Introduction
Apoptosis is a complex biological process relying on
the balance between pro- and anti-apoptotic factors. One of the key
regulators of apoptosis belongs to a family of proteins known as
inhibitor of apoptosis (IAP). Of the known IAPs, X-linked inhibitor
of apoptosis (XIAP) protein is the best characterized and most
potent inhibitor of apoptosis. It blocks the apoptotic signaling
pathway by binding and inhibiting caspase-3, -7 and -9 (1,2).
XAF1 (XIAP-associated factor 1) was first isolated by the yeast
two-hybrid technique and was identified as a novel XIAP binding
partner that may reverse the anti-apoptotic effect of XIAP
(3). Previous studies have
demonstrated that XAF1 expression levels markedly decreased in a
significant number of cancer cell lines (4) and a variety of cancers (5,6). It
sensitizes cells to apoptotic triggers including tumor necrosis
factor-related apoptosis inducing ligand (7), etoposide treatments, 5-fluorouracil,
H2O2, tumor necrosis factor-α (8) and cisplatin (9,10).
Besides anti-apoptotic effects, ectopic overexpression of XAF1 was
reported to suppress migration and tube formation of mouse
endothelial cells in vitro (11), and inhibit angiogenesis and tumor
growth in hepatocellular carcinoma (12). In addition, it has recently been
reported that XAF1 is involved in regulating the balance between
autophagy and apoptosis in dengue virus type-2-infected endothelial
cells (13).
For years, sprouting angiogenesis has been
considered an exclusive underlying mechanism of tumor
vascularization. In 1999, Maniotis et al (14) described the channels formed by
highly aggressive melanoma cells. The term vasculogenic mimicry
(VM) was used to describe the formation of these channels, which do
not form from pre-existing vessels but supply blood to tumor cells.
Furthermore, the tumor cells lining the inner surface of the
channels are directly exposed to the blood flow. Detachment of
these tumor cells enables them to reach the blood stream and
metastasize to other organs (15).
Immunohistochemistry revealed that VM existed in ovarian carcinoma
and correlated with increased incidence of metastases and a reduced
survival rate (16). This may be a
reason why angiogenesis-targeted therapy strategies have limited
efficacy in the treatment of ovarian cancer (17). Vartanian et al (18) provided experimental evidence that
capillary-like structure formation requires apoptotic cell death
via activation of caspase-dependent mechanisms. Their results
suggested that apoptotic signaling pathways may be involved in the
formation of VM. The present study investigated the expression of
XAF1 in 94 advanced epithelial ovarian cancer (EOC) specimens and
assessed whether it was associated with VM. The potential
involvement of XAF1 in regulating proliferation, migration and
invasion of ovarian cancer cells was additionally examined. Our
previous research demonstrated that low XAF1 expression levels was
significantly associated with high microvessel density in ovarian
cancer tissues (19). The
underlying mechanism by which XAF1 expression mediates angiogenesis
of ovarian cancer cells will be elucidated.
Materials and methods
Tissue samples and cell lines
Formalin-fixed, paraffin-embedded tissues were
obtained from the Department of Pathology of Qilu Hospital,
Shandong University (Jinan, China). All patients received no
treatment prior to surgery. EOC diagnosis was determined by two
staff pathologists in the Department of Pathology. Specimens taken
from 94 patients with stage II–IV EOC who received cisplatin-based
combination chemotherapy following surgery were selected for
analysis. The protocol was approved by the Ethics Committee of
Shandong University and all patients were required to provide
written informed consent prior to inclusion. Patient anonymity was
preserved.
The SKOV3 human ovarian cancer cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and maintained in McCoy's 5A medium supplemented with 10%
fetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Immunohistochemistry and scoring
Following deparaffinization and antigen unmasking,
sections were incubated in H2O2 for 10 min to
inhibit endogenous peroxidase activity, and treated with goat serum
(BOSS&Bio Co., Ltd., Beijing, China) for 30 min. Following
this, they were incubated with anti-XAF1 (1:500; cat. no. ab17204;
Abcam, Cambridge, UK) and anti-cluster of differentiation (CD)31
(1:200; cat. no. ab32457; Abcam) primary antibodies overnight at
4°C. Sections were incubated with the corresponding horseradish
peroxidase-conjugated secondary antibody (1:200; cat. no. SAP-9101;
Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 1 h
and subsequently incubated for 2 min at room temperature with
3,3′-diaminobenzidine. Brown-yellow staining of the cytoplasm,
cytoplasmic membrane and cell nucleus was considered positive. The
staining was scored by 2 pathologists independently: 0 (≤5%
positive tumor cells), 1 (6–25% positive tumor cells), 2 (26–50%
positive tumor cells), and 3 (>51% positive tumor cells).
Staining intensity was graded according to the following criteria:
0 (no staining), 1 (weak staining=light yellow), 2 (moderate
staining=yellow brown), and 3 (strong staining=brown). The staining
index was calculated as the product of staining intensity score and
the proportion of positive tumor cells. A staining index score of
>4 was used to define tumors with high expression levels of XAF1
and CD31, and a staining index score of <3 was used to indicate
low expression levels (20).
CD31/periodic acid-Schiff (PAS) double
staining and evaluation of VM
Following CD31 immunohistochemical staining,
sections were treated with 0.5% periodic acid solution for 10 min
and rinsed with distilled water for 2–3 min. Sections were
subsequently incubated in Schiff solution for 15–30 min, rinsed
with distilled water and counterstained with hematoxylin. VM was
defined as channels surrounded by tumor cells with PAS-positive
materials and red blood cells, while CD31 staining was negative
(21).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using Trizol (Thermo Fisher
Scientific, Inc.). A total of 500 ng total RNA per sample was
reversed transcribed to cDNA using PrimeScript™ RT Master Mix
(Takara Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's instructions. PCR was performed using SYBR-Green
Real time PCR Master Mix (Toyobo Co., Ltd., Osaka, Japan) for 40
cycles of 95°C for 5 sec, 58°C for 10 sec and 72°C for 15 sec.
Primer sequences were as follows: XAF1, forward
5′-CTCGGTGTGCAGGAACTGTAAA-3′ and reverse 5′-CAGGAACCGCAGGCAGTAA-3′;
and β-actin, forward 5′-CCAACCGCGAGAAGATGA-3′ and reverse
5′-CCAGAGGCGTACAGGGATAG-3′. All primers were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). The specificity of PCR
products was confirmed by melting curve analysis. Products were
separated on a 1.5% agarose gel, visualized and imaged under
ultraviolet light.
Plasmids and transfection
A pcDNA3-HA-XAF1 plasmid was provided by Dr Douglas
W Leaman of the University of Toledo (Toledo, OH, USA). SKOV3 cells
were transfected with either the parent vector [SKOV3/control
(Con)] or pcDNA3-HA-XAF1 (SKOV3/XAF1) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were incubated at 37°C in a 5%
CO2 incubator for 48 h, following which 800 µg/ml
geneticin (G418; Invitrogen; Thermo Fisher Scientific, Inc.) was
added.
Migration and invasion assays
Cells at a density of 4×104 suspended in
serum-free McCoy's 5A medium (Gibco; Thermo Fisher Scientific,
Inc.) were seeded into inserts of Transwell chambers without
Matrigel. The top chamber contained 0.2% bovine serum albumin with
10% FBS in the bottom chamber. The plate was incubated for 24 h at
37°C. Migrating cells on the bottom of the filters were fixed and
stained with 0.5% crystal violet for 20 min. All experiments were
performed at least in triplicate.
A cell invasion assay was performed using 24-well
BioCoat Matrigel Invasion Chambers (BD Biosciences, Franklin Lakes,
NJ, USA). A total of 5×104 cells were seeded into the
upper chamber, and 600 ml of McCoy's 5A medium supplemented with
20% FBS was added into the lower chamber. Once cells were cultured
in 5% CO2 at 37°C for 24 h, cells that had migrated
through the Matrigel were fixed and stained with 0.5% crystal
violet. All experiments were performed at least in triplicate.
Western blot analysis
Total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer containing 1% protease
inhibitors for 5 min (Beyotime Institute of Biotechnology,
Shanghai, China). Proteins samples (50 µg) were separated by 10%
SDS-PAGE and then transferred to a polyvinylidene fluoride membrane
(Merck KGaA, Darmstadt, Germany). Membranes were incubated with
primary antibodies for 2 h at room temperature, washed twice for 10
min, and then incubated with secondary antibodies for 10 min at
room temperature. Primary antibodies targeting the vascular
endothelial growth factor (VEGF; 1:1,000; cat. no. sc-7269) and
β-actin (1:1,000; cat. no. sc-47778; loading control) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX. USA).
Horseradish peroxidase labeled anti-mouse or anti-rabbit secondary
antibodies (1:2,000; cat. nos. ZB-2301 and ZB2307, respectively)
were purchased from Zhongshan Jinqiao Biotechnology Co., Ltd. Blots
were developed using enhanced chemiluminescence reagent (Super ECL
Plus; Applygen Technologies, Inc., Beijing, China).
Tumor xenograft growth
SKOV3/Con and SKOV3/XAF1 cells (107) in
0.1 ml saline were implanted subcutaneously into the dorsal flank
of 6-week-old female BALB/c-nude mice (n=15/group) from the
Shanghai Experimental Animals Centre of the Chinese Academy of
Sciences (Shanghai, China). All animal studies were carried out
under approved guidelines of the Animal Care and Use Committee of
Shandong University. Tumor dimensions were measured with a caliper
and the volume was calculated using the formula
V=axb2x0.5 (22)
(a=length, b=width of prolate spheroid). Mice were euthanized and
tumors collected after 6 weeks. Tumor sections were processed for
immunohistochemistry and hematoxylin and eosin staining.
Statistical analysis
SPSS software version 13.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Data are present as the
mean ± standard deviation. Differences between groups were
evaluated using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Association between VM and clinical
data in EOC
VM describes the formation of fluid-conducting
channels by highly invasive and genetically deregulated tumor
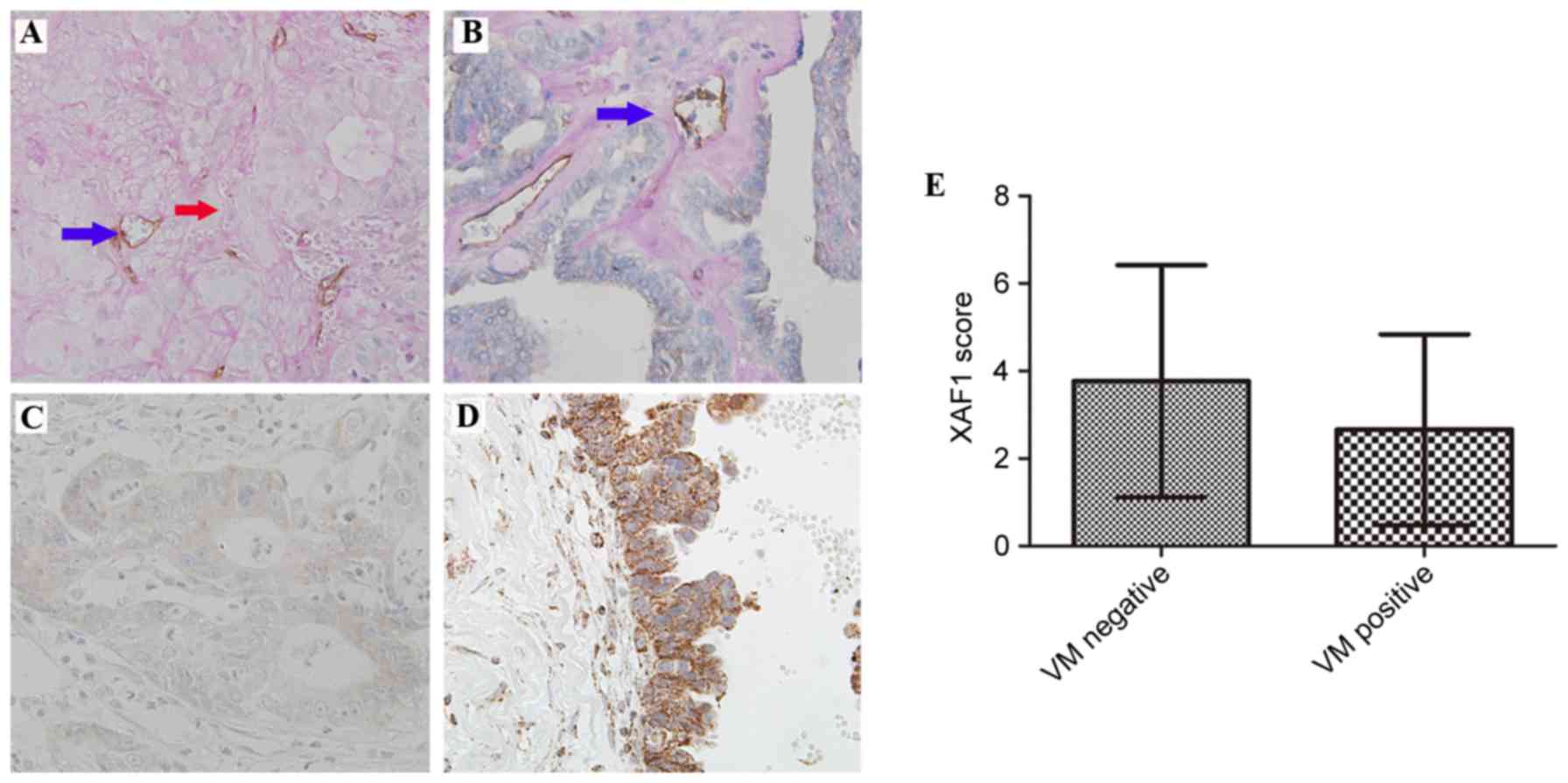
cells. As presented in Fig. 1A, VM
was identified by the presence of red blood cells in vessels lined
by tumor cells (without endothelial cells). A total of 35 (37.2%)
EOC samples were VM positive. CD31 was stained positive in blood
vessels (Fig. 1B).
The association of VM with the clinical data of the
94 EOC samples is presented in Table
I. Presence of VM was associated with increased histological
grade (P=0.030), an International Federation of Gynecology and
Obstetrics (FIGO) stage between III and IV (P=0.098), and a serous
histological type (P=0.124).
 | Table I.Clinical pathological characteristics
of patients with advanced epithelial ovarian cancer. |
Table I.
Clinical pathological characteristics
of patients with advanced epithelial ovarian cancer.
| Variable | Total | VM+ | P |
|---|
| Total cases | 94 | 37.2%
(35/94) |
|
| Age (years) |
|
| 0.197 |
| ≤53 | 38 | 28.9%
(11/38) |
|
|
>53 | 56 | 42.9%
(24/56) |
|
| FIGO stage |
|
| 0.098 |
| II | 26 | 20.7% (6/26) |
|
|
III/IV | 68 | 42.6%
(29/68) |
|
| Histological
grade |
|
| 0.030 |
| 1/2 | 35 | 22.9% (8/35) |
|
| 3 | 59 | 45.8%
(27/59) |
|
| Histological
type |
|
| 0.124 |
|
Serous | 60 | 43.3%
(26/60) |
|
|
Other | 34 | 26.5% (9/34) |
|
| Lymph node
status |
|
| 0.428 |
|
Negative | 36 | 38.9%
(14/36) |
|
|
Positive | 22 | 50.0%
(11/22) |
|
| Not
available | 36 |
|
|
Association between XAF1 expression
levels and VM in EOC sections
Staining of XAF1 was predominantly located in the
cytoplasm of epithelium cells. A total of 37 of 94 (39.4%) of the
samples demonstrated increased protein expression levels of XAF1
(Fig. 1C and D).
Reduced XAF1 expression levels were significantly
associated with presence of VM. XAF1 expression was reduced in 30
of the 35 (85.7%) samples in the VM-positive group and in 27 of the
59 (45.8%) samples in the VM-negative group (P<0.01). As
presented in Fig. 1E, the mean
score of XAF1 expression was reduced in the VM-positive group
compared with the VM-negative group (2.66±2.18 vs. 3.76±2.65,
respectively; P=0.040).
XAF1 overexpression inhibits migration
and invasion of SKOV3 cells
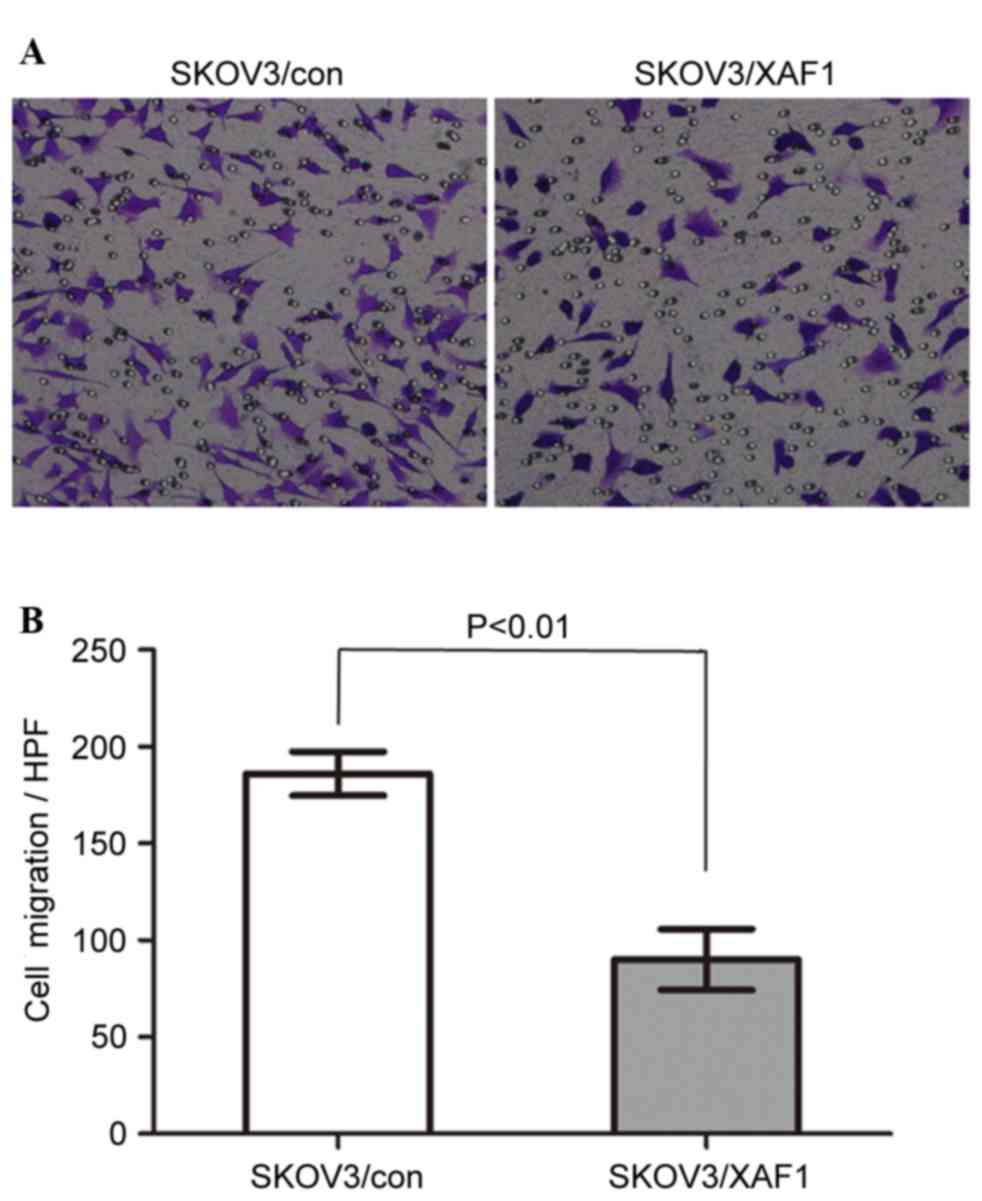
The effect of XAF1 on the motility of ovarian cancer
cells was measured by Transwell migration assays (Fig. 2A and B). SKOV3/XAF1 cells that
migrated through the filter were reduced significantly compared
with the control group. The mean cell count migrated filter
membrane was 122.0±35.64 for SKOV3/Con cells and 58.00±13.04 for
SKOV3/XAF1 cells in one high power field (P<0.01).
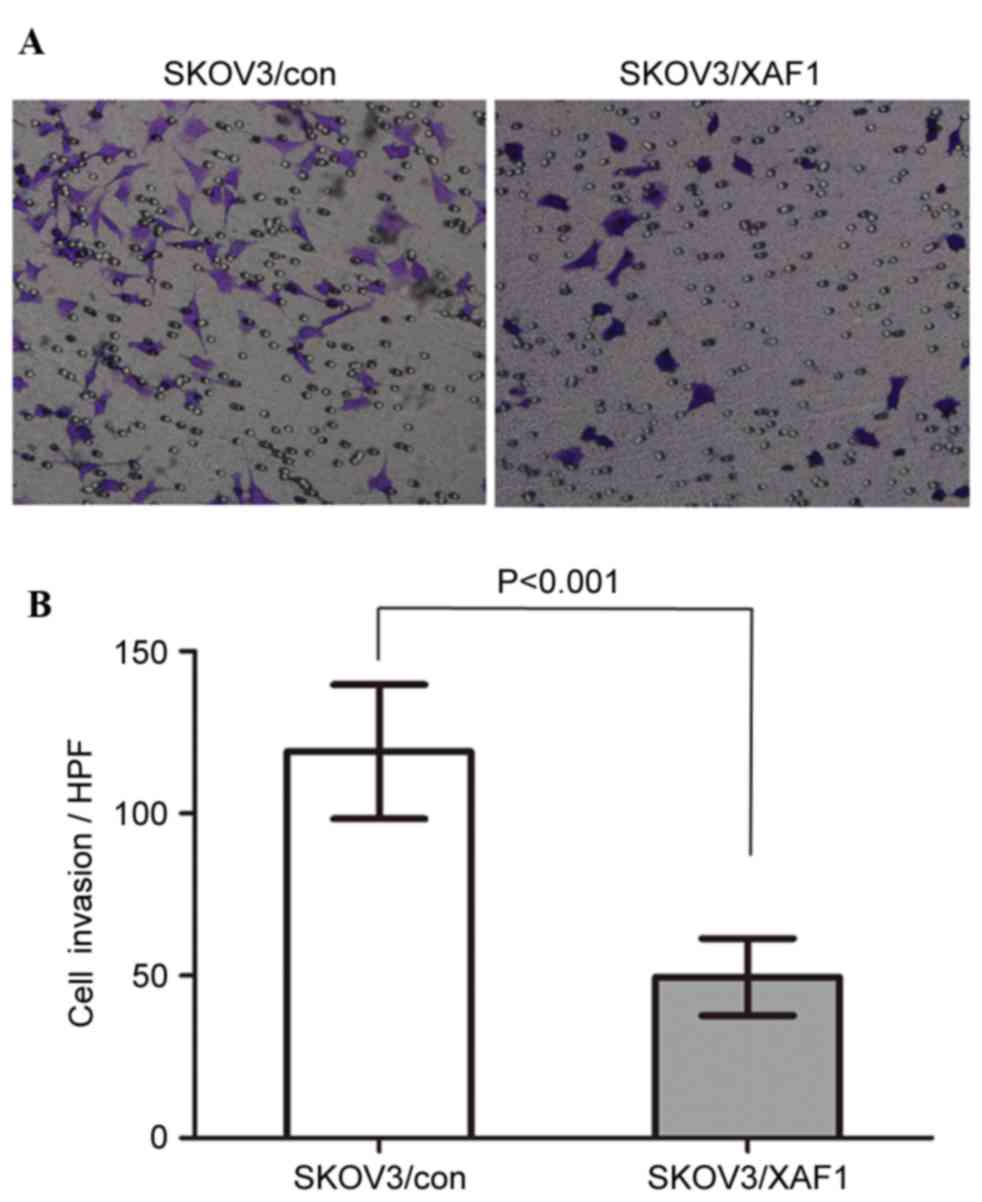
The number of SKOV3/XAF1 cells invaded through the
Matrigel-coated filter was reduced compared with control cells
(Figs. 3A and B). The mean cell
count invaded through the filter was 119.0±20.74 vs. 49.60±11.89
per field for SKOV3/Con and SKOV3/XAF1 cells, respectively
(P<0.001).
XAF1 decreases tumor vasculature in
vivo
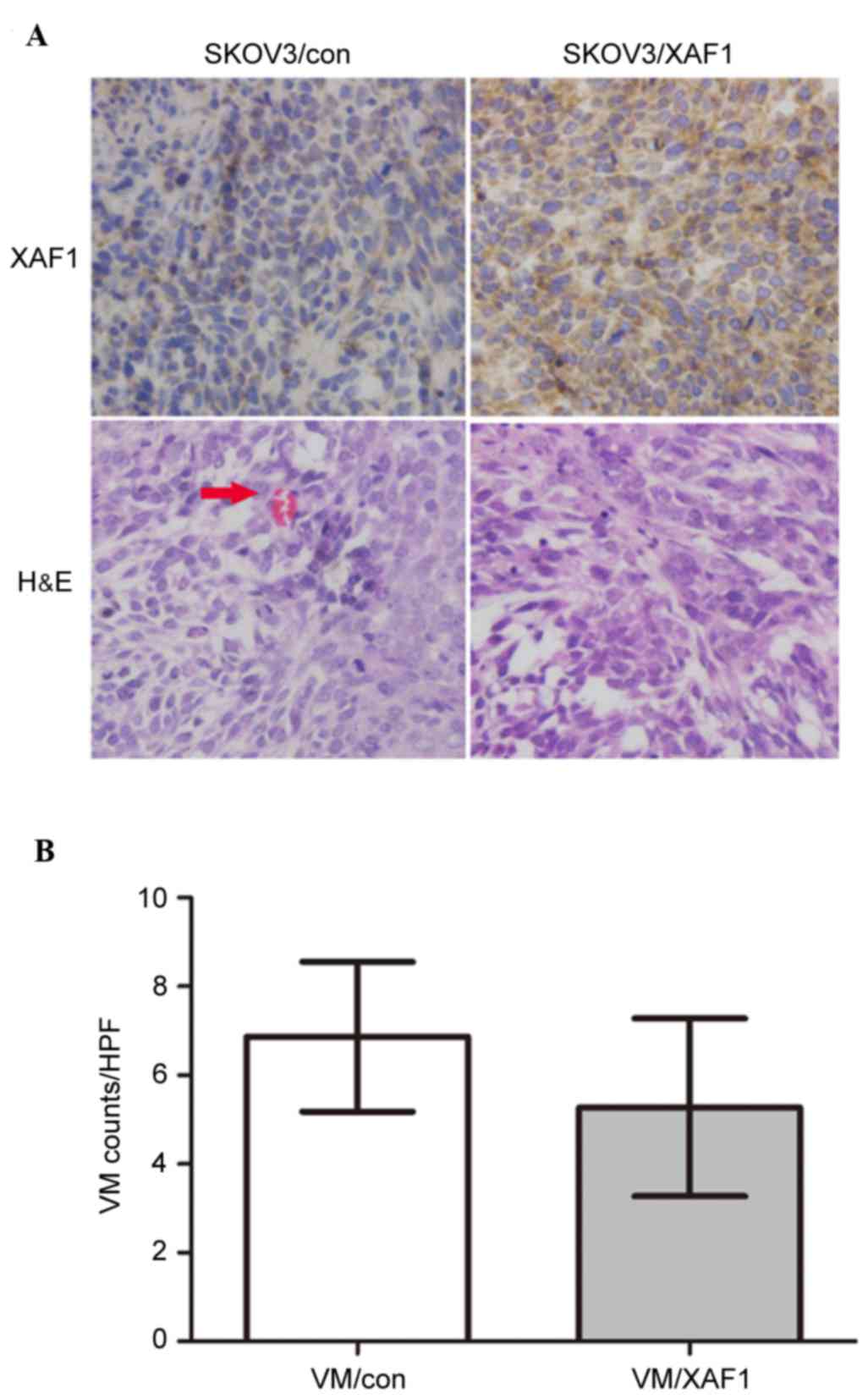
Whether ectopic overexpression of XAF1 decreases
tumor vasculature in vivo was examined. SKOV3/Con and
SKOV3/XAF1 cells were implanted subcutaneously into BALB/c-nude
mice. Tumor volume was monitored every week for up to 6 weeks. By
week 6, mice implanted with control cells exhibited 2-3-fold larger
tumors (data not shown) compared with those implanted with
transfected cells. Immunohistochemistry demonstrated that XAF1
expression was increased in tumor xenografts derived from
XAF1-overexpressing cells compared with those from vector control
cells (Fig. 4A). Tumor xenografts
formed from SKOV3/XAF1 cells exhibited significantly fewer VM
channels compared with those formed from vector controls cells
(Fig. 4B; P=0.037).
XAF1 overexpression inhibits VEGF
protein expression
VEGF protein expression levels were examined in
SKOV3 cells and tumor-bearing mice. XAF1 overexpression resulted in
reduced protein expression levels of VEGF in SKOV3/XAF1 cells
(Fig. 5A) and mice tumor tissues
(Fig. 5B).
Discussion
There are three types of blood supply for cancer:
VM, mosaic vessels and endothelium-dependent vessels. VM provides a
pathway for perfusion independent of angiogenesis. The presence of
VM characteristic structures in patient tumor tissues is associated
with a poor clinical outcome, suggesting a functional contribution
of these networks to tumor progression including ovarian cancer
(16,23–25),
and increased VM channels are associated with poorly differentiated
ovarian cancer cells. Poorly differentiated tumor cells have been
demonstrated to have plasticity and alter their cell markers and
functions in order to adapt to a specific microenvironment
(26–28).
Aggressive tumor features contribute to formation of
tumor cell-lined vasculature in ovarian cancer (29). Our previous study demonstrated that
reduced XAF1 expression was correlated with a high grade of
advanced EOC (19). The present
study demonstrated an association between incidence of VM and
reduced expression of XAF1; the incidence of VM was significantly
increased in XAF1-underexpressing samples. This provides a novel
fundamental mechanism for an effect reported previously that
decreased XAF1 expression is associated with poor prognosis in
numerous types of cancers (30,31).
Restoration of XAF1 expression levels has been
demonstrated to inhibit cell growth of pancreatic cancer (32), and migration and invasion of mouse
endothelial cells (11). However,
this effect on tumor cells has rarely been reported in previous
studies. Using a plasmid-transfection approach, the present study
investigated the direct effect of ectopic overexpression of XAF1 on
the SKOV3 ovarian cancer cell line. In present study,
proliferation, migration and invasion of SKOV3 cells were inhibited
by overexpression of XAF1. This suggested that aggressive tumor
cells serve an important role in VM formation. Consistent with
this, a previous study demonstrated that poorly invasive tumor
cells did not create these networks under identical culture
conditions, even following the addition of conditioned media from
aggressive cell lines (14).
In the present study, XAF1 overexpression resulted
in reduced protein expression of VEGF in SKOV3/XAF1 cells and mouse
tumor tissues. XAF1 has previously been demonstrated to inhibit
angiogenesis and suppress expression of VEGF in hepatocellular
carcinoma (12), and is known to
be important in VM formation. A previous study revealed that
VEGF-silencing induced apoptosis, inhibited proliferation and
suppressed VM in osteosarcoma in vitro (33). Therefore, XAF1 may inhibit
vasculature by suppressing VEGF expression in ovarian cancers.
In conclusion, the present study investigated the
association between XAF1 expression with VM channels in EOC.
Ectopic overexpression of XAF1 was demonstrated to suppress
migration and invasion of SKOV3 cells. These findings may
facilitate the development of novel therapeutic agents for the
treatment of ovarian cancer.
Acknowledgements
The authors would like to thank Dr Douglas W. Leaman
of the University of Toledo (Toledo, OH, USA) for the provision of
the pcDNA3-HA-XAF1 plasmid.
References
|
1
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell-death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Srinivasula SM, Hegde R, Saleh A, Datta P,
Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y
and Alnemri ES: A conserved XIAP-interaction motif in caspase-9 and
Smac/DIABLO regulates caspase activity and apoptosis. Nature.
410:112–116. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liston P, Fong WG, Kelly NL, Toji S,
Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW and Korneluk
RG: Identification of XAF1 as an antagonist of XIAP anti-Caspase
activity. Nat Cell Biol. 3:128–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fong WG, Liston P, Rajcan-Separovic E, St
Jean M, Craig C and Korneluk RG: Expression and genetic analysis of
XIAP-associated factor 1 (XAF1) in cancer cell lines. 70:113–122.
2000.
|
|
5
|
Xing Z, Yu R, Li S and Liu Z: Regulatory
effects of somatostatin on XAF1 in prostate cancer. Regul Peptides.
164:472010. View Article : Google Scholar
|
|
6
|
Kempkensteffen C, Fritzsche FR, Johannsen
M, Weikert S, Hinz S, Dietel M, Riener MO, Moch H, Jung K, Krause
H, et al: Down-regulation of the pro-apoptotic XIAP associated
factor-1 (XAF1) during progression of clear-cell renal cancer. BMC
Cancer. 9:2762009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leaman DW, Chawla-Sarkar M, Vyas K,
Reheman M, Tamai K, Toji S and Borden EC: Identification of
X-linked inhibitor of apoptosis-associated factor-1 as an
interferon-stimulated gene that augments TRAIL Apo2L-induced
apoptosis. J Biol Chem. 277:28504–28511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu SP, Sun YW, Cui JT, Zou B, Lin MC, Gu
Q, Jiang SH, Kung HF, Korneluk RG and Wong BC: Tumor Suppressor
XIAP-Associated factor 1 (XAF1) cooperates with tumor necrosis
factor-related apoptosis-inducing ligand to suppress colon cancer
growth and trigger tumor regression. Cancer. 116:1252–1263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ju WC, Huang GB, Luo XY, Ren WH, Zheng DQ,
Chen PJ, Lou YF and Li B: X-linked inhibitor of
apoptosis-associated factor l (XAFl) enhances the sensitivity of
colorectal cancer cells to cisplatin. Med Oncol. 31:2732014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma B and Wang Y, Zhou X, Huang P, Zhang R,
Liu T, Cui C, Liu X and Wang Y: Synergistic suppression effect on
tumor growth of hepatocellular carcinoma by combining oncolytic
adenovirus carrying XAF1 with cisplatin. J Cancer Res Clin Oncol.
141:419–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiao L, Gu Q, Dai Y, Shen Z, Liu X, Qi R,
Ma J, Zou B, Li Z, Lan HY and Wong BC: XIAP-associated factor 1
(XAF1) suppresses angiogenesis in mouse endothelial cells. Tumour
Biol. 29:122–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu LM, Shi DM, Dai Q, Cheng XJ, Yao WY,
Sun PH, Ding Y, Qiao MM, Wu YL, Jiang SH and Tu SP: Tumor
suppressor XAF1 induces apoptosis, inhibits angiogenesis and
inhibits tumor growth in hepatocellular carcinoma. Oncotarget.
5:5403–5415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Li Y, Qi Y, Zhang Y, Zhang L,
Wang Z, Zhang X and Gui L: Coordinated regulation of autophagy and
apoptosis determines endothelial cell fate during Dengue virus type
2 infection. Mol Cell Biochem. 397:157–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Folberg R and Maniotis AJ: Vasculogenic
mimicry. APMISAPMIS. 112:508–525. 2004. View Article : Google Scholar
|
|
16
|
Gao Y, Zhao X, Gu Q, Wang JY, Zhang SW,
Zhang DF, Wang XH, Zhao N, Gao YT and Sun BC: Correlation of
vasculogenic mimicry with clinicopathologic features and prognosis
of ovarian carcinoma. Zhonghua Bing Li Xue Za Zhi. 38:585–589.
2009.(In Chinese). PubMed/NCBI
|
|
17
|
Tang HS, Feng YJ and Yao L: Angiogenesis,
vasculogenesis, and vasculogenic mimicry in ovarian cancer. Int J
Gynecol Cancer. 19:605–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vartanian AA, Burova OS, Stepanova EV and
Baryshnikov AY: The involvement of apoptosis in melanoma
vasculogenic mimicry. Melanoma Res. 17:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Mao H, Hao Q, Wang Y, Yang Y, Shen
L, Huang S and Liu P: Association of expression of XIAP-associated
factor 1 (XAF1) with clinicopathologic factors, overall survival,
microvessel density and cisplatin-resistance in ovarian cancer.
Regul Pept. 178:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao ZS, Chu YQ, Ye ZY, Wang YY and Tao
HQ: Overexpression of matrix metalloproteinase 11 in human gastric
carcinoma and its clinicopathologic significance. Hum Pathol.
41:686–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sood AK, Seftor EA, Fletcher MS, Gardner
LM, Heidger PM, Buller RE, Seftor RE and Hendrix MJ: Molecular
determinants of ovarian cancer plasticity. Am J Pathol.
158:1279–1288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
23
|
Baeten CI, Hillen F, Pauwels P, de Bruine
AP and Baeten CG: Prognostic role of vasculogenic mimicry in
colorectal cancer. Dis Colon Rectum. 52:2028–2035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K,
Pang JC, Ng HK and Chen ZP: Clinical significance of vasculogenic
mimicry in human gliomas. J Neurooncol. 105:173–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shirakawa K, Wakasugi H, Heike Y, Watanabe
I, Yamada S, Saito K and Konishi F: Vasculogenic mimicry and
pseudo-comedo formation in breast cancer. Int J Cancer. 99:821–828.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Folberg R, Hendrix MJ and Maniotis AJ:
Vasculogenic mimicry and tumor angiogenesis. Am J Pathol.
156:361–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu P, Ning Y, Yao L, Chen M and Xu C: The
proliferation, apoptosis, invasion of endothelial-like epithelial
ovarian cancer cells induced by hypoxia. J Exp Clin Cancer Res.
29:1242010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su M, Feng YJ, Yao LQ, Cheng MJ, Xu CJ,
Huang Y, Zhao YQ and Jiang H: Plasticity of ovarian cancer cell
SKOV3ip and vasculogenic mimicry in vivo. Int J Gynecol Cancer.
18:476–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sood AK, Fletcher MS, Zahn CM, Gruman LM,
Coffin JE, Seftor EA and Hendrix MJ: The clinical significance of
tumor cell-lined vasculature in ovarian carcinoma: Implications for
anti-vasculogenic therapy. Cancer Biol Ther. 1:661–664. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ling ZQ, Lv P, Lu XX, Yu JL, Han J, Ying
LS, Zhu X, Zhu WY, Fang XH, Wang S and Wu YC: Circulating
methylated XAF1 DNA indicates poor prognosis for gastric cancer.
PLoS One. 8:e671952013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SK, Park HJ, Seok H, Jeon HS, Kim JW,
Chung JH, Kwon KH, Woo SH, Lee BW and Baik HH: Missense
polymorphisms in XIAP-associated factor-1 (XAF1) and risk of
papillary thyroid cancer: Correlation with clinicopathological
features. Anticancer Res. 33:2205–2210. 2013.PubMed/NCBI
|
|
32
|
Huang J, Yao W, Zhu Q, Tu SP, Yuan F, Wang
HF, Zhang YP and Yuan YZ: XAF1 as a prognostic biomarker and
therapeutic target in pancreatic cancer. Cancer Sci. 101:559–567.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mei J, Gao Y, Zhang L, Cai X, Qian Z,
Huang H and Huang W: VEGF-siRNA silencing induces apoptosis,
inhibits proliferation and suppresses vasculogenic mimicry in
osteosarcoma in vitro. Exp Oncol. 30:29–34. 2008.PubMed/NCBI
|