Introduction
Epilepsy is a common clinical disease characterized
by abnormal discharge from neurons in the brain. The pathogenesis
of epilepsy remains to be fully elucidated and its causes are
complex. The hippocampus is the site of high concentration of
neurons and has an important role in the pathogenesis of epilepsy
(1). Excitatory and inhibitory
neurotransmitters present in the central nervous system maintain
normal cerebral function, and glutamate is an important excitatory
neurotransmitter in the brain. Glutamate receptor (GluR)
dysfunction may be an important cause of epilepsy (2). Excessive activation of GluRs may
cause neuronal damage, a variety of neurological damage, and
chronic neurodegenerative diseases (3). The
α-am-ino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptor is an important subtype of ionotropic GluRs (4) and is composed of four subunits
(GluR1, 2, 3 and 4). The primary features of AMPA receptors are
determined by GluR2, with GluR2 protein downregulation considered
to be a molecular switch. Blocking or reducing GluR2 expression
forms a calcium-permeable AMPA receptor, which increases
Ca2+ influx and enhances endogenous glutamate
excitotoxicity (5). In the central
nervous system, the GluR2 subunit is highly expressed in various
neurons. Under normal conditions, GluR2 is abundant in synapses.
Following hypoxia, the expression of GluR2 on the surface of the
neuron membrane is significantly decreased, indicating that the
mechanism of blocking Ca2+ influx has weakened. Without
a GluR2 subunit, the Ca2+ permeability of AMPA receptors
increases and a large Ca2+ influx is detected, which may
activate a series of intracellular protein kinases or immediate
early genes. The decreased GluR2 expression induced by epileptic
seizure causes an increase in the Ca2+ permeability of
AMPA receptors, resulting in Ca2+ overload, which is an
important cause of delayed neuronal death in hippocampal neurons
(6). Neuropeptide Y (NPY) contains
36 amino acids and was first extracted from the brain tissue of
pigs by Tatemoto et al (7)
in 1982. It is widely distributed in the central and peripheral
nervous systems (8). In the
central nervous system, NPY concentrations are greatest in the
hippocampus. NPY has a protective effect on cerebellum neuronal
cells cultured in vitro and has been demonstrated to possess
an anti-epileptic effect (9);
however, its protective effects are weakened following blocking of
NPY Y1 and Y2 receptors, suggesting that NPY exerts neuroprotective
effects via these receptors (10).
The present study aimed to investigate the functional alterations
in the GluR2 subunit induced by epileptiform discharge in
hippocampal neurons and to investigate whether NPY affects these
functional alterations.
Materials and methods
Animals and reagents
A total of 64 clean male Sprague-Dawley rats, which
were born within 24 h were provided by the Experimental Animal
Center of Hebei Medical University (animal license no. SCXK (Ji)
2013-1-003; Shijiazhuang, China). The rats were maintained in a 12
h light/dark cycle, humidity of 60±5%, 22±3°C. All rats were
allowed free access to food and water. All animal experimental
procedures were performed in strict accordance with the Guidance
Suggestions for the Care and Use of Laboratory Animals of National
Institutes of Health (U.S.) and the protocol was approved by the
Institutional Animal Care Committee of Hebei Medical University.
Neurobasal medium, B-27, L-glutamine, fetal bovine serum (FBS;
special grade) and Dulbecco's modified Eagle's medium (DMEM)/F-12
medium were purchased from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA); poly-L-lysine and trypsin from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany); and AMPA, NPY and BIBP3226 from
Enzo Life Sciences, Inc. (Farmingdale, NY, USA).
Rabbit anti-microtubule-associated protein 2 (MAP-2)
(cat. no. 17490-1-AP) polyclonal antibody and fluorescein
isothiocyanate (FITC)-labeled goat anti-rabbit immunoglobulin G
(cat. no. SA00003-2) were obtained from ProteinTech Group, Inc.
(Chicago, IL, USA); rabbit anti-phosphorylated (p)-GluR2 (Tyr876)
(cat. no. 4027) antibody and rabbit anti-GluR2 (cat. no. 5306)
antibody from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Mouse anti-β-actin (cat. no. sc-130300) from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Horseradish
peroxidase-conjugated anti-mouse or anti-rabbit secondary
antibodies (cat. nos. 7072 and 7071) from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The random primers were
obtained from Promega Corporation (Madison, WI, USA).
Culture of hippocampal neurons
Hippocampal neurons were primarily cultured as
described by Yang et al (11). Following intraperitoneal injection
of anesthetic (pentobarbital sodium, 3 µl/g), Neonatal
Sprague-Dawley rats aged <24 h were surface sterilized with
disinfectant (75% alcohol) and sacrificed by decapitation and the
brains were extracted and placed in DMEM/F-12 medium at 0°C.
Bilateral hippocampi were harvested under an anatomical microscope.
Following removal of the meninges, the brain tissue was cut into
pieces and immersed in 0.125% trypsin (5X volume of brain tissue).
The samples were digested in a 5% CO2 incubator at 37°C
for 15 min. The digestion was terminated by adding DMEM/F-12 medium
containing 10% serum. All samples were triturated and filtered with
a 200-mesh screen. The resulting cell suspension was adjusted to
~1×105/ml with DMEM/F-12 medium containing 10% FBS,
seeded into 6-well plates with polylysine-coated coverslips (3
ml/well) and placed in a 5% CO2 incubator at 37°C for 24
h. The medium was subsequently replaced with neuronal medium
(Neurobasal medium), supplemented with 2% B-27, 100 U/ml penicillin
and 100 µg/ml streptomycin. Half of the neuronal medium was
replaced with fresh every 3 days, for 7–9 days, following which the
purified neurons were harvested.
Identification of hippocampal
neurons
MAP-2 is a neuron-specific protein and a marker of
neuronal differentiation (12). At
day 9 of in vitro culture, the hippocampal neurons on glass
slides were fixed with 4% paraformaldehyde for 30 min, washed three
times with PBS for 5 min each time, permeabilized with 0.3% Triton
X-100 for 10 min and washed three times with PBS for 5 min each
time. Cells were blocked with 3% goat serum (OriGene Technologies,
Inc., Beijing, China) at room temperature for 30 min and incubated
with MAP-2 antibody (1:100) at 4°C overnight. Following three
washes with PBS for 5 min each time, cells were incubated with a
FITC-conjugated secondary antibody (1:100) at 37°C for 1 h,
followed by a further three washes with PBS for 5 min each time.
Cells were counterstained with Hoechst 33258 for 5 min, washed
three times with PBS for 5 min each time and observed under a
fluorescence microscope.
Group assignment
Following 12 days of in vitro culture,
hippocampal neurons were assigned to the following groups: Control,
Mg2+-free, NPY+Mg2+-free and
BIBP3226+NPY+Mg2+-free. In the control group, neurons
were treated with normal extracellular fluid for 3 h. In the
Mg2+-free group, neurons were treated with
Mg2+-free extracellular fluid for 3 h. In the
NPY+Mg2+-free group, neurons were incubated with cell
culture fluid containing NPY at a final concentration of 1 µmol/l
for 30 min and then with Mg2+-free extracellular fluid
for 3 h. In the BIBP3226+NPY+Mg2+-free group, neurons
were incubated with cell culture fluid containing the NPY Y1
receptor blocker BIBP3226 at a final concentration of 1 µmol/l for
30 min, with NPY at a final concentration of 1 µmol/l for 30 min
and finally with Mg2+-free extracellular fluid for 3 h.
Afterwards, all groups received normal extracellular fluid for 1 h.
Cells were subsequently analyzed using the patch clamp technique,
western blot analysis to measure GluR2 and phosphorylated GluR2
protein expression levels, and RT-qPCR to measure GluR2 mRNA
expression levels.
Normal extracellular fluid comprised: NaCl, 147 mM;
4-(2-hydroxyethyl) −1-piperazineethanesulfonic acid (HEPES), 10 mM;
glucose, 13 mM; KC1, 2 mM; CaCl2, 2 mM; and
MgC12, 2 mM; adjusted to pH 7.3 with 5 mM NaOH; osmotic
pressure 280–320 mM. Mg2+-free extracellular fluid
comprised: NaCl, 147 mM; HEPES, 10 mM; glucose, 13 mM; KC1, 2 mM;
and CaCl2, 2 mM; adjusted to pH 7.3 with 5 mM NaOH;
osmotic pressure 280–320 mM.
Using the patch clamp technique, the action
potential of neurons was recorded in the control,
Mg2+-free and NPY+Mg2+-free groups in
accordance with the method of DeLorenzo et al (13).
Detection of AMPA current
(IAMPA)
IAMPA in neurons was recorded using the
patch clamp technique (14,15).
Cell slides were placed in a 0.3 ml bath and perfused with
extracellular fluid at 2 ml/min to ensure fluid exchange in 2 min.
Cells were observed under an inverted microscope. Neurons with a
distinct stereoscopic outline and smooth surface were used for the
sealing experiment. With a three-dimensional manipulator, a glass
microelectrode with impedance of 1–3 MΩ and pipette solution
(Cs-gluconate, 110 mmol/l; CsCl, 30 mmol/l; HEPES, 10 mmol/l; EGTA,
0.2 mmol/l; NaCl, 8 mmol/l; Mg-ATP, 2 mmol/l; Na3GTP,
0.3 mmol/l; phosphocreatine, 10 mmol/l; pH 7.2 adjusted with NaOH)
was connected to the cell surface. Negative pressure was increased
until the cells ruptured. Capacitive current and series resistance
were compensated and a whole-cell recording created. Voltage was
held at −70 mV for 5 min, following which the intracellular fluid
and pipette solution were completely replaced, and 100 µmol/l AMPA
was administered to induce inward current. This current was
IAMPA. To further verify this, AMPA was measured
following elution and the membrane current returned to normal. When
100 µmol/l AMPA was administered, 10 µmol/l
6-cyano-7-nitroquinoxaline-2,3-dione was used to block the AMPA
receptor and the original inward current could not be detected.
Thus, the recorded current was IAMPA. To exclude error
in different cells, peak current density was measured using
pA/pF.
Western blot analysis of GluR2 protein
expression and phosphorylation levels
Cells were harvested, washed with PBS, lysed with
precooled radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Jiangsu, China) and centrifuged at
12,000 × g at 4°C for 10 min. The supernatant, containing
the total proteins from the hippocampal neurons, was collected.
Protein concentrations were determined by the Lowry method. Total
proteins (100 µg) were separated by electrophoresis on 5% stacking
and 10% separating gels at 100 V for 150 min. Separated proteins
were transferred onto polyvinylidene difluoride membranes.
Membranes were blocked by 5% non-fat milk in 0.01 mol/l PBS at room
temperature for 2 h, incubated with primary antibody at 4°C
overnight (1:1,000), washed three times, incubated with a
horseradish peroxidase-conjugated secondary antibody (1:400) at
37°C for 1 h and washed three times. Protein bands were visualized
with 3,3′-diaminobenzidine (Sangon Biotech Co., Ltd., Shanghai,
China) and the gray values were measured and quantitatively
analyzed with BandScan version 5.0 (Glyko; BioMarin Pharmaceutical,
Inc., San Rafael, CA, USA). The experiments were performed in
triplicate.
RT-qPCR analysis of GluR2 mRNA
expression levels
Following the removal of cell medium, 1 ml
TRIzol® was added to each well. The samples were
triturated and placed in a ribozyme-free centrifuge tube for 5 min.
Subsequently, 0.2 ml chloroform was added to each tube, which were
vigorously agitated for 15 sec, rested for 5 min and centrifuged at
13,800 × g at 4°C for 15 min. The supernatant was
transferred to a new centrifuge tube, treated with an equal volume
of isopropanol and centrifuged at 13,800 × g at 4°C for 10
min. A feathery white precipitate was observed at the bottom of the
tube, the supernatant was discarded and 1 ml 75% ethanol [prepared
with diethyl pyrocarbonate (DEPC)-treated water] was added. The
precipitate was washed and centrifuged at 5,400 × g at 4°C
for 5 min. Following removal of the supernatant, the sample was
air-dried for 3–5 min. RNA was fully dissolved with 20–30 µl of
DEPC-treated water and its purity measured using an ultraviolet
spectrophotometer. cDNA was synthesized using the EasyScript
First-strand cDNA synthesis superMix kit (Beijing TransGen Biotech
Co., Ltd., Beijing, China). Rat GluR2 primer sequences were
synthesized by Promega Corporation (Table I). The reaction mixture was as
follows: 2X UltraSYBR® Mixture (with ROX), 10 µl;
forward primer (10 µmol/l), 1 µl; reverse primer (10 µmol/l), 1 µl;
cDNA, 8 µl. The total reaction volume was 20 µl. qPCR cycling
conditions were as follows: An initial predenaturation step at 95°C
for 10 min, followed by 40 cycles of denaturation at 95°C for 15
sec, annealing at 58°C for 20 sec and extension at 72°C for 27 sec.
Following amplification, results were analyzed with an ABI 7300
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The relative value (RQ value) of target gene expression to
the internal reference gene GAPDH was detected and calculated
(16).
 | Table I.Specific primers for GluR2 and GAPDH
used in reverse transcription-quantitative polymerase chain
reaction. |
Table I.
Specific primers for GluR2 and GAPDH
used in reverse transcription-quantitative polymerase chain
reaction.
| Gene | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| GluR2 | F:
CAAGTTCGCATACCTCTA | 207 |
|
| R:
TTATCCCTTTCACAGTCC |
|
| GAPDH | F:
TGAACGGGAAGCTCACTGG | 120 |
|
| R:
GCTTCACCACCTTCTTGATGTC |
|
Statistical analysis
The data were analyzed with SPSS version 10.0 (SPSS,
Inc., Chicago, IL, USA) using tests for normality and homogeneity
of variance. Data that obeyed normality and homogeneity of variance
were analyzed using analysis of variance for completely random
design. Paired comparison was conducted with the least significant
difference post hoc test. Data were expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
NPY suppresses epileptiform discharges
in hippocampal neurons
Primary cultured hippocampal neurons had axons and
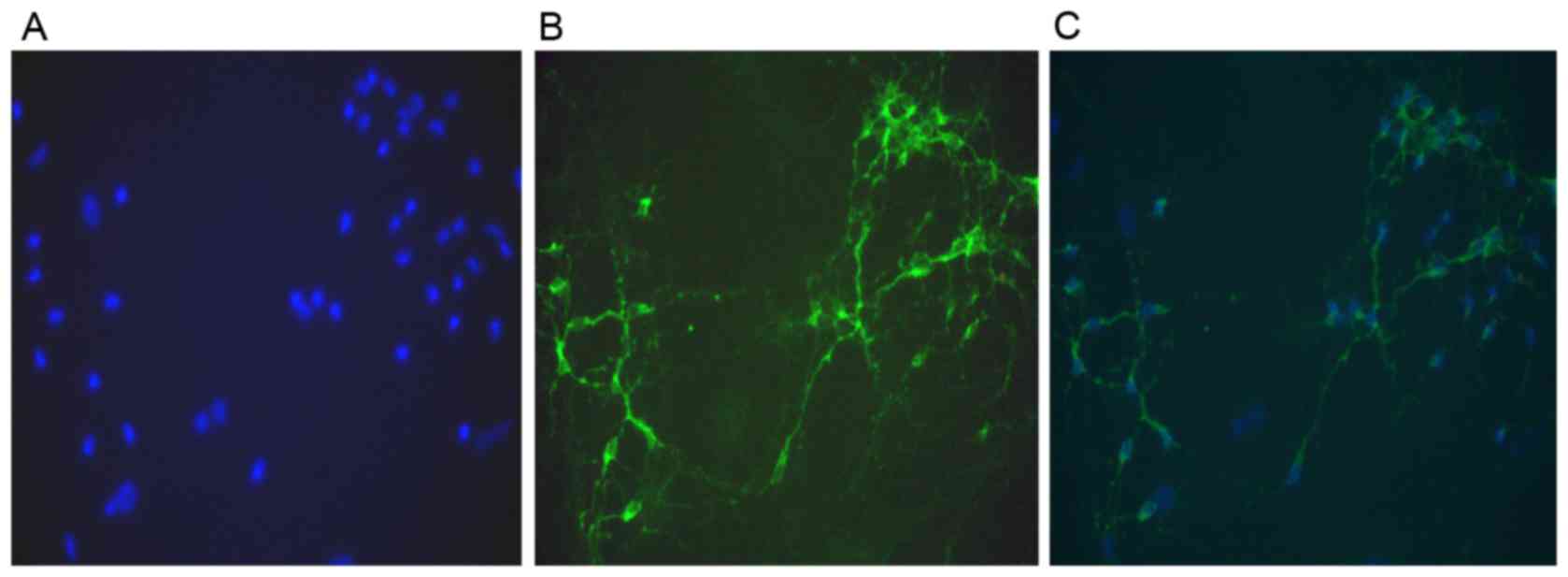
dendrites. Immunostaining of MAP-2 revealed that neuronal purity
was >95% (Fig. 1). The patch
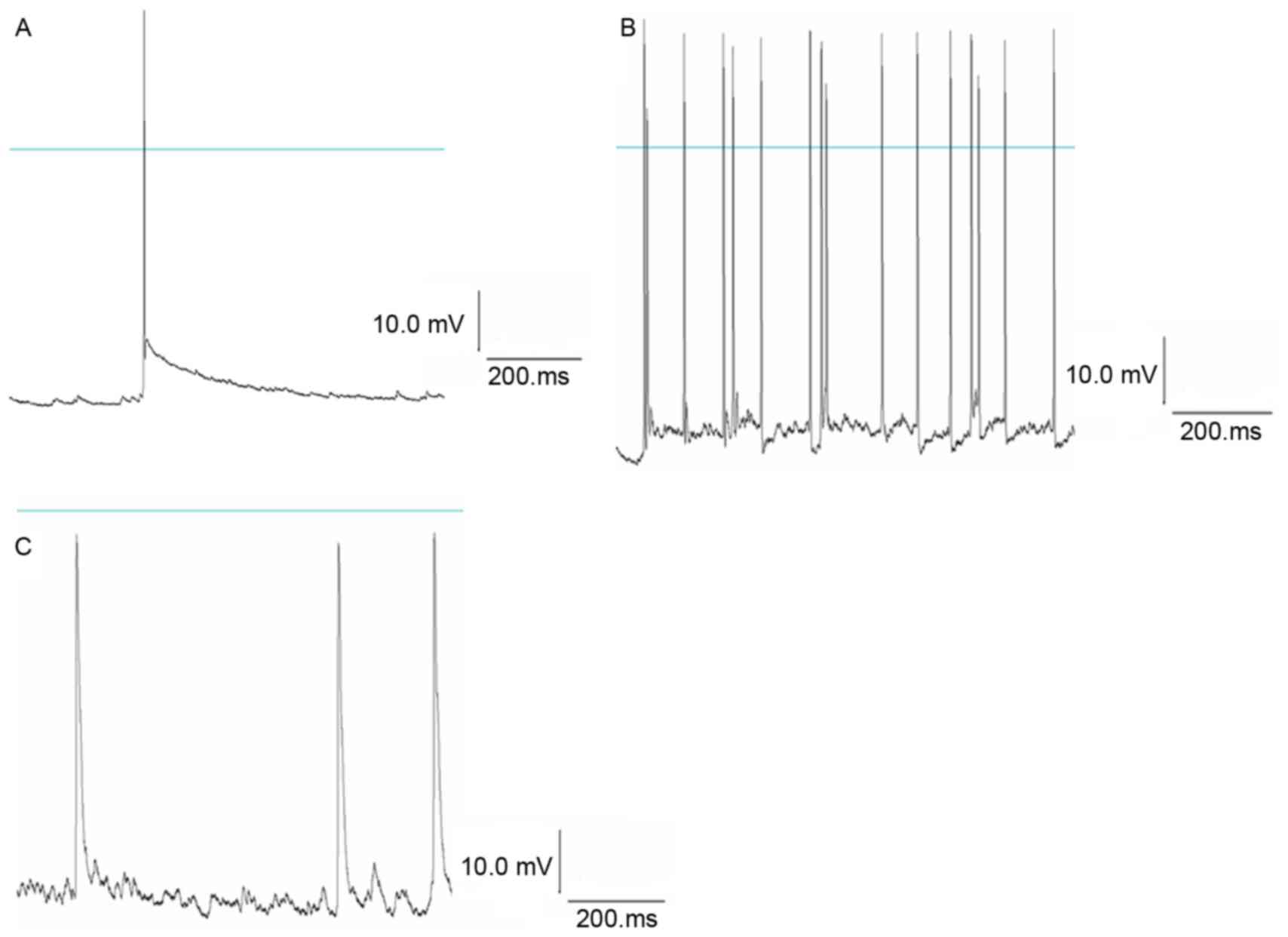
clamp technique records the normal action potential of hippocampal
neurons. Following treatment with Mg2+-free
extracellular fluid for 3 h followed by normal extracellular fluid,
a continuously stable action potential was detected in the neurons;
the frequency and amplitude appeared greater compared with the
control group, indicating spontaneous epileptiform discharges in
the neurons. Following treatment with NPY 1 µmol/l for 30 min,
Mg2+-free extracellular fluid for 3 h and normal
extracellular fluid, the frequency and amplitude of the action
potential were significantly reduced in NPY+Mg2+-free
group compared with the Mg2+-free group (P<0.05;
Fig. 2; Tables II and III). This suggested that NPY
significantly inhibited abnormal discharges in hippocampal
neurons.
 | Table II.Comparison of frequency of action
potential in three groups. |
Table II.
Comparison of frequency of action
potential in three groups.
| Group | Frequency
(number/s) |
|---|
|
Mg2+-free |
13.86±2.19a |
|
NPY+Mg2+-free |
1.89±0.69b |
| Control | 0.85±0.22 |
 | Table III.Comparison of amplitude of action
potential in three groups. |
Table III.
Comparison of amplitude of action
potential in three groups.
| Group | Amplitude (mV) |
|---|
|
Mg2+-free |
81.25±5.18a |
|
NPY+Mg2+-free |
40.06±2.31b |
| Control | 35.56±1.23 |
Epileptiform discharges in hippocampal
neurons inhibit the function of AMPA receptor GluR2 subunit
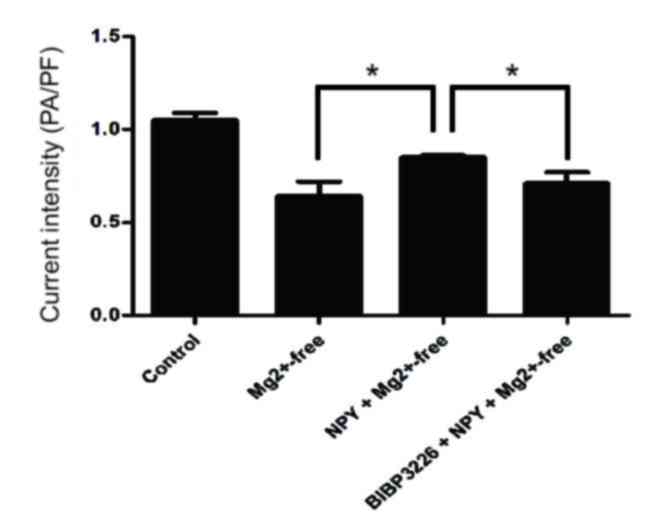
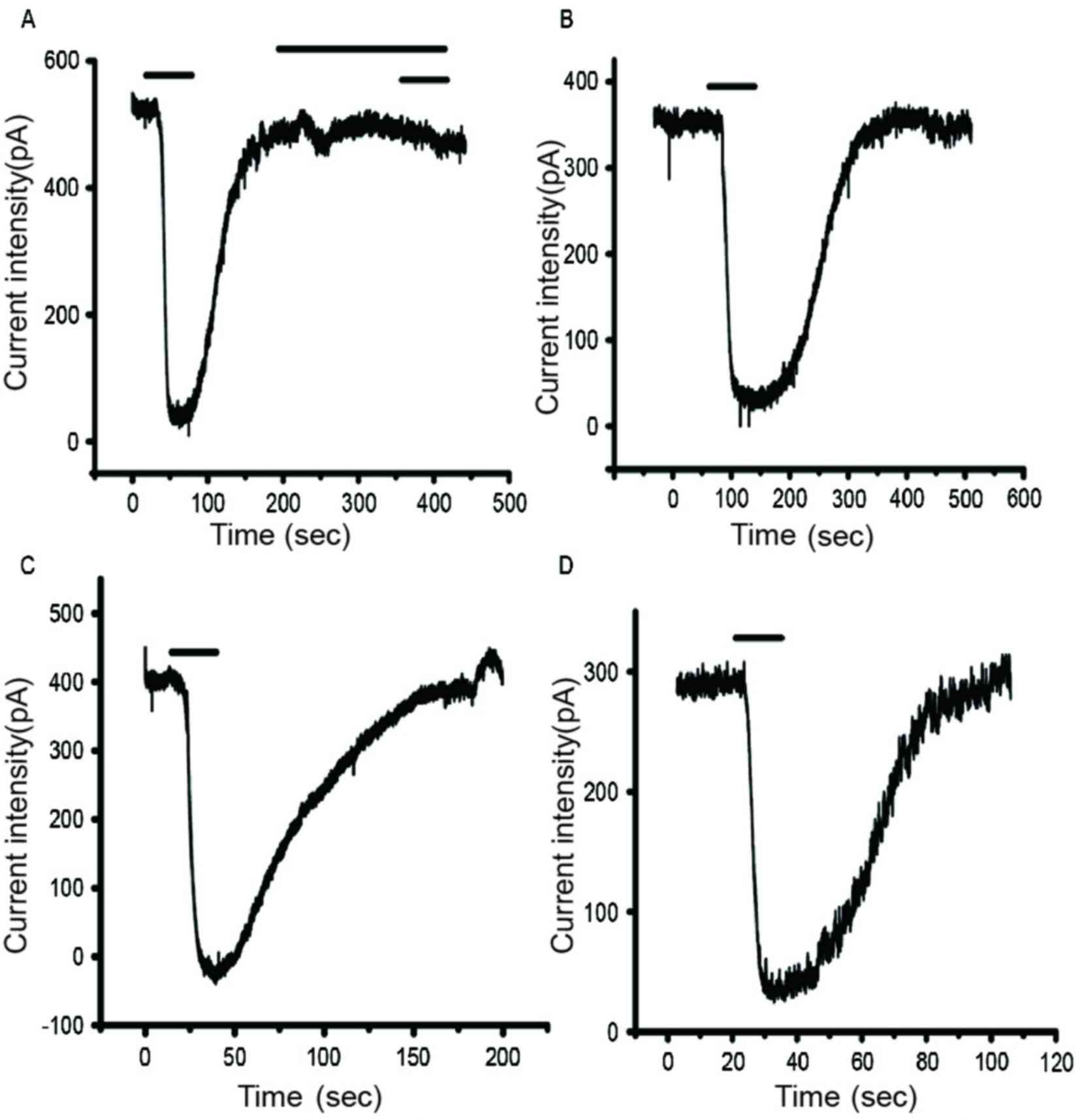
IAMPA detection results demonstrated that
peak current density was significantly reduced in the
Mg2+-free group compared with the control group
(P<0.05; Figs. 3 and 4; Table
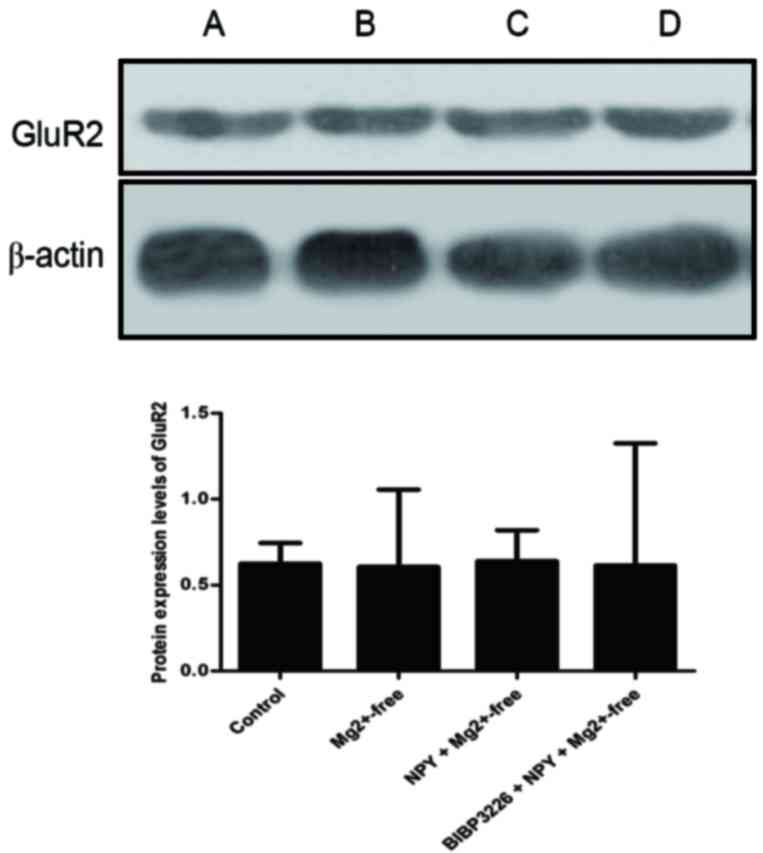
IV). Protein expression levels of GluR2 were slightly reduced
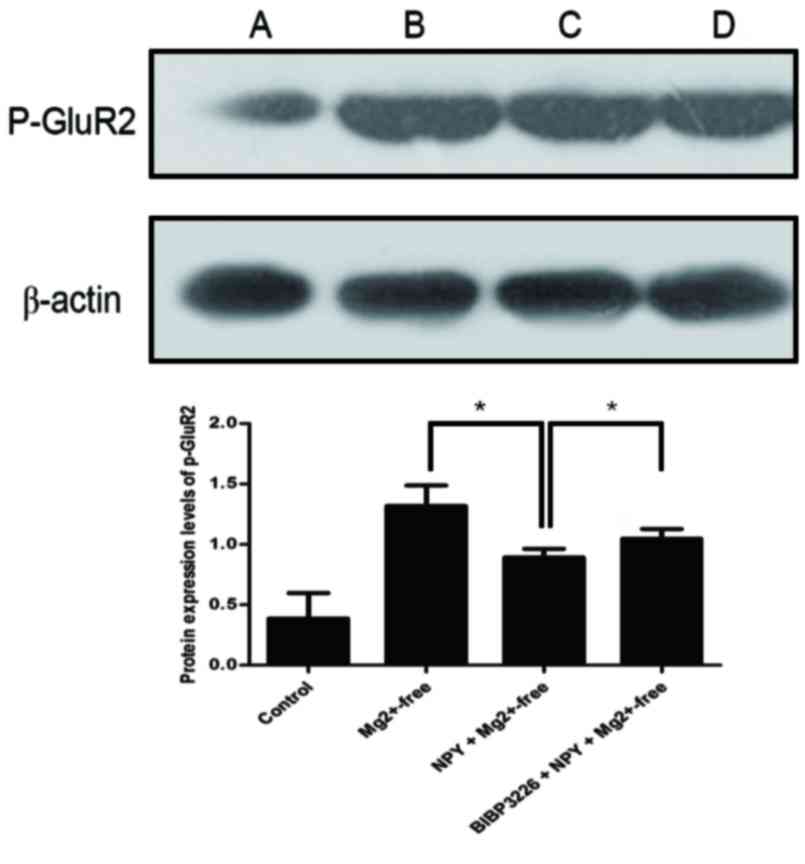
(P>0.05; Fig. 5; Table V) and those of p-GluR2 were
significantly greater (P<0.05; Fig.
6; Table VI) in the
Mg2+-free group compared with the control group. GluR2
mRNA expression levels were significantly reduced in the
Mg2+-free group compared with the control group
(P<0.05; Fig. 7; Table VII). These results suggested that
epileptiform discharges in hippocampal neurons may induce the
suppression of the function of the GluR2 subunit.
 | Table IV.Current density of primary cultured
hippocampal neurons induced by
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid. |
Table IV.
Current density of primary cultured
hippocampal neurons induced by
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
| Group | Current density
(pA/pF) |
|---|
| Control | 1.05±0.04 |
|
Mg2+-free |
0.64±0.08a |
|
NPY+Mg2+-free |
0.85±0.01b |
|
BIBP3226+NPY+Mg2+-free |
0.71±0.06a |
 | Table V.Protein expression levels of GluR2,
as assessed by western blot analysis. |
Table V.
Protein expression levels of GluR2,
as assessed by western blot analysis.
| Group | GluR2 |
|---|
| Control | 0.6241±0.15 |
|
Mg2+-free | 0.6057±0.25 |
|
NPY+Mg2+-free | 0.6397±0.18 |
|
BIBP3226+NPY+Mg2+-free | 0.6146±0.21 |
 | Table VI.Protein expression levels of p-GluR2,
as assessed by western blot analysis. |
Table VI.
Protein expression levels of p-GluR2,
as assessed by western blot analysis.
| Group | p-GluR2 |
|---|
| Control | 0.3879±0.21 |
|
Mg2+-free |
1.3173±0.17a |
|
NPY+Mg2+-free |
0.8918±0.07b |
|
BIBP3226+NPY+Mg2+-free |
1.0483±0.08a |
 | Table VII.mRNA expression levels of GluR2, as
assessed by reverse transcription-quantitative polymerase chain
reaction. |
Table VII.
mRNA expression levels of GluR2, as
assessed by reverse transcription-quantitative polymerase chain
reaction.
| Group | GluR2 |
|---|
| Control | 1.016±0.11 |
|
Mg2+-free |
0.179±0.16a |
|
NPY+Mg2+-free |
0.713±0.08b |
|
BIBP3226+NPY+Mg2+-free |
0.394±0.05a |
NPY relieves the inhibition of GluR2
subunit function induced by epileptiform discharges in hippocampal
neurons
Peak current density was significantly greater in
the NPY+Mg2+-free group compared with the
Mg2+-free group (P<0.05; Figs. 3 and 4; Table
IV). Protein expression levels of GluR2 were slightly increased
(P>0.05; Fig. 5; Table V) and those of p-GluR2 were
significantly reduced (P<0.05; Fig.
6; Table VI) in the
NPY+Mg2+-free group compared with the
Mg2+-free group. GluR2 mRNA expression levels were
significantly greater in the NPY+Mg2+-free group
compared with the Mg2+-free group (P<0.05; Fig. 7; Table VII). These findings indicated
that NPY weakened the inhibition of GluR2 function induced by
epileptiform discharges in neurons.
NPY regulates GluR2 subunit function
possibly via the Y1 receptor
Peak current density was significantly reduced in
the BIBP3226+NPY+Mg2+-free group compared with the
NPY+Mg2+-free group (P<0.05; Figs. 3 and 4; Table
IV). Protein expression levels of GluR2 were slightly reduced
(P>0.05; Fig. 5; Table V) and those of p-GluR2 were
significantly greater (P<0.05; Fig.
6; Table VI) in the
BIBP3226+NPY+Mg2+-free group compared with the
NPY+Mg2+-free group. GluR2 mRNA expression levels were
significantly reduced in the BIBP3226+NPY+Mg2+-free
group compared with the NPY+Mg2+-free group (P<0.05;
Fig. 7; Table VII).
Discussion
Epilepsy is a brain disorder characterized by the
abnormal discharge of neurons in the brain. The pathogenesis of
epilepsy remains unclear and it may be that a variety of factors
contribute to its occurrence. Neuronal loss occurs, and is
accompanied by a large number of abnormal discharge neurons in the
lesion. Ion channel dysfunction in cells causes seizures; this is
the ‘epileptic neuron’ theory (17,18).
The hippocampus is the site of a high concentration of neurons and
possesses an important role in the pathogenesis of epilepsy:
Hippocampal sclerosis is a common cause of temporal lobe epilepsy.
The structure of the nervous system is complex and there are
numerous factors restricting its study in vivo.
Electrophysiological testing, including the patch clamp technique,
is not easy to implement. Hippocampal slices and neuronal cultures
have attracted increasing attention in the study of epilepsy
(19).
Mg2+ serves an important role in
maintaining normal electric activity in the central nervous system
(20). DeLorenzo et al
(13) reported that the removal of
Mg2+ from the cell medium, or treatment with
Mg2+-free extracellular fluid for 3 h, may successfully
induce spontaneous recurrent epileptiform discharges in primary
cultured hippocampal neurons. Such cultured neurons do not possess
real anatomical connections or clinical manifestations, but the
spontaneous recurrent action potential evoked by
Mg2+-free conditions is similar to the
electrophysiological activity during epileptic seizures and
anti-epileptic drugs may prevent such action potentials (21–22).
This technique has been used in studies of the biochemical,
electrophysiological and molecular mechanisms of acquired epilepsy
(23).
NPY is widely distributed in the central nervous
system (cerebral cortex, hippocampus, thalamus, hypothalamus and
brain stem) and the peripheral nervous system. In the central
nervous system, NPY concentration is greatest in the hippocampus.
NPY has an anti-epileptic effect (24,25)
and a protective effect on cerebellum neuronal cells cultured in
vitro. These protective effects are weakened following blocking
of the NPY Y1 and Y2 receptors, suggesting that NPY exerts
neuroprotective effects via the Y1 and Y2 receptors (26–28).
In the present study, epileptiform discharges were detected in
hippocampal neurons of rats following treatment with
Mg2+-free extracellular fluid. Following treatment with
NPY and Mg2+-free extracellular fluid, epileptiform
discharges were notably weakened, indicating that NPY may suppress
epileptiform discharges in neurons.
Excitatory and inhibitory neurotransmitters present
in the central nervous system are responsible for the balance of a
complex network and maintain normal cerebral function. Following
excitatory neurotransmitter increases, or inhibitory
neurotransmitter decreases, the ratio may become unbalanced. GluR
functional alteration is one of the important causes of epilepsy
(29). Excessive activation of
GluRs may cause neuronal damage, a variety of neurological damage,
and chronic neurodegenerative diseases, including cerebral ischemia
and hypoxia, epilepsy and brain trauma. Previous studies have
confirmed that following epilepsy, a massive release of glutamate
and overactivation of its receptors causes a Ca2+
influx, followed by delayed neuronal death and secondary injury
(30,31).
GluR may be divided into metabotropic types (coupled
with G protein) and ionotropic types (containing ion channels). In
accordance with pharmacological properties, molecular
characteristics and electrophysiological properties, ionotropic
GluR may be divided into three subtypes: AMPA receptor,
N-methyl-D-aspartate receptor and kainate receptor (32).
The AMPA receptor, a type of ion channel protein,
may be regulated by membrane potential, glutamate and AMPA. It is
composed of four subunits (GluR1, 2, 3 and 4) encoded by different
genes (33). Due to the low
calcium ion permeability induced by mRNA Q/R site editing, the
relative content of GluR2 subunit in the AMPA receptor determines
the functional properties of AMPA receptors. An AMPA receptor
containing the GluR2 subunit is permeable to monovalent cations
(Na+, K+), but not to divalent cations
(Ca2+); an AMPA receptor without a GluR2 subunit is
highly permeable to Ca2+ (34,35).
The primary features of AMPA receptors are therefore determined by
GluR2 and GluR2 protein downregulation is considered to be a
molecular switch (36). Blocking
or reducing GluR2 expression forms a calcium-permeable AMPA
receptor, increases Ca2+ influx and enhances endogenous
glutamate excitotoxicity.
In the central nervous system, the GluR2 subunit is
highly expressed in various neurons. Under normal conditions, GluR2
is abundant in synapses. Following hypoxia, the content of GluR2 on
the membrane surface of the neuron is significantly decreased and
the number of synapses containing GluR2 reduced, indicating that
the mechanism of blocking Ca2+ influx has markedly
weakened (37).
The results of the present study demonstrated that,
following treatment with Mg2+-free extracellular fluid,
GluR2 subunit mRNA expression levels were decreased, but protein
phosphorylation levels were increased, suggesting that the GluR2
subunit had been reduced or its activity deceased in neuronal
membranes. Abnormal activation of neurons diminished AMPA receptor
GluR2 subunit expression. It has previously been demonstrated that
without a GluR2 subunit, the Ca2+ permeability of AMPA
receptors increases and a large Ca2+ influx may be
detected, able to activate a series of intracellular protein
kinases or immediate early genes (38) and resulting in a decrease in GluR2
subunit expression. Grooms et al (39) hypothesized that the decreased GluR2
expression induced by epileptic seizure caused an increase in the
Ca2+ permeability of AMPA receptors, resulting in
Ca2+ overload, an important cause of delayed neuronal
death in hippocampal neurons. Sanchez et al (40) demonstrated that perinatal
hypoxia-induced seizures increased the Ca2+ permeability
of AMPA receptors.
The present study used a whole cell patch clamp
technique to record IAMPA in neurons cultured in
vitro. AMPA is a selective agonist of the exogenous AMPA
receptor, and is synthesized artificially. AMPA binding to AMPA
receptor depolarizes the cell membrane and opens ion channels.
Results from the present study demonstrated that IAMPA
was reduced in the Mg2+-free group compared with the
control group. The decreased degree of IAMPA was
markedly diminished in the NPY+Mg2+-free group compared
with the Mg2+-free group. This effect was inhibited by
BIBP3226. These results indicated that epileptiform discharges in
cells may induce a reduction in the functions of AMPA
receptors.
The AMPA receptor is a membrane receptor, the
function of which may be regulated by multiple internal and
external cell processes; phosphorylation has the greatest influence
on its function (41). PDZ
domain-containing proteins may interact with the PDZ structural
domain at the GluR2 C terminus following binding to activate
protein kinase C-α, which induces ser880 phosphorylation at the
GluR2 C terminus and endocytosis of the GluR2 complex, reducing the
expression of AMPA receptor GluR2 subunit on the surface of neurons
and thus serving an important role in AMPA receptor expression and
transport (42,43).
IAMPA is the most direct indicator
reflecting electrophysiological alterations in AMPA receptors. A
decrease in IAMPA reflects a reduction in receptor
function. During epileptiform discharges, IAMPA falls,
indicating that AMPA receptor activity is reduced. Based on the
RT-qPCR and western blot analysis results, it is hypothesized that
epileptiform discharges in hippocampal neurons led to the
phosphorylation of the AMPA receptor GluR2 subunit on the membrane
surface and suppressed GluR2 subunit mRNA. The AMPA receptor GluR2
subunit on the membrane surface was transported into cells, so AMPA
receptor activity on the membrane surface diminished. Therefore,
neuron total protein detection did not alter significantly between
groups. The lack of the normal AMPA receptor GluR2 subunit pathway
on the membrane surface causes a high Ca2+ permeability
and may induce a rapid Ca2+ influx, Ca2+
concentration increase and a series of pathological reactions.
IAMPA and GluR2 mRNA expression levels
were significantly greater, but GluR2 protein phosphorylation
levels were significantly reduced in the NPY+Mg2+-free
group compared with the Mg2+-free group. It is
hypothesized that NPY may inhibit the alterations in AMPA receptor
function induced by epileptiform discharges, suggesting that NPY
may have affected AMPA receptor functions and avoided excessive
reduction of IAMPA by regulating the GluR2 subunit. The
inhibitory effect of NPY was suppressed by treatment with the Y1
receptor blocker BIBP3226. NPY exerts different effects by binding
to different receptors; NPY receptors belong to the G
protein-coupled receptor family (44) and contain Y1, Y2, Y3, Y4 and Y5
subtypes in humans. Different NPY receptors are distributed in
different regions of the body; the Y1 receptor is primarily
expressed in the cerebral cortex, amygdala and hippocampus
(45). Previous studies on the
anti-epileptic effect of NPY have primarily focused on Y2 and Y5
receptors (46,47). It is hypothesized that NPY exerted
an anti-epileptic effect in the present study via the Y1 receptor
expressed on the membrane surface of hippocampal neurons. This may
be a pharmacological mechanism underlying the anti-epileptic effect
of NPY.
In conclusion, the present study investigated the
functional alterations in the AMPA receptor GluR2 subunit and the
effect of NPY on these alterations during epileptiform discharges
in hippocampal neurons, to provide a theoretical basis for
specifically blocking pathological process and for the design of
therapeutic agents with low toxicity for the treatment of
epilepsy.
References
|
1
|
Rocha AK, de Lima E, Amaral FG, Peres R,
Cipolla-Neto J and Amado D: Pilocarpine-induced epilepsy alters the
expression and daily variation of the nuclear receptor RORα in the
hippocampus of rats. Epilepsy Behav. 55:38–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muldoon SF, Villette V, Tressard T,
Malvache A, Reichinnek S, Bartolomei F and Cossart R: GABAergic
inhibition shapes interictal dynamics in awake epileptic mice.
Brain. 138:2875–2890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsamis KI, Mytilinaios DG, Njau SN and
Baloyannis SJ: Glutamate receptors in human caudate nucleus in
normal aging and Alzheimer's disease. Curr Alzheimer Res.
10:469–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ettinger AB, LoPresti A, Yang H, Williams
B, Zhou S, Fain R and Laurenza A: Psychiatric and behavioral
adverse events in randomized clinical studies of the noncompetitive
AMPA receptor antagonist perampanel. Epilepsia. 56:1252–1263. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harlow DE, Saul KE, Komuro H and Macklin
WB: Myelin proteolipid protein complexes with αv integrin and AMPA
receptors in vivo and regulates AMPA-dependent oligodendrocyte
progenitor cell migration through the modulation of cell-surface
GluR2 expression. J Neurosci. 35:12018–12032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedman LK, Velísková J, Kaur J, Magrys
BW and Liu H: GluR2(B) knockdown accelerates CA3 injury after
kainate seizures. J Neuropathol Exp Neurol. 62:733–750. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tatemoto K, Carlquist M and Mutt V:
Neuropeptide Y-a novel brain peptide with structural similarities
to peptide YY and pancreatic polypeptide. Nature. 296:659–660.
1982. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woldbye DP and Kokaia M: Neuropeptide Y
and seizures: Effects of exogenously applied ligands.
Neuropeptides. 38:253–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malva JO, Xapelli S, Baptista S, Valero J,
Agasse F, Ferreira R and Silva AP: Multifaces of neuropeptide Y in
the brain-neuroprotection, neurogenesis and neuroinflammation.
Neuropeptides. 46:299–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vezzani A and Sperk G: Overexpression of
NPY and Y2 receptors in epileptic brain tissue: An endogenous
neuroprotective mechanism in temporal lobe epilepsy? Neuropeptides.
38:245–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang S, Zhou W, Zhang Y, Yan C and Zhao Y:
Effects of Liuwei Dihuang decoction on ion channels and synaptic
transmission in cultured hippocampal neuron of rat. J
Ethnopharmacol. 106:166–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Atalay B, Caner H, Can A and Cekinmez M:
Attenuation of microtubule associated protein-2 degradation after
mild head injury by mexiletine and calpain-2 inhibitor. Br J
Neurosurg. 21:281–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeLorenzo RJ, Sombati S and Coulter DA:
Effects of topiramate on sustained repetitive firing and
spontaneous recurrent seizure discharges in cultured hippocampal
neurons. Epilepsia. 41:(Suppl 1). S40–S44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rozov A, Sprengel R and Seeburg PH:
GluA2-lacking AMPA receptors in hippocampal CA1 cell synapses:
Evidence from gene-targeted mice. Front Mol Neurosci. 5:222012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rozov A, Zivkovic AR and Schwarz MK:
Homer1 gene products orchestrate Ca(2+)-permeable AMPA
receptor distribution and LTP expression. Front Synaptic Neurosci.
4:42012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Curtis KM, Gomez LA, Rios C, Garbayo E,
Raval AP, Perez-Pinzon MA and Schiller PC: EF1alpha and RPL13a
represent normalization genes suitable for RT-qPCR analysis of bone
marrow derived mesenchymal stem cells. BMC Mol Biol. 11:612010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dudek FE and Rogawski MA: The epileptic
neuron redux. Epilepsy Curr. 2:151–152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernard C, Anderson A, Becker A, Poolos
NP, Beck H and Johnston D: Acquired dendritic channelopathy in
temporal lobe epilepsy. Science. 305:532–535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kato K, Sekino Y, Takahashi H, Yasuda H
and Shirao T: Increase in AMPA receptor-mediated miniature EPSC
amplitude after chronic NMDA receptor blockade in cultured
hippocampal neurons. Neurosci Lett. 418:4–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Solger J, Heinemann U and Behr J:
Electrical and chemical long-term depression do not attenuate
low-Mg2+-induced epileptiform activity in the entorhinal cortex.
Epilepsia. 46:509–516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Y, Jiang L, Chen H and Zhang X:
Expression of AMPA receptor subunits in hippocampus after status
convulsion. Childs Nerv Syst. 28:911–918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma YX, Yin JB, Fan XT, Xu HW, An N, Wan
ZB, Li ZF, Liu GL, Zhang YH and Yang Hui: Establishment of kainic
acid induced temporal lobe epilepsy in rat and study of its
neurogenesis. Acta Academiae Medicinae Militaris Tertiae.
29:872–875. 2007.
|
|
23
|
Sombati S and Delorenzo RJ: Recurrent
spontaneous seizure activity in hippocampal neuronal networks in
culture. J Neurophysiol. 73:1706–1711. 1995.PubMed/NCBI
|
|
24
|
Furtinger S, Pirker S, Czech T,
Baumgartner C, Ransmayr G and Sperk G: Plasticity of Y1 and Y2
receptors and neuropeptide Y fibers in patients with temporal lobe
epilepsy. J Neurosci. 21:5804–5812. 2001.PubMed/NCBI
|
|
25
|
Sørensen G and Woldbye DP: Mice lacking
neuropeptide Y show increased sensitivity to cocaine. Synapse.
66:840–843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto BK and Raudensky J: The role of
oxidative stress, metabolic compromise, and inflammation in
neuronal injury produced by amphetamine-related drugs of abuse. J
Neuroimmune Pharmacol. 3:203–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cadet JL and Krasnova IN: Molecular bases
of methamphetamine-induced neurodegeneration. Int Rev Neurobiol.
88:101–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nunes AF, Montero M, Franquinho F, Santos
SD, Malva J, Zimmer J and Sousa MM: Transthyretin knockout mice
display decreased susceptibility to AMPA-induced neurodegeneration.
Neurochem Int. 55:454–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoy KC, Huie JR and Grau JW: AMPA receptor
mediated behavioral plasticity in the isolated rat spinal cord.
Behav Brain Res. 236:319–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blair RE, Sombati S, Churn SB and
Delorenzo RJ: Epileptogenesis causes an N-methyl-d-aspartate
receptor/Ca2+-dependent decrease in
Ca2+/calmodulin-dependent protein kinase II activity in
a hippocampal neuronal culture model of spontaneous recurrent
epileptiform discharges. Eur J Pharmacol. 588:64–71. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blair RE, Deshpande LS, Sombati S, Elphick
MR, Martin BR and DeLorenzo RJ: Prolonged exposure to WIN55,212-2
causes downregulation of the CB1 receptor and the development of
tolerance to its anticonvulsant effects in the hippocampal neuronal
culture model of acquired epilepsy. Neuropharmacology. 57:208–218.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gardner SM, Takamiya K, Xia J, Suh JG,
Johnson R, Yu S and Huganir RL: Calcium-permeable AMPA receptor
plasticity is mediated by subunit-specific interactions with PICK1
and NSF. Neuron. 45:903–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Essin K, Nistri A and Magazanik L:
Evaluation of GluR2 subunit involvement in AMPA receptor function
of neonatal rat hypoglossal motoneurons. Eur J Neurosci.
15:1899–1906. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Palmer CL, Cotton L and Henley JM: The
molecular pharmacology and cell biology of
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors.
Pharmacol Rev. 57:253–277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Montero M, Nielsen M, Rønn LC, Møller A,
Noraberg J and Zimmer J: Neuroprotective effects of the AMPA
antagonist PNQX in oxygen-glucose deprivation in mouse hippocampal
slice cultures and global cerebral ischemia in gerbils. Brain Res.
1177:124–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sager C, Tapken D, Kott S and Hollmann M:
Functional modulation of AMPA receptors by transmembrane AMPA
receptor regulatory proteins. Neuroscience. 158:45–54. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colbourne F, Grooms SY, Zukin RS, Buchan
AM and Bennett MV: Hypothermia rescues hippocampal CA1 neurons and
attenuates down-regulation of the AMPA receptor GluR2 subunit after
forebrain ischemia. Proc Natl Acad Sci USA. 100:2906–2910. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Calderone A, Jover T, Mashiko T, Noh KM,
Tanaka H, Bennett MV and Zukin RS: Late calcium EDTA rescues
hippocampal CA1 neurons from global ischemia-induced death. J
Neurosci. 24:9903–9913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grooms SY, Opitz T, Bennett MV and Zukin
RS: Status epilepticus decreases glutamate receptor 2 mRNA and
protein expression in hippocampal pyramidal cells before neuronal
death. Proc Natl Acad Sci USA. 97:3631–3636. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sanchez RM, Koh S, Rio C, Wang C, Lamperti
ED, Sharma D, Corfas G and Jensen FE: Decreased glutamate receptor
2 expression and enhanced epileptogenesis in immature rat
hippocampus after perinatal hypoxia-induced seizures. J Neurosci.
21:8154–8163. 2001.PubMed/NCBI
|
|
41
|
Hanley JG and Henley JM: PICK1 is a
calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J.
24:3266–3278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Y, Venkitaramani DV, Gladding CM,
Zhang Y, Kurup P, Molnar E, Collingridge GL and Lombroso PJ: The
tyrosine phosphatase STEP mediates AMPA receptor endocytosis after
metabotropic glutamate receptor stimulation. J Neurosci.
28:10561–10566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dev KK, Nakanishi S and Henley JM: The PDZ
domain of PICK1 differentially accepts protein kinase C-alpha and
GluR2 as interacting ligands. J Biol Chem. 279:41393–41397. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alvaro AR, Martins J, Costa AC, Fernandes
E, Carvalho F, Ambrósio AF and Cavadas C: Neuropeptide Y protects
retinal neural cells against cell death induced by ecstasy.
Neuroscience. 152:97–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baptista S, Bento AR, Gonçalves J,
Bernardino L, Summavielle T, Lobo A, Fontes-Ribeiro C, Malva JO,
Agasse F and Silva AP: Neuropeptide Y promotes neurogenesis and
protection against methamphetamine-induced toxicity in mouse
dentate gyrus-derived neurosphere cultures. Neuropharmacology.
62:2413–2423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jinde S, Masui A, Morinobu S, Noda A and
Kato N: Differential changes in messenger RNA expressions and
binding sites of neuropeptide Y Y1, Y2 and Y5 receptors in the
hippocampus of an epileptic mutant rat: Noda epileptic rat.
Neuroscience. 115:1035–1045. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Woldbye DP, Nanobashvili A, Sørensen AT,
Husum H, Bolwig TG, Sørensen G, Ernfors P and Kokaia M:
Differential suppression of seizures via Y2 and Y5 neuropeptide Y
receptors. Neurobiol Dis. 20:760–772. 2005. View Article : Google Scholar : PubMed/NCBI
|