Introduction
Osteoporosis is a medical and socioeconomic problem
characterized by the loss of bone mass and mechanical properties,
leading to an increased risk of fracture. A variety of factors may
lead to osteoporosis, and menopause is one of the most common
reasons (1). In postmenopausal
women, decreased estrogen levels cause increased osteoclast
formation and elevated bone resorption, resulting in more rapid
bone loss (2). Therefore,
targeting osteoclast formation and function is one of the
strategies for preventing and treating osteoporosis. Currently,
several drugs aimed at inhibiting osteoclast formation or function
have been used for osteoporosis, including bisphosphonates and
denosumab (3). Aside from these
synthesized compounds or antibodies, natural compounds are
alternative therapeutic agents for the prevention and treatment of
osteoporosis. Several natural products have exhibited an
anti-osteoporotic property, including berberine, kaempferol,
formononetin and osthole (4–7). Our
study group has a long-term interest in evaluating the
pharmacological effect of natural compounds on osteoporosis
(8–11). Sanguinarine [13-methyl-(1,3)
benzodioxole (5,6-c)-1,3-dioxolane (4,5-I) phenanthridinium] is an
alkaloid derived from the roots of Sanguinaria canadensis.
Sanguinarine exhibits multiple pharmacological effects, including
anti-inflammatory, antitumor, antimicrobial, antiplatelet and
antihypertensive properties (12).
The authors previously revealed that sanguinarine inhibited
osteoclast formation and bone resorption by suppressing the tumor
necrosis factor ligand superfamily member 11-induced nuclear
factor-κB and extracellular signal-regulated kinase signaling
pathways (10). However, whether
this natural compound prevents estrogen deficiency-induced bone
loss requires further investigation in vivo. Considering the
potential of sanguinarine for suppressing osteoclast formation
in vitro, and the wide use of this compound in traditional
medicine, the present study was designed to investigate whether
sanguinarine may protect against ovariectomy (OVX)-induced
osteoporosis and, thus, be a potential agent for future clinical
application.
Materials and methods
Ethics statement
The Animal Care and Experiment Committee of Zhejiang
University School of Medicine (Zhejiang, China) approved all
experimental procedures and the study was performed according to
the guidelines for Ethical Conduct in the Care and Use of Nonhuman
Animals in Research by the American Psychological Association
(13).
Media and reagents
Sanguinarine, dimethyl sulfoxide (DMSO) and the
tartrate-resistant acid phosphatase (TRAP) staining kit were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Sanguinarine was dissolved in DMSO and was stored at −20°C. To
prevent photosensitivity, the experiments were performed in the
absence of visible light. Sanguinarine was diluted using PBS so
that DMSO comprised <0.1% of the total volume, to a final
concentration of 2 mg/ml.
Animals and study design
An OVX-induced osteoporosis mouse model was
established to determine the effects of sanguinarine on
osteoporosis in vivo. Healthy 8-week-old female C57BL/6J
mice (n=28; weight, 19.11±0.57 g) received either a sham operation
(n=7) or OVX (n=21) under anesthesia using 8% chloral hydrate. Mice
were housed in an animal facility under temperature (22–24°C) and
humidity (50–60%) controlled conditions, with a 12 h light/dark
cycle and with free access to food and water. The ovary was excised
in OVX-operated mice, and the adipose tissue around the ovary was
excised in sham-operated mice, as described previously (14). Mice were assigned to the following
four groups: Sham with PBS control (sham; n=7; weight, 18.57±0.67
g), OVX with PBS (vehicle; n=7; weight, 19.07±0.45 g), OVX with low
sanguinarine (5 mg/kg; n=7; weight, 19.43±0.61 g), OVX with high
sanguinarine (10 mg/kg; n=7; weight, 19.36±0.57 g). At 1 week
post-operation, all mice were intraperitoneally injected with PBS
or sanguinarine twice a week for 6 weeks. At the end of experiment,
the mice were sacrificed. The left legs were excised and fixed in
4% paraformaldehyde for 48 h under controlled temperature
(22–24°C), for micro-computed tomography (CT) analysis. The right
legs were immediately removed and snap-frozen in liquid nitrogen
for RNA isolation. The uteruses were excised and weighed. In the
sham group the uterine weight was 82.60±15.30 mg, in the OVX groups
the uterine weights were 15.86±1.36, 13.67±2.23 and 11.5±2.68 mg,
in the vehicle, low sanguinarine and high sanguinarine groups,
respectively.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
RT-qPCR was performed to determine the expression of
genes associated with bone metabolism. Total RNA from the tissues
was isolated using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and RT was performed
using 1.0 µg total RNA and the HiFiScript cDNA kit (CWBIO, Beijing,
China), according to the manufacturer's protocol. Amplification
reactions were set up in 20 µl reaction volumes containing
amplification primers and UltraSYBR Mixture (with ROX; CWBIO Co.,
Ltd.), expression was detected by the ABI 7500 Sequencing Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). cDNA
(1 µl) was used in each amplification reaction. The thermocycling
conditions of the reaction were as follows: Activation at 95°C for
10 min; 40 cycles of amplification, 95°C for 10 sec, 60°C for 24
sec and 72°C for 20 sec; final extension at 72°C for 1 min. All the
reactions were performed in triplicate and were normalized to the
housekeeping genes 18S ribosomal RNA (18S) and GAPDH. Expression
was quantified using the ΔΔCq method, as described previously
(15). The primer sequences were
as follows: 18S, 5′-CCTGCGGCTTAATTTGACTC-3′ (forward) and
5′-AACTAAGAACGGCCATGCAC-3′ (reverse); GAPDH,
5′-ACCCAGAAGACTGTGGATGG-3′ (forward) and 5′-CACATTGGGGGTAGGAACAC-3′
(reverse); TRAP, 5′-CCATTGTTAGCCACATACGG-3′ (forward) and
5′-CACTCAGCACATAGCCCACA-3′ (reverse); T-cell immune regulator 1
(Tcirg1), 5′-TGGCTACCGTTCCTATCCTG-3′ (forward) and
5′-CTTGTCCGTGTCCTCATCC-3′ (reverse); cathepsin K (CtsK),
5′-TCCGCAATCCTTACCGAATA-3′ (forward) and
5′-AACTTGAACACCCACATCCTG-3′ (reverse); nuclear factor of activated
T-cells 1 (NFATc1), 5′-TCCACCCACTTCTGACTTCC-3′ (forward) and
5′-CTTCGCCCACTGATACGAG-3′ (reverse); alkaline phosphatase (ALP),
5′-ACTGGCTGTGCTCTCCCTAC-3′ (forward) and 5′-GAAGTTGCCTGGACCTCTCC-3′
(reverse); osteopontin (OPN), 5′-TGATGATGACGATGGAGACC-3′ (forward)
and 5′-GGGACGATTGGAGTGAAAGT-3′ (reverse); runt related
transcription factor 2 (Runx2), 5′-CCTCTGACTTCTGCCTCTGG-3′
(forward) and 5′-ATGAAATGCTTGGGAACTGC-3′ (reverse); ColI
5′-CATCGTGGCTTCTCTGGTCT-3′ (forward) and 5′-CCGTTGAGTCCGTCTTTGC-3′
(reverse); dentin matrix acidic phosphoprotein 1 (Dmp1),
5′-GCTACATTGCTTTGGCTCCT-3′ (forward) and 5′-GGTCACTTCCTGTCCTGCTC-3′
(reverse); phosphate regulating endopeptidase homolog X-linked
(Phex), 5′-CCGAACCAGTGAGGCTATGT-3′ (forward) and
5′-CGAGGGACCAATGTCTTTCA-3′ (reverse); and sclerostin (SOST),
5′-CGTGCCTCATCTGCCTACTT-3′ (forward) and 5′-AGGTCTGCCTCCATTCTCC-3′
(reverse).
Micro-CT scanning
The left tibia of each mouse was fixed with 4%
paraformaldehyde for 48 h under controlled temperature (22–24°C)
and washed with PBS three times, it was subsequently stored in 70%
ethanol until CT scanning. The fixed tissues were analyzed using a
high-resolution micro-CT (SkyScan 1072; Bruker microCT; Bruker
Corporation, Billerica, MA, USA). The scanning protocol was set at
an isometric resolution of 9 µm, and the radiography energy
settings were 80 kV and 80 µA. Following reconstruction, the region
of interest for the tibia was set at 0.5 mm from the proximal
femoral growth plate and was selected for further qualitative and
quantitative analysis. Several structural parameters, including the
trabecular bone volume/total volume (BV/TV), mean trabecular
thickness (Tb.Th), mean trabecular number (Tb.N) and mean
trabecular separation (Tb.Sp) were measured by micro-CT as reported
previously (16).
Histological analysis
The left femur of each mouse was fixed in 4%
paraformaldehyde for 48 h under controlled temperature (22–24°C)
and decalcified in 12% EDTA. Decalcified tissues were
paraffin-embedded and sectioned (thickness, 8 µm). For histological
examination, sections were stained with hematoxylin for 5 min and
with eosin for 2 min under controlled temperature (22–24°C), and
another section was stained with the TRAP staining kit to identify
osteoclasts on the bone surface. Stained sections were imaged using
a photomicroscope (magnification, ×100–200), and the number of
osteoblasts/bone perimeter (N.Ob/B.Pm), empty lacuna rate,
percentage of osteoclast surface/bone surface (Ocs/BS), number of
osteoclasts/field of tissue and microstructure parameters,
including BV/TV, Tb.Th, Tb.N and Tb.Sp, were measured and
quantified using Image Pro-Plus software (version 4.0; Media
Cybernetics, Inc., Rockville, MD, USA) (11).
Statistical analysis
GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA) was used to perform all statistical analyses. Data
are presented as the mean ± standard error of the mean. Statistical
significance was determined by one-way analysis of variance,
followed by the Dunnett's post hoc test to make comparisons between
the groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sanguinarine prevents OVX-induced bone
loss in vivo
Micro-CT was used to determine the
microarchitectural changes of the tibiae (Fig. 1A). The BV of the OVX vehicle group
was significantly reduced compared with the sham group (BV/TV,
11.85±0.42 and 5.14±0.36%, respectively; Fig. 1B). Following treatment with
sanguinarine, a protective effect against OVX-induced bone loss was
observed in a dose-dependent manner. The BV/TV in the low-dose
group was 8.42±0.56%, which was ~30% higher compared with the OVX
vehicle group. In addition, the BV/TV in the high-dose group was
increased by 50% compared with the OVX vehicle group. In addition,
changes in the other bone mineral density indices (Tb.Th, Tb.N,
Tb.Sp) occurred accordingly. The administration of sanguinarine
significantly increased BV/TV and Tb. N, and significantly reduced
Tb. Sp compared with the OVX vehicle group. Furthermore, the four
groups of mice had a similar mean body weight prior to OVX
(Fig. 1C). Despite the same
feeding regimen, the final body weight of mice in the OVX groups
was significantly increased compared with the sham group (Fig. 1D), while the uterine weight of mice
in the OVX groups was significantly lower compared with the sham
group when sacrificed (Fig. 1E).
The results for body and uterine weight upon sacrifice indicate
that the OVX model was established successfully. However, no
difference was observed between the OVX vehicle group and OVX
groups treated with high or low-dose sanguinarine, indicating that
sanguinarine was not able to prevent OVX-induced weight gain and
uterine atrophy. Collectively, the results indicated a protective
effect of sanguinarine against OVX-induced bone loss in
vivo.
 | Figure 1.Sanguinarine prevents
ovariectomy-induced bone loss in vivo. (A) Fixed tibiae were
analyzed by micro-computed tomography and three-dimensional
reconstructed images from each group are presented. (B) BV/TV,
Tb.N, Tb.Sp and Tb.Th were caculated for sham, vehicle, L-S.G. and
H-S.G. groups. (C) Initial (8-weeks-old) and (D) final
(15-weeks-old) body weights of mice. (E) Uteri were dissected and
weighed at the end of the study (15-weeks-old). *P<0.05 and
**P<0.01 vs. vehicle; ##P<0.01 vs. sham. BV/TV,
trabecular bone volume/total volume; Tb.N, trabecular number;
Tb.Sp, mean trabecular separation; Tb.Th, mean trabecular
thickness; L-S.G., low-dose sanguinarine group; H-S.G., high-dose
sanguinarine group. |
Histological analysis indicates that
sanguinarine protects mice from OVX-induced bone loss in vivo
Decalcified bone tissue was analyzed by H&E
staining (Fig. 2A). Consistent
with the micro-CT results, the vehicle group exhibited a
significantly decreased BV compared with the sham group (BV/TV,
4.40±0.24 and 11.56±0.44%, respectively; Fig. 2B). By contrast, sanguinarine at low
and high concentrations prevented OVX-induced bone loss to a
certain extent (BV/TV, 7.42±0.36 and 10.21±0.45% in the low- and
high-dose groups, respectively). In addition, OVX vehicle mice also
exhibited a significantly reduced Tb.N and significantly increased
Tb.Sp compared with the sham group, while sanguinarine
dose-dependently reversed this trend by increasing the Tb.N and
reducing the Tb.Sp compared with the OVX vehicle group (Fig. 2B).
 | Figure 2.Histological analysis demonstrating
that sanguinarine protects mice from ovariectomy-induced bone loss
in vivo. (A) Decalcified tissues were paraffin-embedded and
sectioned for H&E staining. (B) BV/TV, Tb.N, Tb.Sp and Tb.Th
were calculated for sham, vehicle, L-S.G. and H-S.G. groups.
*P<0.05 and **P<0.01 vs. vehicle. H&E, hematoxylin and
eosin; BV/TV, trabecular bone volume/total volume; Tb.N, mean
trabecular number; Tb.Sp, mean trabecular separation; Tb.Th, mean
trabecular thickness; L-S.G., low-dose sanguinarine group; H-S.G.,
high-dose sanguinarine group. |
Sanguinarine inhibits osteoclastic
bone resorption in vivo
TRAP staining was performed to identify osteoclasts
on the bone surface (Fig. 3A),
which revealed a reduced osteoclast number following sanguinarine
treatment, with 53.63±3.02 osteoclasts in the OVX vehicle group and
23.34±2.55 osteoclasts in the sanguinarine high-dose treatment
group. The resorptive area, determined by the Ocs/BS, also
supported the inhibitory effect of sanguinarine on osteoclastic
bone resorption in vivo, with a 10.78±0.82% resorptive area
in the OVX vehicle group and a 4.04±0.38% resorptive area in the
sanguinarine high-dose treatment group (Fig. 3B). In addition, the suppressive
effect of sanguinarine on osteoclasts was further supported by the
osteoclast-specific gene expression profile. qPCR analysis
demonstrated that osteoclastic markers, including TRAP, NFATc1,
Tcirg1 and CtsK, were significantly upregulated in OVX vehicle mice
compared with the sham group (Fig.
3C). The administration of sanguinarine treatment
dose-dependently reduced the upregulation of osteoclast activity
markers in the bone tissue. Therefore, the results indicate that
sanguinarine may protect against OVX-induced osteoporosis by
inhibiting osteoclastic formation and bone resorption in
vivo.
 | Figure 3.Sanguinarine inhibits osteoclastic
bone resorption in vivo. (A) Decalcified tissues were
paraffin-embedded and sectioned for TRAP staining. (B) Number of
Ocs/field of tissue and the Ocs/BS were analyzed. (C) Expression of
Oc-specific genes TRAP, NFATc1, Tcirg1 and CtsK was detected by
quantitative polymerase chain reaction, and the results were
normalized to the expression of 18S and GAPDH. Magnification, 100x.
Yellow arrows, osteoblast; green arrows, Oc lacunae; black arrows,
empty lacunae. *P<0.05 and **P<0.01 vs. vehicle. TRAP,
tartrate-resistant acid phosphatase; Ocs, osteoclasts; Ocs/BS,
percentage of Oc surface/bone surface; NFATc1, nuclear factor of
activated T-cells 1; Tcirg1, T-cell immune regulator 1; CtsK,
cathepsin K; 18S, 18S ribosomal RNA; L-S.G., low-dose sanguinarine
group; H-S.G., high-dose sanguinarine group. |
Sanguinarine promoted osteoblastic
bone formation in vivo
Following H&E staining (Fig. 4A), the N.Ob/B.Pm was calculated to
determine the effect of sanguinarine on osteoblastic bone formation
in vivo (Fig. 4B). The
results demonstrated that the administration of sanguinarine
dose-dependently increased the osteoblast number compared with OVX
vehicle mice (Fig. 4B). The
osteocyte number, osteocyte lacunae number and empty lacunae were
also observed, and the empty lacunae rate was calculated to
determine the effect of sanguinarine on osteocytes (Fig. 4C). The empty lacuna rate was
increased in the vehicle group compared with the sham group
(44.40±2.66 and 13.47±1.88%, respectively). In addition, low- and
high-dose sanguinarine reduced the empty lacuna rate to 26.66±1.02
and 12.76±0.75% in the vehicle and sham groups, respectively. qPCR
analysis further supported the results, which demonstrated that
sanguinarine treatment increased the expression of
osteoblast-specific genes, including Runx2, ALP, OPN and ColI
compared with OVX vehicle mice (Fig.
4D). Furthermore, sanguinarine also increased the expression of
osteocyte-specific genes, including Dmp1, Phex and SOST in
vivo compared with the OVX vehicle group (Fig. 4E). The results indicate that
sanguinarine may increase bone mass by protecting osteoblastic bone
formation.
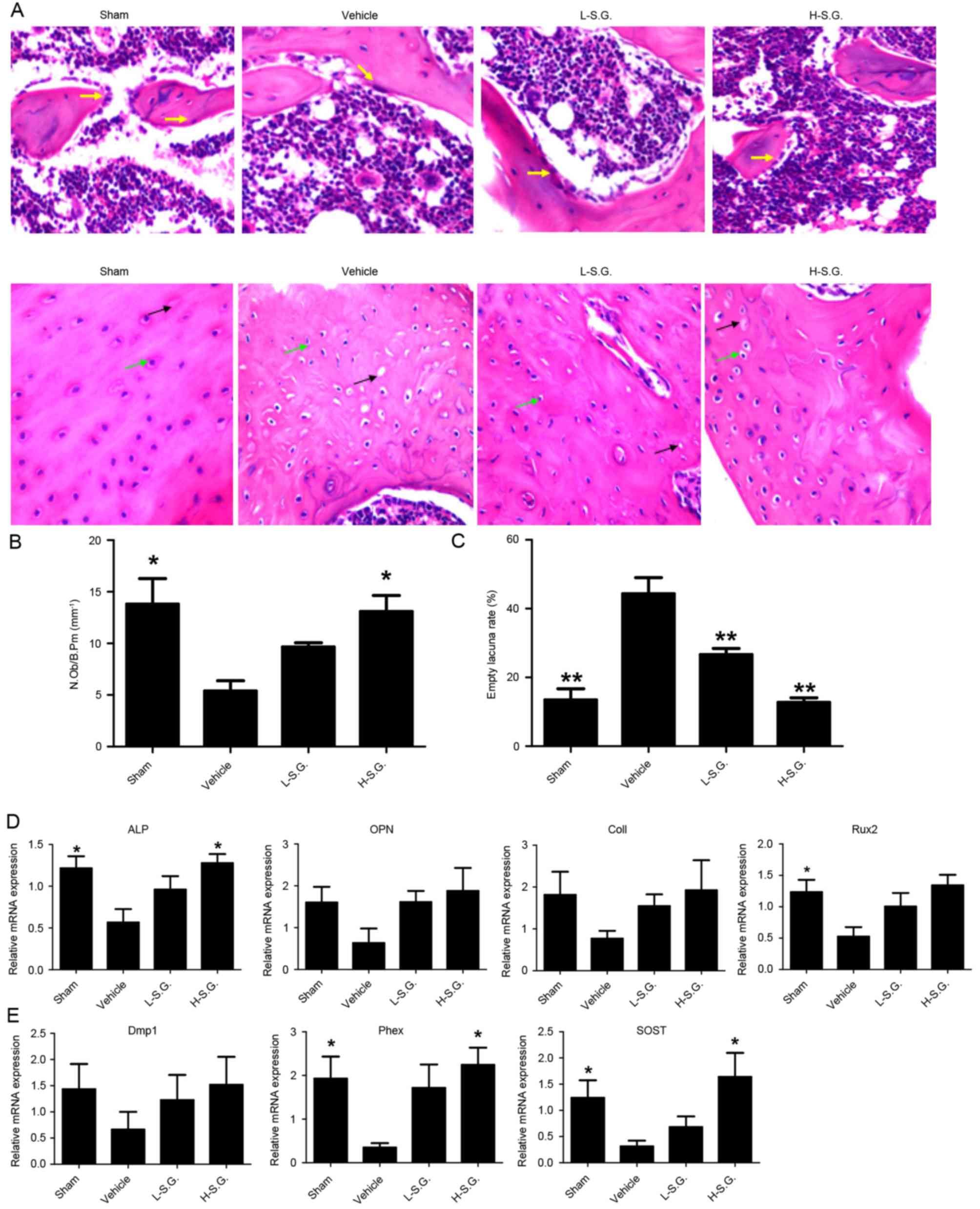 | Figure 4.Sanguinarine promotes osteoblastic
bone formation in vivo. Decalcified tissues were
paraffin-embedded and sectioned for (A) hematoxylin and eosin
staining, and the (B) N.Ob/B.Pm and (C) empty lacuna rate were
calculated. (D) Expression of osteoblast-specific genes, including
Runx2, ALP, OPN and ColI were analyzed using qPCR, and the results
were normalized to the expression of 18S and GAPDH. (E) Expression
of osteocyte-specific genes, including Dmp1, Phex and SOST were
analyzed by qPCR, and the results were normalized to the expression
of 18S and GAPDH. Magnification, 200x. *P<0.05 and **P<0.01
vs. vehicle. N.Ob/B.Pm, number of osteoblasts/bone perimeter;
Runx2, runt related transcription factor 2; ALP, alkaline
phosphatase; OPN, osteopontin; ColI, Type I collagen; qPCR,
quantitative polymerase chain reaction; Dmp1, dentin matrix acidic
phosphoprotein 1; Phex, phosphate regulating endopeptidase homolog
X-linked; SOST, sclerostin; 18S, 18S ribosomal RNA; L-S.G.,
low-dose sanguinarine group; H-S.G., high-dose sanguinarine
group. |
Discussion
Considering the wide use of sanguinarine as a
traditional medicine and its inhibitory effect on osteoclasts in
vitro, it was hypothesized that sanguinarine may have a role in
protecting against osteoporosis in vivo. The present study
revealed that sanguinarine protected mice from OVX-induced
osteoporosis in a dose-dependent manner. This protective effect on
osteoporosis may be due to suppressed osteoclast differentiation,
as well as enhanced bone mineralization from enhanced osteoblast
and osteocyte formation, which was supported by reduced
osteoclast-specific gene expression, and increased osteoblast- and
osteocyte-associated gene expression. Therefore, the results of the
present study indicate a beneficial effect of sanguinarine on bone
loss.
Currently, the treatment for osteoporosis primarily
focuses on bone remodeling performed by osteoclastic bone
resorption and osteoblastic bone formation (17). The coupling process from bone
resorption to formation is important for bone homeostasis (18). Suppressing bone resorption and
enhancing bone formation are favorable options for preventing
osteoporosis. For example, bisphosphonates increase the bone
mineral density and reduce fractures by suppressing bone
resorption, and also by exhibiting the side effect of delayed or
absent fracture healing due to the inhibition of bone formation
(19). Similarly, parathyroid
hormone (PTH) increases bone formation while also stimulating
resorption, which compromises its net anabolic effect on the bones
(20). Therefore, a previous study
proposed combining PTH and bisphosphonates for treating
osteoporosis (21). Strontium
ranelate has a dual effect, it stimulates osteoblast
differentiation and inhibits osteoclast formation and resorption
(22), and it has been used in
clinical settings. Other drugs, including semaphorin 3A,
N-oleoyl-L-serine and follicle stimulating hormone antibody may
decouple bone remodeling; however, investigations are ongoing
(23–25). Therefore, identification of a novel
drug that promotes bone formation and reduces bone resorption
simultaneously may be an appropriate direction for osteoporosis
therapy.
There are several limitations to the present study.
Although the results of the current study indicate that
sanguinarine may affect osteoblasts and osteocytes, the mechanism
of action of sanguinarine on osteoblasts and osteocytes requires
further investigation. Furthermore, as the present study examined
the effect of sanguinarine on bones, the potential side effects on
mice were not investigated. The results of the present study
demonstrated that the natural compound sanguinarine may prevent
OVX-induced bone loss by inhibiting osteoclastic bone resorption
and enhancing osteoblast bone formation in vivo, indicating
that it is a potential option for preventing osteoporosis.
Acknowledgements
This study was supported by the National Nature
Science Fund of China (grant nos. 81171739, 81101378, 81271971,
81271972 and 31270997), Natural Science Fund of Zhejiang Province
(grant no. Y2110372), Funds of Science and Technology Department of
Zhejiang Province (grant no. 2009C03014-1), and Zhejiang Provincial
Program for the Cultivation of High-level Innovative Health
Talents.
References
|
1
|
Sipos W, Pietschmann P, Rauner M,
Kerschan-Schindl K and Patsch J: Pathophysiology of osteoporosis.
Wien Med Wochenschr. 159:230–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weitzmann MN and Pacifici R: Estrogen
deficiency and bone loss: An inflammatory tale. J Clin Invest.
116:1186–1194. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhai YK, Pan YL, Niu YB, Li CR, Wu XL, Fan
WT, Lu TL, Mei QB and Xian CJ: The importance of the prenyl group
in the activities of osthole in enhancing bone formation and
inhibiting bone resorption in vitro. Int J Endocrinol.
2014:9219542014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yogesh HS, Chandrashekhar VM, Katti HR,
Ganapaty S, Raghavendra HL, Gowda GK and Goplakhrishna B:
Anti-osteoporotic activity of aqueous-methanol extract of Berberis
aristata in ovariectomized rats. J Ethnopharmacol. 134:334–338.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee WS, Lee EG, Sung MS and Yoo WH:
Kaempferol inhibits IL-1β-stimulated, RANKL-mediated
osteoclastogenesis via downregulation of MAPKs, c-Fos, and NFATc1.
Inflammation. 37:1221–1230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tyagi AM, Srivastava K, Singh AK, Kumar A,
Changkija B, Pandey R, Lahiri S, Nagar GK, Yadav DK, Maurya R, et
al: Formononetin reverses established osteopenia in adult
ovariectomized rats. Menopause. 19:856–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H,
Ouyang Z, Xu X, Wang W, Fan Q, et al: The effect of enoxacin on
osteoclastogenesis and reduction of titanium particle-induced
osteolysis via suppression of JNK signaling pathway. Biomaterials.
35:5721–5730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouyang Z, Zhai Z, Li H, Liu X, Qu X, Li X,
Fan Q, Tang T, Qin A and Dai K: Hypericin suppresses osteoclast
formation and wear particle-induced osteolysis via modulating ERK
signalling pathway. Biochem Pharmacol. 90:276–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Zhai Z, Liu G, Tang T, Lin Z, Zheng
M, Qin A and Dai K: Sanguinarine inhibits osteoclast formation and
bone resorption via suppressing RANKL-induced activation of NF-κB
and ERK signaling pathways. Biochem Biophys Res Commun.
430:951–956. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu X, Zhai Z, Liu X, Li H, Ouyang Z, Wu C,
Liu G, Fan Q, Tang T, Qin A and Dai K: Dioscin inhibits osteoclast
differentiation and bone resorption though down-regulating the Akt
signaling cascades. Biochem Biophys Res Commun. 443:658–665. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mackraj I, Govender T and Gathiram P:
Sanguinarine. Cardiovasc Ther. 26:75–83. 2008.PubMed/NCBI
|
|
13
|
American Psychological Association:
Guidelines for Ethical Conduct in the Care and Use of Nonhuman
Animals in Research. Washington, DC: American Psychological
Association; 2012, http://www.apa.org/science/leadership/care/guidelines.aspx
|
|
14
|
Thompson DD, Simmons HA, Pirie CM and Ke
HZ: FDA Guidelines and animal models for osteoporosis. Bone.
17:125S–133S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Muller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Das S and Crockett JC: Osteoporosis-a
current view of pharmacological prevention and treatment. Drug Des
Devel Ther. 7:435–448. 2013.PubMed/NCBI
|
|
18
|
Buck DW III and Dumanian GA: Bone biology
and physiology: Part I. The fundamentals. Plast Reconstr Surg.
129:1314–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Odvina CV, Zerwekh JE, Rao DS, Maalouf N,
Gottschalk FA and Pak CY: Severely suppressed bone turnover: A
potential complication of alendronate therapy. J Clin Endocrinol
Metab. 90:1294–1301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zaidi M and Iqbal J: Translational
medicine: Double protection for weakened bones. Nature. 485:47–48.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YF, Zhou CC, Li JH, Luo E, Zhu SS, Feng
G and Hu J: The effects of combined human parathyroid hormone
(1–34) and zoledronic acid treatment on fracture healing in
osteoporotic rats. Osteoporos Int. 23:1463–1474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonnelye E, Chabadel A, Saltel F and
Jurdic P: Dual effect of strontium ranelate: Stimulation of
osteoblast differentiation and inhibition of osteoclast formation
and resorption in vitro. Bone. 42:129–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu LL, Blair H, Cao J, Yuen T, Latif R,
Guo L, Tourkova IL, Li J, Davies TF, Sun L, et al: Blocking
antibody to the β-subunit of FSH prevents bone loss by inhibiting
bone resorption and stimulating bone synthesis. Proc Natl Acad Sci
USA. 109:14574–14579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smoum R, Bar A, Tan B, Milman G,
Attar-Namdar M, Ofek O, Stuart JM, Bajayo A, Tam J, Kram V, et al:
Oleoyl serine, an endogenous N-acyl amide, modulates bone
remodeling and mass. Proc Natl Acad Sci USA. 107:17710–17715. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayashi M, Nakashima T, Taniguchi M,
Kodama T, Kumanogoh A and Takayanagi H: Osteoprotection by
semaphorin 3A. Nature. 485:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|


















