(In Chinese).
|
|
3
|
Shen CZ, Peng MC, Kuang HR and Cheng ZQ:
The effect of Chinese food therapy on the quality of life in
patients with hypertension. Chin J Nurs. 44:510–513. 2009.(In
Chinese).
|
|
4
|
Jiang CX, Cheng JG and Luo T: Nutritional
value analysis of medicinal and edible plant. J Anhui Agri Sci.
43:282–284. 2015.
|
|
5
|
Meng XL, Li HW, Li Y, Yu Q, Wan LL and Guo
C: Advances in Studies on chemical constituents and pharmacological
activities of Gardenia jasminoides. Chinese J New Drug. 20:959–967.
2011.
|
|
6
|
Chen H, Xiao YQ, Li L and Zhang C: Studies
on chemical constituents in fruit of Gardenia jasminoides. Zhongguo
Zhong Yao Za Zhi. 32:1041–1043. 2007.(In Chinese).
|
|
7
|
Chen SC, Shi HY, Yang N and Wang R:
Comparison of main effective constituent content of Gardenia
jasminoides in Chongqing Hou-shan and other domestic areas. China J
Exp Tradit Med Formulae. 18:131–134. 2012.(In Chinese).
|
|
8
|
Thomsen K, Rubin I and Lauritzen M: NO-
and non-NO-, non-prostanoid-dependent vasodilatation in rat sciatic
nerve during maturation and developing experimental diabetic
neuropathy. J Physiol. 543:977–993. 2002. View Article : Google Scholar :
|
|
9
|
Gangula P, Ravella K, Chukkapalli S,
Rivera M, Srinivasan S, Hale A, Channon K, Southerland J and
Kesavalu L: Polybacterial periodontal pathogens alter vascular and
gut BH4/nNOS/NRF2-phase II enzyme expression. PLoS One.
10:e01298852015. View Article : Google Scholar :
|
|
10
|
Simmonds MJ, Detterich JA and Connes P:
Nitric oxide, vasodilation and the red blood cell. Biorheology.
51:121–134. 2014.
|
|
11
|
Zhao X, Wang Q, Li J, Chen F, Qian Y and
Wang R: In vitro antioxidant, anti-mutagenic, anti-cancer and
anti-angiogenic effects of Chinese Bowl tea. J Funct Food.
7:590–598. 2014. View Article : Google Scholar
|
|
12
|
Silva I, Teixeira A, Oliveira J, Almeida
I, Almeida R and Vasconcelos C: Predictive value of vascular
disease biomarkers for digital ulcers in systemic sclerosis
patients. Clin Exp Rheumatol. 33(4 Suppl 91): S127–S130. 2015.
|
|
13
|
Gu X, Li H, Zhu X, Gu H, Chen J, Wang L,
Harding P and Xu W: Inverse correlation between plasma adropin and
ET-1 levels in essential hypertension: A cross-sectional study.
Medicine (Baltimore). 94:e17122015. View Article : Google Scholar :
|
|
14
|
Li X, Qiu J, Pan M, Zheng D, Su Y, Wei M,
Kong X, Sun W and Zhu J: Correlation between congenital heart
disease complicated with pulmonary artery hypertension and
circulating endothelial cells as well as endothelin-1. Int J Clin
Exp Pathol. 8:10743–10751. 2015.
|
|
15
|
Renga B, Cipriani S, Carino A, Simonetti
M, Zampella A and Fiorucci S: Reversal of endothelial dysfunction
by GPBAR1 agonism in portal hypertension involves a AKT/FOXOA1
dependent regulation of H2S generation and endothelin-1. PLoS One.
10:e01410822015. View Article : Google Scholar :
|
|
16
|
Feng EZ, Yang SY, Huang NX, Yin H, Zhang Y
and Tian ZX: Plasma endothelin-1 and nitric oxide correlate with
ligustrazine alleviation of pulmonary artery hypertension in
patients of chronic corpulmonale from high altitude plateau during
acute exacerbation. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
30:532–527. 2014.
|
|
17
|
Basralı F, Nasırcılar Ülker S, Koçer G,
Ülker Karadamar P, Özyurt D, Cengiz M and Şentürk ÜK: Effect of
magnesium on vascular reactivity in NOS inhibition-induced
hypertension. Magnes Res. 28:64–74. 2015.
|
|
18
|
Nieto CI, Cabildo MP, Cornago MP, Sanz D,
Claramunt RM, Torralba MC, Torres MR, Elguero J, García JA, López
A, et al: Fluorination effects on NOS inhibitory activity of
pyrazoles related to curcumin. Molecules. 20:15643–15665. 2015.
View Article : Google Scholar
|
|
19
|
Kubes P and McCafferty DM: Nitric oxide
and intestinal inflammation. Am J Med. 109:150–158. 2000.
View Article : Google Scholar
|
|
20
|
Posa A, Pavo N, Hemetsberger R, Csonka C,
Csont T, Ferdinandy P, Petrási Z, Varga C, Pavo IJ, Laszlo F Jr, et
al: Protective effect of ischaemic preconditioning on
ischaemia/reperfusion-induced microvascular obstruction determined
by on-line measurements of coronary pressure and blood flow in
pigs. Thromb Haemost. 103:450–460. 2010. View Article : Google Scholar
|
|
21
|
Huang HC, Wang SS, Chang CC, Lee FY, Lin
HC, Hou MC, Teng TH, Chen YC and Lee SD: Evolution of
portal-systemic collateral vasopressin response in endotoxemic
portal hypertensive rats. Shock. 32:503–508. 2009. View Article : Google Scholar
|
|
22
|
Pulgar VM, Yamaleyeva LM, Varagic J, McGee
C, Bader M, Dechend R and Brosnihan KB: Functional changes in the
uterine artery precede the hypertensive phenotype in a transgenic
model of hypertensive pregnancy. Am J Physiol Endocrinol Metab.
309:E811–E817. 2015. View Article : Google Scholar :
|
|
23
|
Theodorakis NG, Wang YN, Korshunov VA,
Maluccio MA and Skill NJ: Thalidomide ameliorates portal
hypertension via nitric oxide synthase independent reduced systolic
blood pressure. World J Gastroenterol. 21:4126–4135. 2015.
View Article : Google Scholar :
|
|
24
|
Le Hiress M, Tu L, Ricard N, Phan C,
Thuillet R, Fadel E, Dorfmüller P, Montani D, de Man F, Humbert M,
et al: Proinflammatory signature of the dysfunctional endothelium
in pulmonary hypertension. role of the macrophage migration
inhibitory factor/CD74 complex. Am J Respir Crit Care Med.
192:983–997. 2015. View Article : Google Scholar
|
|
25
|
Zhang JL, Qin YW, Zheng X, Qiu JL, Zhang
LZ, Zhang JR and Bian JL: Relevance of serum soluble E-selectin
level and blood pressure in hypertensive patients. Shanghai Med J.
27:7–9. 2004.
|
|
26
|
Chen J and Li J: Clinical curative effect
and influence on E-selectin, iNOS, eNOS in patient of essential
hypertension with massotherapy. China J Tradit Chinese Med Pharm.
25:1708–1710. 2010.
|
|
27
|
Calò LA, Dal Maso L, Pagnin E, Ravarotto
V, Facco M, Boscaro E, Maiolino G, Pessina AC and Rossi GP: Effect
of olmesartan medoxomil on number and survival of circulating
endothelial progenitor cells and calcitonin gene related peptide in
hypertensive patients. J Hypertens. 32:193–199. 2014. View Article : Google Scholar
|
|
28
|
Wang ZG, Liu JL, Liu Y, Wen SJ, Wen J, Liu
YP, Chen XJ and Wu ZS: Increased plasma vasoactive substances and
antioxidant enzymes levels in prehypertensive patients. Zhonghua
Xin Xue Guan Bing Za Zhi. 35:719–722. 2007.(In Chinese).
|
|
29
|
Nakamura M, Han B, Nunobiki O and Kakudo
K: Adrenomedullin: A tumor progression factor via angiogenic
control. Curr Cancer Drug Targets. 6:635–643. 2006. View Article : Google Scholar
|
|
30
|
Liu K, Deng X, Gong L, Chen X, Wang S,
Chen H, Chen X, Amrit B and He S: The effect of intermedin on
angiotensin II and endothelin-1 induced ventricular myocyte
hypertrophy in neonatal rat. Clin Lab. 59:589–596. 2013. View Article : Google Scholar
|
|
31
|
Sumimoto T, Nishikimi T, Mukai M,
Matsuzaki K, Murakami E, Takishita S, Miyata A, Matsuo H and
Kangawa K: Plasma adrenomedullin concentrations and cardiac and
arterial hypertrophy in hypertension. Hypertension. 30:741–745.
1997. View Article : Google Scholar
|
|
32
|
Oie E, Vinge LE, Yndestad A, Sandberg C,
Grøgaard HK and Attramadal H: Induction of a myocardial
adrenomedullin signaling system during ischemic heart failure in
rats. Circulation. 101:415–422. 2000. View Article : Google Scholar
|
|
33
|
Sano M, Kuroi N, Nakayama T, Sato N, Izumi
Y, Soma M and Kokubun S: Association study of
calcitonin-receptor-like receptor gene in essential hypertension.
Am J Hypertens. 18:403–408. 2005. View Article : Google Scholar
|
|
34
|
Gong YS, Fan XF, Wu XM, Hu LG, Tang CS,
Pang YZ and Qi YF: Changes of intermedin/adrenomedullin 2 and its
receptors in the right ventricle of rats with chronic hypoxic
pulmonary hypertension. Sheng Li Xue Bao. 59:210–214. 2007.(In
Chinese).
|
|
35
|
Jian L, Fa X, Zhou Z and Liu S: Functional
analysis of UMOD gene and its effect on inflammatory cytokines in
serum of essential hypertension patients. Int J Clin Exp Pathol.
8:11356–11363. 2015.
|
|
36
|
Good RB, Gilbane AJ, Trinder SL, Denton
CP, Coghlan G, Abraham DJ and Holmes AM: Endothelial to mesenchymal
transition contributes to endothelial dysfunction in pulmonary
arterial hypertension. Am J Pathol. 185:1850–1858. 2015. View Article : Google Scholar
|
|
37
|
Manhiani MM, Seth DM, Banes-Berceli AK,
Satou R, Navar LG and Brands MW: The role of IL-6 in the
physiologic versus hypertensive blood pressure actions of
angiotensin II. Physiol Rep. 3:pii: e125952015. View Article : Google Scholar
|
|
38
|
Serban D, Leng J and Cheresh D: H-ras
regulates angiogenesis and vascular permeability by activation of
distinct downstream effectors. Circ Res. 102:1350–1358. 2008.
View Article : Google Scholar :
|
|
39
|
Muti LA, Pârvu AE, Crăciun AM, Miron N and
Acalovschi M: Nitro-oxidative stress, VEGF and MMP-9 in patients
with cirrhotic and non-cirrhotic portal hypertension. Clujul Med.
88:140–145. 2015. View Article : Google Scholar :
|
|
40
|
Minarchick VC, Stapleton PA, Sabolsky EM
and Nurkiewicz TR: Cerium dioxide nanoparticle exposure improves
microvascular dysfunction and reduces oxidative stress in
spontaneously hypertensive rats. Front Physiol. 6:3392015.
View Article : Google Scholar :
|
|
41
|
Zhou Y, Zhao L, Zhang Z and Lu X:
Protective effect of enalapril against methionine-enriched
diet-induced hypertension: Role of endoplasmic reticulum and
oxidative stress. Biomed Res Int. 2015:7248762015. View Article : Google Scholar :
|
|
42
|
Zhang S, Yang T, Xu X, Wang M, Zhong L,
Yang Y, Zhai Z, Xiao F and Wang C: Oxidative stress and nitric
oxide signaling related biomarkers in patients with pulmonary
hypertension: A case control study. BMC Pulm Med. 15:502015.
View Article : Google Scholar :
|
|
43
|
Pérez-de-Puig I, Martín A, Gorina R, de la
Rosa X, Martinez E and Planas AM: Induction of hemeoxygenase-1
expression after inhibition of hemeoxygenase activity promotes
inflammation and worsens ischemic brain damage in mice.
Neuroscience. 243:22–32. 2013. View Article : Google Scholar
|
|
44
|
He M, Nitti M, Piras S, Furfaro A,
Traverso N, Pronzato MA and Mann GE: Heme oxygenase-1-derived
bilirubin protects endothelial cells against high glucose-induced
damage. Free Radic Biol Med. 89:91–98. 2015. View Article : Google Scholar
|
|
45
|
Chao HM, Chuang MJ, Liu JH, Liu XQ, Ho LK,
Pan WH, Zhang XM, Liu CM, Tsai SK, Kong CW, et al: Baicalein
protects against retinal ischemia by antioxidation, antiapoptosis,
downregulation of HIF-1α, VEGF, and MMP-9 and upregulation of HO-1.
J Ocul Pharmacol Ther. 29:539–549. 2013. View Article : Google Scholar :
|
|
46
|
Li Z, Wang Y, Man RY and Vanhoutte PM:
Upregulation of heme oxygenase-1 potentiates EDH-type relaxations
in the mesenteric artery of the spontaneously hypertensive rat. Am
J Physiol Heart Circ Physiol. 305:H1471–H1483. 2013. View Article : Google Scholar
|
|
47
|
Imenshahidi M, Hosseinzadeh H and
Javadpour Y: Hypotensive effect of aqueous saffron extract (Crocus
sativus L.) and its constituents, safranal and crocin, in
normotensive and hypertensive rats. Phytother Res. 24:990–994.
2010.
|
|
48
|
Xu GL, Qian ZY, Yu SQ, Gong ZN and Shen
XC: Evidence of crocin against endothelial injury induced by
hydrogen peroxide in vitro. J Asian Nat Prod Res. 8:79–85. 2006.
View Article : Google Scholar
|
|
49
|
Chen L, Luo ZK, Peng GP, Li XH, Liu L,
Sheng XB and Wang ZG: The cardiac systolic and diastolic effects of
genipin-1-β-D-gentiobioside in the experimental heart failure.
Pharm Clin Chinese Mater Med. 29:39–41. 2013.(In Chinese).
|
|
50
|
Wang ZC, Yang XL, Zhang K and Xiao P:
Research on pharmacological activities of gardenoside. J Henan Univ
Sci Technol Med Sci. 30:159–160. 2012.
|
|
51
|
Pang H, Han B, Yu T and Peng Z: The
complex regulation of tanshinone IIA in rats with
hypertension-induced left ventricular hypertrophy. PLoS One.
9:e922162014. View Article : Google Scholar :
|
|
52
|
Wang HT, Liu CF, Tsai TH, Chen YL, Chang
HW, Tsai CY, Leu S, Zhen YY, Chai HT, Chung SY, et al: Effect of
obesity reduction on preservation of heart function and attenuation
of left ventricular remodeling, oxidative stress and inflammation
in obese mice. J Transl Med. 10:1452012. View Article : Google Scholar :
|
|
53
|
Kolwicz SC, MacDonnell SM, Renna BF, Reger
PO, Seqqat R, Rafiq K, Kendrick ZV, Houser SR, Sabri A and Libonati
JR: Left ventricular remodeling with exercise in hypertension. Am J
Physiol Heart Circ Physiol. 297:H1361–H1368. 2009. View Article : Google Scholar :
|
|
54
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany NY). 4:330–349. 2012. View Article : Google Scholar :
|
|
55
|
Liu Y, Wang S, Wang C, Song H, Han H, Hang
P, Jiang Y, Wei L, Huo R, Sun L, et al: Upregulation of M3
muscarinic receptor inhibits cardiac hypertrophy induced by
angiotensin II. J Transl Med. 11:2092013. View Article : Google Scholar :
|
|
56
|
Shi XC, Liu W, Wang J and Zhao YQ: Effect
of the Tianma Gouteng regulating the protein expression of Bax and
Caspase-3 of myocardial tissue in spontaneously hypertensive rats.
Tianjin J Tradi Chinese Med. 33:354–357, (In Chinese).
|
|
57
|
Jing X, Chen SS, Jing W, Tan Q, Yu MX and
Tu JC: Diagnostic potential of differentially expressed homer1,
IL-1β, and TNF-α in coronary artery disease. Int J Mol Sci.
16:535–546. 2014. View Article : Google Scholar :
|
|
58
|
Zhu XY, Chade AR, Krier J, Daghini E, Lavi
E, Guglielmotti A, Lerman A and Lerman LO: The chemokine monocyte
chemoattractant protein-1 contributes to renal dysfunction in swine
renovascular hypertension. J Hypertens. 27:2063–2073. 2009.
View Article : Google Scholar :
|
|
59
|
Fu L, Das B, Mathew S and Shi YB:
Genome-wide identification of Xenopus matrix metalloproteinases:
Conservation and unique duplications in amphibians. BMC Genomics.
10:812009. View Article : Google Scholar :
|
|
60
|
Raffetto JD and Khalil RA: Matrix
metalloproteinases and their inhibitors in vascular remodeling and
vascular disease. Biochem Pharmacol. 75:346–359. 2008. View Article : Google Scholar
|
|
61
|
Givvimani S, Munjal C, Tyagi N, Sen U,
Metreveli N and Tyagi SC: Mitochondrial division/mitophagy
inhibitor (mdivi) ameliorates pressure overload induced heart
failure. PLoS One. 7:e323882012. View Article : Google Scholar :
|
|
62
|
Li W, Mata KM, Mazzuca MQ and Khalil RA:
Altered matrix metalloproteinase-2 and −9 expression/activity links
placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and
vascular remodeling and collagen deposition in hypertensive
pregnancy. Biochem Pharmacol. 89:370–385. 2014. View Article : Google Scholar :
|
|
63
|
Kluknavsky M, Balis P, Puzserova A,
Radosinska J, Berenyiova A, Drobna M, Lukac S, Muchova J and
Bernatova I: (−)-Epicatechin prevents blood pressure increase and
reduces locomotor hyperactivity in young spontaneously hypertensive
rats. Oxid Med Cell Longev. 2016:69490202016. View Article : Google Scholar :
|
|
64
|
Belo VA, Parente JM, Tanus-Santos JE and
Castro MM: Matrix metalloproteinase (MMP)-2 decreases calponin-1
levels and contributes to arterial remodeling in early
hypertension. Biochem Pharmacol. 118:50–58. 2016. View Article : Google Scholar
|
|
65
|
Akinrinde AS, Oyagbemi AA, Omobowale TO,
Asenuga ER and Ajibade TO: Alterations in blood pressure,
antioxidant status and caspase 8 expression in cobalt
chloride-induced cardio-renal dysfunction are reversed by Ocimum
gratissimum and gallic acid in Wistar rats. J Trace Elem Med Biol.
36:27–37. 2016. View Article : Google Scholar
|