Introduction
Wilms' tumor is one of the leading types of cancer
and has become the primary cause of cancer-associated mortality in
pediatric oncology worldwide (1).
Clear cell renal cell carcinoma (CCRCC) is the primary type of
kidney cancer of which 75–85% and 80% of patients are at stage III
and IV at the time of diagnosis, respectively, whereas the mixture
of CCRCC and granular cell carcinoma are the predominant types in
nephroblastoma (2). A previous
study indicated that renal cancer incidence in children has
increased globally in the last 10 years and the mortality rate
remains high (3). In order to
improve the clinical outcome, the underlying molecular mechanisms
of the pathogenesis and progression of nephroblastoma require
elucidation.
MicroRNAs (miRNAs) are a family of ~19–22 bp,
non-coding RNAs that function by regulating gene expression via
binding to target mRNA and inducing mRNA cleavage or translational
inhibition. Previous studies have demonstrated that miRNAs have an
important role in various biological processes, including
development, cell differentiation, proliferation and apoptosis
(4,5). Additionally, abnormal miRNA
expression patterns occur in various types of cancer, although not
all malignancies (4,5). There has been increasing interest in
investigating the importance of miRNA (miR)-590 in cancer
progression. Previous studies reported that miR-590 was upregulated
in hepatocellular carcinoma cells and CCRCC (6,7). A
previous study identified that miR-590 was a novel regulator of
polybromo 1 (PBRM1) in CCRCC (7);
therefore, increases the proliferation and invasion abilities of
epithelial cells. These previous findings may suggest that miR-590
functions as a tumor activator in various types of cancer, although
the contrary results have been obtained in other types of cancer
including lung cancer and T-cell acute lymphoblastic leukaemia
(8,9). Several target genes of miR-590
associated with various types of cancer have been identified:
Programmed cell death 4, transforming growth factor β receptor type
II, and phosphatase and tensin homolog in human hepatocellular
cancer, PBRM1 in renal cancer, and cell adhesion molecule L1-like
(CHL1) in cervical cancer (6–8,10).
However, the effects of miR-590 in CCRCC remain to be
elucidated.
The present study determined that the expression
level of miR-590 was increased in nephroblastoma tissues and the
levels were consistent with the clinical stage of the tissue, which
indicated that miR-590 was involved in the pathogenesis of Wilms'
tumor. miR-590 induced G401 cell proliferation through Wilms' tumor
1 (WT1) as upregulation of miR-590 expression had the same effect
as siRNA-WT1.
Materials and methods
Clinical samples
Clinical Wilms tumor samples were collected from 65
patients (male:female, 34:31; age, 5 months-7 years old) who
underwent positive debulking surgery in the Pediatric Surgery
Department of the First Affiliated Hospital of Suzhou University
(Suzhou, China) between March 2013 and March 2015. The diagnosed
Wilms' tumor tissues were reviewed by an experienced pathologist,
using histological slides, according to the 2007 guidelines of WHO
classification (11). The present
study contained 25 stage I–II, 20 stage III and 20 stage IV renal
cancer samples. Additionally, normal kidney tissue samples were
obtained from 20 patients (male:female, 12:8; age, 5 months-6.5
years old) at the Emergency Department with Kidney Trauma at the
First Affiliated Hospital of Suzhou University between March 2013
and March 2015. The present study was approved by the Ethics
Committee of the Children's Hospital of Soochow University (Suzhou,
China) and informed consent was obtained from the guardians of all
patients.
Plasmid construction
3′-untranslated region (3′-UTR) of human WT1 was
amplified by polymerase chain reaction (PCR) and the PCR fragment
was inserted into the downstream of luciferase coding sequence of
pcDNA3.1-luciferase reporter plasmid (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) between the restriction site of
BamHI and EcoRI. For 3′-UTR mutation, the predicted
miR-590 binding site was randomly scrambled by PCR-mediated
site-specific mutation. For WT1 overexpression in G401 cells, the
coding DNA sequence (CDS) of WT1 was cloned into pCDNA3.1. The
vectors used in the present study were verified by sequencing, and
the primers were as follows: Wild type 3′-UTR of WT1 forward (F)
5′-CGGGATCCCTGGGAGTGTCCTTAGTGT-3′ and reverse (R)
5′-CGGAATTCAACCCCATTCAACCACAG-3′; mutant 3′-UTR of WT1 F
5′-TGACAACTACCATTAGGACTG-3′ and R 5′-ACAGTCCTAATGGTAGTTGTC-3′; CDS
of WT1 F 5′-CCAAGCTTGGCTTTGCTGCTGAGGAC-3′ R
5′-GCTCTAGAACCTCGGGAATGTTAGAC-3′.
Cell culture
G401 cells were purchased from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). G401 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS) at 37°C, 5% CO2 and 100% humidity.
Cell transfection
G401 cells were seeded at a density of
5×106 cells/well 16 h before transfection using DMEM
medium with 1% penicillin/streptomycin. When cells reached 60%
confluence, they were transfected with miRNA mimic, si-RNA WT1
(cat. no. sc-36846; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) or expression vectors using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Briefly, 10 nM miRNA mimic, 100 nM si-RNA, 800 ng plasmid
and Lipofectamine 2000 were diluted in the serum-free medium
respectively at room temperature for 5 min. After 5 min, the
diluted miRNA mimic, si-RNA or plasmid was mixed with the diluted
Lipofectamine 2000 and incubated for 20 min at room temperature.
The mixture was added to cells uniformly at 30 µl/well. Cells were
cultured at 37°C, 5% CO2 for 6 h, the medium was changed
with fresh DMEM supplemented with 10% FBS and 1%
penicillin/streptomycin.
Total RNA preparation and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA extracted from G401 cells and renal
samples with TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) were synthesized by RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.) using the specific miR-590 RT
primer supplied from Guangzhou RiboBio Co., Ltd. (Guangzhou,
China). The qPCR was performed with 20 ng cDNA using 2X UltraSYBR
Mixture kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. U6 was used as a reference gene determined
at the same time. The miRNA qPCR detection primers purchased from
Guangzhou RiboBio Co., Ltd. were used for qPCR analysis for miR-590
and U6. qPCR was performed in triplicate for each experiment. The
cycling conditions were as follows: Initial denaturation at 95°C
for 1 min, 30 cyles of denaturation at 94°C for 30 sec, annealing
at 58°C for 30 sec and extension at 72°C at 10 sec, followed by
72°C for 2 min and 16°C for 5 min. The reaction mixture contained
2X UltraSYBR Mixture (10 µl), primers (forward/reverse, 0.5 µl/0.5
µl) and ddH2O (9 µl).
Luciferase activity assay
For the luciferase activity assay, G401 cells were
cultured in a 24-well plate at a density of 2.5×105
cells/well 1 day prior to the transfection. When cells reached 60%
confluence 200 ng pcDNA3.1-luciferase,
pcDNA3.1-luciferase-WT1-3′UTR or pcDNA3.1-luciferase-WT1-3′UTR
mutant and miR590 mimic or miRNA control plus 10 ng pRLSV40 was
used for transfection using Lipofectamine 2000 according to the
manufacturer's protocol. The medium was changed after 6 h.
Luciferase activity was quantified 36 h after transfection using
Dual Glow Luciferase Assay system (Promega Corporation, Madison,
WI, USA). Firefly luciferase activity was normalized to Renilla
luciferase activity for each well. Three independent experiments
were performed for each group.
5-ethynyl-20-deoxyuridine (EdU)
assays
Briefly, G401 cells were seeded into a 24-well plate
at a density of 5×106 cells/well 1 day prior to
transfection. After 12 h, G401 cells were transfected with 10 nM
miR-590 mimic, 10 nM miRNA control, 10 nM miR-590 inhibitor, 10 nM
miRNA inhibitor, 10 nM si-RNA-WT1, 10 nM si-RNA-control or 800 ng
pCDNA3.1-WT1. The proliferation of G401 cells was quantified in
vitro 24 h after transfection by EdU DNA Proliferation and
Detection kit (Ribobio Co., Ltd.) following the manufacturer's
protocol.
Western blotting
G401 cells were washed with 800 µl 1X PBS 3 times
for 5 min and lysed with 150 µl radioimmunoprecipitation assay per
well. For kidney samples, tissue was homogenized in lysis buffer
and sonicated for 3 min. The protein concentration of each sample
was determined by bicinchoninic acid assay (Beyotime Institute of
Biotechnology, Haimen, China). Proteins (50 µg/lane) were
segregated using 10% SDS-PAGE gel and immunoblotting was performed
using polyclonal antibodies against WT1 (AB10840; 1:2,500; Abcam,
Cambridge, UK) and β-tubulin (AB6040; 1:5,000; Abcam). The two
antibodies were used at 4°C overnight at 1 mg/ml in PBS with 5%
non-fat milk according to the manufacturer's protocol. Then the
PVDF membrane was probed with horseradish peroxidase
(HRP)-conjugated antibodies (1:4,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Finally, immunoreactivity was detected
using Chemiluminescent HRP Substrate reagent (Merck Millipore,
Darmstadt, Germany) and the signal was analyzed using Bio-Rad
ChemiDoc XRS+ (BioRad Laboratories, Inc., Hercules, CA, USA).
β-tubulin was used as an internal control. Western blot analysis
was performed in triplicate for each group.
Statistical analysis
Data are expressed as the mean ± standard deviation.
A one-way analysis of variance followed by Newman-Keuls comparison
post-hoc test was performed using GraphPad Prism, version 5.0
(GraphPad Software, San Diego, CA, USA). P<0.05 was considered
to indicate a statistically significant difference. All of the
experimental values are the means of triplicate independent
repeats.
Results
miR-590 expression in
nephroblastoma
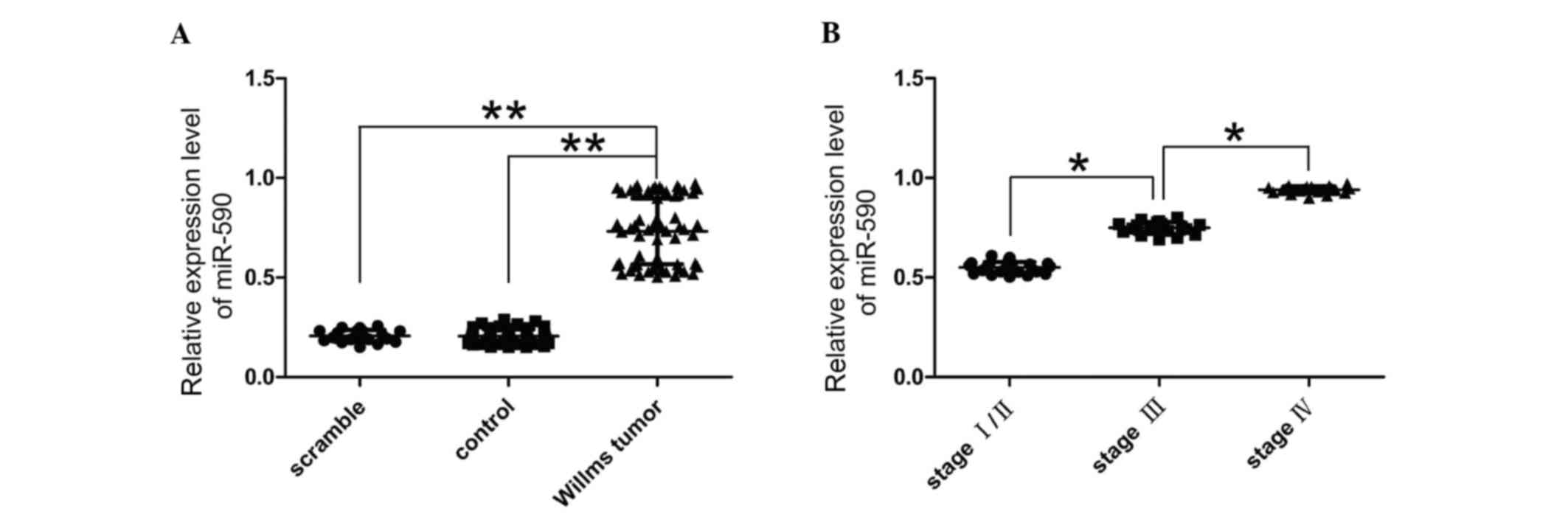
The present study quantified the expression levels
of miR-590 in Wilms' tumor to confirm the involvement of miR-590 in
nephroblastoma using RT-qPCR. miR-590 expression levels were
significantly higher in Wilms' tumor tissues compared with normal
kidney tissue (P<0.05; Fig.
1A). Additionally, the clinical and pathological data of 65
patients with cancer, 25 at stage I/II, 20 at stage III and 20 at
stage IV were also evaluated. The expression level of miR-590 was
quantified in 65 matched tumor samples compared with the adjacent
normal tissues using RT-qPCR. The present study determined that
miR-590 was upregulated in 65 tumor tissues compared with the
paired adjacent normal tissues. miR-590 expression levels were
greater in cancer tissues of patients with stage III or IV tumors
compared with stage I/II (Fig.
1B). Therefore, the present study determined that miR-590
upregulation was associated with the development of cancer.
Prediction of miR-590 binding site in
the 3′-UTR of WT1 mRNA
In order to identify the potential downstream
targets of miR-590, three independent online databases were used,
TargetScan, PicTar and miRBase. Several putative targets were
unanimously forecasted using the aforementioned three databases and
WT1 was selected as the candidate gene as previous studies have
reported its important role in cancer (12–14)
and it carries a putative miR-590 binding site within its 3′-UTR,
which is located at 1155–1162 bp (Fig.
2A).
miR-590 regulates WT1 expression by
binding to its 3′-UTR
In order to verify that miR-590 binds to the
predicted region and the binding leads to translational inhibition,
a luciferase reporter plasmid with either wide-type or mutant
sequence of WT1 mRNA 3′-UTR was cotransfected with miR-590 mimic or
miR-control mimic. Successful overexpression of exogenous miR-590
in G401 cells for 36 h downregulated the activity of the luciferase
reporter, which was inserted with wild-type WT1 3′-UTR, whereas the
activity with mutated WT1 was not affected, indicating that the
mutant target was a potential functional site for miR-590 in WT1
3′-UTR (Fig. 2B and C). In order
to determine whether miR-590 has a functional role in WT1
downregulation, G401 cells were treated with miR-590 mimic and
western blot analysis indicated that miR-590 overexpression led to
a reduction in WT1 protein levels (Fig. 2D). These findings indicated that
miR-590-mediated suppression of WT1 expression via binding to 3-UTR
of WT1.
miR-590 promotes cell
proliferation
As miR-590 expression was upregulated in Wilms'
tumor, which indicated its potential role in the pathogenesis of
Wilms' tumor. The effect of increased miR-590 expression on cell
proliferation was evaluated. In order to investigate this, miR-590
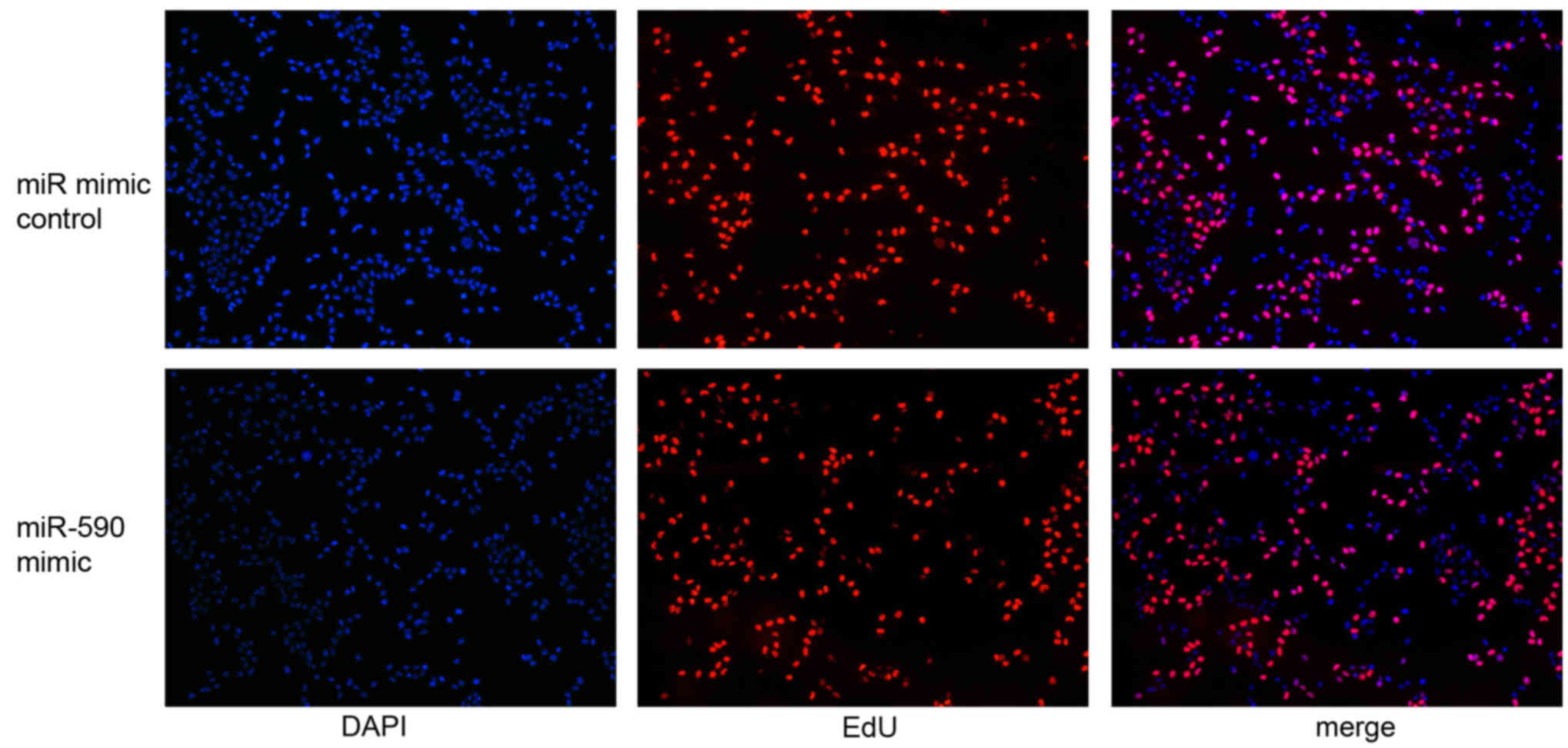
mimics were transfected into the G401 cells and the effect of
miR-590 expression on cell proliferation was analyzed using EdU
assay. miR-590 expression level was markedly upregulated in G401
cells treated with miR-590 mimics compared with control mimics, as
determined by RT-qPCR (P<0.01; 100-fold). Additionally, cells
labeled with EdU were immunostained for EdU (Fig. 3, red), whereas the nuclei were
stained with DAPI (Fig. 3, blue).
The EdU assay data demonstrated that the overexpression of miR-590
promoted cell proliferation 48 h after transfection (P<0.05;
Fig. 3).
WT1 siRNA transfection leads to G401
cell proliferation
The aforementioned results indicated the potential
role of miR-590 in the regulation of G401 cell proliferation and
WT1 gene expression. Therefore, the importance of WT1 as a
functional target of miR-590 was determined in G401 cell
proliferation. To determine the effect of WT1 on cell
proliferation, siRNA was used to downregulate WT1 expression
levels. WT1 si-RNA markedly reduced WT1 protein expression levels
in G401 cells compared with the control siRNA. Following
downregulation of WT1 expression levels, a marked increase in
proliferation occurred 48 h after treatment with siRNA.
Additionally, the statistical analysis indicated that the number of
cells was markedly different between two groups (Fig. 4). These findings suggested that WT1
affected the proliferation in G401 cells.
Antiproliferative effect of WT1 on
G401 cells in vitro
The present study determined that miR-590 expression
was upregulated in Wilms' tumor, was associated with Wilms' tumor
clinical stage, and that miR-590 directly suppressed the expression
of WT1. Therefore, the current study aimed to determine whether an
increase in WT1 expression may provide an explanation for the
observed effects of miR-590 overexpression. WT1 overexpression with
pCDNA3.1-WT1 transfection was performed, followed by EdU assay. The
successful overexpression of WT1 in G401 cells was confirmed by
western blot analysis (Fig. 5).
The effect of WT1 treatment for 48 h on cell proliferation is
presented in Fig. 6. Exposure to
WT1 led to a reduction in cell proliferation compared with the
control group. Therefore, WT1 inhibition may partly elucidate the
proliferative effects of miR-590 on G401 cells and WT1 may be
involved in the miR-590-mediated proliferation in G401 cells.
Discussion
Previous studies have identified that miRNAs are key
in novel pathways of carcinogenesis (4,5,15).
miRNAs function either as tumor suppressors or oncogenes. Aberrant
expression of miR-590 has been identified in various types of human
cancers, including acute myeloid leukemia (16), hepatocellular carcinoma (6), cervical cancer (10) and kidney cancer (7). Additionally, previous studies have
suggested that miR-590 upregulation may be associated with cancer
metastasis (6,7). A previous study demonstrated that the
miR-590 expression level was significantly higher in recurrent
compared with primary tumors in GC cells and tissues (17). Therefore, miR-590 may act as a
tumor promoter in some malignant diseases; however, in order to
elucidate the specific functions, further investigation is
required.
The present study demonstrated that the endogenous
miR-590 expression level was significantly upregulated in Wilms'
tumor tissue compared with normal renal tissue, which was
consistent with a previous study reporting high miR-590 levels in
kidney cancer tissues (7).
Subsequently gain-of-function and loss-of-function approaches were
used by transfection with miR-590 mimics or miR-590 inhibitor in
G401 cells to determine the function of miR-590. Change in the rate
of cell proliferation is one of the key phenotypes observed in
malignant transformation, which may lead to a human cancer
mortality rate as high as 90% (18,19).
The findings of the current study indicated that the overexpression
of miR-590 increased G401 cell proliferation, having a tumor
promoter role in Wilms' tumor development.
Several biological targets of miR-590 have been
identified. For example, miR-590 downregulates PBRM1 expression,
leading to increased cell proliferation and invasion in CCRCC
(7) and promotes cervical cancer
cell growth and invasion by targeting CHL1 (10). The present study demonstrated that
miR-590 inhibited WT1 expression by targeting the 3′-UTR,
indicating that WT1 is a direct target of miR-590. As the Wilms'
tumor transcription factor (WT1) was originally classified as a
tumor suppressor, miR-590 may be a tumor activator, inversely
associated with its specific target gene.
Wilms' tumor has an incidence of ~1 in 10,000 live
births and is one of the most common solid tumors occurring in
childhood in the developed world (20–22).
The mean age of patients when diagnosed is 43–48 months, depending
on gender (women express clearer clinical symptoms thus are likely
to be diagnosed earlier) and 95% of patients are diagnosed by 10
years of age (20,21). Tumors often occur unilaterally in
90–95% of cases (in a single kidney); however, they may also occur
bilaterally. The bilateral patients present clinical symptoms 12
months earlier compared with unilateral patients (22–24).
According to previous studies, ~2% of cases have an affected
relative and Wilms' tumor predisposition segregates in families as
an autosomal dominant trait (23,24).
The WT1 tumor suppressor factors, which were involved in the
development of nephroblastoma, were the first tumor repressor genes
to be cloned (25,26). Observational data from Wilms'
tumors and acute myeloid leukemia combined with improved knowledge
of temporal and spatial expression levels of WT1 in nephrogenesis
and hematopoiesis suggest that functional loss of WT1 has an
important role in a subset of these malignant diseases (27–30).
However, in vivo and in vitro experimental data
indicated that functional loss of WT1 may result in differing
phenotypic consequences, including apoptosis, quiescence or
proliferation, depending on the differentiation status of the cell
(31–33). In the present study, the knockdown
of WT1 expression by siRNA-WT1 had a proliferative effect on G401
cells, similar effect to overexpression of miR-590. WT1
upregulation may rescue the cell proliferative phenotype of G401
cells via siRNA-WT1 (34).
Therefore, WT1 may be one of the key mediators of G401 suppression
by miR-590. A previous study suggested that WT1 may be involved in
the progression of cancer (29).
As WT1 is a target gene of miR-590, the suppression of WT1
expression may be due to miR-590 overexpression in Wilms'
tumor.
In conclusion, the results of the present study are
consistent with the hypothesis that miR-590 may promote G401 cell
proliferation via downregulation of it specific target gene, WT1 as
miR-590 expression level increased in Wilms' tumor tissues compared
with normal kidney tissues. A direct and functional target of
miR-590, WT1, is downregulated and associated with cell
proliferation in G401. Further investigations should be undertaken
and directed toward complete understanding of the underlying
molecular mechanism of the miRNA upregulation within tumorigenesis,
in order to develop promising strategies for targeted therapy of
Wilms' tumor in the future.
Acknowledgements
The present study was supported by a grant from the
Science and Technology Program in Suzhou (grant no. sys201434) to
Professor Hong Zhu.
References
|
1
|
Tournade MF, Com-Nougué C, Voûte PA,
Lemerle J, de Kraker J, Delemarre JF, Burgers M, Habrand JL,
Moorman CG, Bürger D, et al: Results of the sixth international
society of pediatric oncology Wilms' tumor trial and study: A
risk-adapted therapeutic approach in Wilms' tumor. J Clin Oncol.
11:1014–1023. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Graf N, van Tinteren H, Bergeron C, Pein
F, van den Heuvel-Eibrink MM, Sandstedt B, Schenk JP, Godzinski J,
Oldenburger F, Furtwängler R and de Kraker J: Characteristics and
outcome of stage II and III non-anaplastic Wilms' tumour treated
according to the SIOP trial and study 93-01. Eur J Cancer.
48:3240–3248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidoff AM, Giel DW, Jones DP, Jenkins
JJ, Krasin MJ, Hoffer FA, Williams MA and Dome JS: The feasibility
and outcome of nephron-sparing surgery for children with bilateral
Wilms' tumor. The St Jude children's research hospital experience:
1999–2006. Cancer. 112:2060–2070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akkina S and Becker BN: MicroRNAs in
kidney function and disease. Transl Res. 157:236–240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Xiang G, Wang Y, Zhang L, Yang X,
Cao L, Peng H, Xue P and Chen D: MicroRNA-590-5p regulates
proliferation and invasion in human hepatocellular carcinoma cells
by targeting TGF-β RII. Mol Cells. 33:545–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao X, Tang C, Xiao S, Fu C and Yu P:
Enhancement of proliferation and invasion by MicroRNA-590-5p via
targeting PBRM1 in clear cell renal carcinoma cells. Oncol Res.
20:537–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shan X, Miao Y, Fan R, Qian H, Chen P, Liu
H, Yan X, Li J and Zhou F: MiR-590-5P inhibits growth of HepG2
cells via decrease of S100A10 expression and inhibition of the Wnt
pathway. Int J Mol Sci. 14:8556–8569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu T, Nie F, Yang X, Wang X, Yuan Y, Lv
Z, Zhou L, Peng R, Ni D, Gu Y, et al: MicroRNA-590 is an
EMT-suppressive microRNA involved in the TGF-β signaling pathway.
Mol Med Rep. 12:7403–7411. 2015.PubMed/NCBI
|
|
10
|
Chu Y, Ouyang Y, Wang F, Zheng A, Bai L,
Han L, Chen Y and Wang H: MicroRNA-590 promotes cervical cancer
cell growth and invasion by targeting CHL1. J Cell Biochem.
115:847–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tongaonkar HB, Qureshi SS, Kurkure PA,
Muckaden MA, Arora B and Yuvaraja TB: Wilms Tumor: An Update.
Indian J Urol. 23:458–466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Menssen HD, Siehl JM and Thiel E: Wilms
tumor gene (WT1) expression as a panleukemic marker. Int J Hematol.
76:103–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Gao P, Lin F, Long M, Weng Y,
Ouyang Y, Liu L, Wei J, Chen X, He T, et al: Wilms' tumour
suppressor gene 1 (WT1) is involved in the carcinogenesis of Lung
cancer through interaction with PI3K/Akt pathway. Cancer Cell
Intern. 13:1142013. View Article : Google Scholar
|
|
14
|
Tatsumi N, Oji Y, Tsuji N, Tsuda A,
Higashio M, Aoyagi S, Fukuda I, Ito K, Nakamura J, Takashima S, et
al: Wilms' tumor gene WT1-shRNA as a potent apoptosis-inducing
agent for solid tumors. Int J Oncol. 32:701–711. 2008.PubMed/NCBI
|
|
15
|
Zhang J and Ma L: MicroRNA control of
epithelial-mesenchymal transition and metastasis. Cancer Metast
Rev. 31:653–662. 2012. View Article : Google Scholar
|
|
16
|
Favreau AJ and Sathyanarayana P:
miR-590-5p, miR-219-5p, miR-15b and miR-628-5p are commonly
regulated by IL-3, GM-CSF and G-CSF in acute myeloid leukemia. Leuk
Res. 36:334–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jalava SE, Urbanucci A, Latonen L,
Waltering KK, Sahu B, Jänne OA, Seppälä J, Lähdesmäki H, Tammela TL
and Visakorpi T: Androgen-regulated miR-32 targets BTG2 and is
overexpressed in castration-resistant prostate cancer. Oncogene.
31:4460–4471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yifru S and Muluye D: Childhood cancer in
Gondar University Hospital, Northwest Ethiopia. BMC Res Notes.
8:4742015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ochicha O, Gwarzo AK and Gwarzo D:
Pediatric malignancies in Kano, Northern Nigeria. World J Pediatr.
8:235–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu A, Heck JE, Ribeiro KB, Brennan P,
Boffetta P, Buffler P and Hung RJ: Wilms' tumour: A systematic
review of risk factors and meta-analysis. Paediatr Perinat
Epidemiol. 24:449–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scélo G and Brennan P: The epidemiology of
bladder and kidney cancer. Nat Clin Prac Urol. 4:205–217. 2007.
View Article : Google Scholar
|
|
22
|
Schüz J, Schmidt LS, Kogner P, Lähteenmäki
PM, Pal N, Stokland T and Schmiegelow K: Birth characteristics and
Wilms tumors in children in the Nordic countries: A register-based
case-control study. Int J Cancer. 128:2166–2173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beckwith JB, Kiviat NB and Bonadio JF:
Nephrogenic rests, nephroblastomatosis and the pathogenesis of
Wilms' tumor. Pediatr Pathol. 10:1–36. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beckwith JB: Nephrogenic rests and the
pathogenesis of Wilms tumor: Developmental and clinical
considerations. Am J Med Genet. 79:268–273. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dallosso AR, Hancock AL, Brown KW,
Williams AC, Jackson S and Malik K: Genomic imprinting at the WT1
gene involves a novel coding transcript (AWT1) that shows
deregulation in Wilms' tumours. Hum Mol Genet. 13:405–415. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Menke AL, van der Eb AJ and Jochemsen AG:
The Wilms' tumor 1 gene: Oncogene or tumor suppressor gene? Int Rev
Cytol. 181:151–212. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amini Nik S, Hohenstein P, Jadidizadeh A,
Van Dam K, Bastidas A, Berry RL, Patek CE, Van der Schueren B,
Cassiman JJ and Tejpar S: Upregulation of Wilms' tumor gene 1 (WT1)
in desmoid tumors. Int J Cancer. 114:202–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oji Y, Suzuki T, Nakano Y, Maruno M,
Nakatsuka S, Jomgeow T, Abeno S, Tatsumi N, Yokota A, Aoyagi S, et
al: Overexpression of the Wilms' tumor gene W T1 in primary
astrocytic tumors. Cancer Sci. 95:822–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo
T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, et al:
Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T
lymphocytes by WT1 peptide vaccine and the resultant cancer
regression. Proc Natl Acad Sci USA. 101:13885–13890. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang L, Han Y, Suarez Saiz F and Minden
MD: A tumor suppressor and oncogene: The WT1 story. Leukemia.
21:868–876. 2007.PubMed/NCBI
|
|
31
|
Hohenstein P and Hastie ND: The many
facets of the Wilms' tumour gene, WT1. Hum Mol Genet 15 Spec No.
2:R196–R201. 2006. View Article : Google Scholar
|
|
32
|
Xu C, Wu C, Xia Y, Zhong Z, Liu X, Xu J,
Cui F, Chen B, Røe OD, Li A and Chen Y: WT1 promotes cell
proliferation in non-small cell lung cancer cell lines through
up-regulating cyclin D1 and p-pRb in vitro and in vivo. PLoS One.
8:e688372013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Algar EM, Khromykh T, Smith SI, Blackburn
DM, Bryson GJ and Smith PJ: A WT1 antisense oligonucleotide
inhibits proliferation and induces apoptosis in myeloid leukaemia
cell lines. Oncogene. 12:1005–1014. 1996.PubMed/NCBI
|
|
34
|
Davies JA, Ladomery M, Hohenstein P,
Michael L, Shafe A, Spraggon L and Hastie N: Development of an
siRNA-based method for repressing specific genes in renal organ
culture and its use to show that the Wt1 tumour suppressor is
required for nephron differentiation. Hum Mol Genet. 13:235–246.
2004. View Article : Google Scholar : PubMed/NCBI
|