Introduction
Cells maintain their numbers by dividing, repairing,
growing and dying through the cell cycle under normal conditions.
However, an abnormal recovery mechanism following damage to cells
may result in cell death. These cells may be genetically modified,
grow excessively and become a malfunctioning cell mass, or cancer
(1,2). Cancers are one of the most common
conditions globally, with >1.5 million treated for cancer in
2010, according to the US National Cancer Institute (Rockville, MD,
USA). The South Korean National Cancer Information Center reported
that ~1 in every 3 people will develop cancer at some point in
their lifetime (3). The incidence
of all patients with cancers was estimated to be 445.3/100,000
people in 2012.
Cancer cells divide rapidly, proliferate and invade
surrounding tissues and organs, which eventually malfunction and
are destroyed. Because of these cancer cell characteristics, it is
difficult to treat cancer by only killing cancer cells without side
effects affecting normal cells. Cancer treatment is divided into
chemotherapy, radiation therapy and surgery (4). Chemotherapy involves drugs that kill
or weakens cancer cells directly, however it is expensive, and the
courses are long in duration. Chemotherapy often causes a variety
of side effects, such as excruciating pain, anemia, decreased
numbers of white blood cells and platelets, vomiting, diarrhea,
reproductive disorders and chronic fatigue (5,6).
Thus, new methods to improve the clinical response to cancer
chemotherapy with drugs that have fewer or no side effects are
required.
Flavonoids are secondary plant metabolites and are
biologically active polyphenolic compounds (7,8).
Among the various types of flavonoids, luteolin
(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromenone) is a flavone
in many substances, including celery, broccoli, green pepper,
parsley and thyme (9). Several
cellular and molecular biology studies have demonstrated that
luteolin may possess anticancer activities (10,11).
Luteolin has a strong inhibitory effect on the nuclear factor-κB
pathway, which is continuously active in cancer cells (12). Proliferation of many kinds of
cancer cells has been previously inibited by luteolin, including
lung (A549), colon (HCT116), liver (HepG2), breast (MCF7/6), tongue
(SCC-4), cervix (HeLa) and leukemia (HL-60) cells (13–19).
Nevertheless, inhibiting cancer cell proliferation is a block in
the development of new anticancer agents, because the details of
the molecular mechanism remain unclear. Therefore, the authors
tested the rat PC12 adrenal medulla pheochromocytoma cell line,
which characteristic of neuroblastic and eosinophilic cells, as a
model to examine the induction of endoplasmic reticulum (ER)
stress-mediated apoptosis.
The ER is a membrane-bound intercellular organelle
where lipid biosynthesis, post-translational modification, folding,
processing and trafficking of secreted and membrane-bound proteins
occurs (20). ER molecular
chaperones serve central roles, and the binding immunoglobulin
protein (BiP) is the most representative chaperone; therefore, it
can be used as an ER stress marker. The cellular response to ER
stress is called the unfolded protein response (UPR), in which
three ER stress sensors, inositol requiring enzyme 1 (IRE1),
PKR-like ER kinase (PERK) and activating transcription factor 6
(ATF6), are downstream components of ER chaperones (21–24).
Although luteolin-induced ER stress has been reported, little is
known about the mechanisms of luteolin-induced apoptosis. In the
current study, PC12 cells were used to understand the molecular
mechanism of luteolin-induced activation of the UPR pathway. The
results hope to provide clues for the therapeutic effects of
luteolin on apoptosis through ER stress.
Materials and methods
Cell culture
PC12 cells were purchased from American Type Culutre
Collection (cat. no. CRL-1721; Manassas, VA, USA) and cultured on
collagen-coated flasks in 85% RPMI-1640 medium, supplemented with
25 mM HEPES buffer, 10% heat-inactivated horse serum, 5%
heat-inactivated fetal bovine serum, 2 mM L-glutamine, 1 mM sodium
pyruvate, 1 g/l d-(+)-glucose, 25 µg/ml streptomycin and 25 U/ml
penicillin (all Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The cells were maintained in a humidified incubator at 37°C
in a 5% CO2 atmosphere, and the medium was changed every
2 days. The cells were rinsed with 1X DPBS and detached with 0.25%
trypsin/EDTA (both Gibco; Thermo Fisher Scientific, Inc.). After
centrifugation at 1,000 × g for 5 min, the cells were subcultured
in 25 cm2 flasks using 1:2-1:4 subcultivation ratios and
were photographed every 24 h with an inverted microscope. Cells
were passaged twice weekly. The 80% confluent monolayer of PC12
cells was treated with luteolin at the indicated doses and times.
Total RNA from cultured cells was extracted using an RNA isolation
reagent (TRI-Reagent; Ambion; Thermo Fisher Scientific, Inc.) and
measured using the Nanodrop 1000 spectrophotometer (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). The resulting RNA was used
for the following reverse transcription-quantitative polymerase
chain reaction experiments.
MTT assay
Cell viability measurements by MTT assay. Growth and
viability of PC12 cells were determined using MTT from
Sigma-Aldrich; Merck KGaA (Darmadst, Germany). The cells were
seeded in 96-well plates (at 60 to 80% confluency) and treated with
luteolin for 0 (control), 4 and 16 h. MTT solution (0.5 mg/ml) was
added to each well, and the plates were incubated for an additional
4 h at 37°C. Following removal of the medium, the formazan crystals
were solubilized in DMSO (Sigma-Aldrich; Merck KGaA). Color
development was monitored at 595 nm with a reference wavelength of
650 nm using the Sunrise™ microplate reader (Tecan Trading AG,
Männedorf, Switzerland).
Hoechst 33342 staining
Following treatment with luteolin, PC12 cells were
incubated for 30 min with Hoechst 33342 (Molecular Probes; Thermo
Fisher Scientific, Inc.) loading dye and washed three times in
ice-cold 1X PBS. Following staining for 10 min, the stained cells
were monitored using a fluorescence microscope (Axio Scope A1;
Zeiss GmbH, Jena, Germany) at 340 nm.
DNA fragmentation assay
Cells were lysed in 100 µl 10 mM Tris-HCl buffer (pH
7.4) containing 10 mM EDTA and 0.5% Triton X-100. Following
centrifugation for 5 min at 16,000 × g, the supernatant was treated
with RNase A and proteinase K (Promega Corporation, Madison, WI,
USA). Subsequently, 20 µl of 5 M NaCl and 120 µl isopropanol were
added and kept on ice for 1 h. Following centrifugation for 15 min
at 16,000 × g, the DNA pellets were dissolved in 20 µl TE buffer
(10 mM Tris-HCl and 1 mM EDTA). The DNA samples were loaded onto a
0.7% agarose gel and observed using a UV source after ethidium
bromide (Sigma-Aldrich; Merck KGaA) staining.
Semiquantitative reverse
transcription-polymerase chain reaction (RT-PCR)
RT-PCR conditions included 30 cycles of the
following: 94°C for 30 sec, 58°C for 30 sec and 72°C for 1 min (10
min in the final cycle) using the below primers with Taq DNA
polymerase (Solgent Co., Ltd., Daejeon, Korea). The RT-PCR primers
were supplied by Bioneer Corporation (Daejeon, Korea). All
chemicals were purchased from Sigma-Aldrich; Merck KGaA. The RT-PCR
primers are as follows: IRE1 forward, 5′-ACCACCAGTCCATCGCCATT-3′
and reverse, 5′-CCACCCTGGACGGAAGTTTG-3′; ATF6 forward,
5′-CTAGGCCTGGAGGCCAGGTT-3′ and reverse, 5′-ACCCTGGAGTATGCGGGTTT-3′;
PERK forward, 5′-GGTCTGGTTCCTTGGTTTCA-3′ and reverse,
5′-TTCGCTGGCTGTGTAACTTG-3′; BiP forward, 5′-AGTGGTGGCCACTAATGGAG-3′
and reverse, 5′-TCTTTTGTCAGGGGTCGTTC-3′; β-actin forward,
5′-ACATCAAATGGGGTGATGCT-3′ and reverse, 5′-AGGAGACAACCTGGTCCTCA-3′.
The figure presented the results of a representative experiment
from three experiments with different samples.
Western blot analysis
PC12 cells were scraped and lysed by adding SDS
sample buffer [62.5 mM Tris-HCl (pH 6.8), 6% (w/v) SDS, 30%
glycerol and 125 mM DTT. Protein concentration was determined as
described previously (25).
Protein (15 µg) was separated by 10% SDS-PAGE gel electrophoresis.
The proteins were transferred to a nitrocellulose membrane, and the
membranes were blocked by the 5% skim milk in 0.1% TBST (TBS with
0.1% Tween-20) for 1 h at room temperature and incubated with the
primary antibodies (all 1:1,000 dilution) overnight at 4°C. The
rabbit anti-eIF2α antibody (cat. no. sc-133132), eIF2α-P antibody
(cat. no. sc-133132p) and goat anti-actin antibody (cat. no.
sc-1616-r) were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The horseradish peroxidase-conjugated
anti-rabbit (cat. no. sc-2004), anti-goat (cat. no. sc-2020) and
anti-mouse (cat. no. sc-2005) IgG secondary antibodies were
obtained from Santa Cruz Biotechnology, Inc. and were reacted with
a 1:1,000 dilution for 1 h at room temperature. Goat anti-actin
antibody was used to standardize the quantity of sample proteins.
The mouse anti-ATF6 antibody (cat. no. NBP1-40256) was obtained
from Novus Biologicals, LLC (Littleton, CO, USA). The blots were
developed using an enhanced chemiluminescence western blotting
detection system kit (Amersham; GE Healthcare Life Sciences,
Chalfont, UK). Experiments were performed in triplicate and the
protein bands were quantified using ImageJ software (version 1.48;
https://imagej.nih.gov/ij/).
Statistical analysis
All statistical analyses were performed using the
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Analysis of
variance and Tukey-honest significant difference post hoc tests
were performed to analyze the statistical significance. P<0.05
was considered to indicate a statistically significant difference.
Data are expressed as the mean ± standard deviation, or as median
values, in accordance with Gaussian distribution. All variables
included in the regression analysis respected a linear
distribution; when necessary, variables were linearized and checked
for normality.
Results and discussion
Luteolin (Fig. 1A)
is a flavone present in parsley, artichoke, celery and green pepper
(26,27). These plants have long been used in
traditional medicine to treat a broad range of diseases. Luteolin
inhibits growth of many cell types (28). The preliminary results using PC12
cells demonstrated morphological differences between
luteolin-treated and control cells, as well as an increase in the
number of floating cells in the medium (data not shown). This
finding suggested that luteolin leads to cell death. The authors
then tested the effects of 100 µM luteolin on cell viability in the
MTT assay following 4 and 16 h of treatment. Luteolin treatment
inhibited the PC12 cell growth time-dependently, and
luteolin-treated cell growth was reduced by half, following 4 h
(Fig. 1B). This result suggested
that apoptosis was induced based on cell shrinkage and extensive
detachment of the cells (29). A
total of two experiments were performed to determine whether
luteolin induces chromatin condensation and DNA fragmentation,
which are hallmarks of apoptosis. As a result, different nuclei
were observed in cells treated with and without luteolin following
Hoechst 33342 staining (Fig. 1C).
Next, inter-nucleosomal DNA fragmentation increased
time-dependently, which is the typical ladder pattern of apoptosis
(Fig. 1D). These results indicated
that luteolin-induced inhibition of cell growth is associated with
induction of apoptosis in PC12 cells.
The UPR in mammalian cells comprises three separate
ER stress sensors. These are downstream components of ER
chaperones, which transmit stress signals from the ER to the
nucleus. IRE1 activates the endonuclease domains, which cleave
X-box DNA-binding protein (XBP) mRNA, generating an activated form
of the XBP1 protein (30).
Activating PERK results in phosphorylation of the eIF2α subunit and
inhibits translation initiation (31). ATF6 is cleaved at the cytosolic
face of the membrane in response to ER stress, and the resulting
N-terminal cytoplasmic domain subsequently binds to both the ER
stress-response element and ATF6 sites, to enhance expression of ER
molecular chaperone genes (32,33).
Binding immunoglobulin protein (BiP) binds transiently to newly
synthesized proteins in the ER when a cell is ER stress-free.
However, stimulating ER stress induces interactions with misfolded,
underglycosylated and unassembled proteins through activation of
ATF6, IRE1 and PERK (34). BiP
eventually regulates the equilibrium between cell survival and
apoptosis (35). Moreover, BiP is
a principal regulator of ER stress signaling and survival in
ER-stressed cells. ER stress-induced apoptosis is a key
pathological event in various cancer cells (36,37).
To understand whether luteolin-induced apoptosis is
associated with ER stress, changes in the expression of BiP ATF6,
IRE1 and PERK were evaluated under luteolin-treated conditions in
PC12 cells. As presented in Fig.
2, treating PC12 cells with luteolin (10, 50 and 100 µM) for 16
h altered BiP mRNA expression (Fig.
2A), and it decreased in response to 100 µM luteolin following
1, 4, 8, 16 and 24 h of treatment (Fig. 2B). Expression of ATF6 and IRE1
tended to increase, whereas PERK expression decreased as luteolin
concentration was increased (Fig.
2C). The expression of all of the ER stress sensors decreased
over time reduced (Fig. 2D). In
summary, luteolin downregulated transcription of BiP and the ER
stress sensors gradually in a time-dependent manner, and
upregulated ATF6 and IRE1 mRNA expression in a dose-dependent
manner.
 | Figure 2.Expression of (A and B) ER chaperones
and (C and D) ER stress sensors at different luteolin doses and
times. PC12 cells were treated with luteolin (10, 50, 100 and 200
µM; panels A and C) for 1, 4, 8, 16 and 24 h (panels B and D). The
revere transcription-polymerase chain reaction results were
measured three times. Data is presented as the mean ± standard
deviation. *P<0.05 and **P<0.01 vs. C (control). ER,
endoplasmic reticulum; BiP, immunoglobulin heavy-chain binding
protein; ATF6, activating transcription factor 6; IRE1,
inositol-requiring kinase 1; PERK, PKR (protein kinase regulated by
RNA)-like ER-associated kinase; C, control. |
Furthermore, the authors investigated activation of
the ER stress sensors in response to luteolin treatment, such as
ATF6 fragmentation, eIF2-α phosphorylation and XBP1 mRNA splicing.
Under ER stress conditions, ATF6 is transported from the ER to the
Golgi complex, where it is sequentially cleaved by site 1 and site
2 proteases (38). An
anti-ATF6-specific antibody that recognizes a 50 kDa cleaved
fragment form of ATF6 was used to understand activation of ATF6
under ER stress conditions. Upon ER stress, PERK phosphorylates
eIF2-α to reduce biosynthesis of total mRNAs. Thus, we measured
eIF2α phosphorylation levels by western blotting to detect ER
stress conditions. The ER stress-mediated splicing of XBP1 is a
prerequisite for IRE1 activation, which controls the intensity of
XBP1 splicing (removing a 26-bp segment from the full-length XBP-1
mRNA) (39). RT-PCR analysis was
used to determine the unspliced and spliced isoforms of XBP1 mRNA.
PC12 cells were treated with 100 µM luteolin for 4 and 16 h
(Fig. 3). Although luteolin did
not induce ATF6 fragmentation (Fig.
3A), it gradually upregulated eIF2-α phosphorylation (Fig. 3B) and XBP1 mRNA splicing (Fig. 3C), suggesting that luteolin
triggered the UPR signaling pathway by activating the ER stress
sensors, except for ATF6. Phosphotidyl inositol 3-kinase (PI3 K) is
a key regulator of many cellular processes, including cell
survival, proliferation and differentiation (40). Under resting conditions, PI3K is
composed of p85 and p110 (41).
p85 interacts with other proteins (small GTPase cdc42, nuclear
receptor co-repressor and CD148) that complex and serve significant
roles in other cell signal pathways (42). Park et al (43) demonstrated that p85 interacts with
the spliced form of XBP-1 (XBP-1s), which increases both XBP-1 s
activity and the nuclear import of XBP-1s under ER stress
conditions. Thus, the authors examined whether phosphorylation of
p85 under ER stress conditions is related to luteolin stimulation.
As demonstrated in Fig. 3D,
short-term treatment (4 h) with luteolin induced higher levels of
phosphorylated p85 than those of long-term treatment (24 h). The
reason is unclear, but treatment with LY294002 upregulated
phosphorylated p85. These data suggested that inhibition of PI3 K
is associated with p85 phosphorylation through ER stress.
 | Figure 3.Luteolin controls endoplasmic
reticulum stress sensors. (A) Cells were treated with 100 µM
luteolin for 4 and 16 h. Cell lysates were subjected to western
blotting with mouse anti-ATF6 monoclonal antibody. (B) Western
blotting was performed using anti-eIF2-α antibody and eIF2-α-P
antibody against cells treated for different times (1, 4 and 16 h).
(C) The revere transcription-polymerase chain reaction analysis was
performed using mRNAs from Fig.
3A. The resulting PCR product was further digested by
PstI to reveal a restriction site that was lost following
XBP1 splicing under ER stress. The resulting XBP1 cDNA products
were revealed on a 2% agarose gel. Unspliced XBP1 mRNA produced the
two lower bands indicated by arrows (upper, 290 bp and lower, 183
bp). The spliced XBP1 mRNA indicated by a bold arrow. (D) Western
blotting was performed using a p85 antibody and p85-P antibody
against cells treated for 4 and 24 h. ATF6, activating
transcription factor; eIF2-alpha, translation initiation factor
eIF2-α; eIF2-alpha-P, phosphorylated form of translation initiation
factor eIF2α; XBP1, X-box binding protein 1; p85,
phosphatidylinositol 3-kinase 85 kDa regulatory subunit alpha;
p85-P, phosphorylated form of the phosphatidylinositol 3-kinase 85
kDa regulatory subunit alpha; C, control. |
If early cellular responses fail to maintain ER
homeostasis, ER stress activates the UPR to stimulate the apoptosis
pathway for cell survival. The authors investigated whether
luteolin induces both apoptosis and autophagy through the UPR, as
the exact mechanisms of the induction of ER apoptosis and autophagy
remain elusive. However, signaling through ER stress trigger
several regulators associated with the apoptosis pathway during
prolonged ER stress (44). A link
between the UPR and autophagy has been presented; as
phosphorylation of eIF2α modulate autophagy and ER stress-inducible
drugs induce autophagy (45). The
present results indicated that luteolin induced transcriptional
expression of pro-apoptotic Bax and Bad but decreased
anti-apoptotic Bcl-2 and Bcl-xl (Fig.
4A). Luteolin strongly downregulated Lc3 and Beclin
transcription levels (Fig. 4B),
suggesting that luteolin upregulates apoptosis and downregulates
autophagy in PC12 cells. In conclusion, although the underlying
mechanism of luteolin in cell death is still unclear, the present
study demonstrated luteolin regulates apoptosis and autophagy.
Therefore, it may serve as a novel strategy to treat cancer by
regulating the UPR signal.
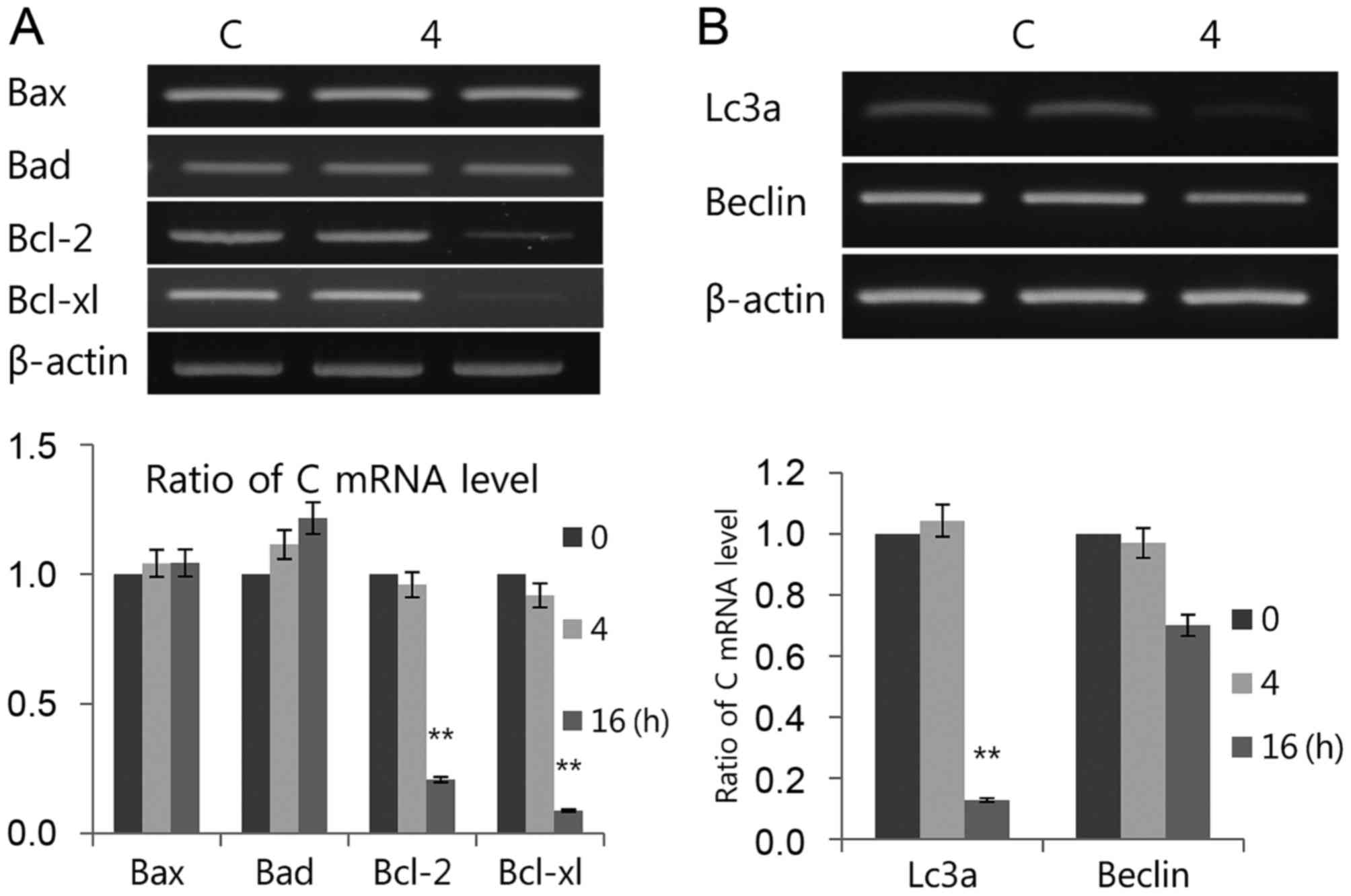 | Figure 4.Luteolin induces (A) apoptosis and
(B) autophagy. The mRNAs used for both tests were the same as those
in Fig. 3A. Bax, Bad, mRNA levels
of Bcl-2, Bcl-xl, LC3 and Beclin-1 were measured by RT-PCR. The
RT-PCR results were measured three times. Data is presented as the
mean ± standard deviation. β-actin was used as the loading control.
**P<0.01 vs. C (control). Bax, Bcl-2-associated X; Bad,
Bcl-2-associated death promoter; Bcl-2, B-cell lymphoma 2; Bcl-xl,
B-cell lymphoma/leukemia-x long; LC3, microtubule-associated
protein light chain 3; Beclin-1, coiled-coil moesin-like BCL2
interacting protein; RT-PCR, semiquantitative revere
transcription-polymerase chain reaction; C, control. |
In summary, luteolin treatment markedly induced
apoptosis via the unfolded protein response, including ER
chaperones and ER stress sensors, in PC12 cells. It is thought that
further understanding of the biological mechanisms underlying
luteolin-induced apoptosis may be useful in the prevention and
treatment of chronic or acute neurological disorders such as
Alzheimer's disease and Parkinson's disease as well as their
symptoms (back pain) and signs (aphasia), and also neurological
syndromes such as Aicardi syndrome.
Acknowledgements
The present study was supported by the research fund
of Chungnam National University.
References
|
1
|
Sage EK, Thakrar RM and Janes SM:
Genetically modified mesenchymal stromal cells in cancer therapy.
Cytotherapy. 18:1435–1445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharpe M and Mount N: Genetically modified
T cells in cancer therapy: Opportunities and challenges. Dis Model
Mech. 8:337–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung KW, Won YJ, Kong HJ, Oh CM, Cho H,
Lee DH and Lee KH: Cancer statistics in korea: Incidence,
mortality, survival, and prevalence in 2012. Cancer Res Treat.
47:127–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Runowicz CD, Leach CR, Henry NL, Henry KS,
Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge
SB, Jacobs LA, et al: American cancer society/american society of
clinical oncology breast cancer survivorship care guideline. J Clin
Oncol. 34:611–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Overbeek A, van den Berg MH, van Leeuwen
FE, Kaspers GJ, Lambalk CB and van Dulmen-den Broeder E:
Chemotherapy-related late adverse effects on ovarian function in
female survivors of childhood and young adult cancer: A systematic
review. Cancer Treat Rev. 53:10–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davoodi S, Mohammadzadeh Z and Safdari R:
Mobile phone based system opportunities to home-based managing of
chemotherapy side effects. Acta Inform Med. 24:193–196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crozier A, Burns J, Aziz AA, Stewart AJ,
Rabiasz HS, Jenkins GI, Edwards CA and Lean ME: Antioxidant
flavonols from fruits, vegetables and beverages: Measurements and
bioavailability. Biol Res. 33:79–88. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
López-Lázaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Somerset SM and Johannot L: Dietary
flavonoid sources in australian adults. Nutr Cancer. 60:442–449.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YJ, Lim T, Han MS, Lee SH, Baek SH,
Nan HY and Lee C: Anticancer effect of luteolin is mediated by
downregulation of TAM receptor tyrosine kinases, but not
interleukin-8, in non-small cell lung cancer cells. Oncol Rep.
37:1219–1226. 2017.PubMed/NCBI
|
|
11
|
Yan H, Wei P, Song J, Jia X and Zhang Z:
Enhanced anticancer activity in vitro and in vivo of luteolin
incorporated into long-circulating micelles based on DSPE-PEG2000
and TPGS. J Pharm Pharmacol. 68:1290–1298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia Z, Nallasamy P, Liu D, Shah H, Li JZ,
Chitrakar R, Si H, McCormick J, Zhu H, Zhen W and Li Y: Luteolin
protects against vascular inflammation in mice and
tnf-alpha-induced monocyte adhesion to endothelial cells via
suppressing IΚBα/NF-κB signaling pathway. J Nutr Biochem.
26:293–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang X, Wang H, Fan L, Wu X, Xin A, Ren H
and Wang XJ: Luteolin inhibits Nrf2 leading to negative regulation
of the Nrf2/ARE pathway and sensitization of human lung carcinoma
A549 cells to therapeutic drugs. Free Radic Biol Med. 50:1599–1609.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi RX, Ong CN and Shen HM: Luteolin
sensitizes tumor necrosis factor-alpha-induced apoptosis in human
tumor cells. Oncogene. 23:7712–7721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yee SB, Choi HJ, Chung SW, Park DH, Sung
B, Chung HY and Kim ND: Growth inhibition of luteolin on hepG2
cells is induced via p53 and Fas/Fas-ligand besides the TGF-β
pathway. Int J Oncol. 47:747–754. 2015.PubMed/NCBI
|
|
16
|
Attoub S, Hassan AH, Vanhoecke B, Iratni
R, Takahashi T, Gaben AM, Bracke M, Awad S, John A, Kamalboor HA,
et al: Inhibition of cell survival, invasion, tumor growth and
histone deacetylase activity by the dietary flavonoid luteolin in
human epithelioid cancer cells. Eur J Pharmacol. 651:18–25. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SF, Yang WE, Chang HR, Chu SC and
Hsieh YS: Luteolin induces apoptosis in oral squamous cancer cells.
J Dent Res. 87:401–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang L, Li Y, Chen WY, Zeng S, Dong LN,
Peng XJ, Jiang W, Hu M and Liu ZQ: Breast cancer resistance
protein-mediated efflux of luteolin glucuronides in HeLa cells
overexpressing UDP-glucuronosyltransferase 1A9. Pharm Res.
31:847–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng AC, Huang TC, Lai CS, Kuo JM, Huang
YT, Lo CY, Ho CT and Pan MH: Pyrrolidine dithiocarbamate inhibition
of luteolin-induced apoptosis through up-regulated phosphorylation
of Akt and caspase-9 in human leukemia HL-60 cells. J Agric Food
Chem. 54:4215–4221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pereira DM, Valentão P, Correia-da-Silva
G, Teixeira N and Andrade PB: Translating endoplasmic reticulum
biology into the clinic: A role for ER-targeted natural products?
Nat Prod Rep. 32:705–722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rutkowski DT and Kaufman RJ: A trip to the
ER: Coping with stress. Trends Cell Biol. 14:20–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mori K: Signalling pathways in the
unfolded protein response: Development from yeast to mammals. J
Biochem. 146:743–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noble JE: Quantification of protein
concentration using UV absorbance and Coomassie dyes. Methods
Enzymol. 536:17–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin LC, Pai YF and Tsai TH: Isolation of
luteolin and luteolin-7-O-glucoside from dendranthema morifolium
ramat Tzvel and their pharmacokinetics in rats. J Agric Food Chem.
63:7700–7706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai X, Lu W, Ye T, Lu M, Wang J, Huo J,
Qian S, Wang X and Cao P: The molecular mechanism of
luteolin-induced apoptosis is potentially related to inhibition of
angiogenesis in human pancreatic carcinoma cells. Oncol Rep.
28:1353–1361. 2012.PubMed/NCBI
|
|
28
|
Tuorkey MJ: Molecular targets of luteolin
in cancer. Eur J Cancer Prev. 25:65–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Flusberg DA and Sorger PK: Surviving
apoptosis: Life-death signaling in single cells. Trends Cell Biol.
25:446–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Li Q, She T, Li H, Yue Y, Gao S,
Yan T, Liu S, Ma J and Wang Y: IRE1α-XBP1 signaling pathway, a
potential therapeutic target in multiple myeloma. Leuk Res.
49:7–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Axten JM: Protein kinase R (PKR)-like
endoplasmic reticulum kinase (PERK) inhibitors: A patent review
(2010–2015). Expert Opin Ther Pat. 27:37–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimata Y and Kohno K: Endoplasmic
reticulum stress-sensing mechanisms in yeast and mammalian cells.
Curr Opin Cell Biol. 23:135–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Welihinda AA, Tirasophon W and Kaufman RJ:
The cellular response to protein misfolding in the endoplasmic
reticulum. Gene Expr. 7:293–300. 1999.PubMed/NCBI
|
|
34
|
Moreno JA and Tiffany-Castiglioni E: The
chaperone Grp78 in protein folding disorders of the nervous system.
Neurochem Res. 40:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dandekar A, Mendez R and Zhang K: Cross
talk between ER stress, oxidative stress, and inflammation in
health and disease. Methods Mol Biol. 1292:205–214. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Breckenridge DG, Germain M, Mathai JP,
Nguyen M and Shore GC: Regulation of apoptosis by endoplasmic
reticulum pathways. Oncogene. 22:8608–8618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clarke HJ, Chambers JE, Liniker E and
Marciniak SJ: Endoplasmic reticulum stress in malignancy. Cancer
Cell. 25:563–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martins AS, Alves I, Helguero L, Domingues
MR and Neves BM: The unfolded protein response in homeostasis and
modulation of mammalian immune cells. Int Rev Immunol. 35:457–476.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vanhaesebroeck B, Stephens L and Hawkins
P: PI3K signalling: The path to discovery and understanding. Nat
Rev Mol Cell Biol. 13:195–203. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohsaka Y and Nomura Y: Rat white
adipocytes activate p85/p110 PI3K and induce PM GLUT4 in response
to adrenoceptor agonists or aluminum fluoride. Physiol Int.
103:35–48. 2016.PubMed/NCBI
|
|
42
|
Vogt PK, Hart JR, Gymnopoulos M, Jiang H,
Kang S, Bader AG, Zhao L and Denley A: Phosphatidylinositol
3-kinase: The oncoprotein. Curr Top Microbiol Immunol. 347:79–104.
2010.PubMed/NCBI
|
|
43
|
Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung
J, Ueki K and Ozcan U: The regulatory subunits of PI3K, p85alpha
and p85beta, interact with XBP-1 and increase its nuclear
translocation. Nature Med. 16:429–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stefani IC, Wright D, Polizzi KM and
Kontoravdi C: The role of ER stress-induced apoptosis in
neurodegeneration. Curr Alzheimer Res. 9:373–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee WS, Yoo WH and Chae HJ: ER stress and
autophagy. Curr Mol Med. 15:735–745. 2015. View Article : Google Scholar : PubMed/NCBI
|


















