Introduction
Telomerase is an RNA nuclear protease that is
composed of human telomerase reverse transcriptase (hTERT), human
telomerase RNA and a number of associated proteins (1). Telomerase RNA has a short template
element that directs the synthesis of telemetric repeats at the end
of chromosomes, maintains chromosomal stability, stabilizes
telomere length and may promote cancer progression and cell
immortality (2). Telomerase
activity, which is absent or weakly detected in the majority of
human somatic cells, is elevated in immortalized cell lines, stem
and germ cells and in ~85% of human cancers, including esophageal
cancer (3–5). Esophageal cancer is the eighth most
common type of malignancy and is the sixth leading cause of
cancer-associated mortality worldwide (6). Incidence rates of esophageal squamous
cell carcinoma have been increasing in certain Asian countries. In
particular, areas of China contain the highest incidence rates of
esophageal cancer in the world (7). Esophageal cancer is the fourth most
frequently diagnosed cancer and is the fourth most common cause of
cancer-associated mortality in China (8). Despite numerous advances in diagnosis
and treatment, the 5-year survival rate for patients diagnosed with
esophageal cancer ranges between 15 and 20% due to the aggressive
nature of this type of malignancy (9). Therefore, telomerase activity may
represent a useful diagnostic marker for human esophageal cancer
and may be a potential target for pharmacological intervention.
Azidothymidine (AZT) is a thymidine analog used in
the treatment of acquired immune deficiency syndrome (AIDS). It is
phosphorylated to AZT-triphosphate (AZT-TP) by a thymidine kinase
enzyme, and in this form, it is incorporated into viral DNA where
it acts as a false substitute for viral reverse transcription (RT)
and blocks chain elongation. AZT-TP has a high affinity for RT and
a low affinity for DNA polymerases α, β and γ. The identification
that the hTERT component of telomerase is a functional catalytic
RT, prompted studies to inhibit telomerase with RT viral
inhibitors, including AZT (10).
AZT was demonstrated to inhibit the activity of telomerase and cell
growth in various tumor cells in vitro, including those
derived from human cancers of the bladder, colon, ovarian,
parathyroid, breast and liver (11–16).
In the present study, TE-11 cells were treated with AZT and the
effect on telomerase activity, cell proliferation, cell cycle
progression and DNA damage were investigated. The results suggested
that AZT may be a possible clinical therapy for esophageal
cancer.
Materials and methods
Cell culture and treatments
TE-11 cells, a cell line derived from a patient with
human esophageal cancer, were purchased from the American Type
Culture Collection and were maintained at 37°C and 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM),
supplemented with 10% fetal bovine serum and 100 U/ml
penicillin/streptomycin. AZT was purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) and dissolved in PBS.
MTT assay
A total of 1×104 cells/well were seeded
in a 96-well plate. Cells were treated with 2, 20, 100 and 200 µM
AZT for 24, 48, 72 and 96 h. The wells were subsequently replaced
with 10 µl/well MTT solution (5 mg/ml) and incubated at 37°C for 4
h. The supernatant was removed and 100 µl/well dimethyl sulfoxide
was added for 15 min. The spectrometric absorbance at a wavelength
of 490 nm was measured on a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA). As a control, TE-11 cells were
additionally treated with DMEM alone.
Measurement of telomerase
activity
TE-11 cells (2×106) were cultured in
6-well plates and treated with 2, 20, 100 and 200 µM AZT for 48 h
at 37°C. Lysates were prepared by treating cells for 30 min on ice
with lysis buffer [10 mM Tris-HCl (pH 7.5), 1.5 mM MgCl2, 1 mM
EGTA, 1% 3-((3-cholamidopropyl)
dimethylammonio)-1-propanesulfonate, 10% glycerol, 5 mM
β-mercaptoethanol and 0.1 mM phenylmethane sulfonyl fluoride].
Lysates were centrifuged at 15,000 × g for 20 min at 4°C.
Supernatants were collected and the protein concentrations were
determined using a Bicinchoninic Assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Lysates were subsequently diluted to 10
mg/ml. Telomerase activity was measured by polymerase chain
reaction (PCR) and the telomeric repeat amplification (TRAP) assay.
The PCR reaction mixture was prepared using TRAP reaction buffer
[20 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 63 mM KCl, 0.005% Tween-20
and 1 mM EGTA]. The PCR reaction mixture was comprised of 312.5 µM
dNTP, 0.625 µM telomerase substrate (TS) primer, the reverse primer
for amplification (CX) and 1 U hot-start Taq DNA polymerase, in a
total volume of 50 µl. In addition, each reaction mixture contained
0.625 µM internal control primer (NT) and 0.01 aM internal control
template (TSNT) for amplification of a 36 bp internal standard. A
total of 1 µl lysate was subsequently added to this mixture, which
was placed in a thermal cycler. Primers were as follows:
5′-AATCCGTCGAGCAGAGTT-3′ for TS; 5′-CCCTTACCCTTACCCTTACCCTAA-3′ for
CX; 5′-ATCGCTTCTCGGCCTTTT-3′ for NT and
5′-AATCCGTCGAGCAGAGTTAAAAGGCCGAGAAGCGAT-3′ for TSNT. Cycling
conditions were as follows: An initial telomerase extension step at
30°C for 30 min, followed by 35 cycles of denaturation at 95°C for
30 sec, annealing at 50°C for 30 sec and extension at 72°C for 1
min. For each sample, a total 10 µl PCR product was loaded onto 10%
polyacrylamide gels and subjected to electrophoresis at 180–200 V
for ~1 h in Tris/Borate/EDTA buffer. The gel was stained with
GelRed™ (Biotium, Inc., Hayward, CA, USA) and visualized under an
ultraviolet (UV) illuminator to determine telomerase activity. An
internal control was included and was evident by a 36 bp PCR
product. TE-11 cells cultured in DMEM medium alone were utilized as
a control, and the band intensities were quantified with ImageJ
software (version 1.47; National Institutes of Health, Bethesda,
MD, USA).
Cell cycle analysis
Following treatment with 2, 20, 100 and 200 µM AZT
for 48 h, 2×106 cells were washed twice with PBS.
Following an overnight fixation in 70% ethanol at 4°C, cells were
harvested by centrifugation at 200 × g for 10 min at room
temperature and washed twice with PBS. Cells were subsequently
stained with propidium iodide and analyzed on the BD FACScan™
system using BD Accuri C6 Software version 1.0.264.21 (BD
Biosciences, Franklin Lakes, CA, USA).
Comet assay
TE-11 cells were cultured for 48 h in a 6 well plate
and treated with 20, 100 and 200 µM AZT for 48 h. Additionally,
cells treated with DMEM alone served as an untreated control, and
cells treated with UV served as a positive control. The Comet assay
was performed under alkaline conditions. Cells were resuspended in
DMEM at a concentration of 1×105/ml and were combined
with molten LMAgarose (Trevigen, Gaithersburg, MD, USA) (at 37°C)
at a ratio of 1:10, prior to pipetting 75 µl onto CometSlides™
(Trevigen). Slides were stored in the dark at 4°C for 30 min and
immersed in alkaline solution (0.25 M NaOH containing 0.1 µM EDTA;
pH 12.6) at 4°C for 2 h. Slides were gently removed from the lysis
buffer and rinsed with distilled H2O. Slides were placed in freshly
prepared alkaline solution at pH>13 for 30 min at room
temperature. Gel electrophoresis was performed at 1 V/cm for 30
min. Subsequently, slides were washed in 70% ethanol, stained with
GelRed™ (Biotium, Inc.) and analyzed under a
fluorescence microscope at ×400 magnification.
Western blot analysis
TE-11 cells were lysed with radioimmunoprecipitation
assay buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% TritonX-100, 1%
sodium deoxycholate, 0.1% SDS, 2 mM sodium pyrophosphate, 25 mM
β-glycerophosphate, 1 mM EDTA, 1 mM Na3VO4)
following treatment with 20, 100 and 200 µM AZT for 48 h. Protein
concentrations were determined using the Bicinchoninic Assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Heat-denatured
protein samples (20 mg/lane) were loaded onto 10% gels and
subjected to electrophoresis prior to transfer onto polyvinylidene
fluoride membranes. The membranes were incubated with 5% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature. The membranes were then incubated at 4°C overnight
with the following primary antibodies: Rabbit
anti-phosphorylated-checkpoint kinase 2 [pChk2 (Thr68); cat. no.
ab85743; 1:1,000; Abcam, Cambridge, MA, USA], mouse anti-γ-H2A
histone family member X (γ-H2AX; cat. no. ab180651; 1:1,000; Abcam)
and mouse anti-β-actin (cat. no. sc-8432; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Subsequently, membranes were
probed with goat anti-rabbit-horseradish peroxidase (−HRP; cat. no.
sc2004; 1:5,000; Santa Cruz Biotechnology, Inc.) or
goat-anti-mouse-HRP (cat. no. sc2005; 1:5,000; Santa Cruz
Biotechnology, Inc.) secondary antibodies for 1 h at room
temperature. Proteins were detected using the Enhanced
Chemiluminescence Detection reagent (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three experiments. Statistical differences were analyzed using
one-way analysis of variance followed by Tukey's post-hoc test via
GraphPad Prism software version 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA) or Microsoft Excel 2007 software (Microsoft
Corporation, Redmond, WA, USA).
Results
Inhibition of TE-11 cell proliferation
by AZT
TE-11 cell proliferation was inhibited following
treatment with 20, 100 and 200 µM AZT for 0, 24, 48, 72 and 96 h,
as determined by an MTT assay (Fig.
1). However, treatment with 2 µM of AZT did not have an
inhibitory effect on TE-11 cells. Therefore, the inhibitory effect
of AZT was time- and dose-dependent (Fig. 1; Table
I).
 | Table I.Percentage inhibition of AZT on the
growth of TE-11 cells. |
Table I.
Percentage inhibition of AZT on the
growth of TE-11 cells.
|
| AZT concentration,
µM |
|---|
|
|
|
|---|
| Time, h | 2 | 20 | 100 | 200 |
|---|
| 24 | 4.80 | −2.48 | 0.08 | 16.98a |
| 48 | −4.86 | 40.03b | 52.20b | 57.97b |
| 72 | −5.58 | 42.21b | 56.37b | 65.87b |
| 96 | −8.38 | 45.99b | 67.54b | 76.64b |
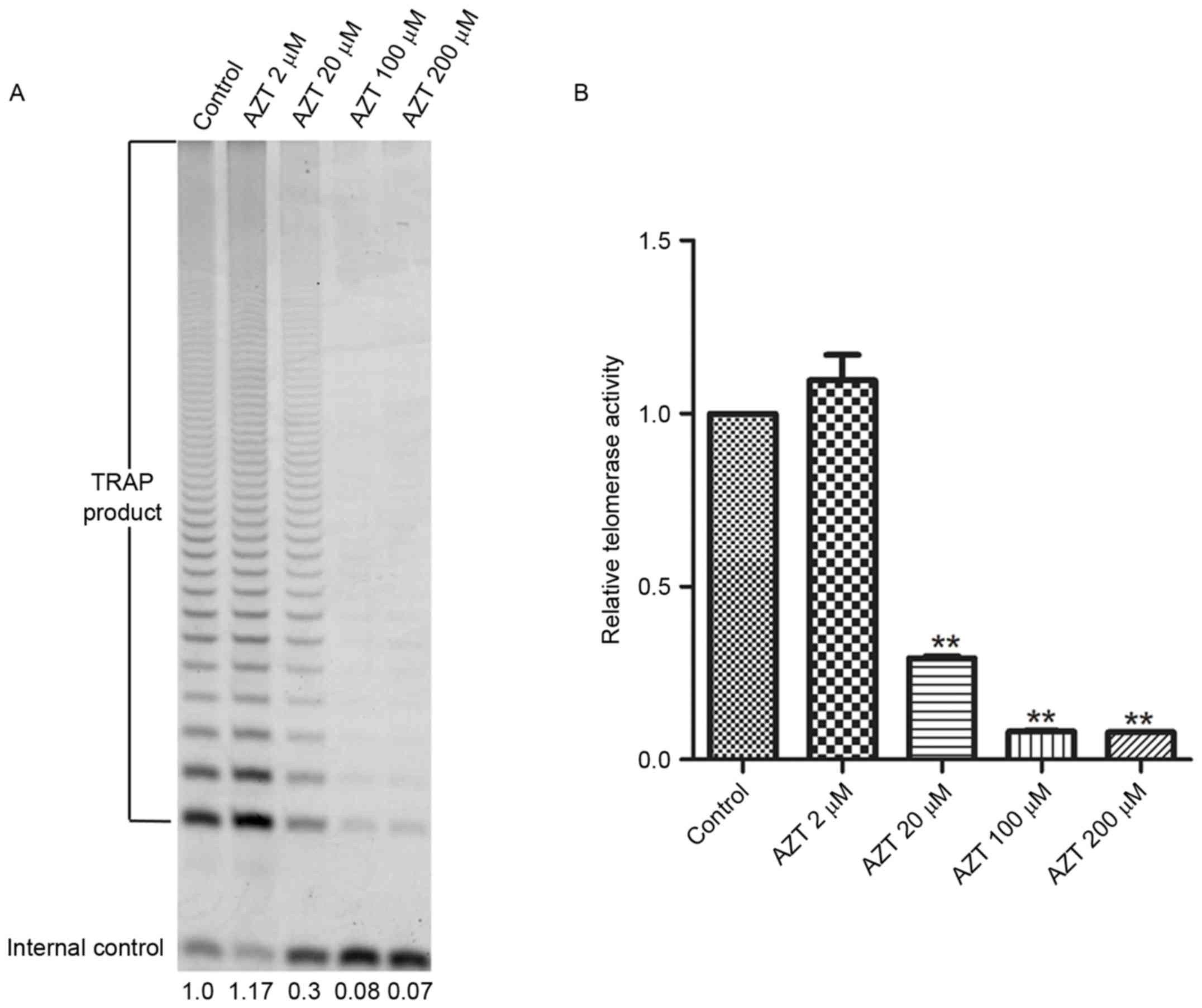
Inhibition of telomerase activity in
TE-11 cells by AZT
The effect of AZT on telomerase activity in TE-11
cells was determined by a TRAP assay. Treatment with 20, 100 and
200 µM AZT for 48 h resulted in a dose-dependent decrease in
telomerase activity (Fig. 2A and
B) compared with the control. However, there was no significant
difference in activity following treatment with 2 µM AZT.
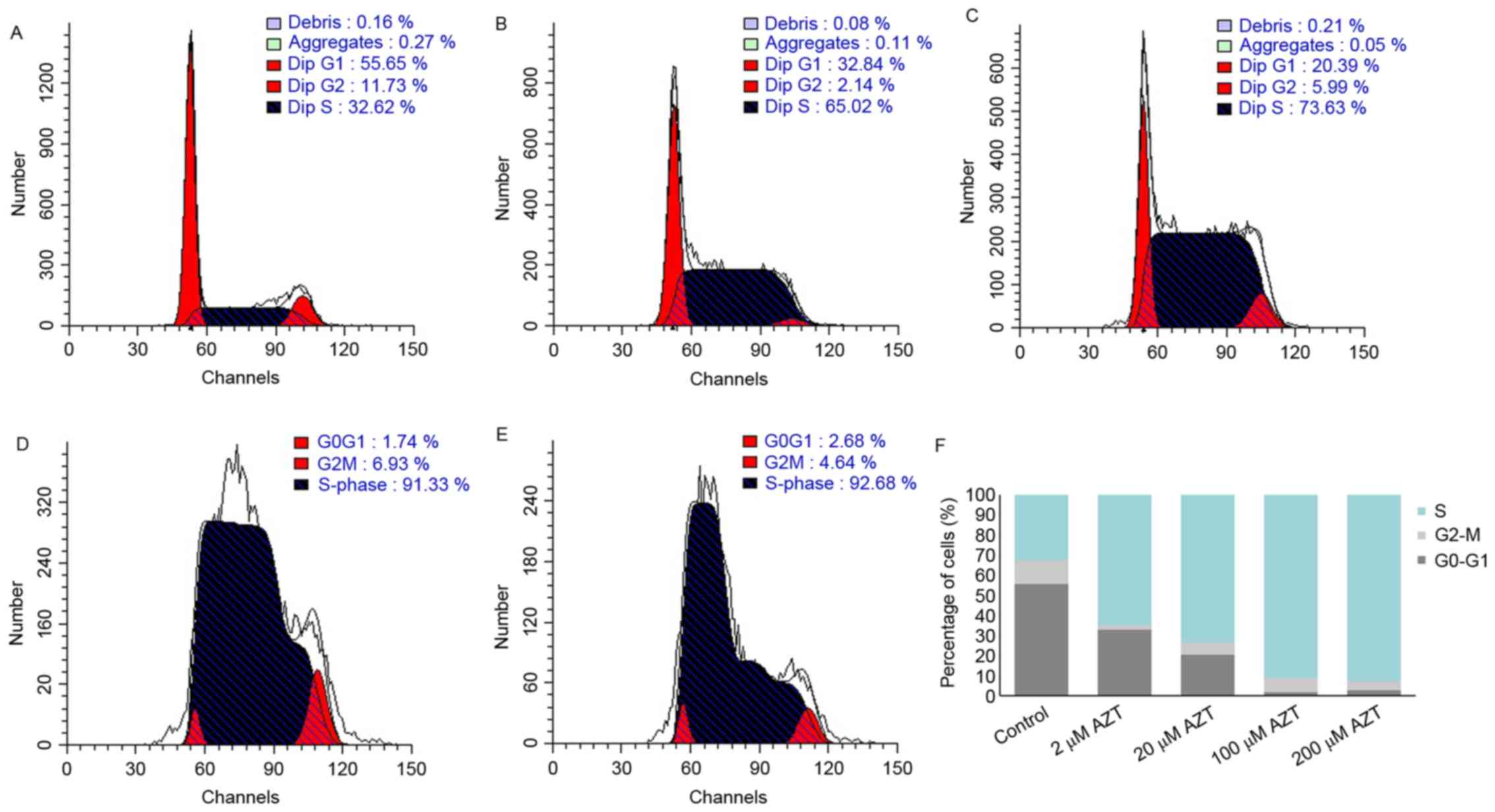
Effect of AZT on cell cycle
progression
The effect of AZT on cell cycle distribution was
assessed by flow cytometric analysis. Representative cell cycle
profiles of TE-11 cells treated with 2, 20, 100 and 200 µM AZT for
48 h are demonstrated in Fig.
3A-E. Treatment with AZT led to a marked dose-dependent
decrease in the percentage of G1/G0 phase cells and a marked
dose-dependent increase in S-phase cells, compared with the control
(Fig. 3F).
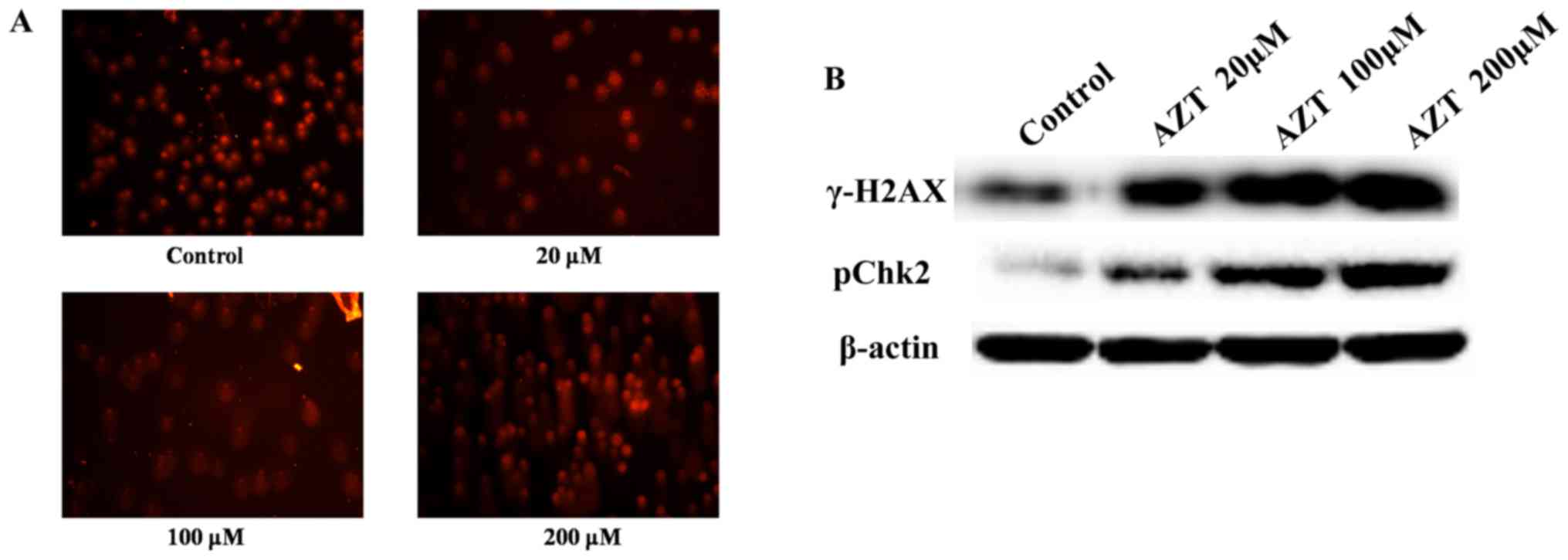
Effect of AZT on DNA damage
Degradation of DNA is an irreversible event
following apoptotic cascade events (17). To investigate whether treatment
with AZT may induce DNA degradation, a comet assay was performed.
As demonstrated in Fig. 4A,
treatment of TE-11 cells with increasing concentrations of AZT for
48 h resulted in significant DNA damage compared with control
cells; the comet tails of the treated cells demonstrate DNA
migration out of the nucleus due to DNA breakage and loss of
structure. Consistent with comet assay results, western blot
analysis revealed that treatment with increasing concentrations of
AZT resulted in enhanced expression levels of γ-H2AX and pChk2
(Fig. 4B), which are markers of
the DNA damage response (DDR) pathway. In conclusion, AZT may
induce DNA damage in TE-11 cells.
Discussion
The majority of cancer cells have been reported to
exhibit enhanced telomerase activity, whereas healthy somatic cells
generally exhibit a low level of telomerase activity. Previously,
telomerase activity has been identified in tumor initiating cells
(3,18). Telomerase has been reported to be
expressed in 86.2% esophageal carcinoma tissues; however, healthy
esophageal tissue did not express it (19). Therefore, this suggests that there
may be a window for telomerase inhibition-based treatment.
Repressing telomerase activity may limit cell growth and induce
apoptosis. Therefore, telomerase is an attractive target for cancer
therapy and various strategies that target telomerase have been
applied in clinical practice (20,21).
Telomerase serves a role in abnormal proliferation
of tumor cells. A reverse transcriptase inhibitor, AZT, has been
utilized in the treatment for AIDS-associated Kaposi sarcoma,
Kaposi sarcoma-associated primary effusion lymphoma,
Epstein-Barr-associated lymphoma, primary central nervous system
lymphoma and adult T cell leukemia (22). AZT has been reported to regress
tumors in phase I and II clinical trials, as a single agent or in
combination with other drugs for gastrointestinal cancers,
pancreatic cancer and various advanced malignancies (23–27).
By interacting with hTERT, AZT causes a series of events, including
telomere shortening, cell cycle blockade, termination of cellular
replication and inhibition of cell growth (10). Chemotherapy is a conventional
treatments for esophageal cancer, and is considered to be an
essential therapeutic strategy (28). Compared with chemotherapy, AZT may
serve as a promising drug as it directly inhibits telomerase
activity and causes little injury to healthy cells, and therefore
may be less toxic. In the present study, the effects of AZT on
TE-11 cells were investigated at various concentrations and time
points. Results demonstrated that AZT inhibited telomerase activity
and proliferation, delayed cell cycle progression and induced
apoptosis of human esophageal cancer TE-11 cells in vitro.
The results of the MTT assay revealed that AZT inhibits the growth
of cancer cells, which provides an experimental foundation for
esophageal tumor therapy. The inhibitory effect was time- and
dose-dependent, which suggested that these were important
parameters for the treatment of esophageal cancer cells. Treatment
of TE-11 cells with AZT resulted in a dose-dependent decrease in
telomerase activity and cells in the G1/G0 phase of the cell cycle,
and a dose-dependent increase in cells in S-phase. These findings
were consistent with previous reports, which demonstrated that AZT
arrested NIH3T3 fibroblasts and SGC-7901 gastric cancer cells in S
and G2/M phase (29,30).
Telomerase has been implicated in DNA double strand
break (DSB) repair (31,32). To investigate whether AZT affects
DNA damage in TE-11 cells, a comet assay was performed following
treatment with AZT at various concentrations. DNA damage was
enhanced with increasing concentrations of AZT. The expression
levels of γ-H2AX and pChk2, which are markers of the DDR pathway
(33), were enhanced following
treatment with AZT. Treatment with AZT leads to a reduction in
telomerase activity, shortening of telomeres, end-to-end fusions
and chromosome instability. In addition, a reduction in telomerase
activity interferes with correct rejoining of DSB ends, causes a
deficiency in DNA repair and induces apoptosis (31). Telomerase activity is directly
associated with protection against cell death, and therefore the
inhibition of telomerase in cancer cells leads to apoptosis
(12,14,15).
Telomerase as target for cancer treatment has great
potential (34). It has been
reported that AZT synergistically interacts with other treatment
modalities, including chemotherapy agents (35,36).
Therefore, AZT may be a novel strategy for the treatment of cancer,
including those derived from the esophagus. However, the mechanism
by which AZT inhibits cell growth and arrests cell cycle
progression in esophageal cancer requires further
investigation.
References
|
1
|
Blackburn EH: Telomerases. Annu Rev
Biochem. 61:113–129. 1992. View Article : Google Scholar
|
|
2
|
Urquidi V, Tarin D and Goodison S: Role of
telomerase in cell senescence and oncogenesis. Ann Rev Med.
51:65–79. 2000. View Article : Google Scholar
|
|
3
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar
|
|
4
|
Pal J, Gold JS, Munshi NC and Shammas MA:
Biology of telomeres: Importance in etiology of esophageal cancer
and as therapeutic target. Transl Res. 162:364–370. 2013.
View Article : Google Scholar
|
|
5
|
Li C, Wu MY, Liang YR and Wu XY:
Correlation between expression of human telomerase subunits and
telomerase activity in esophageal squamous cell carcinoma. World J
Gastroenterol. 9:2395–2399. 2003.
|
|
6
|
Mao WM, Zheng WH and Ling ZQ:
Epidemiologic risk factors for esophageal cancer development. Asian
Pac J Cancer Prev. 12:2461–2466. 2011.
|
|
7
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar
|
|
8
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar :
|
|
9
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar :
|
|
10
|
Gomez DE, Armando RG and Alonso DF: AZT as
a telomerase inhibitor. Front Oncol. 2:1132012. View Article : Google Scholar :
|
|
11
|
Ji HJ, Rha SY, Jeung HC, Yang SH, An SW
and Chung HC: Cyclic induction of senescence with intermittent AZT
treatment accelerates both apoptosis and telomere loss. Breast
Cancer Res Treat. 93:227–236. 2005. View Article : Google Scholar
|
|
12
|
Li H, Song T, Xu W, Yu Y, Xin X and Hui D:
Effect of 3′-Azido-3′-deoxythymidine (AZT) on telomerase activity
and proliferation of HO-8910 cell line of ovarian cancer. Int J
Biomed Sci. 2:34–40. 2006.
|
|
13
|
Sun YQ, Guo TK, Xi YM, Chen C, Wang J and
Wang ZR: Effects of AZT and RNA-protein complex (FA-2-b-beta)
extracted from Liang Jin mushroom on apoptosis of gastric cancer
cells. World J Gastroenterol. 13:4185–4191. 2007. View Article : Google Scholar :
|
|
14
|
Fang JL and Beland FA: Long-term exposure
to zidovudine delays cell cycle progression, induces apoptosis, and
decreases telomerase activity in human hepatocytes. Toxicol Sci.
111:120–130. 2009. View Article : Google Scholar :
|
|
15
|
Falchetti A, Franchi A, Bordi C, Mavilia
C, Masi L, Cioppi F, Recenti R, Picariello L, Marini F, Del Monte
F, et al: Azidothymidine induces apoptosis and inhibits cell growth
and telomerase activity of human parathyroid cancer cells in
culture. J Bone Miner Res. 20:410–418. 2005. View Article : Google Scholar
|
|
16
|
Pressacco J and Erlichman C: Combination
studies with 3′-azido-3′-deoxythymidine (AZT) plus ICI D1694.
Cytotoxic and biochemical effects. Biochem Pharmacol. 46:1989–1997.
1993. View Article : Google Scholar
|
|
17
|
Bröker LE, Kruyt FA and Giaccone G: Cell
death independent of caspases: A review. Clin Cancer Res.
11:3155–3162. 2005. View Article : Google Scholar
|
|
18
|
Terali K and Yilmazer A: New surprises
from an old favourite: The emergence of telomerase as a key player
in the regulation of cancer stemness. Biochimie. 121:170–178. 2016.
View Article : Google Scholar
|
|
19
|
Yu HP, Xu SQ, Lu WH, Li YY, Li F, Wang XL
and Su YH: Telomerase activity and expression of telomerase genes
in squamous dysplasia and squamous cell carcinoma of the esophagus.
J Surg Oncol. 86:99–104. 2004. View Article : Google Scholar
|
|
20
|
Shay JW and Wright WE: Telomerase: A
target for cancer therapeutics. Cancer Cell. 2:257–265. 2002.
View Article : Google Scholar
|
|
21
|
Zvereva MI, Zatsepin TS, Azhibek DM,
Shubernetskaya OS, Shpanchenko OV and Dontsova OA: Oligonucleotide
inhibitors of telomerase: Prospects for anticancer therapy and
diagnostics. Biochemistry (Mosc). 80:251–259. 2015. View Article : Google Scholar
|
|
22
|
Datta A, Bellon M, Sinha-Datta U,
Bazarbachi A, Lepelletier Y, Canioni D, Waldmann TA, Hermine O and
Nicot C: Persistent inhibition of telomerase reprograms adult
T-cell leukemia to p53-dependent senescence. Blood. 108:1021–1029.
2006. View Article : Google Scholar :
|
|
23
|
Posner MR, Darnowski JW, Weitberg AB,
Dudley MN, Corvese D, Cummings FJ, Clark J, Murray C, Clendennin N,
Bigley J, et al: High-dose intravenous zidovudine with
5-fluorouracil and leucovorin. A phase I trial. Cancer.
70:2929–2934. 1992. View Article : Google Scholar
|
|
24
|
Marchbanks K, Dudley MN, Posner MR and
Darnowski J: Pharmacokinetics and pharmacodynamics of high-dose
zidovudine administered as a continuous infusion in patients with
cancer. Pharmacotherapy. 15:451–457. 1995.
|
|
25
|
Clark J, Sikov W, Cummings F, Browne M,
Akerley W, Wanebo H, Weitberg A, Kennedy T, Cole B, Bigley J, et
al: Phase II study of 5-fluoruracil leucovorin and azidothymidine
in patients with metastatic colorectal cancer. J Cancer Res Clin
Oncol. 122:554–558. 1996. View Article : Google Scholar
|
|
26
|
Miller KD, Loehrer PJ, Gonin R, Weber G,
Ansari R, Pletcher W, McClean J, Spiridonidis CH and Mortimer J: A
phase II study of weekly oral methotrexate and zidovudine (AZT) in
advanced adenocarcinoma of the pancreas and hepatocellular
carcinoma. Invest New Drugs. 14:207–212. 1996. View Article : Google Scholar
|
|
27
|
Falcone A, Lencioni M, Brunetti I, Pfanner
E, Allegrini G, Antonuzzo A, Andreuccetti M, Malvaldi G, Danesi R,
Del Tacca M and Conte PF: Maximum tolerable doses of intravenous
zidovudine in combination with 5-fluorouracil and leucovorin in
metastatic colorectal cancer patients. Clinical evidence of
significant antitumor activity and enhancement of
zidovudine-induced DNA single strand breaks in peripheral nuclear
blood cells. Ann Oncol. 8:539–545. 1997. View Article : Google Scholar
|
|
28
|
Lin SH and Chang JY: Esophageal cancer:
Diagnosis and management. Chin J Cancer. 29:843–854. 2010.
View Article : Google Scholar
|
|
29
|
Fang JL, McGarrity LJ and Beland FA:
Interference of cell cycle progression by zidovudine and lamivudine
in NIH 3T3 cells. Mutagenesis. 24:133–141. 2009. View Article : Google Scholar
|
|
30
|
Sun L and Wang X: Effects of allicin on
both telomerase activity and apoptosis in gastric cancer SGC-7901
cells. World J Gastroenterol. 9:1930–1934. 2003. View Article : Google Scholar :
|
|
31
|
Zhou FX, Liao ZK, Dai J, Xiong J, Xie CH,
Luo ZG, Liu SQ and Zhou YF: Radiosensitization effect of zidovudine
on human malignant glioma cells. Biochem Biophys Res Commun.
354:351–356. 2007. View Article : Google Scholar
|
|
32
|
Nugent CI, Bosco G, Ross LO, Evans SK,
Salinger AP, Moore JK, Haber JE and Lundblad V: Telomere
maintenance is dependent on activities required for end repair of
double-strand breaks. Curr Biol. 8:657–660. 1998. View Article : Google Scholar
|
|
33
|
Oka K, Tanaka T, Enoki T, Yoshimura K,
Ohshima M, Kubo M, Murakami T, Gondou T, Minami Y, Takemoto Y, et
al: DNA damage signaling is activated during cancer progression in
human colorectal carcinoma. Cancer Biol Ther. 9:246–252. 2010.
View Article : Google Scholar :
|
|
34
|
Chen Z and Corey DR: Telomerase
inhibitors: A new option for chemotherapy. Adv Cancer Res.
87:31–58. 2003. View Article : Google Scholar
|
|
35
|
Chen C, Zhang Y, Wang Y, Huang D, Xi Y and
Qi Y: Synergic effect of 3′-azido-3′-deoxythymidine and arsenic
trioxide in suppressing hepatoma cells. Anticancer Drugs.
22:435–443. 2011. View Article : Google Scholar
|
|
36
|
Mattson DM, Ahmad IM, Dayal D, Parsons AD,
Aykin-Burns N, Li L, Orcutt KP, Spitz DR, Dornfeld KJ and Simons
AL: Cisplatin combined with zidovudine enhances cytotoxicity and
oxidative stress in human head and neck cancer cells via a
thiol-dependent mechanism. Free Radic Biol Med. 46:232–237. 2009.
View Article : Google Scholar
|