Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an
aggressive hematologic disease (1), accounting for ~15 and 25% of ALL in
pediatric and adult patients, respectively (2). Despite progress in treatment and
children having an approximate cure rate of 90%, the cure rate of
adults is <50%, even with hematopoietic stem cell
transplantation (3). In adult
patients with ALL who have achieved complete remission, the
majority relapse, and few patients are cured even when treated
using the best therapeutic regimens currently available. Therefore,
more effective treatment is urgently required for this disease.
The 26S proteasome is vital in eukaryotic cell
function and viability. It is responsible for several integral
cellular processes, including the timely degradation of cell cycle
regulator proteins and transcription factors, and the maintenance
of cellular homeostasis, all of which are essential for cell
proliferation, differentiation apoptosis and angiogenesis (4). The proteasome has consequently become
an attractive target for therapeutic intervention in cancer
chemotherapy. Bortezomib (Velcade; PS-341), a dipeptide boronic
acid analog and the first clinically available proteasome
inhibitor, has been widely applied to treat multiple myeloma
(5). The drug exhibits selectivity
towards the proteasome of cancer cells, rendering it with a
distinct therapeutic advantage. Preclinical studies have shown that
bortezomib also induces apoptosis in acute leukemia and solid tumor
cells (6). However, as a single
agent, the clinical results of bortezomib in patients with acute
myeloid leukemia and pediatric refractory ALL are less encouraging
(7). Accumulating clinical studies
have indicated that the effect of bortezomib combined with
anthracycline drugs, including idarubicin and doxorubicin, is
likely to be active with acceptable toxicity in hematological
malignancies, including acute leukemia (8–10).
Daunorubicin belongs to the anthracycline family, and is one of the
major antitumor agents in the treatment of myeloid leukemia
(11). Daunorubicin kills leukemic
cells by the inhibition of topoisomerase II, induction of DNA
damage and generation of reactive oxygen species (ROS) (12). However, whether the combination of
daunorubicin and bortezomib is also effective in T-ALL cells
remains to be elucidated.
In the present study, the cytotoxicities against
T-ALL cells of bortezomib and daunorubicin, alone and in
combination were compared. It was found that cotreatment of
daunorubicin and bortezomib was more effective, compared with
either agent used alone at inducing T-ALL cell death, and it was
demonstrated that the mitochondrial pathway was important in this
process.
Materials and methods
Cells and drug treatments
The T-ALL Jurkat and Molt-4 cells and Burkitt
lymphoma Daudi cells (both from American Type Culture Collection;
ATCC, Manassas, VA, USA) were maintained at 37°C in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
HyClone, Logan, UT, USA) in a humidified atmosphere containing 95%
air and 5% CO2. Primary ALL cells were harvested from bone marrow
samples of 5 patients with T-ALL in Ruijin Hosptial (Shanghai,
China), from October 2013 to July 2014 (patient information is
shown in Table I). Bone marrow
mononuclear cells were isolated using Ficoll-Paque isolation
solution and centrifugation for 30 min at 400 × g at 25°C and
re-suspended in RPMI-1640 medium supplemented with 10% FBS. The
study protocol was approved by the local institution review board
at Shanghai Jiaotong University School of Medicine (Shanghai,
China) and informed consent was obtained from the patients. Cells
were seeded at 5×105 cells/ml in 6-well plates. Jurkat cells were
treated with 10 nM bortezomib (Velcade; Millennium Pharmaceutical,
Cambridge, MA, USA) or 1 µM daunorubicin hydrochloride
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and Molt-4 cells
were treated with 15 nM bortezomib or 15 nM daunorubicin
hydrochloride, alone or in combination, for 24 h or 48 h in a
humidified atmosphere containing 95% air and 5% CO2.
 | Table I.Information of patients with T-cell
acute lymphoblastic leukemia. |
Table I.
Information of patients with T-cell
acute lymphoblastic leukemia.
| Number | Sex | Age (years) | Blast (%) | Chromosome | White blood cells
(×109/l) |
|---|
| 1 | Male | 16 | 95.5 | ND | 15.4 |
| 2 | Female | 37 | 85.0 | ND | 2.2 |
| 3 | Female | 24 | 91.5 | 51-53,XX,3p-,
del(6), | 22.1 |
| 4 | Female | 14 | 95.0 | 46,XX | 15.6 |
| 5 | Female | 37 | 90.0 |
46,XX,3p+,del(9), | 32.3 |
Cell proliferation assays
The cells were seeded at 2–3×105 cells/ml
in a 6-well plate and were treated with the appropriate drugs. The
cells were counted every 12 h using a Beckman Coulter counter
(Beckman Coulter, Inc., Fullerton, CA, USA) and cellular morphology
was evaluated using light microscopy with Wright's staining. The
experiment was performed in duplicate at least three times
independently.
Apoptotic assays
For the apoptotic assays, 2×106 cells were washed
twice with phosphate-buffered saline (PBS), and then labeled using
Annexin V-fluorescein isothiocyanate and propidium iodide (PI)
according to the manufacturer's protocol (BD Pharmingen, San Diego,
CA, USA). Fluorescence intensity was measured using flow cytometry
(BD Biosciences, Franklin Lakes, NJ, USA). Data were analyzed using
CellQuest software (version 3.3; BD Biosciences). For each
analysis, 10,000 events were recorded. The experiment was performed
in duplicate at least three times independently.
Determination of mitochondrial
membrane potential (ΔΨm)
For measurement of alterations in ΔΨm, the cells
(1×106) were incubated with 100 µg/ml Rhodamine123 for
30 min at room temperature with 50 µg/ml PI. Fluorescence intensity
was measured using flow cytometry. The experiment was performed at
least three times independently in triplicate.
Western blot analysis
The cells were washed with PBS and lysed with lysis
buffer containing 62.5 mM Tris-HCl (pH 6.8), 100 mM DTT, 2% SDS and
10% glycerol by boiling at 100°C for 5 min. The cell lysates were
centrifuged at 20,000 × g for 10 min at 4°C, and proteins in the
supernatants were quantified using a bicinchoninic acid protein
assay (Merck KGaA). Protein extracts (10–20 µg) were equally loaded
to an 8–14% SDS-polyacrylamide gel, electrophoresed, and
transferred onto nitrocellulose membrane (GE Healthcare Life
Sciences, Buckinghamshire, UK). The blots were stained with 0.2%
Ponceau S red to ensure equal protein loading. Following blocking
with 5% nonfat milk in PBS, the membranes were probed with
antibodies against caspase-3 (cat. no. 9662), caspase-8 (cat. no.
9746), caspase-9 (cat. no. 9502), Bim (cat. no. 2933), Bcl-2 (cat.
no. 4223) and Bcl-xl (cat. no. 2764), overnight at 4°C (all at
1:1,000 dilution; Cell Signaling Technology, Inc., Danvers, MA,
USA), then followed by incubation with horseradish peroxidase
(HRP)-linked secondary antibodies (1:2,000 dilution; Cell Signaling
Technology, Inc.) for 1 h at room temperature. The signals were
detected using a chemiluminescence phototope-HRP kit (Cell
Signaling Technology, Inc.) according to the manufacturer's
protocol. As necessary, the blots were stripped and re-probed with
anti-β-actin antibody (1:5,000 dilution; cat. no. PM053; Medical
and Biological Laboratories Co., Ltd., Nagoya, Japan) as an
internal control. All experiments were repeated three times.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). Student's
t-test was used to compare differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference. Synergistic interactions between bortezomib and
daunorubicin were analyzed by the Chou-Talalay method using
CalcuSyn software version 2.1 (Biosoft, Cambridge, UK) (13). A combination index (CI) of <1,
equal to 1, and >1 indicate synergistic, additive and
antagonistic effects, respectively.
Results
Bortezomib and daunorubicin
cotreatment promotes late apoptosis of Jurkat and Molt-4 cells
The present study first examined the possible
combinatorial effect of bortezomib and daunorubicin against Jurkat
and Molt-4 cells. The Jurkat cells were treated with 1 µM
daunorubicin in the presence or absence of 10 nM bortezomib.
Bortezomib and daunorubicin alone caused a 24.9±0.74 and a
72.6±2.17% reduction in the proliferation of Jurkat cells 48 h post
treatment, respectively. The combined use of the two agents
resulted in a marked decrease in the proliferation of the Jurkat
cells (90.1±3.23%; Fig. 1A), with
similar results observed in the Molt-4 cells (Fig. 1B).
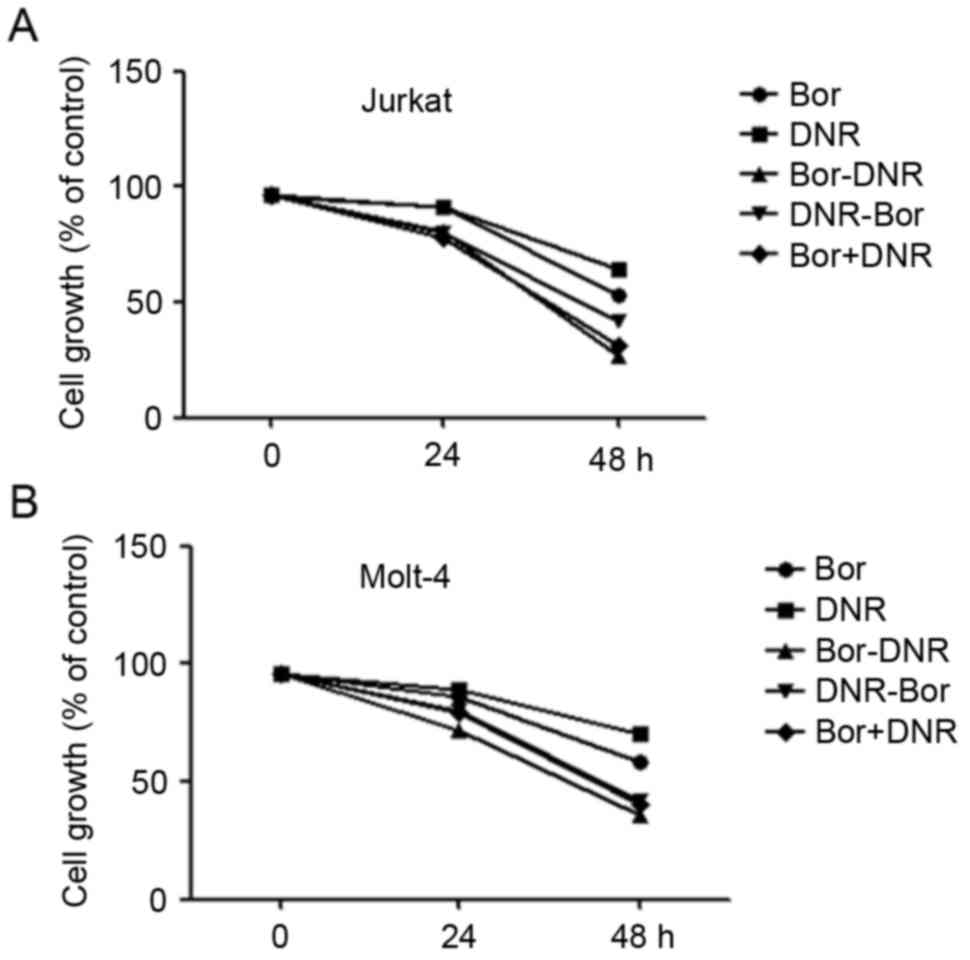
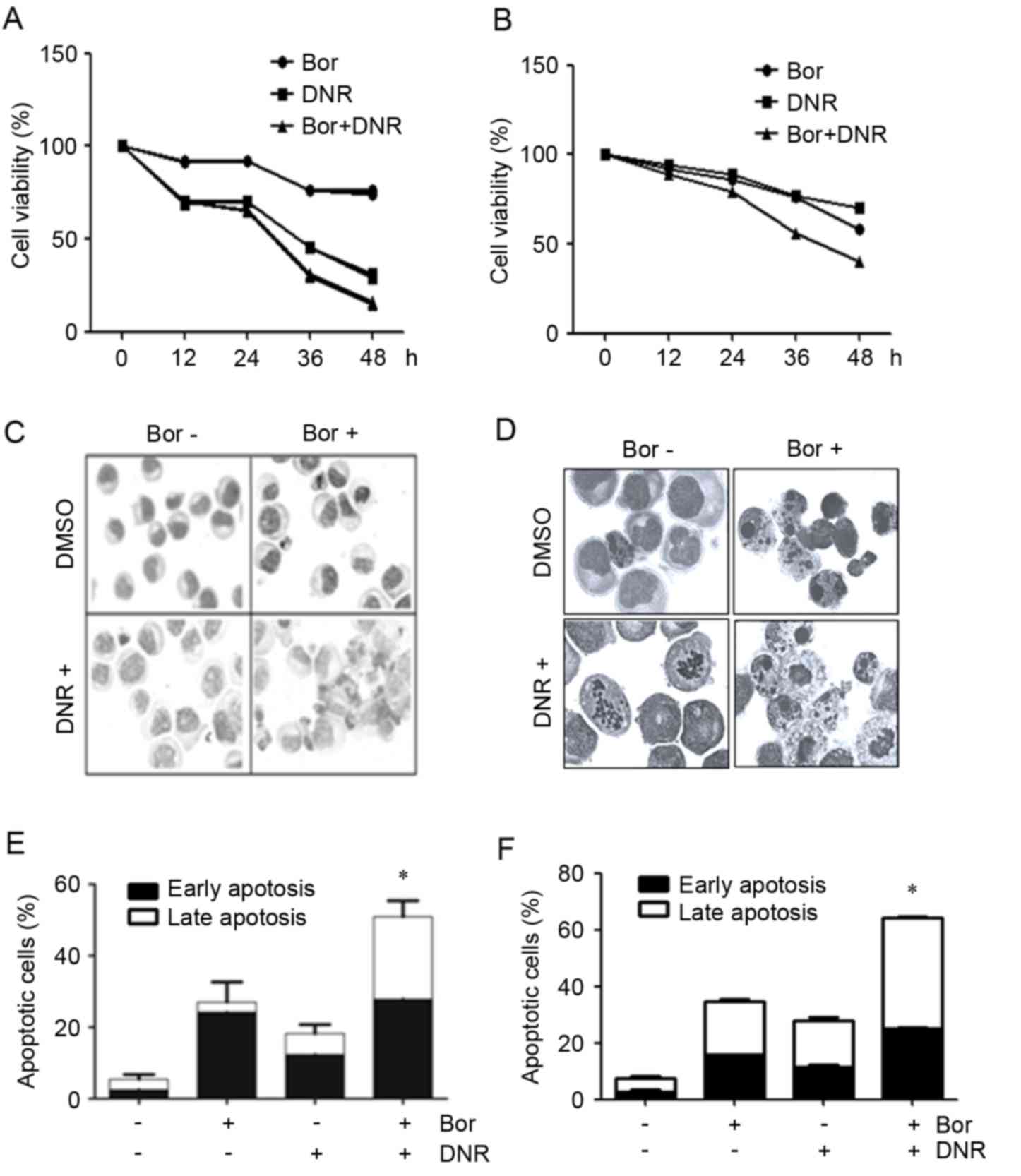 | Figure 1.Effects of Bor+DNR on the
proliferation of Jurkat and Molt-4 cells. (A) Jurkat cells were
treated with 10 nM Bor with or without 1 µM DNR, and (B) Molt-4
cells were treated with 15 nM Bor, with or without 15 nM DNR for
12, 24, 36 and 48 h, respectively, and cell viability was
determined. The morphology of the (C) Jurkat and (D) Molt-4 cells
treated with Bor, with or without DNR, was examined using Wright's
staining (magnification, ×100). Apoptotic cells were determined for
(E) Jurkat and (F) Molt-4 cells treated with Bor, with or without
DNR, for 24 h, by Annexin V/propidium iodide staining. All values
are expressed as the mean ± standard deviation. Each experiment was
performed at least 3 times independently. *P<0.05, compared with
Bor or DNR treatment. Bor, bortezomib; DNR, daunorubincin. |
Wright's staining of the Jurkat cells treated with
bortezomib (10 nM) and daunorubicin (1 µM) alone or in combination
for 24 h showed that an induction of apoptosis was observed in the
combination group, with the appearance of cell shrinkage, nuclear
condensation and the formation of apoptotic bodies (Fig. 1C and D). Flow cytometric analysis
revealed that, following treatment for 24 h, the percentage of
early apoptotic Jurkat cells treated with bortezomib was 24.2±6.35%
(P<0.05, vs. control) whereas the percentage of late apoptotic
Jurkat cells was 2.8±5.65% (P>0.05, vs. control; Fig. 1E). The percentage of early
apoptotic Jurkat cells treated with daunorubicin was 12.4±2.75%
(P<0.05, vs. control) whereas the percentage of late apoptotic
Jurkat cells was 5.8±2.55% (P>0.05, vs. control; Fig. 1E). The combination of daunorubicin
and bortezomib caused no apparent increase in the percentage of
early apoptotic cells, compared with the cells treated with
daunorubicin alone (27.9±4.81%; P>0.05), however, a significant
increase in the percentage of late apoptotic cells was observed
(23.0±4.51%; P<0.05, vs. daunorubicin or bortezomib alone;
Fig. 1E). Similar results were
observed in the Molt-4 cells (Fig.
1F). These data indicated that the combination of daunorubicin
and bortezomib enhanced the late apoptosis of Jurkat and Molt-4
cells.
To determine whether these two drugs synergistically
inhibited the proliferation of leukemia cells, the CI values were
calculated using the Chou-Talalay method. As shown in Tables II–IV, daunorubicin and bortezomib
cotreatment had a synergistic effect on the Jurkat and Molt-4, but
not on the Daudi cells.
 | Table II.Combination index values of
bortezomib and daunorubicin in Jurkat cells. |
Table II.
Combination index values of
bortezomib and daunorubicin in Jurkat cells.
| Dose bortezomib
(nM) | Dose daunorubicin
(µM) | Growth inhibition
(%) | Combination
index |
|---|
| 10.0 | 1.00 | 52 | 0.31398 |
| 10.0 | 1.25 | 58 | 0.33839 |
| 10.0 | 1.50 | 64 | 0.34315 |
| 15.0 | 10.00 | 70 | 0.29281 |
| 15.0 | 10.25 | 84 | 0.12266 |
| 15.0 | 10.50 | 78 | 0.40632 |
| 20.0 | 1.00 | 40 | 0.66699 |
| 20.0 | 1.25 | 62 | 0.47163 |
| 20.0 | 1.50 | 70 | 0.38763 |
 | Table IV.Combination index values of
bortezomib and daunorubicin in Daudi cells. |
Table IV.
Combination index values of
bortezomib and daunorubicin in Daudi cells.
| Dose bortezomib
(nM) | Dose daunorubicin
(nM) | Growth inhibition
(%) | Combination
index |
|---|
| 10.0 | 40.0 | 51 | 1.13996 |
| 10.0 | 50.0 | 64 | 0.90496 |
| 10.0 | 60.0 | 67 | 1.03848 |
| 15.0 | 40.0 | 53 | 1.12142 |
| 15.0 | 50.0 | 68 | 0.82208 |
| 15.0 | 60.0 | 63 | 1.40427 |
| 20.0 | 40.0 | 61 | 0.93591 |
| 20.0 | 50.0 | 70 | 0.83512 |
| 20.0 | 60.0 | 71 | 0.99927 |
To determine whether the administration sequence
affected the combination effect of daunorubicin and bortezomib,
daunorubicin or bortezomib was added prior to, or following, the
other drug. As shown in Fig. 2A and
B, bortezomib treatment followed by daunorubicin was more
effective, compared with daunorubicin treatment followed by
bortezomib, or with the two drugs simultaneously.
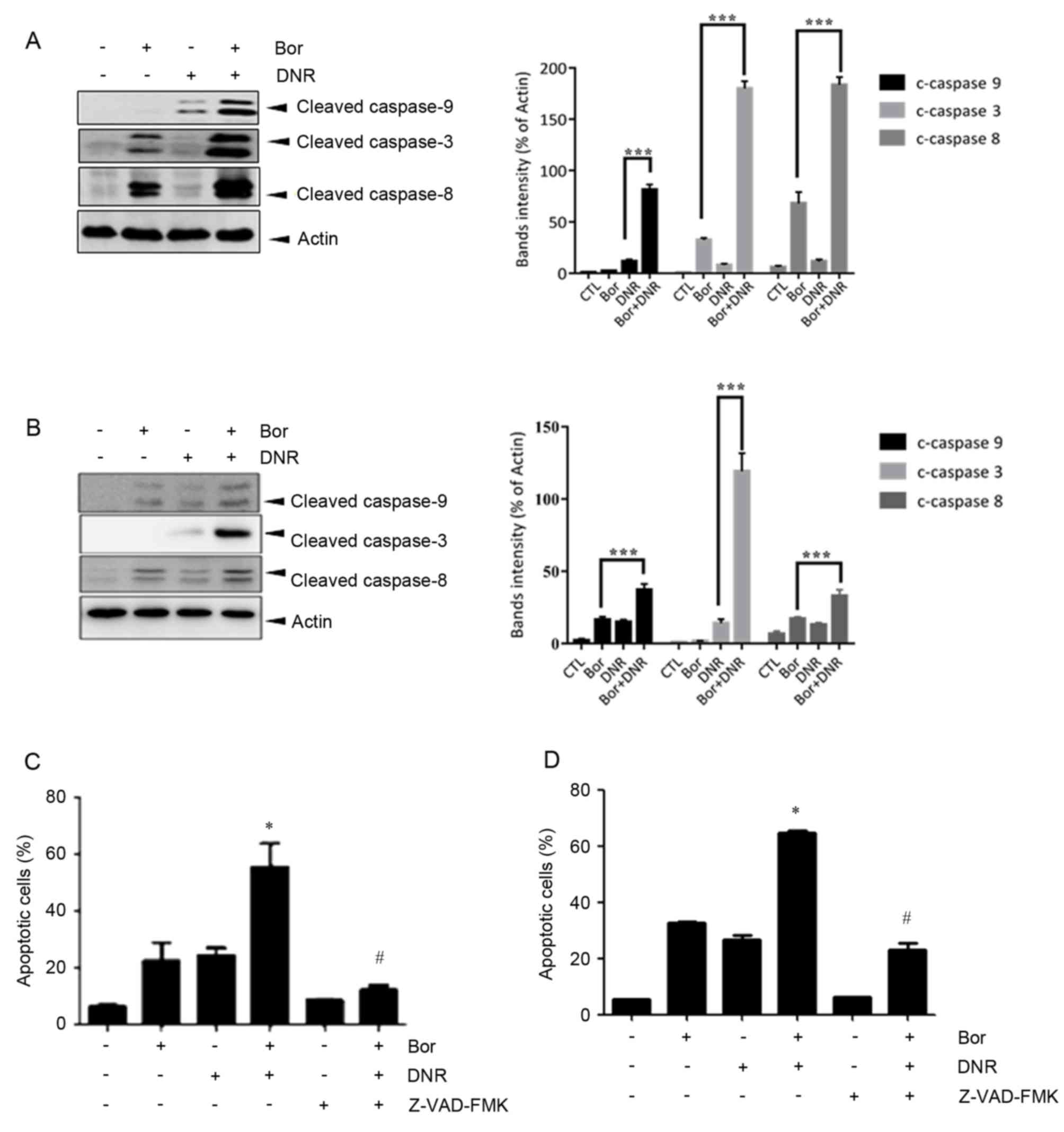
Bortezomib promotes cell death by
activating the caspase cascade
To investigate the mechanisms underlying
bortezomib+daunorubicin-induced cell death in the Jurkat and Molt-4
cells, the present study examined the activation of caspase using
western blot analysis. It was found that cotreatment of the Jurkat
(Fig. 3A) and Molt-4 (Fig. 3B) cells with daunorubicin and
bortezomib significantly increased the levels of cleaved caspase-3,
-8 and -9. To determine the role of caspase activation, the Jurkat
(Fig. 3C) and Molt-4 (Fig. 3D) cells were treated with
bortezomib and daunorubicin in the presence or absence of the
broad-spectrum caspase inhibitor, Z-VAD-FMK (10 µM). The results of
the flow cytometric analysis revealed that Z-VAD-FMK significantly
attenuated the cell death induced by cotreatment with bortezomib
and daunorubicin (P<0.05). These findings suggested that
bortezomib and daunorubicin promoted apoptosis through activating
caspase-3, -8 and -9.
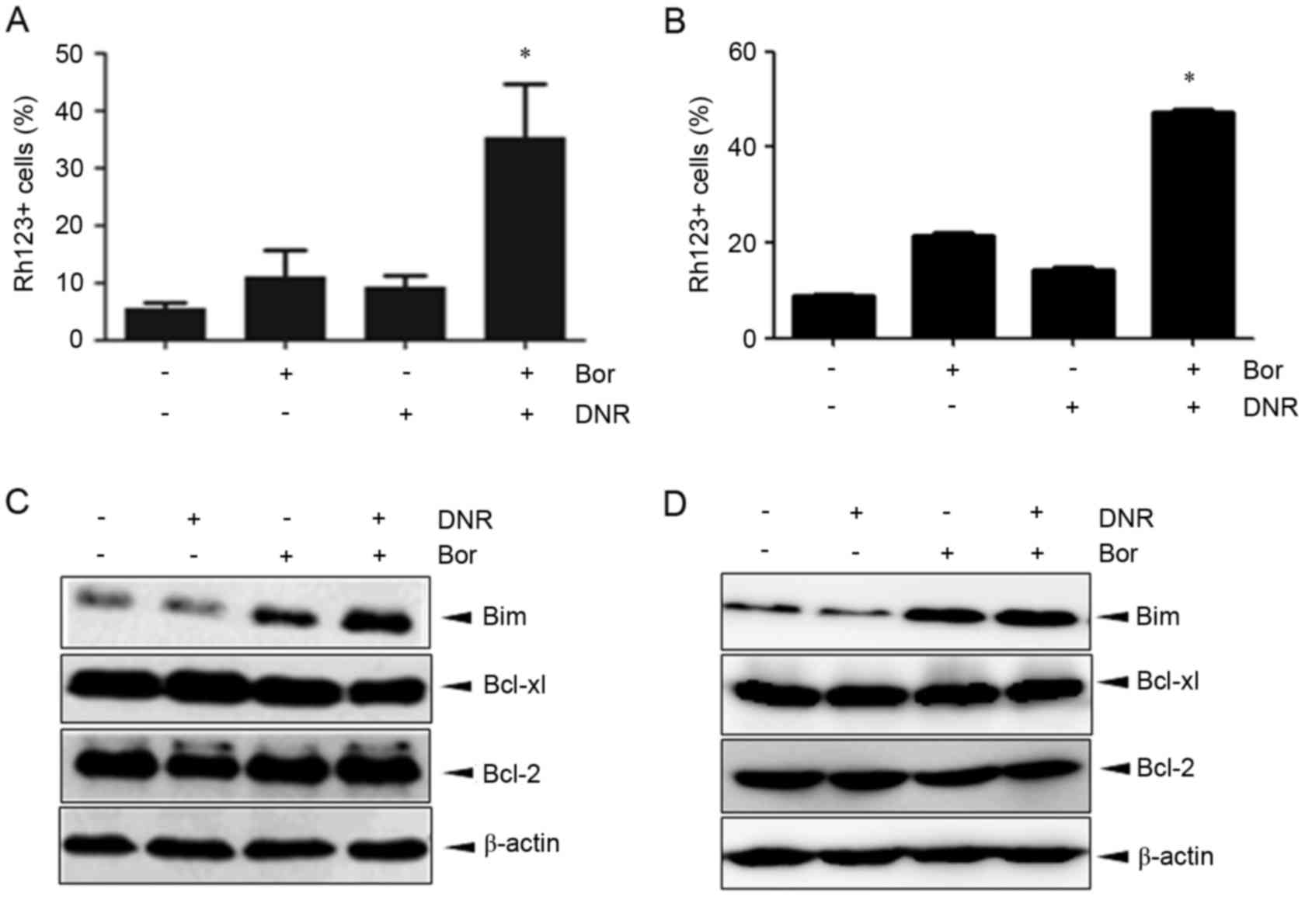
Bortezomib+daunorubicin causes marked
dissipation of ΔΨm and increases the expression of Bim
The mitochondrial pathway is critical in the
execution of apoptotic cell death, and the collapse of ΔΨm is an
early event in the mitochondrial cell death pathway. The present
study examined alterations in ΔΨm using rhodamine123/PI staining.
Marked dissipation of ΔΨm was observed in the Jurkat cells
cotreated with bortezomib and daunorubicin at 24 h (35.4±9.3%),
which was significantly higher, compared with that in the Jurkat
cells treated with bortezomib (11.1±4.6%) or daunorubicin
(9.3±2.0%) alone (Fig. 4A).
Similar results were observed in Molt-4 cells (Fig. 4B). Alterations in the expression of
the Bcl-2 family proteins, Bcl-2, Bcl-xl and Bim, were also
examined. In the Jurkat cells, daunorubicin alone had no apparent
effect, whereas bortezomib upregulated the expression of Bim.
However, the combination of bortezomib and daunorubicin caused a
marked increase in the expression of Bim at 24 h (Fig. 4C). By contrast, the combination
regimen exerted no noticeable effect on the expression of Bcl-2 or
Bcl-xl (Fig. 4C). Similar results
were observed in the Molt-4 cells (Fig. 4D). These data indicated that the
combination effect of bortezomib+daunorubicin was associated with
the collaspe of ΔΨm, in which Bim may be involved.
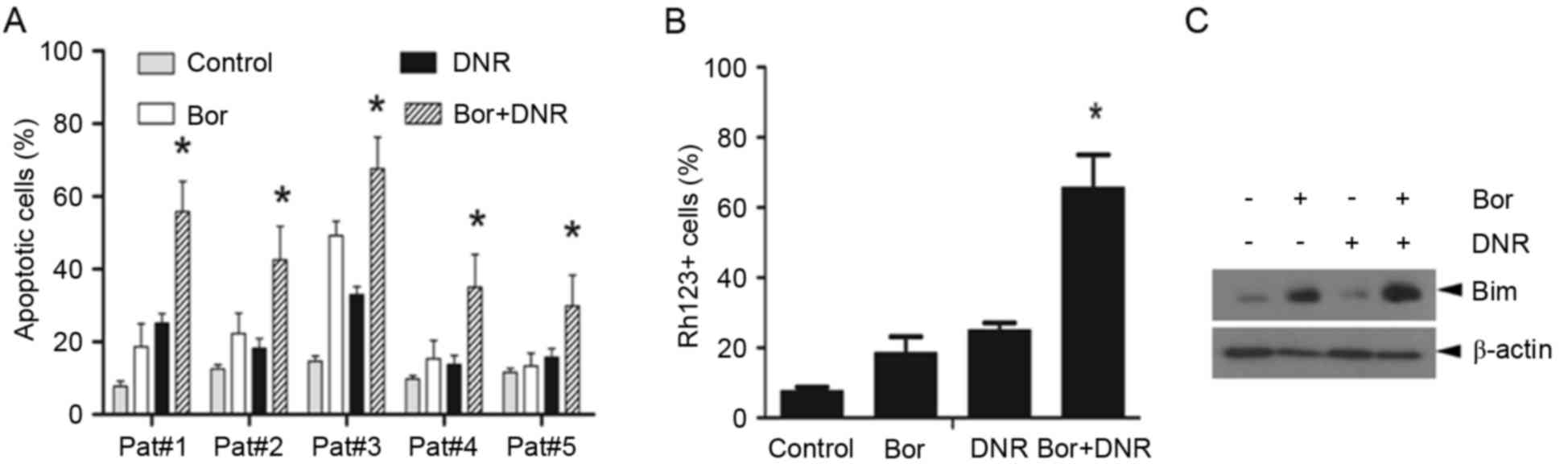
Bortezomib and daunorubicin in
combination are effective in inducing the apoptosis of primary
T-ALL cells
To determine whether the combination effect of
bortezomib and daunorubicin was limited to Jurkat cells, five
primary T-ALL cells were treated with daunorubicin (500 nM) in the
presence or absence of bortezomib (10 nM) for 24 h. As shown in
Fig. 5A, bortezomib+daunorubicin
caused a significantly higher apoptotic rate, compared with either
agent used alone (P<0.05). In addition, flow cytometric analyses
showed that bortezomib or daunorubicin alone caused only modest
mitochondrial injury, whereas the combination of bortezomib and
daunorubicin resulted in more prominent mitochondrial injury
(Fig. 5B). Furthermore, the
western blot assays showed that the combination treatment markedly
increased the expression of Bim in primary ALL cells, compared with
either bortezomib or daunorubicin alone (Fig. 5C). Together, these findings
indicated that the combination of bortezomib and daunorubicin
exerted more marked cytotoxicity against primary ALL cells by
disrupting the ΔΨm.
Discussion
T-ALL has a poor prognosis due to its intrinsic
chemoresistance and severe immunosuppression. Although traditional
chemotherapeutic regimens have shown improved response rate, they
have failed to achieve a significant effect on long-term survival
rates (2). Therefore, novel
strategies are required for the treatment of T-ALL. In the present
study, it was demonstrated that bortezomib and daunorubicin
cotreatment induced apoptosis in T-ALL cells via disrupting the
ΔΨm.
Human leukemic cells express abnormally high levels
of proteasomes, compared with normal peripheral blood cells, and
they are significantly more sensitive to proteasome inhibition,
compared with normal bone marrow progenitor cells or peripheral
blood lymphocytes (6,14,15).
Clinical trials are underway to assess the efficacy of bortezomib
in several human malignancies, including leukemia (10). Available data suggest that
bortezomib as a single drug may only yield minor clinical benefits
in patients with leukemia (7,16).
However, bortezomib has been shown to enhance the efficacy of
several conventional therapies and may overcome resistance to
conventional anticancer drugs, including belinostat, SAHA,
gemcitabine and imatinib, and the effects in combination studies
appear promising (10,17–24).
In the present study, using human T-ALL Jurkat and Molt-4 cells as
a model, it was demonstrated that bortezomib and daunorubicin
cotreatment was more effective, compared with either agent used
alone at inducing apoptosis, as reflected by the activation of
caspase-3, -8 and -9, and the appearance of apoptotic morphology.
Notably, it was demonstrated that this combination effect was also
present in primary T-ALL cells. It is well known that standard
chemotherapy is inhibitory to normal hematopoietic cells and
frequently results in severe myelosuppression, and anthracyclines
can cause cumulative dose-dependent cardiotoxicity (25). Thus, the combination of bortezomib
and daunorubicin at a lower dose may reduce their dose-associated
side effect but increasing their efficacy. Similar to the findings
obtained in the present study, Koyama et al reported that
bortezomib and doxrubicin also induced apoptosis in T-ALL cell
lines (26). However, the
combination effect of these drugs on primary leukemia cells was not
investigated.
The mitochondrial and cell death receptor apoptotic
pathways are two major apoptotic cell death pathways. It has been
shown that mitochondrial signaling exerts a critical role in
bortezomib-induced apoptosis (27–30).
The present study found that the combination of these two agents
caused extensive loss of ΔΨm, indicating the involvement of the
mitochondrial apoptotic pathway. Consistent with this, bortezomib
and daunorubicin cotreatment enhanced the collapse of ΔΨm in
primary T-ALL leukemia cells. The cell death receptor pathway may
also be activated by cotreatment of bortezomib and daunorubicin, as
evidenced by the activation of caspase-8.
An important event in the mitochondrial apoptotic
pathway is mitochondrial outer membrane permeabilization, which is
primarily mediated and controlled by the Bcl-2 family members
(31). When mitochondrial outer
membrane permeabilization occurs, it precipitates cell death
through either the release of molecules involved in apoptosis or
the loss of mitochondrial functions essential for cell survival.
The present study determined the effect of bortezomib daunorubicin
cotreatment on several Bcl-2 family members. The bortezomib
daunorubicin cotreatment markedly increased the proapoptotic
regulator protein, Bim, in the Jurkat and primary ALL cells, but
exerted minimal effect on the expression of Bcl-2 or Bcl-xl. Bim is
a member of the BH3-only protein family, which mediates cell death
from physiologic stimuli, including cytokine deprivation and
signals from activated oncogenes. The upregulation of Bim triggers
the release of cytochrome c from the mitochondria and the
onset of apoptosis (32). The
results of the present study indicated that Bim may be important in
bortezomib+daunorubicin-induced cell death. Consistent with this,
several reports have shown that Bim-targeting contributes to the
bortezomib-based combination regime (33–35).
However, whether Bim contributed to bortezomib+daunorubicin-induced
mitochondria impairment, and how cotreatment with bortezomib and
daunorubicin upregulated the expression of Bim required further
investigation. BH3-interacting domain death agonist (Bid), another
proapoptotic Bcl-2 family member, may also be involved in this
process (36,37). As shown in Fig. 2, bortezomib and daunorubicin
cotreatment induced the activation of caspase 8. Caspase 8 can
cleave Bid into t-Bid, which then causes mitochondrial outer
membrane permeabilisation. This leads to the mitochondrial release
of apoptogenic proteins, including cytochrome c.
In conclusion, the present demonstrated that
bortezomib cooperated with daunorubicin to induce the apoptosis of
Jurkat and Molt-4 cells, and primary T-ALL cells, in which the
mitochondrial apoptotic pathway was pivotal. These findings provide
a rationale for use of the combination of bortezomib and
daunorubicin in the treatment T-ALL in future preclincal and
clinical investigations.
Acknowledgements
This study was supported by the Shanghai Commission
of Science and Technology (grant nos. 10411966900 and 15401901800),
the National Natural Science Foundation of China (grant nos.
81170508, 31100980, 81570118 and 81570112) and the Innovation
Program of Shanghai Municipal Education Commission (grant no.
13YZ028).
References
|
1
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bazarbachi A, Ghez D, Lepelletier Y, Nasr
R, de Thé H, El-Sabban ME and Hermine O: New therapeutic approaches
for adult T-cell leukaemia. Lancet Oncol. 5:664–672. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marks DI and Rowntree C: Management of
adults with T-cell lymphoblastic leukemia. Blood. 129:1134–1142.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson DE: The ubiquitin-proteasome
system: Opportunities for therapeutic intervention in solid tumors.
Endocr Relat Cancer. 22:T1–T17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kouroukis TC, Baldassarre FG, Haynes AE,
Imrie K, Reece DE and Cheung MC: Bortezomib in multiple myeloma:
Systematic review and clinical considerations. Curr Oncol.
21:e573–e603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orlowski RZ and Kuhn DJ: Proteasome
inhibitors in cancer therapy: Lessons from the first decade. Clin
Cancer Res. 14:1649–1657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horton TM, Pati D, Plon SE, Thompson PA,
Bomgaars LR, Adamson PC, Ingle AM, Wright J, Brockman AH, Paton M
and Blaney SM: A phase 1 study of the proteasome inhibitor
bortezomib in pediatric patients with refractory leukemia: A
children's oncology group study. Clin Cancer Res. 13:1516–1522.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertaina A, Vinti L, Strocchio L, Gaspari
S, Caruso R, Algeri M, Coletti V, Gurnari C, Romano M, Cefalo MG,
et al: The combination of bortezomib with chemotherapy to treat
relapsed/refractory acute lymphoblastic leukaemia of childhood. Br
J Haematol. 176:629–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Howard DS, Liesveld J, Phillips GL II,
Hayslip J, Weiss H, Jordan CT and Guzman ML: A phase I study using
bortezomib with weekly idarubicin for treatment of elderly patients
with acute myeloid leukemia. Leuk Res. 37:1502–1508. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Messinger YH, Gaynon PS, Sposto R, van der
Giessen J, Eckroth E, Malvar J and Bostrom BC: Therapeutic Advances
in Childhood Leukemia & Lymphoma (TACL) Consortium: Bortezomib
with chemotherapy is highly active in advanced B-precursor acute
lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia
& Lymphoma (TACL) study. Blood. 120:285–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Löwenberg B, Ossenkoppele GJ, van Putten
W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Maertens J,
Jongen-Lavrencic M, von Lilienfeld-Toal M, et al: High-dose
daunorubicin in older patients with acute myeloid leukemia. N Engl
J Med. 361:1235–1248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pommier Y, Leo E, Zhang H and Marchand C:
DNA topoisomerases and their poisoning by anticancer and
antibacterial drugs. Chem Biol. 17:421–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crawford LJ and Irvine AE: Targeting the
ubiquitin proteasome system in haematological malignancies. Blood
Rev. 27:297–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soligo D, Servida F, Delia D, Fontanella
E, Lamorte G, Caneva L, Fumiatti R and Lambertenghi Deliliers G:
The apoptogenic response of human myeloid leukaemia cell lines and
of normal and malignant haematopoietic progenitor cells to the
proteasome inhibitor PSI. Br J Haematol. 113:126–135. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du XL and Chen Q: Recent advancements of
bortezomib in acute lymphocytic leukemia treatment. Acta Haematol.
129:207–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai Y, Chen S, Wang L, Pei XY, Kramer LB,
Dent P and Grant S: Bortezomib interacts synergistically with
belinostat in human acute myeloid leukaemia and acute lymphoblastic
leukaemia cells in association with perturbations in NF-κB and Bim.
Br J Haematol. 153:222–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lieu C, Chow L, Pierson AS, Eckhardt SG,
O'Bryant CL, Morrow M, Tran ZV, Wright JJ and Gore L: A phase I
study of bortezomib, etoposide and carboplatin in patients with
advanced solid tumors refractory to standard therapy. Invest New
Drugs. 27:53–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walker AR, Klisovic RB, Garzon R, Schaaf
LJ, Humphries K, Devine SM, Byrd JC, Grever MR, Marcucci G and Blum
W: Phase I study of azacitidine and bortezomib in adults with
relapsed or refractory acute myeloid leukemia. Leuk Lymphoma.
55:1304–1308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schelman WR, Traynor AM, Holen KD, Kolesar
JM, Attia S, Hoang T, Eickhoff J, Jiang Z, Alberti D, Marnocha R,
et al: A phase I study of vorinostat in combination with bortezomib
in patients with advanced malignancies. Invest New Drugs.
31:1539–1546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Messinger Y, Gaynon P, Raetz E, Hutchinson
R, Dubois S, Glade-Bender J, Sposto R, van der Giessen J, Eckroth E
and Bostrom BC: Phase I study of bortezomib combined with
chemotherapy in children with relapsed childhood acute
lymphoblastic leukemia (ALL): A report from the therapeutic
advances in childhood leukemia (TACL) consortium. Pediatr Blood
Cancer. 55:254–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Z, Pan XF, Wu FQ, Ma LY, Liu DP, Liu Y,
Feng TT, Meng FY, Liu XL, Jiang QL, et al: Synergy between
proteasome inhibitors and imatinib mesylate in chronic myeloid
leukemia. PLoS One. 4:e62572009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang QL, Wang L, Zhang YW, Jiang XX, Yang
F, Wu WL, Janin A, Chen Z, Shen ZX, Chen SJ and Zhao WL: The
proteasome inhibitor bortezomib interacts synergistically with the
histone deacetylase inhibitor suberoylanilide hydroxamic acid to
induce T-leukemia/lymphoma cells apoptosis. Leukemia. 23:1507–1514.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hui KF, Leung YY, Yeung PL, Middeldorp JM
and Chiang AK: Combination of SAHA and bortezomib up-regulates
CDKN2A and CDKN1A and induces apoptosis of Epstein-Barr
virus-positive Wp-restricted Burkitt lymphoma and lymphoblastoid
cell lines. Br J Haematol. 167:639–650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schimmel KJ, Richel DJ, van den Brink RB
and Guchelaar HJ: Cardiotoxicity of cytotoxic drugs. Cancer Treat
Rev. 30:181–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koyama D, Kikuchi J, Hiraoka N, Wada T,
Kurosawa H, Chiba S and Furukawa Y: Proteasome inhibitors exert
cytotoxicity and increase chemosensitivity via transcriptional
repression of Notch1 in T-cell acute lymphoblastic leukemia.
Leukemia. 28:1216–1226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song IS, Kim HK, Lee SR, Jeong SH, Kim N,
Ko KS, Rhee BD and Han J: Mitochondrial modulation decreases the
bortezomib-resistance in multiple myeloma cells. Int J Cancer.
133:1357–1367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berges C, Haberstock H, Fuchs D, Sadeghi
M, Opelz G, Daniel V and Naujokat C: Proteasome inhibition
activates the mitochondrial pathway of apoptosis in human
CD4+ T cells. J Cell Biochem. 108:935–946. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grandjenette C, Schnekenburger M, Karius
T, Ghelfi J, Gaigneaux A, Henry E, Dicato M and Diederich M:
5-aza-2′-deoxycytidine-mediated c-myc down-regulation triggers
telomere-dependent senescence by regulating human telomerase
reverse transcriptase in chronic myeloid leukemia. Neoplasia.
16:511–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Voortman J, Checinska A, Giaccone G,
Rodriguez JA and Kruyt FA: Bortezomib, but not cisplatin, induces
mitochondria-dependent apoptosis accompanied by up-regulation of
noxa in the non-small cell lung cancer cell line NCI-H460. Mol
Cancer Ther. 6:1046–1053. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akiyama T, Dass CR and Choong PF:
Bim-targeted cancer therapy: A link between drug action and
underlying molecular changes. Mol Cancer Ther. 8:3173–3180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wirth M, Stojanovic N, Christian J, Paul
MC, Stauber RH, Schmid RM, Häcker G, Krämer OH, Saur D and
Schneider G: MYC and EGR1 synergize to trigger tumor cell death by
controlling NOXA and BIM transcription upon treatment with the
proteasome inhibitor bortezomib. Nucleic Acids Res. 42:10433–10447.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen S, Zhang Y, Zhou L, Leng Y, Lin H,
Kmieciak M, Pei XY, Jones R, Orlowski RZ, Dai Y and Grant S: A
Bim-targeting strategy overcomes adaptive bortezomib resistance in
multiple myeloma through a novel link between autophagy and
apoptosis. Blood. 124:2687–2697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pigneux A, Mahon FX, Moreau-Gaudry F,
Uhalde M, de Verneuil H, Lacombe F, Reiffers J, Milpied N, Praloran
V and Belloc F: Proteasome inhibition specifically sensitizes
leukemic cells to anthracyclin-induced apoptosis through the
accumulation of Bim and Bax pro-apoptotic proteins. Cancer Biol
Ther. 6:603–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Premkumar DR, Jane EP, DiDomenico JD,
Vukmer NA, Agostino NR and Pollack IF: ABT-737 synergizes with
bortezomib to induce apoptosis, mediated by Bid cleavage, Bax
activation, and mitochondrial dysfunction in an Akt-dependent
context in malignant human glioma cell lines. J Pharmacol Exp Ther.
341:859–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Unterkircher T, Cristofanon S, Vellanki
SH, Nonnenmacher L, Karpel-Massler G, Wirtz CR, Debatin KM and
Fulda S: Bortezomib primes glioblastoma, including glioblastoma
stem cells, for TRAIL by increasing tBid stability and
mitochondrial apoptosis. Clin Cancer Res. 17:4019–4030. 2011.
View Article : Google Scholar : PubMed/NCBI
|