Introduction
G-protein-coupled receptors (GPCRs) constitute one
of the largest receptor superfamilies. By binding to different
ligands, GPCRs activate different G subunits, which subsequently
induce different intracellular signaling pathways and lead to a
number of diverse biological effects (1,2). In
general, GPCRs contain an extracellular amino terminus, a
seven-transmembrane helix, and an intracellular C-terminus
(3). The extracellular and
transmembrane regions are primarily involved in ligand recognition,
while the cytoplasmic region participates in the interaction with
G-proteins or additional proteins (4). The C-termini of GPCRs differ in terms
of their length and structure, implying their functional importance
and complexity (5). The C-terminus
is indispensable for the functions of GPCRs, including cell surface
localization (6), G-protein
coupling (7), agonist-driven
internalization (8) and signal
transduction (9). Therefore,
mutations in the C-termini of GPCRs have been associated with a
variety of dysfunctions and disease states (10,11).
The human orexin 2 receptor (OX2R), also known as
the hypocretin receptor, is a GPCR and exhibits an equal binding
affinity for its two ligands, orexin-A and orexin-B (12). OX2R is involved in a number of
diverse physiological actions, including control of feeding and
energy homeostasis (13), control
of the sleep-wake cycle (14),
regulation of cardiovascular functions (15), and the neuroendocrine system
(16). Thus, OX2R is implicated as
a potential therapeutic target in various disorders associated with
OX2R mutations. The mRNA sequence of OX2R contains 1,843 bases that
are translated into 444 amino acids (aa), and its C-terminus
contains 78 residues. OX2R may interact with multiple G-proteins
(Gs, Gq and Gi) and stimulate
cyclic adenosine monophosphate (cAMP) and inositol trisphosphate
production (17). Despite its
functional implications, no comprehensive studies have been
conducted to date to determine the role of the C-terminus in
governing the surface expression and signaling of OX2R.
Basic experiments to identify functional domains in
the C-termini of GPCRs usually focus on truncations of the
C-terminus. Therefore, in the present study, 3 C-terminal
truncation mutants of OX2R were employed to determine which
segments are involved in its cell surface expression and signal
transduction. The three mutants were truncated at the 368 (HA-M1),
the 384 (HA-M2), and the 414 aa positions (HA-M3), respectively
(Fig. 1A). The effects of these
mutations on the normal function of OX2R were subsequently
evaluated. The results may enhance the current understanding of the
OX2R complex, and provide a theoretical basis for elucidating the
molecular mechanisms underlying OX2R-associated diseases.
Materials and methods
Materials
Orexin-A and orexin-B were purchased from Phoenix
Pharmaceuticals (St. Joseph, MO, USA). Lipofectamine 2000 and
geneticin (G418) were obtained from Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Forskolin and
3-isobutyl-1-methylxanthine (IBMX) were obtained from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). Primary
antibodies against hemagglutinin (HA; cat no. 3724), phosphorylated
(p)-extracellular signal-regulated kinase 1/2 (ERK1/2; cat no.
9101), and ERK1/2 (cat no. 4696) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Anti-β-actin primary antibody
(cat no. TA-09), polyclonal goat anti-rabbit (cat no. ZB-2301) or
anti-mouse secondary antibodies (cat no. ZB-2305) were obtained
from ZSGB-BIO (Beijing, China).
Generation of expression
constructs
The following 3 truncated OX2R C-terminal region
constructs were generated: M1, terminating at Ser368 to remove the
intracellular C-terminal tail; M2, terminating at Leu384 to leave a
truncated intracellular C-terminal tail; M3, terminating at Phe414
to leave an intracellular C-terminal tail. The oligonucleotides
5′-CCCAAGCTTATGTCCGGCACCAAATTGGAGG-3′ and
5′-CGCGGATCCCTATTTTCCACTGAGAAAATTATAAAT-3′ introduced a stop codon
following serine 368. The oligonucleotide
5′-CGCGGATCCCTATCCAAGGCAACAGCAAGAAAACGC-3′ introduced a stop codon
following Leu384. The oligonucleotide
5′-CGCGGATCCCTAATCAAAGTTGCTGATTTGAGTGGT-3′ introduced a stop codon
following Phe414. The full-length human OX2R gene was cloned in the
pcDNA3.1 vector and was used as a template. The amplified gene
fragments were cloned into the HindIII and BamHI restriction sites
of the pcDNA3.1 (+) vector. All constructs were confirmed by DNA
sequencing using the DNA Analyzer ABI 3730 xl (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following primer
sequences were used: T7 promoter, 5′-TAATACGACTCACTATAGGG-3′; and
bovine growth hormone reverse primer, 5′-TAGAAGGCACAGTCGAGG-3′.
To generate HA-tagged OX2R fragments, the sequence
encoding the HA epitope tag (YPYDVPDYA) was inserted into the
pcDNA3.1-OX2R and the 3 truncated C-terminal OX2R constructs at the
N-terminus by polymerase chain reaction. The resulting constructs
(HA-OX2R, HA-M1, HA-M2, and HA-M3) were confirmed by sequencing by
Sangon Biotech Co., Ltd. (Shanghai, China).
Cell culture and transfection
HEK293 cells were cultured in minimum essential
medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal
calf serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 5%
CO2 incubator. To generate cell lines stably expressing
HA-OX2R, HA-M1, HA-M2, and HA-M3, HEK293 cells were transfected
with the plasmid constructs using Lipofectamine® 2000
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h following transfection, the culture
media was removed and replaced with media containing G418 (0.5
mg/ml). Individual clones that survived 2 weeks were cultured in
positive selection medium for 8 weeks. Expression of HA-OX2R,
HA-M1, HA-M2, and HA-M3 was assessed by western blotting using an
anti-HA antibody (dilution, 1:1,000), as described below. For some
experiments, transient transfection was performed (as described
below).
Enzyme-linked immunosorbent assay
(ELISA) to assess total and surface expression of mutants
Cells stably expressing HA-OX2R, HA-M1, HA-M2, and
HA-M3 were plated into 96-well plates at a density of
104 cells/well and incubated for 24 h. Cells were then
fixed in 4% paraformaldehyde prepared in phosphate-buffered saline
(PBS) at 37°C for 1 h, with or without 0.25% Triton X-100 for total
HA or surface HA expression analysis, respectively. Cells were
subsequently washed three times with PBS, and nonspecific binding
sites were blocked with blocking buffer (3% dry milk) at room
temperature for 1 h. Cells were incubated with a primary polyclonal
anti-HA antibody (dilution, 1:500) overnight at 4°C, and then
washed three times with PBS. The following day, cells were
incubated with a horseradish peroxidase-conjugated goat anti-rabbit
immuniglobulin (Ig) G secondary antibody (dilution, 1:2,000) for 1
h at 37°C, and then washed three times with PBS.
3,3′,5,5′-tetramethylbenzidine substrate (200 µl; Sigma-Aldrich;
Merck Millipore) was added and cells were incubated for 30 min at
37°C. The enzymatic reaction was stopped with 2N
H2SO4 solution. Finally, each well was
measured using an iMark™ Microplate Absorbance Reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 450
nm.
cAMP production assay
Stably transfected cells were cultured in 12-well
plates at a density of 5×105 cells/well and preincubated
in stimulation buffer consisting of Dulbecco's modified Eagle's
medium with 500 µM IBMX and 10 mM MgCl2, at 37°C for 20
min. Cells were then stimulated with buffer containing either
orexin-A (0.1–1,000 nM), orexin-B (0.1–1,000 nM) for 10 min at
37°C. cAMP levels were determined using a Cyclic AMP assay kit
(Cell Signaling Technology, Inc.) according to the manufacturer's
instructions. Optical density at 450 nm was measured using a
microplate reader.
Inositol phosphate (IP) accumulation
assay
Stably transfected cells were plated in 24-well
plates at a density of 2×105 cells/well. The following
day, cells were treated with orexin-A (100 nM) or orexin-B (100 nM)
prepared in stimulation buffer containing 10 mM HEPES, 1 mM
CaCl2, 4.2 mM KCl, 0.5 mM MgCl2, 146 mM NaCl,
50 mM LiCl and 5.5 mM glucose (pH 7.4), for 1 h at 37°C. Cells were
then lysed with 2.5% lysis reagent (Beyotime Institute of
Biotechnology, Shanghai, China) in 5% CO2 at 37°C for 30
min, according to the manufacturer's protocol. IP levels were
measured using a Human Inositol Triphosphate ELISA kit (cat no.
MBS024296; MyBioSource, San Diego, CA, USA), according to the
manufacturer's protocol. The optical density at 490 nm was measured
using a microplate reader.
Calcium release
Calcium signals in cells were detected using the
Fluo-4 NW Calcium assay kit (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Stably
transfected cells at a density of 104 cells/well were
cultured in a black poly-D-lysine-coated 96-well plate for 24 h.
The following day, cells were stimulated for 5 min with 100 nM
orexin-A or orexin-B, washed twice with assay buffer, and 100 µl
loading dye solution was added to each well. Cells were
subsequently incubated at 37°C for 30 min, and then at room
temperature for an additional 30 min. The plates were washed three
times with assay buffer. Fluorescence was measured using a TriStar
LB 941 dual luciferase reporter-ready microplate reader (Berthold
Technologies GmbH, Bad Wildbad, Germany), at an excitation
wavelength of 485 nm and emission wavelength of 525 nm.
Internalization of OX2R and truncation
mutants
HEK293 cells were plated at a density of
2×104 cells in 96-well plates. Cells transiently
transfected with HA-OX2R, HA-M1, HA-M2, or HA-M3 was co-transfected
with pcDNA3.1-β-arrestin1 or pcDNA3.1-β-arrestin2 using
Lipofectamine 2000 according to the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific, Inc.).
pcDNA3.1-β-arrestin1/β-arrestin2 recombinant plasmids were kindly
provided by Professor Karin Eidne (QEII Medical Centre, Nedlands,
Australia). Following further incubation with 100 nM orexin-A or
orexin-B for 10, 30 and 60 min, cells were fixed for 30 min at 37°C
using 4% paraformaldehyde prepared in PBS. Cells were then washed
with PBS and blocked at room temperature for 1 h using 3% dry milk.
Cells were subsequently incubated with a primary polyclonal anti-HA
antibody (dilution, 1:500) overnight at 4°C. The following day,
cells were washed three times with PBS, and incubated with a
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (dilution, 1:2,000) for 1 h at room temperaure. Cells were
then washed and incubated with the 3,3′,5,5′-tetramethylbenzidine
substrate (Sigma-Aldrich; Merck Millipore) for 30 min. Finally, the
optical density was measured at a wavelength of 450 nm using a
microplate reader.
Western blotting
Stably transfected cells at a density of
1×105 cells/well were cultured in 6-well plates until
they reached 90% confluence. Following serum starvation for 3 h,
cells were stimulated with 100 nM orexin-A or 100 nM orexin-B for
10 min, immediately washed with ice-cold PBS, and lysed in
radioimmunoprecipitation assay lysis buffer for 30 min at 4°C. The
supernatants were collected by centrifugation at 13,400 × g at 4°C
for 30 min, and the protein concentration was determined using a
bicinchoninic assay kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). Equal amounts of extracted protein samples (50 mg) were
separated by 10% SDS-PAGE and transferred onto a polyvinylidene
difluoride membrane. The membrane was blocked with 5% skimmed milk
at room temperature for 1 h and incubated with a primary
anti-p-ERK1/2 (Thr202/Tyr204) antibody (Cell Signaling Technology,
Inc.; dilution, 1:1,000) at 4°C overnight. The following day, the
membrane was washed three times with TBS containing 0.1% Tween-20
(TBST), and then incubated with a horseradish peroxidase-conjugated
anti-rabbit secondary antibody (dilution, 1:2,000) for 1 h at room
temperature. The membrane was subsequently washed three times in
TBST and signals were detected by enhanced chemiluminescence (ECL)
using an ECL detection reagent (Beyotime Institute of
Biotechnology). As a loading control, the membranes were stripped
of the anti-p-ERK1/2 antibody using stripping buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China;
SW3020), and labeled with an anti-total (t)ERK1/2 antibody (Cell
Signaling Technology, Inc.; dilution, 1:1,000) at 4°C overnight.
Finally, the gray value of bands was calculated using ImageJ
software version 2.1.4.6 (National Institutes of Health, Bethesda,
MD, USA). The results are expressed as the p-ERK/t-ERK ratio.
β-actin was used as the loading control.
Statistical analysis
Quantitative data are presented as the mean ±
standard error of the mean. GraphPad Prism software (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze the
results with multiple group comparisons. One-way analysis of
variance followed by a Tukey's post hoc test was used for multiple
group comparisons, and P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times, and representative experiments are shown.
Results
The C-terminus of OX2R is important
for its cell surface expression
The C-terminus has been repeatedly demonstrated to
be an indispensable region of GPCRs that govern their cell surface
expression (6). In the present
study, the effect of C-terminal truncation on the surface
expression of OX2R was assessed using an ELISA. The cell surface
expression of the three truncated receptors was significantly lower
when compared with that of the wild-type receptor (Fig. 1B). The HA-M3 mutant, containing a
30-aa deletion at position 415–444 of the C-terminus, exhibited an
18.66% reduction in cell surface expression (Fig. 1B). Cell surface expression of the
HA-M2 mutant, containing a 60-aa deletion at position 385–444, and
the HA-M1 mutant, containing a 76-aa deletion at position 369–444,
were decreased by 29.51% and 28.73%, respectively (Fig. 1B). Expression of HA-M2 was lower
than that of HA-M3, implying that the 60 residues at position
385–444 demonstrate a remarkable cumulative effect on the surface
expression of the OX2R receptor. No significant difference in
expression was observed between HA-M2 and HA-M1 mutants, which
indicates that the aa residues at position 369–384 demonstrate no
obvious effect on OX2R surface expression.
C-terminus of OX2R significantly
affects downstream effectors
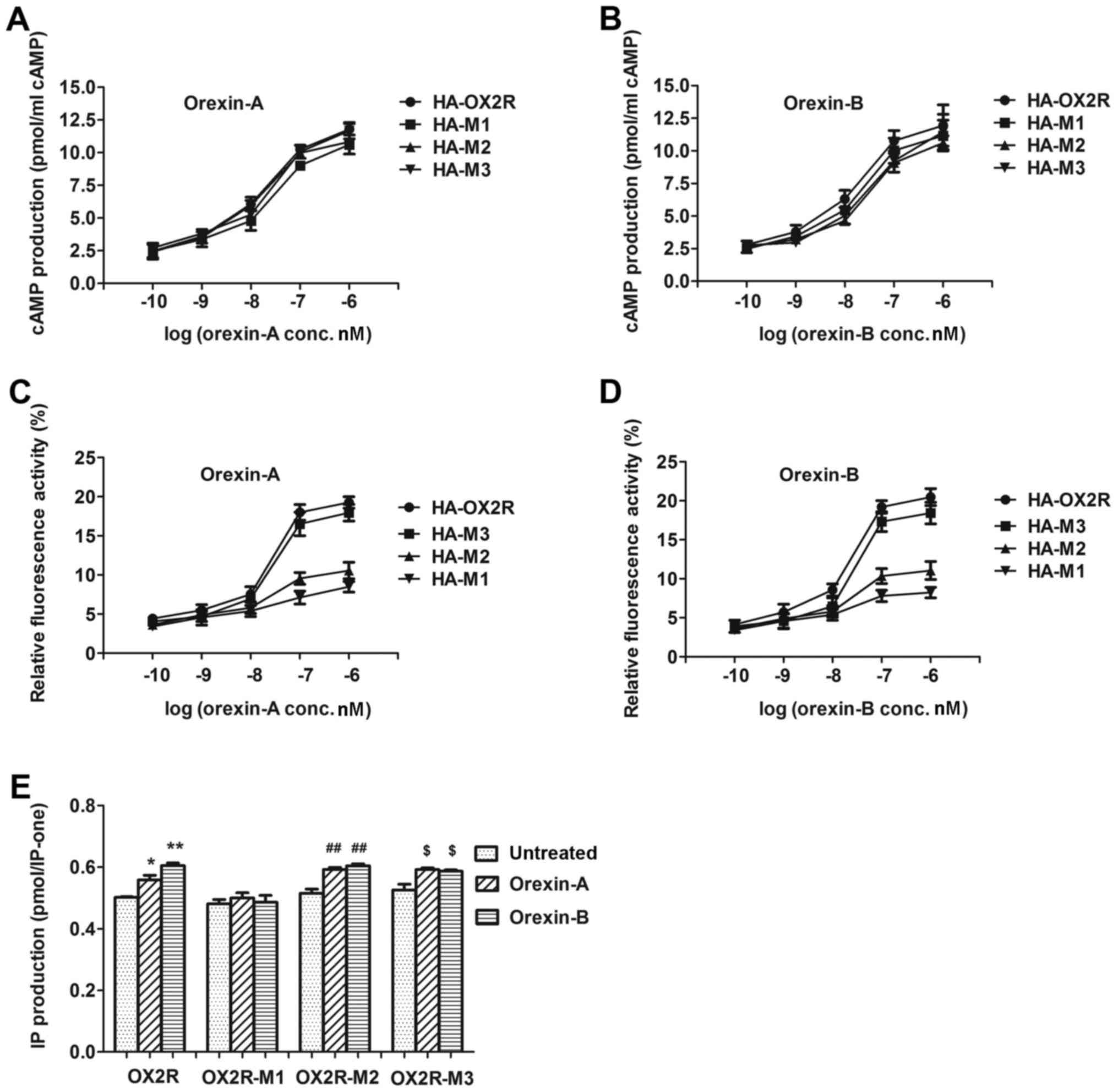
The production of cAMP was detected following
stimulation of cells stably expressing OX2R and the three truncated
receptors with various concentrations of orexin-A (0.1–1,000 nM) or
orexin-B (0.1–1,000 nM). cAMP production was not significantly
altered among orexin-A or orexin-B-treated cells transfected with
the truncated receptors and cells transfected with wild-type OX2R.
The half maximal effective concentration (EC50) values
for orexin A-treated cells were as follows: HA-M1, 294.1 nM; HA-M2,
142.9 nM; HA-M3, 281.6 nM (Fig.
2A). The EC50 values for orexin-B-treated cells were
as follows: HA-M1, 202.2 nM; HA-M2, 302.5 nM; and HA-M3, 352.2 nM
(Fig. 2B). These results indicated
that the 76-aa in the 369–444 C-terminal region demonstrated no
significant influence on cAMP production.
Following stimulation with orexin-A and orexin-B,
Ca2+ release was not significantly altered among cells
expressing HA-M3 and those expressing full-length HA-OX2R, which
demonstrated that the 30 aa sequence at position 415–444 was not
responsible for affecting Ca2+ release (Fig. 2C and D). Nevertheless,
Ca2+ release was markedly lower in cells expressing
HA-M1 and HA-M2 when compared with cells expressing HA-OX2R. The
EC50 values for orexin-A-treated cells were as follows:
HA-M1, 255.2 nM; HA-M2, 250.5 nM; HA-M3, 123.9 nM (Fig. 2C). The EC50 values for
orexin-B-treated cells were as follows: HA-M1, 253.9 nM; HA-M2,
233.5 nM; 147.1 nM for HA-M3 (Fig.
2D). These results indicated that the 30-aa sequence at
position 385–414, which was deleted in HA-M2, and the 16-aa
sequence at position 369–384, which was deleted in HA-M1, (residues
369–414) significantly influenced Ca2+ release.
To assess the effect of OX2R C-terminal truncation
on Gq subunit protein coupling, the abilities of OX2R
and the three truncated receptors in stimulating IP production were
examined. Following stimulation with 100 nM orexin-A or 100 nM
orexin-B, IP production was significantly higher in cells
expressing HA-M3 and HA-M2 when compared with their respective
non-simulated controls (Fig. 2E).
This indicated that the 30-aa sequence at position 415–444, which
was deleted in HA-M3, and the 30-aa sequence at position 385–414,
which was deleted in HA-M2, significantly influenced IP production.
By contrast, IP production in cells expressing HA-M1 was not
significantly altered between orexin-A or orexin-B-simulated and
non-simulated cells (Fig. 2E),
indicating that the 16-aa sequence at position 369–384, did not
influence IP production.
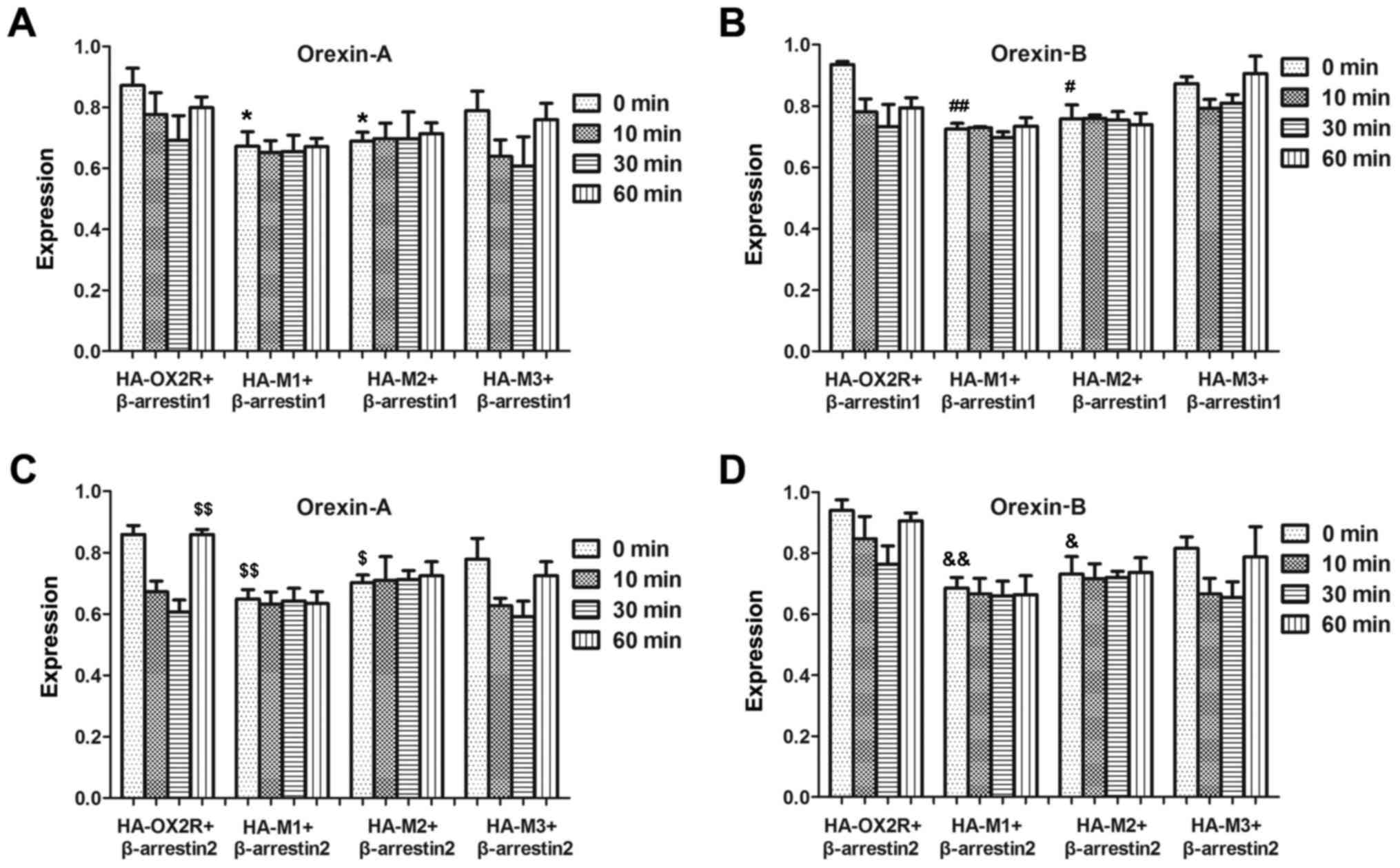
C-terminus of OX2R is important for
receptor internalization
β-arrestin family members mediate desensitization of
many GPCRs by uncoupling the stimulated receptors from their
cognate G-proteins (18,19). To assess whether OX2R and the
C-terminal truncation mutants differ in their ability to recruit
arrestin proteins, their physical associations with β-arrestin1 and
β-arrestin2 were tested using an ELISA. Following co-transfection
with β-arrestin1, internalization of HA-M3 after stimulation with
orexin-A or orexin-B, was not significantly affected at all time
points examined (Fig. 3A and B),
which indicated that the 30-aa sequence at position 415–444 was not
important for receptor internalization. However, internalization of
HA-M2 following stimulation with orexin-A or orexin-B following
transfection with β-arrestin1, which lacked an additional 30-aa
sequence at the 385–414 position, was significantly affected
between 0 and 60 min compared with the full-length OX2R (Fig. 3). This suggests that the 30-aa
sequence may be important for receptor internalization.
Internalization of HA-M1 and HA-M2 in β-arrestin2-transfected cells
following treatment with orexin-A or -B were not significantly
different with each other, which suggests that residues 369–384 of
the OX2R C-terminal domain may not be important for receptor
internalization (Fig. 3).
Similarly, this effect was observed following co-transfection with
β-arrestin2 and stimulation with orexin-A (Fig. 3C) or orexin-B (Fig. 3D). Therefore, the results suggest
that the 385–414 region is the major site for physical association
with arrestin.
C-terminus of OX2R affects ERK1/2
phosphorylation
In order to determine the effect of the C-terminal
region of OX2R on ERK1/2 phosphorylation, the protein expression
levels of p-ERK were determined following transfection of cells
with the three C-terminal truncation mutations and a full-length
OX2R C-terminal sequence. Stably transfected cells were treated
with 100 nM orexin-A or orexin-B for 10 min and the level of ERK1/2
phosphorylation was detected. As shown in Fig. 4, ERK1/2 phosphorylation was
significantly lower in orexin-A and orexin-B-treated cells stably
transfected with HA-M1 when compared with the full-length sequence.
By contrast, ERK1/2 phosphorylation was not significantly different
in cells stably transfected with HA-M2 and HA-M3 when compared with
those transfected with the full-length sequence (Fig. 4). The results indicate that the
16-aa sequence at position 369–384 in OX2R may influence ERK1/2
phosphorylation and potentially the downstream signaling
pathway.
 | Figure 4.Expression of p-ERK1/2 in cells
expressing OX2R and 3 truncation mutants. (A) Western blot analysis
of p-ERK1/2 expression and (B) quantification of band intensities
in HA-OX2R, HA-M1, HA-M2 and HA-M3-transfected cells following
stimulation with 100 nM orexin-A for 10 min. (C) Western blot
analysis of p-ERK1/2 expression and (D) quantification of band
intensities in HA-OX2R, HA-M1, HA-M2 and HA-M3-transfected cells
following stimulation with 100 nM orexin-B. ERK1/2 phosphorylation
was significantly lower in cells expressing HA-M1 when compared
with HA-OX2R-transfected cells. However, no significant difference
in ERK1/2 phosphorylation was observed between cells expressing
HA-M2, HA-M3 and HA-OX2R. **P<0.01 vs. HA-OX2R. ERK1/2,
extracellular signal-regulated kinase 1/2; p-ERK, phosphorylated
ERK; t-ERK, total-ERK; OX2R, orexin 2 receptor; HA,
hemagglutinin. |
Discussion
The C-terminus is known to be involved in mediating
the cell surface expression of GPCRs. For instance, serial
truncation of the C-C chemokine receptor type 5 may result in
progressive loss of its cell surface expression (20). Tetsuka et al (21) demonstrated that C-terminal mutants
of melanin-concentrating hormone receptor 1 (MCH1R) exhibit
progressively reduced expression levels when compared with the
wild-type protein. When progressive truncations were introduced at
the C-terminus of the gonadotropin-releasing hormone receptor
(GnRH-R), the stop331 and stop337 mutants exhibited 40% cell
surface expression when compared with wild-type GnRH-R (22). Alanine residues at the C-terminus
are necessary for the cell surface expression of the glucagon-like
peptide-2 receptor (23). In order
to investigate the effect of the C-terminus on the cell surface
expression of OX2R in the present study, three truncation mutants
of OX2R were constructed. The three mutant receptors lacking aa
sequences at the C-terminus exhibited significantly reduced
expression at the cell surface. Cell surface expression was not
significantly altered between HA-M2 and HA-M1 mutants, which
indicated that the aa sequence at position 369–384 demonstrated no
obvious effect on the surface expression of this receptor.
Therefore, aa sequences in the 385–444 region of the OX2R
C-terminus are a key determinant for the localization or stability
of the receptor at the cell surface. This domain must therefore
contain sites essential for cell surface expression, and future
studies will focus on identifying and mutating these sites in order
to confirm their functional role.
Following activation of a GPCR, several dispersed
loci facilitate internalization. Phosphorylation of aa sequences at
the C-terminus is required for interaction with β-arrestin, leading
to receptor internalization. The precise molecular nature of GPCR
internalization is complicated and involves numerous protein
partners (24,25). Using a series of truncation and
deletion mutants, Chaki et al (26) demonstrated that the 310–327 region
is required for internalization of the rat angiotensin II type 1A
receptor. The non-mammalian GnRH-R shows rapid desensitization
(22) and agonist-induced
internalization (27) when aa
sequences are phosphorylated in its C-terminal tail. The C-terminus
of the human prostaglandin E2 receptor subtype EP4 contains 38
Ser/Thr phosphorylation sites. These residues are necessary for
β-arrestin1 recruitment and agonist-induced internalization
(28). The results of the present
study demonstrated that the aa sequences at position 385–414 of the
OX2R C-terminal domain may be the major site for agonist-induced
internalization. This domain contains ten potential Ser/Thr
phosphorylation sites important for coupling to β-arrestin, leading
to internalization (29,30). The results of the current study are
consistent with those presented by Golan et al (25), which demonstrated that putative
Ser/Thr clusters in the C-terminus of OX2R are involved in
receptor-β-arrestin-ubiquitin complex formation.
The signal transduction of GPCRs is primarily
mediated by the third intracellular loop and the C-terminal
sequence (31). Over the past few
years, a number of studies have increasingly highlighted the
importance of the C-terminal sequence for intracellular signal
transduction. Lehmann et al (19) analyzed two C-terminal mutants of
MCH1R, one of which contained a 36-aa deletion, while the other
possessed a 32-aa deletion. The sequence between Phe318 and Arg321
was observed to be responsible for signal transduction. In
addition, mutation of either Arg319 or Lys320, but not Arg321,
significantly reduced Ca2+ influx (21). For the glucagon-like peptide-2
receptor, the major C-terminal sequence is dispensable for cAMP
accumulation, ERK1/2 activation and endocytosis (23). Tang et al (17) reported that OX2R may activate the
ERK1/2 signaling pathway via Gq, Gs, and
Gi subunits; with Gq being the major
mediator. In the present study, the OX2R truncation mutations
demonstrated no significant effects on cAMP production. This result
is consistent with the finding that the C-terminal tail of one
family B GPCR is not required for coupling to the Gs
subunit (32). However, the OX2R
truncation mutations demonstrated a marked effect on IP production
and Ca2+ release in the current study. This implied that
the C-terminus of OX2R may be indispensable for the Gq
signaling pathway. In addition, residues 369–384 at the C-terminus
influenced the expression of p-ERK1/2. Willars et al
(27) demonstrated that orexin A
(100 nM) increased the labeling of OX2R with Gs and
GI subunits, however the same effect was not observed
for Go and Gq subunits. In addition, the
authors indicated that OX2R binds to Gs and
Gi subunits in the human fetal adrenal cortex, whereas a
shift towards Gq and less towards Gi occurs
in the adult adrenal gland (28).
Therefore, the authors of the present study hypothesized that the
coupling of orexin receptors to multiple G proteins may be diverse
in different cell lines and under different stimulus
conditions.
The present study demonstrated that aa residues in
the C-terminus serve an important role in the expression and signal
transduction of OX2R. In addition, the aa residues in different
locations possess evidentially different roles. The aa residues in
the C-terminus of OX2R were analyzed using bioinformatics. A series
of conserved residues were identified, which reportedly serve an
indispensable role in receptor expression and the downstream
signaling pathways (unpublished data). In future studies,
C-terminal mutants will be constructed whereby one or several of
the conserved residues are mutated, in order to determine the
effects of these mutations on the expression and signal
transduction of the OX2R receptor.
Acknowledgements
The present work was supported by grants from the
National Nature Science Foundation of China (grant nos. 81501018
and 31271243) and the Shandong Province Natural Science Foundation
(grant no. ZR2013CQ031) and by a starting grant for Doctor of
Jining Medical University.
Glossary
Abbreviations
Abbreviations:
|
aa
|
amino acids
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
|
GnRH-R
|
gonadotropin-releasing hormone
receptor
|
|
GPCR
|
G-protein-coupled receptor
|
|
HA
|
hemagglutinin
|
|
IBMX
|
3-isobutyl-1-methylxanthine
|
|
IP
|
inositol phosphate
|
|
OX2R
|
orexin 2 receptor
|
References
|
1
|
Bockaert J and Pin JP: Molecular tinkering
of G protein-coupled receptors: An evolutionary success. Embo J.
18:1723–1729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Croft W, Hill C, McCann E, Bond M,
Esparza-Franco M, Bennett J, Rand D, Davey J and Ladds G: A
physiologically required G protein-coupled receptor
(GPCR)-regulator of G protein signaling (RGS) interaction that
compartmentalizes RGS activity. J Biol Chem. 288:27327–27342. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu ZL, Saldanha JW and Hulme EC:
Seven-transmembrane receptors: Crystals clarify. Trends Pharmacol
Sci. 23:140–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wistrand M, Käll L and Sonnhammer EL: A
general model of G protein-coupled receptor sequences and its
application to detect remote homologs. Protein Sci. 15:509–521.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fortin JP, Zhu Y, Choi C, Beinborn M,
Nitabach MN and Kopin AS: Membrane-tethered ligands are effective
probes for exploring class B1 G protein-coupled receptor function.
Proc Natl Acad Sci USA. 106:8049–8054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson A and Kanamarlapudi V: Distinct
regions in the C-Terminus required for GLP-1R cell surface
expression, activity and internalisation. Mol Cell Endocrinol.
413:66–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng Y, Liu T, Li XQ, Liu Y, Zhu XY,
Jankovic J, Pan TH and Wu YC: Neuroprotection by Orexin-A via
HIF-1α induction in a cellular model of Parkinson's disease.
Neurosci Lett. 579:35–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lowther KM, Uliasz TF, Götz KR, Nikolaev
VO and Mehlmann LM: Regulation of constitutive GPR3 signaling and
surface localization by GRK2 and β-arrestin-2 Overexpression in
HEK293 cells. PLoS One. 8:e653652013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsushima Y, Sato T, Yamada C, Ito M,
Suzuki Y, Ogawa E, Sukegawa I, Sukegawa J, Fukunaga K and
Yanagisawa T: Interaction of PICK1 with C-terminus of growth
hormone- releasing hormone receptor (GHRHR) modulates trafficking
and signal transduction of human GHRHR. J Pharmacol Sci.
122:193–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shim JY, Ahn KH and Kendall DA: Molecular
basis of cannabinoid CB1 receptor coupling to the G protein
heterotrimer Gαiβγ: identification of key CB1 contacts with the
C-terminal helix α5 of Gαi. J Biol Chem. 288:32449–32465. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gandía J, Fernández-Dueñas V, Morató X,
Caltabiano G, González-Muñiz R, Pardo L, Stagljar I and Ciruela F:
The Parkinson's disease-associated GPR37 receptor-mediated
cytotoxicity is controlled by its intracellular cysteine-rich
domain. J Neurochem. 125:362–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ammoun S, Holmqvist T, Shariatmadari R,
Oonk HB, Detheux M, Parmentier M, Akerman KE and Kukkonen JP:
Distinct recognition of OX1 and OX2 receptors by orexin peptides. J
Pharmacol Exp Ther. 305:507–514. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baccari MC: Orexins and gastrointestinal
functions. Curr Protein Pept Sci. 11:148–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mavanji V, Perez-Leighton CE, Kotz CM,
Kotz CM, Billington CJ, Parthasarathy S, Sinton CM and Teske JA:
Promotion of wakefulness and energy expenditure by Orexin-A in the
ventrolateral preoptic area. Sleep. 38:1361–1370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kannan H, Shirasaka T, Watanabe S, Yu NS,
Kuitake T and Takasaki M: Central action of orexins on sympathetic
outflow and cardiovascular function with a focus on the
paraventricular nucleus of the hypothalamus. Masui. 56:30–39.
2007.(In Japanese). PubMed/NCBI
|
|
16
|
Kaminski T and Smolinska N: Expression of
orexin receptors in the pituitary. Vitam Horm. 89:61–73. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang J, Chen J, Ramanjaneya M, Punn A,
Conner AC and Randeva HS: The signalling profile of recombinant
human orexin-2 receptor. Cell Signal. 20:1651–1661. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kara E, Crépieux P, Gauthier C, Martinat
N, Piketty V, Guillou F and Reiter E: A phosphorylation cluster of
five serine and threonine residues in the C-terminus of the
follicle-stimulating hormone receptor is important for
desensitization but not for beta-arrestin-mediated ERK activation.
Mol Endocrinol. 20:3014–3026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lehmann A, Kliewer A, Schutz D, Nagel F,
Stumm R and Schulz S: Carboxyl-terminal multi-site phosphorylation
regulates internalization and desensitization of the human sst2
somatostatin receptor. Mol Cell Endocrinol. 387:44–51. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Venkatesan S, Petrovic A, Locati M, Kim
YO, Weissman D and Murphy PM: A membrane-proximal basic domain and
cysteine cluster in the C-terminal tail of CCR5 constitute a
bipartite motif critical for cell surface expression. J Biol Chem.
276:40133–40145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tetsuka M, Saito Y, Imai K, Doi H and
Maruyama K: The basic residues in the membrane-proximal C-terminal
tail of the rat melanin-concentrating hormone receptor 1 are
required for receptor function. Endocrinology. 145:3712–3723. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blomenröhr M, Heding A, Sellar R, Leurs R,
Bogerd J, Eidne KA and Willars GB: Pivotal role for the cytoplasmic
carboxyl-terminal tail of a nonmammalian gonadotropin-releasing
hormone receptor in cell surface expression, ligand binding, and
receptor phosphorylation and internalization. Mol Pharmacol.
56:1229–1237. 1999.PubMed/NCBI
|
|
23
|
Estall JL, Koehler JA, Yusta B and Drucker
DJ: The glucagon-like peptide-2 receptor C terminus modulates
beta-arrestin-2 association but is dispensable for ligand-induced
desensitization, endocytosis, and G-protein-dependent effector
activation. J Biol Chem. 280:22124–22134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Innamorati G, Giannone F, Guzzi F, Rovati
GE, Accomazzo MR, Chini B, Bianchi E, Schiaffino MV, Tridente G and
Parenti M: Heterotrimeric G proteins demonstrate differential
sensitivity to beta-arrestin dependent desensitization. Cell
Signal. 21:1135–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Golan M, Schreiber G and Avissar S:
Antidepressants, beta-arrestins and GRKs: From regulation of signal
desensitization to intracellular multifunctional adaptor functions.
Curr Pharm Des. 15:1699–1708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaki S, Guo DF, Yamano Y, Ohyama K, Tani
M, Mizukoshi M, Shirai H and Inagami T: Role of carboxyl tail of
the rat angiotensin II type 1A receptor in agonist-induced
internalization of the receptor. Kidney Int. 46:1492–1495. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Willars GB, Heding A, Vrecl M, Sellar R,
Blomenröhr M, Nahorski SR and Eidne KA: Lack of a C-terminal tail
in the mammalian gonadotropin-releasing hormone receptor confers
resistance to agonist-dependent phosphorylation and rapid
desensitization. J Biol Chem. 274:30146–30153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Neuschäfer-Rube F, Hermosilla R, Rehwald
M, Rönnstrand L, Schülein R, Wernstedt C and Püschel GP:
Identification of a Ser/Thr cluster in the C-terminal domain of the
human prostaglandin receptor EP4 that is essential for
agonist-induced beta-arrestin1 recruitment but differs from the
apparent principal phosphorylation site. Biochem J. 379:573–585.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiu Y, Loh HH and Law PY: Phosphorylation
of the delta-opioid receptor regulates its beta-arrestins
selectivity and subsequent receptor internalization and adenylyl
cyclase desensitization. J Biol Chem. 282:22315–22323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Q, Dewi DA, Liu W, Bee MS and
Schonbrunn A: Distinct phosphorylation sites in the SST2A
somatostatin receptor control internalization, desensitization, and
arrestin binding. Mol Pharmacol. 73:292–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Conchon S, Barrault MB, Miserey S, Corvol
P and Clauser E: The C-terminal third intracellular loop of the rat
AT1A angiotensin receptor plays a key role in G protein coupling
specificity and transduction of the mitogenic signal. J Biol Chem.
272:25566–25572. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Conner M, Hicks MR, Dafforn T, Knowles TJ,
Ludwig C, Staddon S, Overduin M, Günther UL, Thome J and Wheatley
M: Functional and biophysical analysis of the C-terminus of the
CGRP-receptor; a family B GPCR. Biochemistry. 47:8434–8444. 2008.
View Article : Google Scholar : PubMed/NCBI
|