Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common cancer worldwide, representing over half a
million incidents every year (1,2).
Epidemiological studies have revealed an association between
exposure to carcinogens (such as tobacco and alcohol), infection by
oncogenic human papillomavirus types 16 and 18 (HPV16 and HPV18,
respectively) and the increased risk of HNSCC development (3). Currently, the treatment of HNSCC
consists of surgery followed by postoperative chemo- and/or
radiotherapy (4,5). However, the 5-year mortality rate of
patients with HNSCC has not improved, despite the advanced
treatment methods (6).
Cancer cells, including HNSCC, are characterized by
increased telomerase activity. This enzymatic complex is active in
~80 to 90% of all cancer types and is responsible for the
lengthening of telomeres (7).
Telomeres, as highly specialized nucleoproteins located at the end
of chromosomes, provide genomic stability, integrity and
immortalization of cells. The human telomeric sequence is composed
of hexamer repeats (5′-TTAGGG-3′) at the 3′ end strand. Human
telomeres have ~500 to 2,000 copies of hexamer repetitions, giving
rise to 3,000 to 12,000 base pairs (8–11).
Telomerase activity is controlled by a number of
mechanisms, including alternative splicing, posttranslational
modifications and activating or inhibiting factors. Thus, the
transcriptional regulation of the human telomerase reverse
transcriptase (hTERT) gene, followed by the regulation of
telomerase activity, is crucial (12). Highly recurrent point mutations in
the hTERT promoter have been reported in a number of human
malignancies such as melanoma and glioma. This mutation is located
at −146 bp (C250T) from the ATG start site of the hTERT gene
(13). This mutation generates
de novo E-Twenty six (ETS)/ternary complex factors
transcription-binding sites. This process results in increased
hTERT expression and it has been proposed as a novel mechanism of
activating telomerase in malignant cells (13,14).
A previous study investigating telomerase and telomere length,
indicated that their regulation may be controlled by factors
released during carcinogenesis such as hormones and cytokines,
including adiponectin and interleukin (IL)-6 (15). These changes, if they are present
in the human organism even in the local area (cancer initiation at
a single cell level), may be reflected by alterations in the whole
organism including leukocytes (16).
Considering the results of our previous studies
(16), it was hypothesized that
short telomere length and a hTERT mutation in leukocytes may be
evaluated as markers of prognosis and tumors presenting at a very
early stage of carcinogenesis. Thus, telomere length measurements
in leukocytes may be an effective, non-invasive method for the
predictive assessment of carcinogenesis. Therefore, the present
study focused on the potential application of telomere length and
hTERT mutation analysis as prognostic parameters of cancer
progression in HNSCC.
Materials and methods
Patients
The study group consisted of 46 patients (31 males
and 15 females; age 21–88 years, median=67, mean=63) who were
histologically diagnosed with HNSCC at various stages, and
different anatomical sites based on World Health Organization
criteria (Table I). All patients
were recruited between April 2015 and June 2016 from the Department
of Head and Neck Surgery at the Poznan University of Medical
Sciences and The Greater Poland Cancer Centre in Poznan, Poland.
The control group comprised of 49 healthy blood donors from Poznan
Regional Blood Center. The patients were Caucasians and the
majority were from the same region of Poland (Greater Poland). The
study protocol was approved by the Ethics Committee of the Poznan
University of Medical Sciences (Decision no. 446/15) and written
informed consent was provided by the participating individuals.
 | Table I.Characteristics of the present study's
cohort. |
Table I.
Characteristics of the present study's
cohort.
| Characteristic | Total number (n) |
|---|
| Healthy
individuals | 49 |
| TERT
promoter status [n(%)] |
|
|
T/T | 13 (26.5) |
|
C/T | 29 (59.2) |
|
C/C | 7 (14.3) |
| Cancer patients | 61 |
| Age
(years) |
|
|
Median | 63 |
|
Mean | 62 |
|
Range | 37 to 88 |
| Sex
[n(%)] |
|
|
Male | 49 (80.3) |
|
Female | 12 (19.7) |
| Tumor
stage (TNM classification) [n(%)] |
|
|
T1 | 11 (18.0) |
|
T2 | 22 (36.1) |
|
T3 | 17 (27.9) |
|
T4 | 11 (18.0) |
|
Anatomical site (n) |
|
|
Mouth (including
lip) | 25 |
|
Voice box
(larynx) | 25 |
|
Nose and
sinuses | 5 |
|
Throat
(pharynx) | 6 |
| TERT
promoter status [n(%)] |
|
|
T/T | 23 (37.7) |
|
C/T | 16
(26.2)a |
|
C/C | 22
(36.1)b |
| Total study cohort
(n) | 110 |
Exclusion criteria
Following the study protocol, patients with local
recurrences, second primary tumors and were HPV positive were
excluded from experimental groups. Patients with a previous history
of chemo- or radiotherapy were also excluded.
DNA isolation
DNA was extracted from 300 µl peripheral blood
mononuclear cells (PBMCs) using a Wizard Genomic DNA Purification
kit (Promega Corporation, Madison, WI, USA) according to
manufacturer's protocol. DNA was then stored at −20°C until
analysis.
Quantitative polymerase chain reaction
(qPCR)
Assessment of relative telomere length
Telomere length was assessed using 2 pairs of
primers, one telomere-specific and one a single copy gene-specific
(albumin) (Table II), as
previously described (16–18). Briefly, specific primers with an
efficiency close to 100% (98±2%), were used in a SYBR Green-based
assay with FastStart Essential SYBR Green I Master (Roche Applied
Science, Penzberg, Germany). Initial denaturation and polymerase
activation (hot start) was performed at 95°C for 10 min. The signal
was detected during 45 cycles of 95°C for 10 sec, 61°C for 10 sec
and 72°C for 10 sec. Melting analysis (65 to 95°C; 0.2°C
resolution) was performed at the end of the reaction to verify
specificity of the product. Telomere length was assessed using a
qPCR system (LC 96; Roche Diagnostics, Basel, Switzerland), and
calculated using the 2−ΔΔCq method (19).
 | Table II.Primer sequences for quantitative
polymerase chain reaction. |
Table II.
Primer sequences for quantitative
polymerase chain reaction.
| Primer name | Sequence
(5′-3′) | Amplicon length
(base pairs) |
|---|
| ALB_F | TTT GCA GAT GTC AGT
GAA AGA GA | 69 |
| ALB_R |
TGGGGAGGCTATAGAAAATAAGG |
|
| Telo_F |
ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT | 91 |
| Telo_R |
GTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA |
|
| TERT_HRM_F |
CTGCCCCTTCACCTTCCAG | 159 |
| TERT_HRM_R |
AGCGCTGCCTGAAACTCG |
|
High resolution melting (HRM) mutation
analysis
To identify the C250T hTERT promoter mutation, HRM
analysis using specific primers was performed (sequences listed in
Table II). The reaction was
optimized to 10 ng genomic DNA, 0.8 µM of each primer, 2.5 mM
magnesium chloride and 5 µl of LightCycler 480 High Resolution
Melting Master Mix (2X; Roche Applied Science, Penzberg, Germany),
in a total volume of 10 µl. The initial denaturation and polymerase
activation (hot start) was performed at 95°C for 10 min. The signal
was detected during another 45 cycles of 95°C for 10 sec, 57°C for
15 sec and 72°C for 10 sec. HRM analysis (65 to 95°C; 0.07°C
resolution) at the end of the reaction was performed to identify
different variants of the hTERT mutation. The HRM analysis was
performed using a qPCR system (LC 96; Roche Diagnostics, Basel
Switzerland).
Sequencing
A total of 10% of samples were verified by
sequencing using the BigDye v3.1 kit according to the
manufacturer's instructions (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and separation by ethanol
extraction using the ABI Prism 3130XL (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in a 36 cm capillary and a POP7
polymer.
Statistical analysis
Statistical analysis was performed using Student's
t-test, two-way analysis of variance, χ2 and Fisher's
exact tests, which were calculated using GraphPad Prism version 5
(GraphPad Software, Inc., La Jolla, CA, USA). Data is presented as
the mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference.
Results
Telomere length assessment
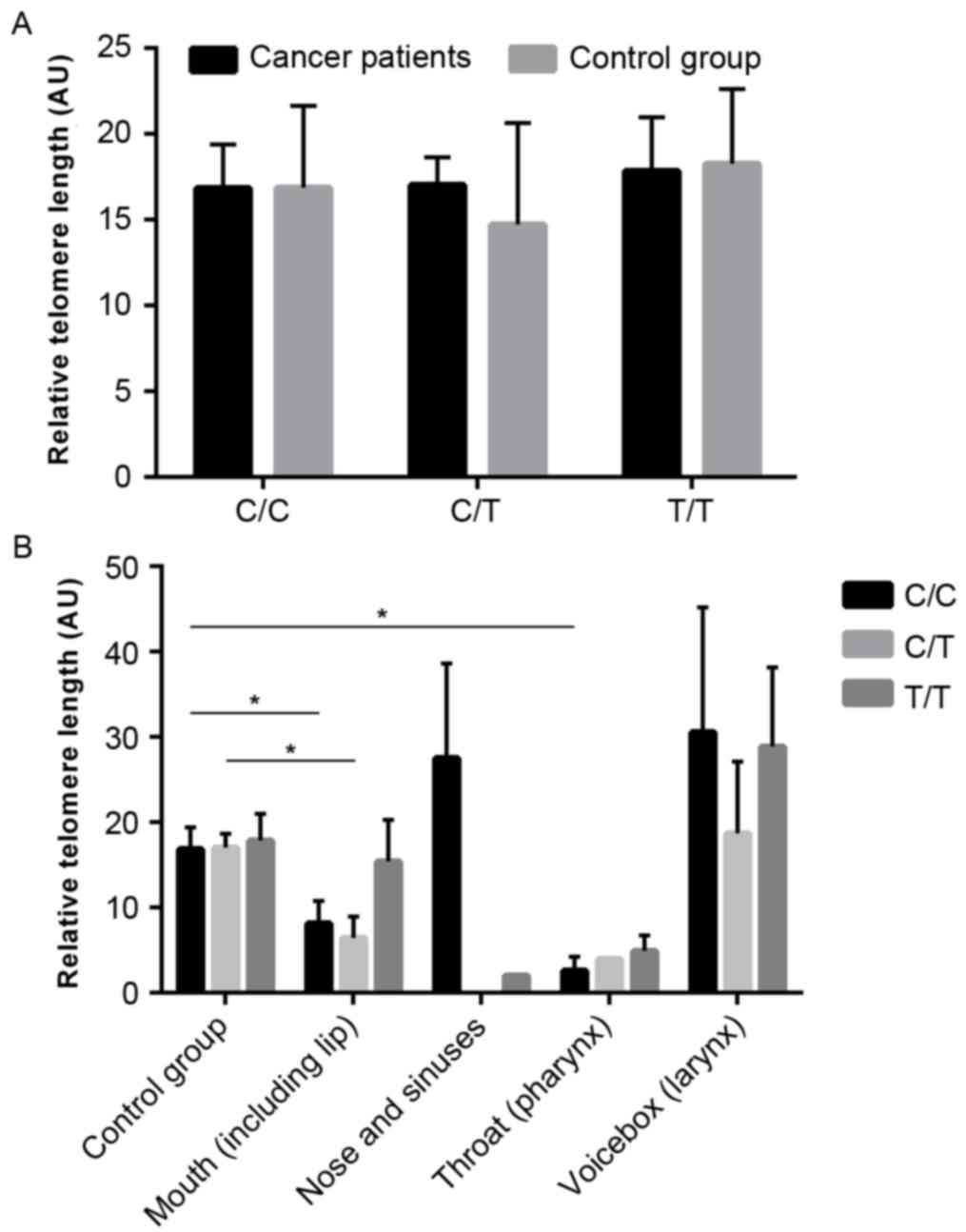
The average relative telomere length (AU) in the
cancer and control groups (14.06±2.109 and 19.06±1.801,
respectively) was evaluated, and no significant difference was
observed (P=0.787; Fig. 1A).
Telomere length in leukocytes in relation to tumor invasion
according to the TNM Classification of Malignant Tumors (TNM) was
also determined. Telomeres in leukocytes from individuals with T2
HNSCC cancer were significantly shorter when compared with those of
healthy individuals (6.329±1.864 and 19.06±1.801, respectively;
P=0.0001; Fig. 1B). There was also
a significant difference in telomere length between T2 and T3
patients (6.329±1.864 and 16.94±3.301, respectively; P=0.0063;
Fig. 1B), and T2 and T4 patients
(6.329±1.864 and 26.3±7.615, respectively; P=0.0028; Fig. 1B). In addition, there was trend
towards shortened telomeres in the first two tumor invasion stages
(P=0.0003; Fig. 1B).
The analysis of telomere lengths in different
anatomical sites of patients with HNSCC demonstrated that there was
a significant difference between healthy individuals and patients
affected by mouth cancer (19.06±1.801 and 10.76±2.365,
respectively; P=0.0083; Fig. 1C)
and pharyngeal cancer (19.06±1.801 and 3.985±1.03, respectively;
P=0.0063; Fig. 1C).
TERT promoter C250T mutation
assessment-TNM classification
The hTERT promoter mutation was identified in 36% of
the patients with HNSCC, while 27% of healthy individuals also
carried the mutation (Table I and
Fig. 2A). However, the
heterozygous variant was observed in 26% of the HNSCC group and 59%
of the control group (P<0.0001; Table I and Fig. 2A). Notably, when compared with the
control group (14%), 38% of the cancer types had the C wild-type
allele (P≤0.0001; Table I and
Fig. 2A).
The analysis of the C250T mutations indicated that
there is a significant association between the frequency of the
homozygous mutation and the grade of the tumor (T1=27%; T2=36%;
T3=35%; T4=46%; P≤0.0001; Fig.
2B).
An opposite trend was identified in the case of the
wild-type allele as there was a decreasing frequency of the wild
type C allele with increasing tumor advancement (P≤0.0001), and a
significantly higher frequency of the wild type allele in T1=55%,
T2=36%, T3=35% and T4=27% when compared with the control group
(14%; P≤0.0001; Fig. 2B).
TERT promoter C250T mutation
assessment-anatomical sites
The C250T mutation was identified in 40% of patients
with mouth cancer (P=0.001; Fig.
2C) and in 20% of patients with nose and sinuses cancer
(P=0.0018; Fig. 2C), compared with
27% of healthy individuals. In addition, a nonsignificant trend for
increased incidence of the C250T mutation was observed in
pharyngeal cancer (50%) and laryngeal cancer (32%) compared with
healthy individuals (Fig. 2C).
Differences in C allele status were also revealed.
In the control group, 14% of samples contained the wild type
homozygous allele (Fig. 2C); by
comparison, 44% of mouth cancer (P=0.001), 80% of nose and sinuses
cancer (P=0.0018), 33% of pharyngeal cancer and 24% of laryngeal
cancer were homozygous for the wild type allele (Fig. 2C).
Comparisons between C250T variant and telomere
length revealed no significant differences between patients with
HNSCC and the healthy control group (Fig. 3A). However, it was revealed that
telomeres were significantly shorter in patients with hTERT-wild
and heterozygous mouth cancer than in the control group (P=0.0401
and P=0.0252, respectively; Fig.
3B). Significantly shorter telomeres were also observed in
hTERT-wild (P=0.026) pharyngeal cancer. The results exhibited a
trend suggesting that hTERT-mutant laryngeal tumors have longer
telomeres than the control group, however the differences were not
significant (28.861±8.198 and 17.849±3.115, respectively; P=0.195;
Fig 3B).
Discussion
In the present study, the frequency of the C250T
hTERT promoter mutation in patients with HNSCC, identification of
the mutation associated with telomere length and the association
between cancer advancement and telomere length and mutation status
was determined.
There have been a number of studies that have
correlated telomere length in leukocytes and cancer risk, however
only a few focused on head and neck cancer. Zhang et al
(20) demonstrated that short
telomere length in PBMCs is strongly associated with a moderately
increased risk of oropharyngeal squamous cell carcinoma however,
not with increased risk of oral cavity cancer. Associations between
telomere length and a higher risk of HPV16-positive oropharyngeal
carcinoma and tumor HPV16 status were also revealed (20). Bau et al (21) observed that short leukocyte
telomere length was associated with an increased risk of developing
oral premalignant lesions and precursors of oral squamous cell
carcinoma. However, the opposite was revealed by Liu et al
(22) who reported that relative
telomere length may not be important in HNSCC carcinogenesis. A
correlation between mouth cancer and pharyngeal cancer, and
significantly shorter telomeres when compared to the control group
was identified in the present study. Similar results were obtained
by Bau et al (21) and
Zhang et al (20). These
studies demonstrated that short telomeres were a consistent feature
of oral and pharyngeal cancer. Notably, the present study
demonstrated that leukocytes from individuals with early stage T2
HNSCC tumors are characterized by shorter telomeres than leukocytes
from healthy donors. The expected increase in the length of
telomeres was observed in leukocytes from T3 and T4 patients when
compared with T2 individuals. There is evidence that suggests that
the advancement of the tumors may correlate with long telomeres and
poor prognosis (23). Increased
telomere length indicates that telomere maintenance may also be
important for the progression of HNSCC.
The presence of the C250T hTERT promoter mutation
results in increased hTERT expression, for which a novel mechanism
of activating telomerase in malignant cells has been proposed
(24). Only a few studies have
described the correlation between hTERT mutation status and the
risk of HNSCC. In the present study, hTERT promoter mutations were
identified in 36% of patients with HNSCC. Similar results have been
obtained by Vinothkumar et al (25). The correlation between two hot spot
mutations and the patient's clinicopathological phenotype was
analyzed by the authors and a relatively high frequency of hTERT
hot spot mutations in oral squamous cell carcinoma was identified.
Qu et al (26) observed
hTERT promoter mutations in a large group of laryngeal cancers. A
strong association between these mutations and poor survival rates
in patients with laryngeal cancer was demonstrated as an
independently variable with respect to tumor localization or tumor
invasion. An increased frequency of hTERT mutations in laryngeal
and pharyngeal cancers was also established in the present study. A
significantly higher frequency of the hTERT mutation was revealed
in patients with mouth cancer. Notably, the opposite result was
observed in nose and sinuses cancer. In addition, a significantly
higher frequency of the hTERT mutation was observed in more advance
tumors with increasing tendency. The results highlight the
potential influence of the hTERT promoter mutation in the
progression of HNSCC. Notably, trends towards the decreasing
frequency of wild allele with increasing tumor advancement, and a
significantly higher frequency of C allele in T1, T2 and T3 tumors
when compared to the control group were also described.
By contrast with the present study, the majority of
previous studies in the head and neck area were focused on thyroid
cancer. Vingare et al (24)
demonstrated that the hTERT promoter mutations are relatively
frequent in specific types of human cancers including thyroid
cancer. Liu et al (27)
also revealed that hTERT promoter mutations are common,
particularly in follicular thyroid cancer and BRAF
mutation-positive papillary thyroid cancer, and are associated with
aggressive clinicopathological phenotypes. In addition, the
mutation in the hTERT promoter was associated with decreased
survival in patients with papillary thyroid carcinoma (28), and the hTERT promoter mutation is
particularly prevalent in aggressive thyroid cancers (29). Notably, the present study revealed
a trend with an increased frequency of the wild allele in mouth,
nose and sinuses, pharyngeal and laryngeal cancers when compared to
healthy individuals.
In addition, the results revealed significantly
shorter telomeres in hTERT-wild and heterozygous mouth cancer, and
in hTERT-wild pharyngeal cancer, when compared with the control
group. To the best of our knowledge this association has not
previously been identified in HNSCC. These results indicate that
the hTERT promoter mutation may increase or decrease telomere
length depending on the tumor localization and advancement of
cancer.
In conclusion, the results demonstrated that the
C250T hTERT promoter mutation may represent a common event during
carcinogenesis in patients with HNSCC, which together with telomere
length assessment may be one of the molecular markers of HNSCC
progression. The present study also revealed an association between
short telomeres and the presence of the C250T mutation, which
depends on the anatomical site of cancer and tumor invasion status.
However, long or short telomeres in the PBMCs of patients with
HNSCC do not necessarily indicate the presence or absence of the
hTERT mutation, therefore the two parameters should be considered
to characterize patient status. The present study has highlighted
that there should be a greater emphasis on using the length of
telomeres in PBMCs as biomarkers of HNSCC. Future studies should be
performed with long-term follow ups and larger study groups to
determine the clinical importance of C250T hTERT promoter mutation
status in the absence and/or presence of short telomeres.
Acknowledgements
The present study was supported by grants from the
National Science Centre, Poland (grant no. 2015/17/N/NZ5/00686) and
The Greater Poland Cancer Centre (grant no.
9/11/2015/PRB/WCO/21).
References
|
1
|
Thomas SM and Grandis JR: The current
state of head and neck cancer gene therapy. Hum Gene Ther.
20:1565–1575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suh Y, Amelio I, Guerrero Urbano T and
Tavassoli M: Clinical update on cancer: Molecular oncology of head
and neck cancer. Cell Death Dis. 5:e10182014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hashibe M, Brennan P, Benhamou S,
Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova
E, Fernandez L, et al: Alcohol drinking in never users of tobacco,
cigarette smoking in never drinkers, and the risk of head and neck
cancer: Pooled analysis in the International Head and Neck Cancer
Epidemiology Consortium. J Natl Cancer Inst. 99:777–789. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szeszko B, Osowiecka K, Rucińska M,
Wasilewska-Teśluk E, Gliński K and Kepka L: Smoking during
radiotherapy for head and neck cancer and acute mucosal reaction.
Rep Pract Oncol Radiother. 20:299–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vojtíšek R, Ferda J and Fínek J:
Effectiveness of PET/CT with (18)F-fluorothymidine in the staging
of patients with squamous cell head and neck carcinomas before
radiotherapy. Rep Pract Oncol Radiother. 20:210–216. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashibe M, Brennan P, Chuang SC, Boccia S,
Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova
E, et al: Interaction between tobacco and alcohol use and the risk
of head and neck cancer: Pooled analysis in the international head
and neck cancer epidemiology consortium. Cancer Epidemiol
Biomarkers Prev. 18:541–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen CH and Chen RJ: Prevalence of
telomerase activity in human cancer. J Formos Med Assoc.
110:275–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McEachern MJ, Krauskopf A and Blackburn
EH: Telomeres and their control. Annu Rev Genet. 34:331–358. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hug N and Lingner J: Telomere length
homeostasis. Chromosoma. 115:413–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bibby MC: An introduction to telomeres and
telomerase. Mol Biotechnol. 24:295–301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suchorska WM, Augustyniak E and Łukjanow
M: Genetic stability of pluripotent stem cells during anti-cancer
therapies. Exp Ther Med. 11:695–702. 2016.PubMed/NCBI
|
|
12
|
Chan S and Blackburn EH: Telomeres and
telomerase. Philos Trans R Soc Lond B Biol Sci. 359:109–121. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda
C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL,
Giovanella BC, et al: TERT promoter mutations occur frequently in
gliomas and a subset of tumors derived from cells with low rates of
self-renewal. Proc Natl Acad Sci USA. 110:6021–6026. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jangard M, Zebary A, Ragnarsson-Olding B
and Hansson J: TERT promoter mutations in sinonasal malignant
melanoma: A study of 49 cases. Melanoma Res. 25:185–188. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan S, Shukla S, Sinha S and Meeran SM:
Role of adipokines and cytokines in obesity-associated breast
cancer: Therapeutic targets. Cytokine Growth Factor Rev.
24:503–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barczak W, Rozwadowska N, Romaniuk A,
Lipińska N, Lisiak N, Grodecka-Gazdecka S, Książek K and Rubiś B:
Telomere length assessment in leukocytes presents potential
diagnostic value in patients with breast cancer patients. Oncol
Lett. 11:2305–2309. 2016.PubMed/NCBI
|
|
17
|
Cawthon RM: Telomere length measurement by
a novel monochrome multiplex quantitative PCR method. Nucleic Acids
Res. 37:e212009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gruszecka A, Kopczyński P, Cudziło D,
Lipińska N, Romaniuk A, Barczak W, Rozwadowska N, Totoń E and Rubiś
B: Telomere shortening in down syndrome patients-when does it
start? DNA Cell Biol. 34:412–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Sturgis EM, Dahlstrom KR, Wen J,
Liu H, Wei Q, Li G and Liu Z: Telomere length in peripheral blood
lymphocytes contributes to the development of HPV-associated
oropharyngeal carcinoma. Cancer Res. 73:5996–6003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bau DT, Lippman SM, Xu E, Gong Y, Lee JJ,
Wu X and Gu J: Short telomere lengths in peripheral blood
leukocytes are associated with an increased risk of oral
premalignant lesion and oral squamous cell carcinoma. Cancer.
119:4277–4283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Ma H, Wei S, Li G, Sturgis EM and
Wei Q: Telomere length and TERT functional polymorphisms are not
associated with risk of squamous cell carcinoma of the head and
neck. Cancer Epidemiol Biomarkers Prev. 20:2642–2645. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh BK, Kim H, Park YN, Yoo JE, Choi J, Kim
KS, Lee JJ and Park C: High telomerase activity and long telomeres
in advanced hepatocellular carcinomas with poor prognosis. Lab
Invest. 88:144–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vinagre J, Almeida A, Pópulo H, Batista R,
Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al:
Frequency of TERT promoter mutations in human cancers. Nat Commun.
4:21852013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vinothkumar V, Arunkumar G, Revathidevi S,
Arun K, Manikandan M, Rao AK, Rajkumar KS, Ajay C, Rajaraman R,
Ramani R, et al: TERT promoter hot spot mutations are frequent in
Indian cervical and oral squamous cell carcinomas. Tumour Biol.
37:7907–7913. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu Y, Dang S, Wu K, Shao Y, Yang Q, Ji M,
Shi B and Hou P: TERT promoter mutations predict worse survival in
laryngeal cancer patients. Int J Cancer. 135:1008–1010. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G,
Murugan AK, Guan H, Yu H, Wang Y, et al: TERT promoter mutations
and their association with BRAF V600E mutation and aggressive
clinicopathological characteristics of thyroid cancer. J Clin
Endocrinol Metab. 99:E1130–E1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
George JR, Henderson YC, Williams MD,
Roberts DB, Hei H, Lai SY and Clayman GL: Association of TERT
promoter mutation, but not braf mutation, with increased mortality
in PTC. J Clin Endocrinol Metab. 100:E1550–E1159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr Relat
Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|