Introduction
Pre-eclampsia is a pregnancy-specific syndrome
characterized by hypertension and significant proteinuria developed
at or after 20 weeks of pregnancy in a previously normotensive,
non-proteinuric patient (1,2). It
affects ~5–10% of all pregnancies worldwide and is a major cause of
maternal and perinatal morbidity and mortality in developed
countries (1,3). Although the etiology of pre-eclampsia
is still not fully clear, strong evidence supports the involvement
of inadequate trophoblast invasion, failed remodeling of the spiral
arteries, imbalance of angiogenic and antiangiogenic factors and
endothelial cell dysfunction (1–5).
Consequently, elucidating disorder factors of placental
angiogenesis is crucial in understanding the pathophysiological
process of pre-eclampsia.
Follicle stimulating hormone (FSH), an anterior
pituitary gonadotroph-derived heterodimeric glycoprotein which
binds G protein-coupled FSH receptor (FSHR), is promoted by
hypothalamic decapeptide gonadotrophin-releasing hormone and serves
a critical role in hypothalamic-pituitary-gonadal axis (6,7).
Traditional considerations manifest the physiological structure and
function of the FSH/FSHR system within gonads that stimulates
growth of follicles and synthesis of estrogens in the ovary or
promotes spermatogenesis in the testes (6,8).
However, previous reports revealed that the FSH/FSHR system serves
an important role in extragonadal tissues and organs, including
prostanoid synthesis of bovine cervix (8,9),
electrical activity of the mouse myometrium (10,11),
and some organs unrelated to reproduction (12). For instance, FSHR expression of
osteoclast is related to bone resorption, which aggravates
periodontitis-related alveolar bone loss without estrogen
deficiency (12–14). Its relevance to angiogenesis has
been also reported well in previous research (15,16).
Studies indicated that the FSHR was expressed in the endothelial
cells of human placental chorionic villi and umbilical vein at term
(15). Stilley et al
(8,15) revealed that FSH promoted the
formation of endothelial tubes and other angiogenic processes
without increasing secretion of vascular endothelial growth factor
(VEGF). Furthermore, the study indicated that the
haplo-insufficiency of the feto-placental FSHR impaired the growth
of the mouse placenta (8). In
addition, Radu et al (17)
determined that the FSHR was selectively expressed on the
endothelium of blood vessels in a wide range of tumors, and
endothelial FSHR expression in breast cancer was associated with
vascular remodeling at tumor peripheries (18). In light of the evidence pointing to
roles of FSHR in the angiogenesis, the authors hypothesized that
abnormal expression of the FSH/FSHR system would present in the
placentas of women with pre-eclampsia.
To verify the hypothesis, the authors examined
placental mRNA and protein expression and localization of FSH and
FSHR by RT-qPCR and immunohistochemistry methods in normotensive
control and pre-eclamptic women. Additionally, serum levels of
maternal FSH were also tested using chemiluminescence immunoassay.
The results indicated that FSHR mRNA and protein levels were
significantly decreased in the placentas of women with
pre-eclampsia.
Materials and methods
Patients
The present study was approved by the Ethical
Committee of Nanchang University (Nanchang, China) and Jiangxi
Province People's Hospital (Nanchang, China), and informed consent
was obtained from each participant. Placentas were collected from
pregnancies with: (1) Normal
pregnancy (maternal blood pressure <140/90 mmHg, absence of
proteinuria and no medical complications), (2) severe pre-eclampsia (new-onset
hypertension, defined as systolic blood pressure of >160 mmHg or
diastolic blood pressure of >110 mmHg, with at least two
measurements, accompanying by significant proteinuria >5 g/24 h
or 3+ by dipstick in two random samples collected at >4 h
interval after 20 weeks of gestation). Those who developed renal
disease, gestational diabetes, spontaneous abortion, transient
hypertension of pregnancy, intrauterine fetal death, fetal
chromosomal abnormalities, congenital abnormalities, pregnancies
conceived by fertility treatment, or had hereditary history and
smoking and alcohol the history were not included in the study. The
clinical characteristics of patients and controls are presented in
Table I. Tissues were collected
from the villous tree within 1 h following delivery. To minimize
blood contamination, each piece of tissue was intensively washed in
Dulbecco's phosphate-buffered saline (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Tissue samples were then
snap-frozen and stored at −80°C.
 | Table I.Clinical characteristics of study
population. |
Table I.
Clinical characteristics of study
population.
| Clinical
characteristic | Normal
pregnancy | Pre-eclampsia | P-value |
|---|
| Case (n) | 25 | 20 |
|
| Maternal age (mean
± SD, years) | 27.3±4.2 | 29.3±3.9 | 0.37 |
| Gestational age at
delivery (mean ± SD, weeks) | 37.7±2.4 | 34.7±2.6 | 0.013 |
| Birth weight (mean
± SD, g) | 3347.6±311.6 | 2806.5±665.3 | <0.001 |
| Maternal height
(cm) | 163.1±7.8 | 160.9±6.4 | 0.314 |
| Maternal weight
(kg) | 60.2±4.5 | 62.1±5.0 | 0.175 |
| Body mass
index | 22.2±1.4 | 23.0±2.1 | 0.133 |
| Systolic blood
pressure (mean ± SD, mmHg) | 106.3±11.3 | 165.4±11.9 | <0.001 |
| Diastolic blood
pressure (mean ± SD, mmHg) | 69.3±10.2 | 109.1±13.4 | <0.001 |
| Fetal sex
(male/female) | 14/11 | 9/11 | >0.05 |
| Serum FSH (mean ±
SD, mIU/ml) | 2.92±0.95 | 2.79±0.63 | 0.61 |
RNA isolation and RT-qPCR
Total RNA was extracted from placental tissues with
the RNAiso Plus solution (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocol. RNA (2 µg) samples
were reverse-transcribed into single-stranded cDNA in a 25 µl
reaction mixture, containing 4 µl 5X reaction buffer, 1 µl RNase
inhibitor, 2 µl 10 mM dNTP, 1 µl reverse transcriptase and 1 µl 10
µM primers (Takara Biotechnology Co., Ltd.). RT-qPCR was then
performed in a 20 µl reaction volume containing 10 µl 2X Brilliant
SYBR Green Mix (Takara Biotechnology Co., Ltd.), 2 µl template
cDNA, 0.5 µM primers, and 300 nM reference dyes using the ABI
thermal cycler 7500 (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermal cycling conditions were 95°C for 30 sec,
followed by 40 cycles at 94°C for 5 sec, 60°C for 34 sec. Melting
curve analysis and agarose gel electrophoresis were conducted
following the RT-qPCR assays to monitor PCR product purity. The
results were analyzed using ABI Prism 7500 software (Applied
Biosystems; Thermo Fisher Scientific, Inc.). 18S rRNA was used for
normalization (19). The following
primers were used: FSH sense, 5′-CCACTTGGTGTGCTGGCTACT-3′, and
antisense, 5′-GGCCTGGCTGGGTCCTTATA-3′; FSHR sense,
5′-GCCATGCTGCCAGTGTCAT-3′, and antisense, 5′-GAGGGCAGCTGCAAAAGC-3′;
18S sense, 5′-GCTGAGAAGACGGTCGAACT-3′, and antisense,
5′-TTAATGATCCTTCCGCAGGT-3′.
Immunohistochemistry
Tissues were fixed in Bouin's solution, dehydrated,
and embedded in paraffin. Tissue sections were deparaffinized, and
rehydrated in a graded series of ethanol solutions. Endogenous
peroxidase activity was blocked by incubating the sections in 3%
hydrogen peroxide in PBS for 10 min. Nonspecific binding was
blocked with 5% BSA in PBS for 30 min. Then, the sections were
incubated in rabbit anti-FSHR (1:200; ab113421; Abcam, Cambridge,
UK), rabbit anti-CD31 (1:300; ZM-0044; ZSGB-Bio, Beijing, China)
and mouse anti-FSH monoclonal antibody (1:150; ZA-0264; ZSGB-Bio)
overnight at 4°C. Following washing in PBS, the sections were
incubated with a horseradish peroxidase-conjugated secondary
antibody (1:200; ZM-0003; ZSGB-Bio) for 50 min at 37°C. The primary
antibody was visualized with fresh diaminobenzidine solution,
together with counter-staining with Harris' hematoxylin. In some
sections, the primary antibodies were omitted or replaced with
rabbit or mouse pre-immune IgG as a negative control.
Analyses of immunohistochemical
staining
Images were captured in three sections per sample
using digital camera head DS-Fi1 (Nikon Corporation, Tokyo, Japan),
and analyses of immunohistochemical staining were taken using
NIS-ELEMENTF analysis system (Nikon Corporation). Under the same
magnification (×100) and light intensity, each slide was measured
in randomly selected eight fields. Mean values of optical density
for positive cells were calculated using sections from normal
pregnancy and severe pre-eclampsia.
Hormone measurements
Blood samples were collected into clotting tubes
between 7:30 a.m. and 8:30 a.m. from the cubital vein during the
routine visits at the end of gestation. Blood was centrifuged at
2,000 × g for 20 min at 4°C and then stored at −80°C until the
assay. The serum concentration of FSH was measured using a
chemiluminescence immunoassay kit (BD-2003; DPC Biermann GmbH, Bad
Nauheim, Germany). The intra-and interassay coefficients of
variation did not exceed 10%. The cross-reactivities with other
peptides and steroid hormones did not exceed 4%. The detection
limitation of the FSH kit is 0.2 mIU/ml.
Statistical analysis
Data were presented as means ± standard deviation.
Statistical analysis was performed by independent-samples t-test
for parametric and Wilcoxon test for nonparametric data to
determine the significance of the differences. Additionally, the
chi-squared test was used to examine fetal sex. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS software (version,
13.0; SPSS, Inc., Chicago, IL, USA).
Results
Clinical data analysis
Compared with normal pregnancy, the gestational age
of women with pre-eclampsia was 3 weeks shorter at delivery
(P=0.013), and gained less weight during their pregnancies
(P<0.001). However, there was no significant difference in serum
concentrations of FSH between normal pregnancy and pre-eclampsia
(P=0.61; Table I).
FSHR expression in human
placentas
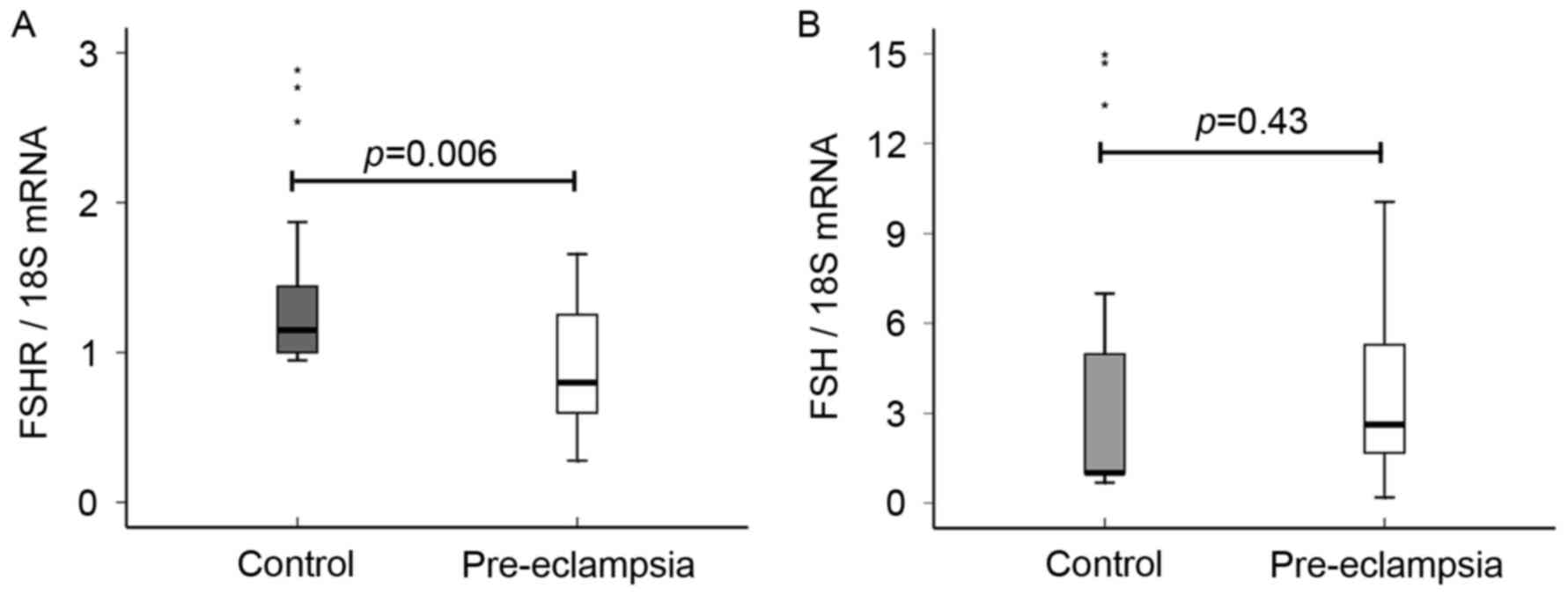
RT-qPCR results indicated that expression level of
placental FSHR mRNA in pre-eclamptic samples was significantly
lower than that of the normal sample (1.27±0.56, 0.92±0.42;
P=0.006; Fig. 1A). The authors
then analyzed the spatiotemporal expression of the FSHR protein in
the placental tissues by immunohistochemistry method (Fig. 2). Immunostaining results from
normal pregnant samples demonstrated that the FSHR protein was
strongly expressed in endothelial cells of blood vessels in the
chorionic villi (confirmed by CD31 staining, Fig. 2A and B), moderately expressed in
the chorionic stromal cells, but not expressed in trophoblast cells
of term placenta (Fig. 2D and E).
Compared to the normal control group, the staining intensity of the
FSHR-positive area was significantly lower in the placental villi
of pre-eclampsia (P=0.0018; Fig.
3A), in accordance with the RT-qPCR results.
FSH expression in human placentas
RT-qPCR analysis revealed that no significant
difference was observed in the expression levels of placental FSH
mRNA between normal pregnancy and pre-eclampsia (3.22±2.93,
3.88±2.95; P=0.43; Fig. 1B).
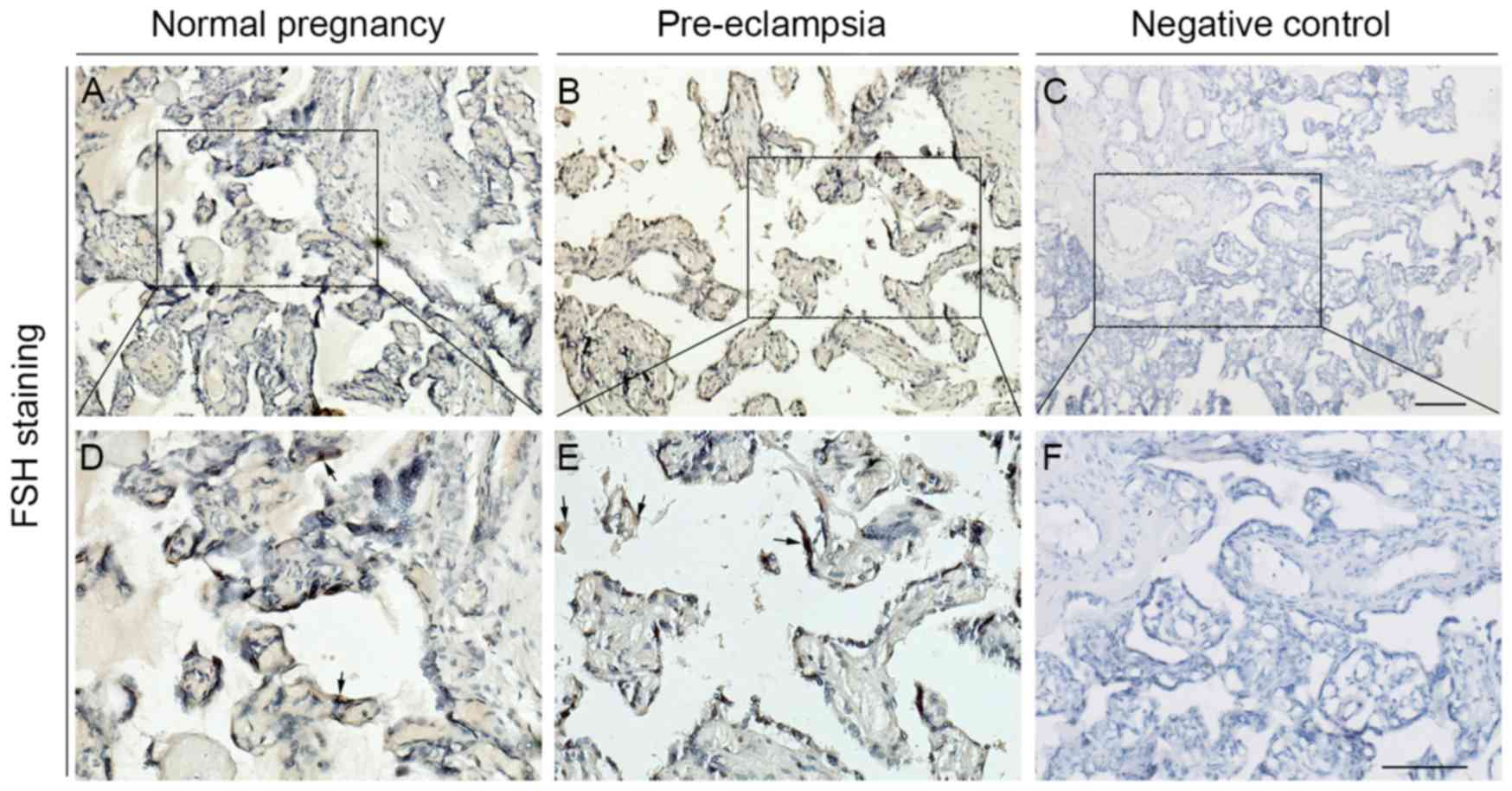
Furthermore, immunohistochemical analysis also verified the above
results. Immunostaining results indicated that expression level of
the FSH protein was generally low in the cytotrophoblasts and
syncytiotrophoblasts, blood vessel and stroma of placental villi
(Fig. 4A-F). Compared to the
normal control group, the staining intensity of the FSH-positive
area was a little stronger in the placental villi of pre-eclampsia,
but no significant difference was identified (P=0.199; Fig. 3B).
Discussion
The hypothesis that was investigated in the present
study involved whether placental dysfunction associated with
pre-eclampsia correlates with altered FSH and FSHR mRNA and protein
expressions. The current results indicated that decreased FSHR mRNA
and protein levels in placental tissues derived from pre-eclamptic
women compared to those with uncomplicated pregnancies. No
significant difference was demonstrated in serum FSH levels and
expression levels of placental FSH mRNA and protein between normal
pregnancy and pre-eclampsia.
In women, FSH serves an important role during the
growth and development of ovarian follicles, including granulosa
cell function and production of estrogens from androgen substrates
(6,7). It is generally believed that level of
pituitary FSH in the peripheral blood is suppressed during
pregnancy (20). Faiman et
al (20) observed only low
levels of radio-immunoassayable serum FSH throughout human
pregnancy, and its concentrations averaged 0.39 mIU/ml. The results
identified that the level of serum FSH averaged 2.92 mIU/ml during
the third trimester, which is consistent with Penny, Olambiwonnu
and Frasier's results (21,22).
However, Jaffe, Lee and Midgley (23) indicated that 76% of 45 pregnant
subjects displayed values >6 mIU/ml and only 4% of subject's
values <3 mIU/ml (22,23). The difference between these results
may have attributed to the different sampling times and numbers,
assay methods and FSH antibody used in these studies. In addition,
the current data indicated that no significant differences were
identified in levels of serum FSH between normal pregnancies and
pre-eclampsia, suggesting that locally produced FSH at the
maternal-fetal interface may exert its physiological effects
through paracrine ways. Stilley et al (8) revealed that both FSHB mRNA (encoding
the FSHβ subunit) and CGA mRNA (encoding the common FSHα subunit)
are present in the placental tissue, uterine deciduas and
myometrium (24–26). The present RT-qPCR and
immunostaining results also indicated that FSH was expressed in
term placental tissues, but no significant difference was observed
in the expression levels of placental FSH mRNA and protein between
normal pregnancy and pre-eclampsia.
Previous studies have indicated that the FSHR is
expressed in endothelial cells of placental blood vessels and FSH
could promote angiogenesis of human umbilical vein endothelial
cells through the FSHR (8,15,27).
The results indicated that expression levels of placental FSHR were
significantly reduced in pregnancies complicated by pre-eclampsia.
It suggests that decreased FSHR expression could contribute to
aberrant angiogenesis and trophoblast development associated with
pre-eclampsia. FSH stimulates angiogenesis possibly via a different
mechanism (26). Fatima et
al (28) demonstrated that FSH
could upregulate mRNA and proteins of VEGF, fibroblast growth
factors 2, and their receptors in vitro and in vivo
in luteal cells of buffaloes. High/mid-dose FSH significantly
stimulated VEGF secretion in the slow-growing follicles at 5%
O2 environments (16).
However, recombinant human FSH directly stimulates angiogenesis
without VEGF secretion in FSHR-expressing endothelial cells by the
PI3 K/AKT signaling pathway (15).
In addition, the FSHR is selectively expressed on the surface of
the blood vessels of a wide range of tumors (17,18,29–31).
FSHR expression of endothelial cells may be involved in the
proliferation of tumor tissues in this particular location, and
could promote angiogenesis by inducing VEGF and VEGF receptor 2
signaling in tumor endothelial cells (17,30).
Interestingly, relatively recent genetic studies
identified an association of single nucleotide polymorphisms in the
FSHR gene to preterm birth, polycystic ovary syndrome and premature
ovarian failure (32–35). However, no significant association
was identified in the comparison of genotypes and allele of the
FSHR gene, rs1394205, with pre-eclampsia in a Chinese population
with a small sample size (~100) reported (32). To confirm the association of FSHR
gene polymorphisms with pre-eclampsia, further genetic studies in
other populations with larger sample sizes and denser markers are
required for further investigation.
It should be noted that the present study has
potential limitations. Firstly, the number of patients studied was
relatively small, so further studies employing large numbers of
samples are required to confirm the findings. Secondly, the authors
did not completely assess all of the factors related to placental
angiogenesis. Some known confounding factors, such as soluble
Fms-like tyrosine kinase-1, soluble endoglin and VEGF were not
included (36,37). Finally, the design of the present
study does not allow us to define if altered levels of placental
FSHR mRNA and protein represent a response to abnormal placentation
or its cause. Also, these events may be a component of an
adaptation to placental hypoxia that incorporates other angiogenic
factors as well.
Overall, the current findings indicated that the
expression levels of placental FSHR mRNA and protein were
significantly decreased in pregnancies complicated by
pre-eclampsia. These results indicated that decreased FSHR
expression could contribute to aberrant angiogenesis and
trophoblast development associated with pre-eclampsia. In order to
determine whether similar differences antedate the clinical onset
of the disease, future longitudinal studies are needed to trace the
mRNA and protein expression of FSHR in first and second trimester
placenta and determine whether the results are the cause or
effect.
Acknowledgments
The present study work was supported by the National
Natural Science Foundation of China (grant nos. 81671486, 81270668,
30960118 and 81460226) and the 555 Project of Jiangxi Province Gan
Po Excellence and Jiangxi Province and Nanchang University
Postgraduate Innovation Project (grant nos. cx2015176 and
cx2016355). The authors are grateful to all mothers who donated
their placentas for the current study.
References
|
1
|
Young BC, Levine RJ and Karumanchi SA:
Pathogenesis of preeclampsia. Annu Rev Pathol. 5:173–192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji L, Brkić J, Liu M, Fu G, Peng C and
Wang YL: Placental trophoblast cell differentiation: Physiological
regulation and pathological relevance to preeclampsia. Mol Aspects
Med. 34:981–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Escudero C, Celis C, Saez T, Martin San S,
Valenzuela FJ, Aguayo C, Bertoglia P, Roberts JM and Acurio J:
Increased placental angiogenesis in late and early onset
pre-eclampsia is associated with differential activation of
vascular endothelial growth factor receptor 2. Placenta.
35:207–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armant DR, Fritz R, Kilburn BA, Kim YM,
Nien JK, Maihle NJ, Romero R and Leach RE: Reduced expression of
the epidermal growth factor signaling system in preeclampsia.
Placenta. 36:270–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kappou D, Sifakis S, Androutsopoulos V,
Konstantinidou A, Spandidos DA and Papantoniou N: Placental mRNA
expression of angiopoietins (Ang)-1, Ang-2 and their receptor Tie-2
is altered in pregnancies complicated by preeclampsia. Placenta.
35:718–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Liu H, Chen X, Chen PH, Fischer
D, Sriraman V, Yu HN, Arkinstall S and He X: Structure of
follicle-stimulating hormone in complex with the entire ectodomain
of its receptor. Proc Natl Acad Sci USA. 109:12491–12496. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernard DJ, Fortin J, Wang Y and Lamba P:
Mechanisms of FSH synthesis: What we know, what we don't, and why
you should care. Fertil Steril. 93:2465–2485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stilley JA, Christensen DE, Dahlem KB,
Guan R, Santillan DA, England SK, Al-Hendy A, Kirby PA and Segaloff
DL: FSH receptor (FSHR) expression in human extragonadal
reproductive tissues and the developing placenta, and the impact of
its deletion on pregnancy in mice. Biol Reprod. 91:742014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizrachi D and Shemesh M:
Follicle-stimulating hormone receptor and its messenger ribonucleic
acid are present in the bovine cervix and can regulate cervical
prostanoid synthesis. Biol Reprod. 61:776–784. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Celik O, Tagluk ME, Hascalik S, Elter K,
Celik N and Aydin NE: Spectrotemporal changes in electrical
activity of myometrium due to recombinant follicle-stimulating
hormone preparations follitropin alfa and beta. Fertil Steril.
90:(Suppl 4). S1348–S1356. 2008. View Article : Google Scholar
|
|
11
|
Hascalik S, Celik O, Tagluk ME, Yildirim A
and Aydin NE: Effects of highly purified urinary FSH and human
menopausal FSH on uterine myoelectrical dynamics. Mol Hum Reprod.
16:200–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang
Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, et
al: FSH directly regulates bone mass. Cell. 125:247–260. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu LL, Blair H, Cao J, Yuen T, Latif R,
Guo L, Tourkova IL, Li J, Davies TF, Sun L, et al: Blocking
antibody to the β-subunit of FSH prevents bone loss by inhibiting
bone resorption and stimulating bone synthesis. Proc Natl Acad Sci
USA. 109:14574–14579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Cheng Y, Fan M, Chen D and Bian Z:
FSH aggravates periodontitis-related bone loss in ovariectomized
rats. J Dent Res. 89:366–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stilley JA, Guan R, Duffy DM and Segaloff
DL: Signaling through FSH receptors on human umbilical vein
endothelial cells promotes angiogenesis. J Clin Endocrinol Metab.
99:E813–E820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fisher TE, Molskness TA, Villeda A,
Zelinski MB, Stouffer RL and Xu J: Vascular endothelial growth
factor and angiopoietin production by primate follicles during
culture is a function of growth rate, gonadotrophin exposure and
oxygen milieu. Hum Reprod. 28:3263–3270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Radu A, Pichon C, Camparo P, Antoine M,
Allory Y, Couvelard A, Fromont G, Hai MT and Ghinea N: Expression
of follicle-stimulating hormone receptor in tumor blood vessels. N
Engl J Med. 363:1621–1630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Planeix F, Siraj MA, Bidard FC, Robin B,
Pichon C, Sastre-Garau X, Antoine M and Ghinea N: Endothelial
follicle-stimulating hormone receptor expression in invasive breast
cancer and vascular remodeling at tumor periphery. J Exp Clin
Cancer Res. 34:122015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faiman C, Ryan RJ, Zwirek SJ and Rubin ME:
Serum FSH and HCG during human pregnancy and puerperium. J Clin
Endocrinol Metab. 28:1323–1329. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Penny R, Olambiwonnu NO and Frasier SD:
Follicle stimulating hormone (FSH) and luteinizing hormone-human
chorionic gonadotropin (LH-HCG) concentrations in paired maternal
and cord sera. Pediatrics. 53:41–47. 1974.PubMed/NCBI
|
|
22
|
Parlow AF, Daane TA and Dignam WJ: On the
concentration of radioimmunoassayable FSH circulating in blood
throughout human pregnancy. J Clin Endocrinol Metab. 31:213–214.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jaffe RB, Lee PA and Midgley AR Jr: Serum
gonadotropins before, at the inception of, and following human
pregnancy. J Clin Endocrinol Metab. 29:1281–1283. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Winn VD, Haimov-Kochman R, Paquet AC, Yang
YJ, Madhusudhan MS, Gormley M, Feng KT, Bernlohr DA, McDonagh S,
Pereira L, et al: Gene expression profiling of the human
maternal-fetal interface reveals dramatic changes between
midgestation and term. Endocrinology. 148:1059–1079. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dezso Z, Nikolsky Y, Sviridov E, Shi W,
Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ,
Guryanov A, et al: A comprehensive functional analysis of tissue
specificity of human gene expression. BMC Biol. 6:492008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eyster KM, Klinkova O, Kennedy V and
Hansen KA: Whole genome deoxyribonucleic acid microarray analysis
of gene expression in ectopic versus eutopic endometriusm. Fertil
Steril. 88:1505–1533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reisinger K, Baal N, McKinnon T, Münstedt
K and Zygmunt M: The gonadotropins: Tissue-specific angiogenic
factors? Mol Cell Endocrinol. 269:65–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fatima LA, Evangelista MC, Silva RS,
Cardoso AP, Baruselli PS and Papa PC: FSH up-regulates angiogenic
factors in luteal cells of buffaloes. Domest Anim Endocrinol.
45:224–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siraj MA, Pichon C, Radu A and Ghinea N:
Endothelial follicle stimulating hormone receptor in primary kidney
cancer correlates with subsequent response to sunitinib. J Cell Mol
Med. 16:2010–2016. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siraj A, Desestret V, Antoine M, Fromont
G, Huerre M, Sanson M, Camparo P, Pichon C, Planeix F, Gonin J, et
al: Expression of follicle-stimulating hormone receptor by the
vascular endothelium in tumor metastases. BMC Cancer. 13:2462013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Renner M, Goeppert B, Siraj MA, Radu A,
Penzel R, Wardelmann E, Lehner B, Ulrich A, Stenzinger A, Warth A,
et al: Follicle-stimulating hormone receptor expression in soft
tissue sarcomas. Histopathology. 63:29–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Tong XH, Sun CJ and Zhang WY:
Study of follicle-stimulating hormone receptor and tyrosine
hydroxylase polymorphisms and pre-eclampsia in Chinese Han
population. Zhonghua Yi Xue Za Zhi. 90:1213–1215. 2010.(In
Chinese). PubMed/NCBI
|
|
33
|
Katari S, Wood-Trageser MA, Jiang H,
Kalynchuk E, Muzumdar R, Yatsenko SA and Rajkovic A: Novel
inactivating mutation of the FSH receptor in two siblings of Indian
origin with premature ovarian failure. J Clin Endocrinol Metab.
100:2154–2157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu XQ, Xu SM, Liu JF, Bi XY, Wu YX and Liu
J: Association between FSHR polymorphisms and polycystic ovary
syndrome among Chinese women in north China. J Assist Reprod Genet.
31:371–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma L, Chen Y, Mei S, Liu C, Ma X, Li Y,
Jiang Y, Ha L and Xu X: Single nucleotide polymorphisms in
premature ovarian failure-associated genes in a Chinese Hui
population. Mol Med Rep. 12:2529–2538. 2015.PubMed/NCBI
|
|
36
|
Brownfoot FC, Tong S, Hannan NJ, Hastie R,
Cannon P, Tuohey L and Kaitu'u-Lino TJ: YC-1 reduces placental
sFlt-1 and soluble endoglin production and decreases endothelial
dysfunction: A possible therapeutic for preeclampsia. Mol Cell
Endocrinol. 413:202–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Tao YM, Cheng XY, Zhu TF, Chen ZF,
Yao H and Su LX: Vascular endothelial growth factor affects
dendritic cell activity in hypertensive disorders of pregnancy. Mol
Med Rep. 12:3781–3786. 2015.PubMed/NCBI
|