Introduction
Fulminant hepatitis remains a major health problem
worldwide, and this condition may occur from multiple origins,
including viral hepatitis, autoimmune hepatitis, drug-induced liver
injury, metabolic disease and circulatory disturbances (1). Immune-mediated mechanisms appear to
have a central role in acute hepatitis, however, detailed
mechanisms have not previously been elucidated (2,3). Due
to the lack of specific treatments for fulminant hepatitis, plasma
exchange is usually provided to patients with this condition
(1). In addition, liver
transplantation, usually from living donors, is scheduled when
clinical improvement is not achieved with these conventional
medical treatments (1,4).
Concanavalin A (ConA) injections in mice result in
serious, immune-mediated liver injury similar to that observed in
human viral, autoimmune and fulminant cases of hepatitis (5,6).
ConA injection results in an increase in the serum concentrations
of several inflammatory cytokines, including tumor necrosis
factor-α, interleukin-6 and interferon-γ, which contribute to the
development of hepatitis (5–7). In
the present study, a ConA-induced mouse hepatitis model was used to
investigate the effectiveness of galectin-9 (Gal-9) as a novel
therapeutic target in fulminant hepatitis. Gal-9 is a
β-galactoside-binding lectin belonging to the galectin family, and
was first identified as an eosinophil chemoattractant and
activation factor (5). Gal-9 has
been previously reported to induce apoptosis in T cells,
particularly in CD4 + Th1 and Th17 cells, and this lectin
additionally exhibits a stimulatory effect on regulatory T cell
activity (6–8). Notably, Gal-9 has been investigated
as a potential therapeutic agent for various autoimmune diseases
(8). Furthermore, Gal-9 has
previously been demonstrated to exhibit anti-allergenic (9) and anti-tumor (10) effects. However, there are few
reports demonstrating the effects of Gal-9 in fulminant
hepatitis.
miRNAs are small, non-coding RNAs, and their number
within the human genome is predicted to be ~1,000 (11). There are >2,000 genes encoding
human miRNAs that are registered in miRBase (http://www.mirbase.org) (12). It has been demonstrated that the
expression of miRNAs is associated with various liver diseases,
including hepatitis and hepatocellular carcinoma (13–15).
However, miRNA expression profiles following Gal-9 administration
in fulminant hepatitis remain to be elucidated. Therefore, the
present study aimed to investigate if Gal-9 ameliorates fulminant
hepatitis and to identify specific miRNAs associated with the
effects of Gal-9 in hepatitis.
Materials and methods
Mice and treatments
Animal experiments were performed according to the
guidelines of the Animal Care and Use Committee for Kagawa
University (Takamatsu, Japan). Ethical approval was obtained from
the Animal Care and Use Committee for Kagawa University. A total of
56 male BALB/c mice, at 7 weeks of age, 21–26 g, were purchased
from Japan SLC, Inc. (Hamamatsu, Japan). The mice were housed
together in a temperature-controlled environment under a 12 h:12 h
day:night cycle (light from 07:00 to 19:00) at 22±1°C with freely
available food and water throughout the experiment. Mice were given
a single intravenous injection of ConA (Merck KGaA, Darmstadt,
Germany) at a dose of 35 mg/kg body weight, and the animals were
sacrificed 24 h following ConA administration or were observed for
48 h while checking for survival every 6 h. In the Gal-9 treated
group (n=17), 00 µg Gal-9 (Department of Immunology, Faculty of
Medicine, Kagawa University, Takamatsu, Japan) per mouse was
injected subcutaneously immediately following the administration of
ConA. Controls were treated with only PBS (n=10), only Gal-9 (n=10)
or PBS with ConA (n=19).
Analysis of liver enzymes in mice
Alanine aminotransferase (ALT) activities were
measured by L-Type AST.J2 (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan) and analyzed by an enzymatic colorimetric test (Bio
Majesty JCA-BM8060; JEOL Ltd., Tokyo, Japan) according to Japan
Society of Clinical Chemistry transferable method (16).
Histological analyses
Livers and spleens from individual mice were fixed
for ~48 h in 10% formalin at room temperature (22±1°C). Tissue
samples were dehydrated by ethanol and xylene. After embedding in
paraffin wax, sections with a thickness of 5 µm were cut by Shikoku
Cytopathological Laboratory (Takamatsu, Japan) and stained with
hematoxylin and eosin to determine the degree of necrosis. Image
analysis of the necrotic area was performed using a light
microscope, (BX51; Olympus Corporation, Tokyo, Japan) with cellSens
version 1.14 (Olympus Corporation). The sections of livers were
also stained using the Masson Trichrome staining procedure (Merck
KGaA), according to the manufacturer's protocol, to examine the
progression of fibrosis.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assays
To investigate levels of apoptosis,
paraffin-embedded sections (5 µm) also fixed in 10% formalin as
above, were stained by TUNEL assays using the TACS®2
TdT-DAB In Situ Apoptosis Detection kit (Trevigen, Inc.,
Gaithersburg, MD, USA), according to the manufacturer's
protocol.
Immunohistochemistry
Liver paraffin sections also fixed in 10% formalin
(5 µm) were incubated in 3% H2O2 in 20%
methanol for 10 min to remove endogenous peroxidase activity. The
sections were then treated with 2.5% normal goat serum for 30 min
and incubated with rat anti-Ly-6G (ab25377; 1:100; Abcam,
Cambridge, UK) and rabbit anti-CD11b (ab75476; 1:200; Abcam)
primary antibodies in 2.5% normal goat serum overnight at 4°C.
Sections were subsequently incubated with ImmPRESS™ Reagent HRP
Anti-Rat IgG (MP-7404, ready-to-use) and ImmPRESS™ Reagent
Anti-Rabbit IgG (MP-7401, ready-to-use) secondary antibodies (both
from Vector Laboratories, Inc., Burlingame, CA, USA), and a DAB
Peroxidase (HRP) Substrate kit (Vector Laboratories, Inc.).
Counterstaining were performed by Mayer's hematoxylin solution
(Wako Pure Chemical Industries, Ltd.) to stain nuclei. Positive
cells were counted in 10 high-power fields/section using a light
microscope, at a magnification of ×400.
Global miRNA expression profiling
Total RNA was extracted from mouse liver samples
with the miRNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocol. Following the quality and
quantitation of RNA measurement with an RNA 6000 Nano kit (Agilent
Technologies, Inc., Santa Clara, CA, USA), the samples (250 ng
RNA/sample) were labeled using a miRCURY LNA™ microRNA Hi-Power
Labeling kit (Hy3; Exiqon A/S, Vedbaek, Denmark) according to the
manufacturer's protocol and subsequently hybridized onto a
3D-Gene® mouse miRNA Oligo chip (version 19; Toray
Industries, Inc., Tokyo, Japan) according to the manufacturer's
protocol. Scanning was performed with the 3D-Gene®
Scanner 3000 (Toray Industries, Inc.). The 3D-Gene®
extraction version 1.2 software (Toray Industries, Inc.) was used
to read the raw intensities of the image. To determine changes in
miRNA expression between Gal-9-treated and control samples, the raw
data was analyzed using GeneSpring GX software, version 10.0
(Agilent Technologies, Inc.).
Statistical analysis
All analyses were performed using GraphPad Prism
version 6.0 for Windows (GraphPad Software, Inc., La Jolla, CA,
USA). Survival rate was analyzed using the log-rank test. Unpaired
comparisons between groups were performed using the Mann-Whitney U
test. Differentially expressed miRNAs were also determined with the
Mann-Whitney U test. Hierarchical clustering was performed using
the farthest neighbor method employing the absolute uncentered
Pearson's correlation coefficient as a metric. A heat map was
produced using the relative expression intensity for each miRNA, in
which the base-2 logarithm of the intensity was median centered for
each row. P<0.05 was considered to indicate a statistically
significant difference and data are presented as mean ± standard
deviation.
Results
Gal-9 prolongs overall survival in
ConA-treated mice
Notably, in ConA-treated mice, the overall survival
rate was significantly increased in Gal-9-treated mice compared
with control mice treated with ConA + PBS (P<0.05; Fig. 1). No deaths were observed in the
PBS-only and Gal-9-only-treated groups.
Gal-9 attenuates liver injury in
ConA-treated mice
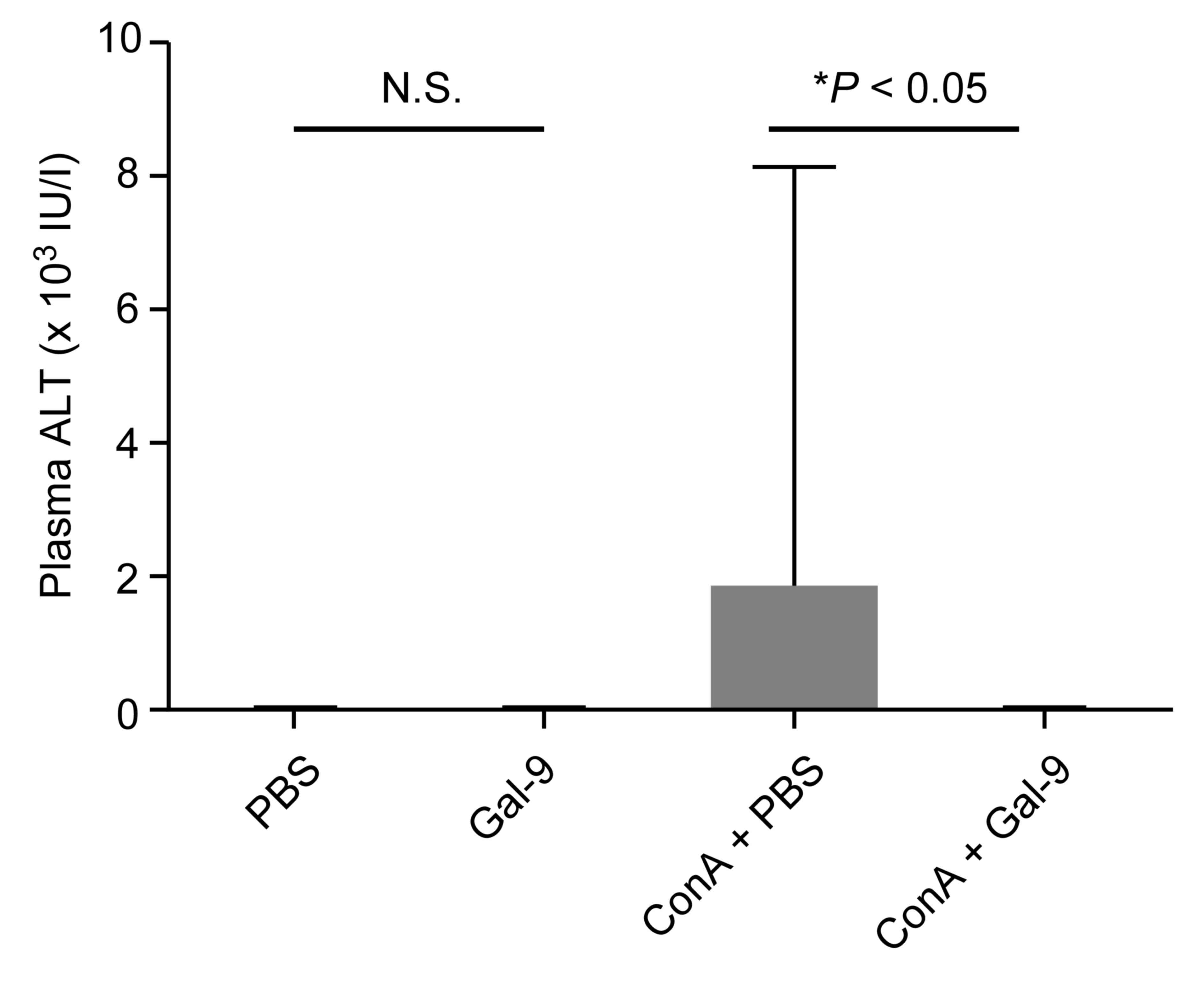
To determine the effects of Gal-9 on severe liver
injury, liver enzymes were examined in the plasma of each group.
Plasma ALT levels were significantly diminished in ConA-treated
mice co-treated with Gal-9 compared with ConA-treated mice
co-treated with PBS. No significant alteration in plasma ALT levels
(within normal limits, <42 U/l) was observed between PBS-only
and Gal-9-only groups (Fig. 2).
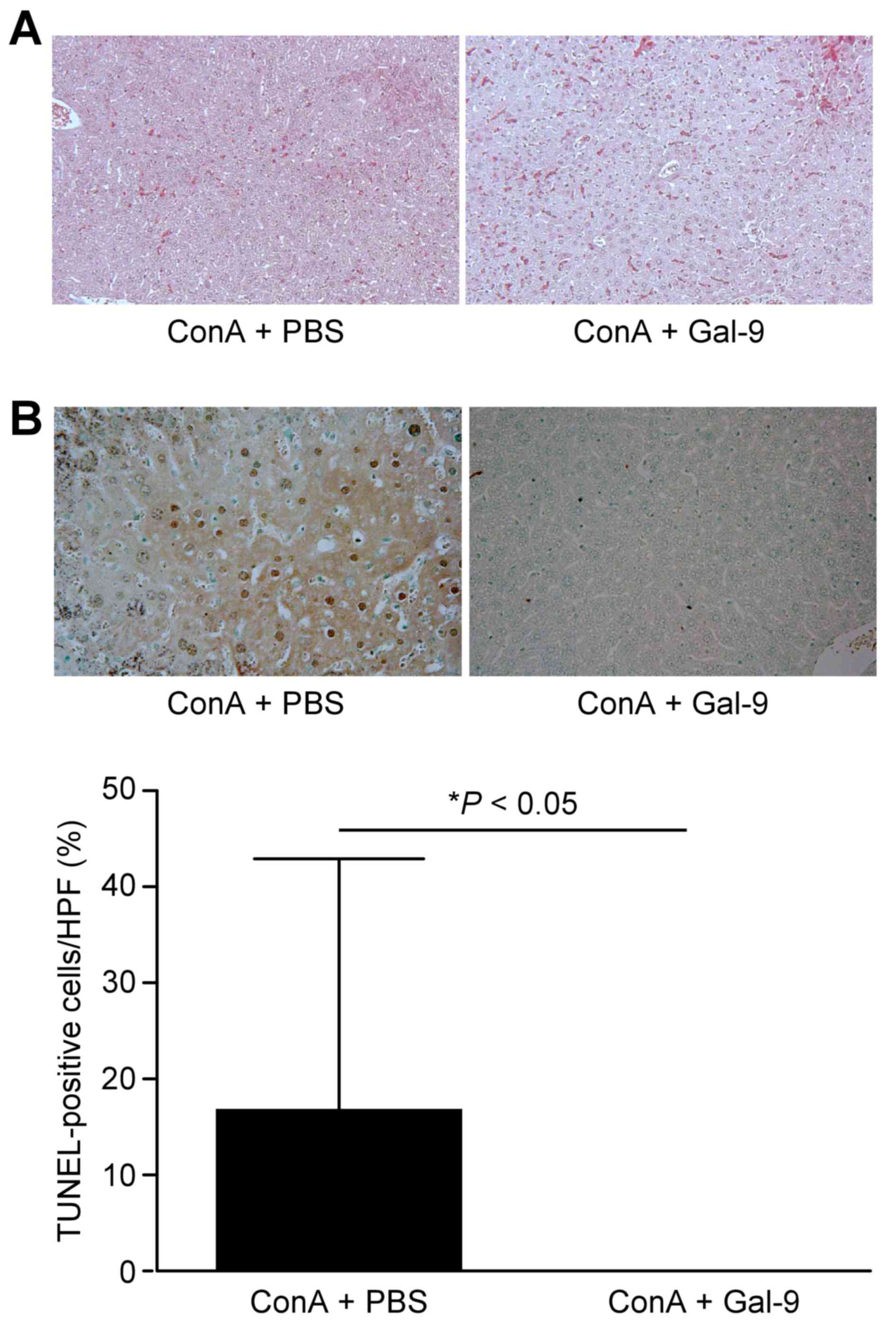
Administration of ConA induced necrosis in the liver and congestion
of blood in the spleens of ConA + PBS control mice. However,
treatment in both the Gal-9 only and ConA + Gal-9 groups resulted
in internal organs that were almost normal. Histologically, the
necrotic areas in the livers of ConA + Gal-9-treated mice were
significantly smaller compared with the ConA + PBS group
(P<0.05; Fig. 3). In addition,
no fibril formation was detected in either of these groups using
Masson's trichrome staining (Fig.
4A).
Gal-9 prevents apoptosis in
ConA-treated mice
Apoptotic cells were detected by TUNEL assay in the
livers of ConA-treated mice, with or without Gal-9 co-treatment.
Although apoptotic cells were observed in the livers without Gal-9
treatment, as illustrated in Fig.
4B, fewer apoptotic cells were detected in Gal-9-treated mice
(P<0.05); ~16.5% of hepatocytes were TUNEL-positive per field in
the livers of mice without Gal-9 treatment (Fig. 4B).
Gal-9 reduces local neutrophil and
macrophage infiltration
In ConA-induced hepatitis, the present study
performed immunohistochemical staining for Ly-6G and CD11b
expression. Gal-9 prevented neutrophil (Ly-6G; P<0.05; Fig. 5A) and CD11b-positive macrophage
(P<0.05; Fig. 5B) infiltration
compared with the ConA + PBS control group.
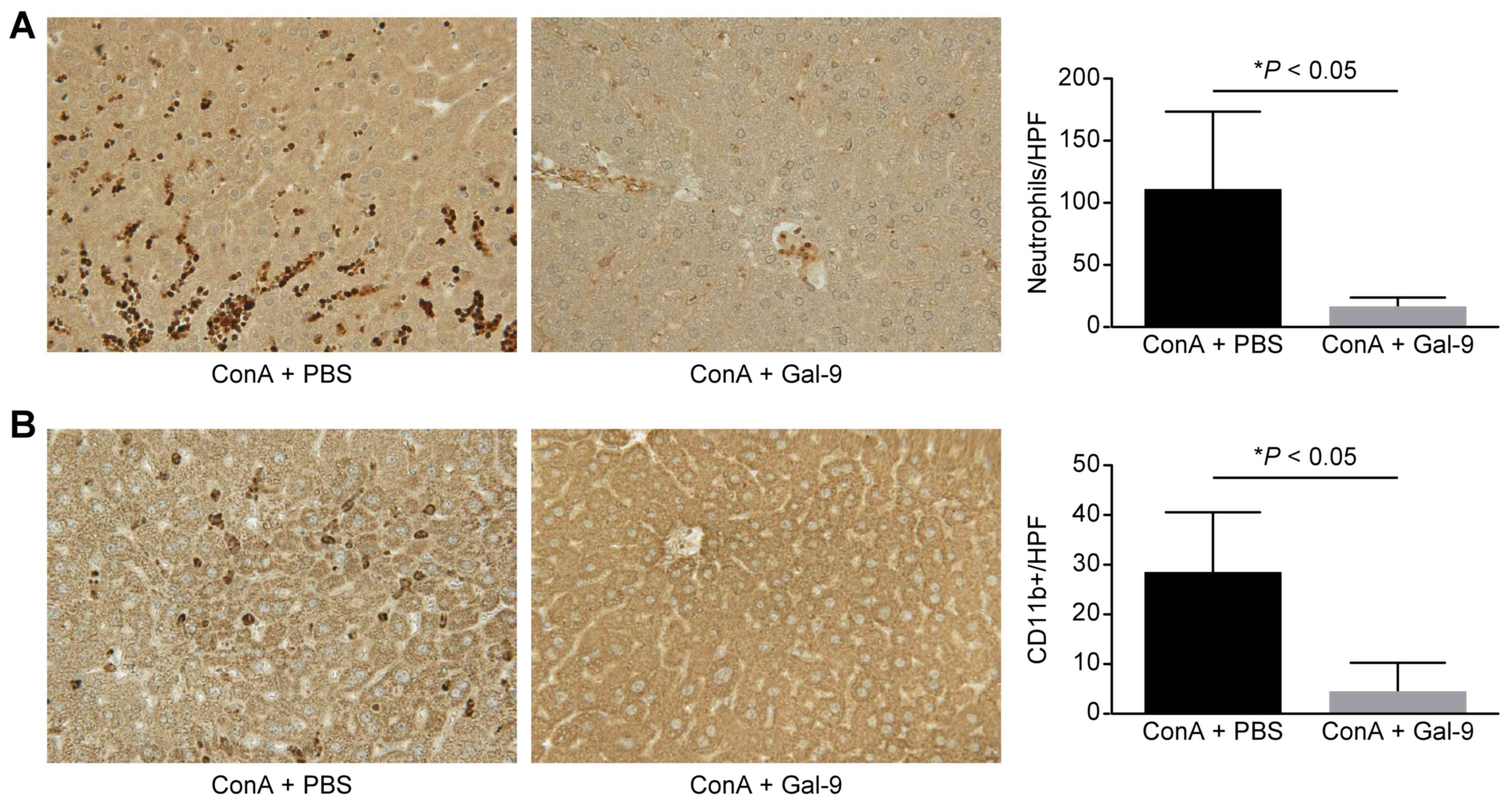 | Figure 5.Gal-9 prevents the accumulation of
neutrophils (Ly-6G) and macrophages (CD11b) in ConA-induced
hepatitis. (A) Left, immunohistochemistry of neutrophils in
representative liver sections. Original magnification, ×200. Right,
quantification of neutrophils per HPF (magnification, ×400). (B)
Left, immunohistochemistry of macrophages in representative liver
sections. Original magnification, ×200. Right, quantification of
macrophages per HPF (magnification, ×400). The infiltration of
neutrophils and macrophages in Gal-9 treated mice was reduced
compared with control animals. Data are presented as the mean +
standard deviation. Gal-9, galectin-9; ConA, concanavalin A; HPF,
high power field. |
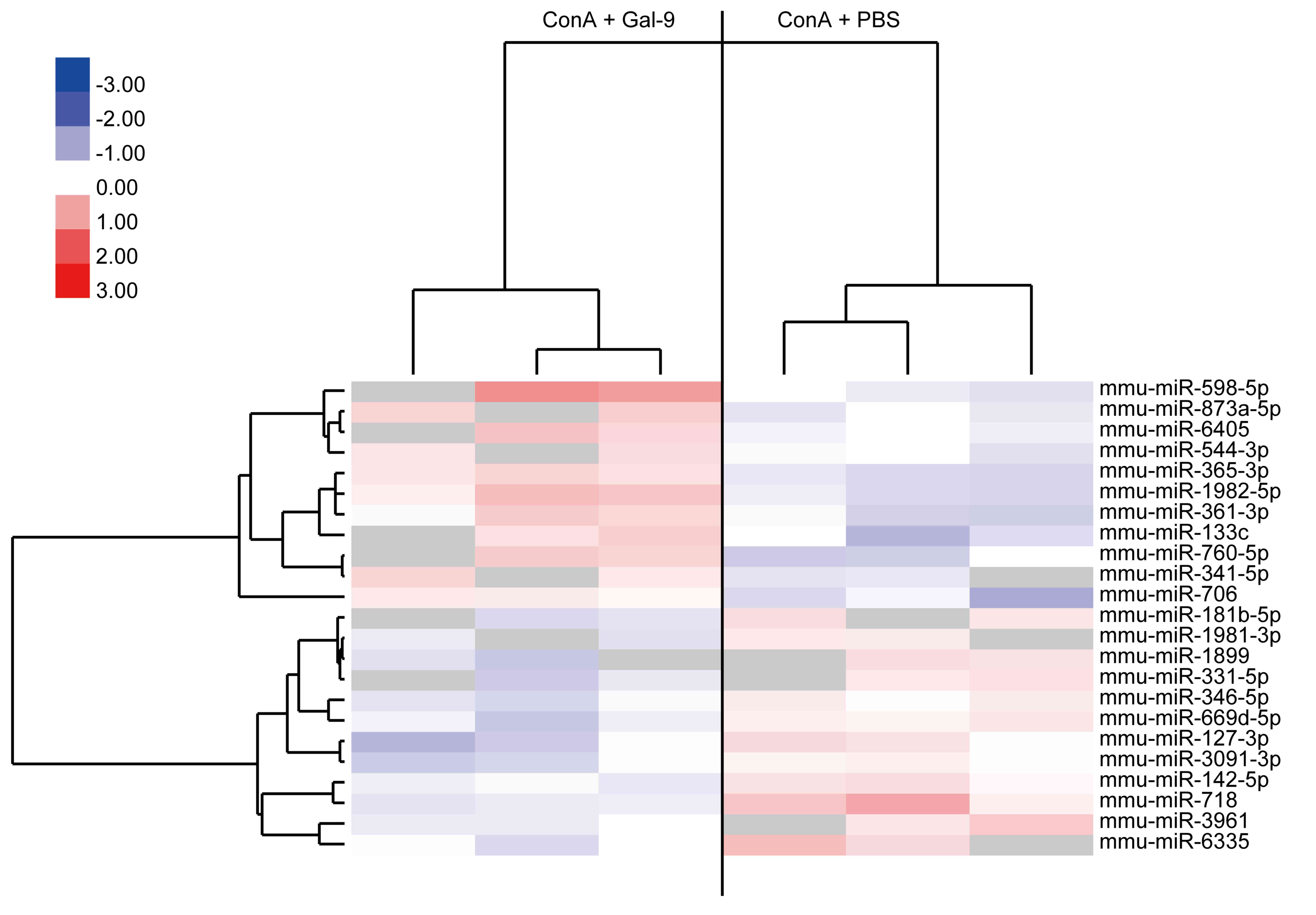
miRNA expression profiles in
ConA-treated mouse livers with or without Gal-9 co-treatment
As presented in Table
I, 11 miRNAs were significantly upregulated 24 h following
Gal-9 treatment, whereas 12 miRNAs were significantly downregulated
(P<0.05; Table I). Unsupervised
hierarchical clustering analysis using Pearson's correlation
demonstrated that Gal-9-treated mice clustered separately from the
control mice (Fig. 6).
 | Table I.Alterations in miRNA expression in
liver cells from mice treated with ConA in presence or absence of
Gal-9. |
Table I.
Alterations in miRNA expression in
liver cells from mice treated with ConA in presence or absence of
Gal-9.
| A, Upregulated
miRNAs |
|---|
|
|---|
| miRNA | P-value | Fold-change,
Gal-9/control | Chromosomal
localization |
|---|
| mmu-miR-598-5p | 0.002 | 2.507±1.188 | 8p23.1 |
|
mmu-miR-1982-5p | 0.019 | 1.823±0.293 | – |
| mmu-miR-760-5p | 0.020 | 1.777±0.840 | 1p22.1 |
| mmu-miR-133c | 0.044 | 1.704±0.808 | – |
| mmu-miR-365-3p | 0.002 | 1.636±0.116 | – |
| mmu-miR-361-3p | 0.034 | 1.613±0.227 | Xq21.2 |
|
mmu-miR-873a-5p | 0.006 | 1.576±0.743 | 9p21.1 |
| mmu-miR-6405 | 0.016 | 1.533±0.731 | – |
| mmu-miR-341-5p | 0.041 | 1.519±0.721 | – |
| mmu-miR-706 | 0.038 | 1.513±0.063 | 14 |
| mmu-miR-544-3p | 0.033 | 1.363±0.644 | – |
|
| B, Downregulated
miRNAs |
|
| miRNA | P-value | Fold change,
Gal-9/control | Chromosomal
localization |
| mmu-miR-718 | 0.045 | 0.564±0.016 | X |
| mmu-miR-1899 | 0.018 | 0.576±0.275 | – |
| mmu-miR-6335 | 0.042 | 0.621±0.071 | – |
| mmu-miR-127-3p | 0.041 | 0.629±0.140 | 14q32.2 |
|
mmu-miR-181b-5p | 0.009 | 0.633±0.299 | – |
| mmu-miR-331-5p | 0.042 | 0.634±0.304 | 12q22 |
| mmu-miR-3961 | 0.041 | 0.676±0.047 | – |
|
mmu-miR-1981-3p | 0.008 | 0.728±0.344 | – |
|
mmu-miR-669d-5p | 0.030 | 0.732±0.106 | 9 |
|
mmu-miR-3091-3p | 0.044 | 0.749±0.116 | – |
| mmu-miR-142-5p | 0.025 | 0.755±0.042 | 17q22 |
| mmu-miR-346-5p | 0.029 | 0.763±0.086 | 10q23.2 |
Discussion
Fulminant hepatitis has various origins, including
viral hepatitis, autoimmune hepatitis, drug-induced liver injury,
metabolic disease and circulatory disturbances (1). Novel treatments for chronic
hepatitis, including hepatitis B and C, have been developed and
have been demonstrated to be effective (17–19).
However, the current treatments for fulminant hepatitis are
inadequate. For example, antiviral therapy is not sufficient for
the treatment of fulminant hepatitis B, as this virus is not
directly cytopathic and the mechanisms of fulminant hepatitis B
have been suggested to be primarily immune-mediated (20). Currently, no specific treatment is
available for fulminant hepatitis and therefore novel curative
immunological approaches are critical for fulminant hepatitis
therapy.
The present study demonstrated that a single
injection of Gal-9 improved the survival rates of ConA-treated
mice. Gal-9 treatment also reduced local neutrophil and macrophage
infiltration. Gal-9 is a ligand of T cell immunoglobulin and mucin
protein 3 (6), and it induces Th1
cell apoptosis, thereby inhibiting Th1 immunity and leading to
peripheral tolerance (21).
Furthermore, Gal-9 results in selective apoptosis of ConA-activated
CD4-positive T cells and ameliorates ConA-induced hepatitis
(22). These reports indicate that
Gal-9 may regulate immune responses to induce apoptosis in T
cells.
In the current study, fewer apoptotic hepatocytes
were observed in livers treated with Gal-9 compared with those not
treated with Gal-9. Indeed, it has been reported that Gal-9
suppresses hepatocyte apoptosis via the inhibition of toll-like
receptor 4 expression in ischemia-reperfusion injury (23,24).
Hepatocyte apoptosis was previously demonstrated to be
significantly increased in hepatitis (25,26),
including viral hepatitis and nonalcoholic steatohepatitis, and has
been demonstrated to be associated with disease severity (25,26).
These results indicate that Gal-9 may suppress hepatocyte apoptosis
even in severe liver injury.
Notably, Gal-9 induces cancer cell apoptosis and
inhibits the proliferation of various human cancer cell types,
including melanoma (10),
hepatocellular carcinoma (27) and
cholangiocarcinoma (28). The fact
that Gal-9 suppresses hepatocyte apoptosis in liver injury and
induces cancer cell apoptosis appears paradoxical. However, Gal-9
may alter its mechanism of action depending upon the situation and
the necessity to maintain hepatic homeostasis.
The present study identified miRNAs associated with
the effects of Gal-9 in fulminant hepatitis, and miR-361 and
miR-133 were upregulated in the livers of Gal-9-treated mice
compared with control mice. Kanitz et al (29) reported vascular endothelial growth
factor (VEGF) A as a putative target of miR-361. In addition,
miR-361 regulates endothelial progenitor cell functioning by
targeting VEGF (30). VEGF is a
potent stimulator of angiogenesis and also contributes to
inflammation via plasma extravasation (31). Furthermore, miR-133, which was also
upregulated by Gal-9 in the current study, exhibits anti-apoptotic
effects by targeting caspase-9 (32) and enhances myoblast proliferation
by repressing serum response factor (12). Therefore, upregulation of miR-361
may induce anti-inflammatory effects, while miR-133 may induce
anti-apoptotic effects and hepatocyte proliferation in the
ConA-treated mice that received Gal-9.
According to the results of the present study,
miR-181 and miR-127 were significantly downregulated by Gal-9
administration in ConA-treated mice. It has been reported that
miR-181 is increased during early T cell development and
subsequently downregulated in mature CD4 T cells, which include Th1
and Th2 effector cells. Specifically, miR-181 functions to enhance
T cell receptor (TCR) signaling strength by inhibiting multiple
phosphatases that negatively regulate the TCR signaling cascade
(33,34). Downregulation of miR-181 also
impairs TCR sensitivity by increasing dual specificity phosphatase
6 activity (35). In addition,
miR-127 is downregulated by Gal-9, which facilitates hepatocyte
proliferation in liver regeneration by releasing the pro-oncogene
B-cell lymphoma 6 protein and SET domain-containing protein 8
(36). The reports indicate that
downregulation of miR-181 and miR-127 by Gal-9 may induce
anti-inflammatory effects and enhance hepatocyte proliferation.
In conclusion, the results of the present study
demonstrated that Gal-9 ameliorated fulminant hepatitis,
potentially by regulation of apoptosis and inflammation,
stimulation of hepatocyte proliferation and alteration of the
expression of miRNAs involved in these events. However, further
studies are required to fully elucidate these mechanisms.
Acknowledgements
Dr Toshiro Niki and Dr Mitsuomi Hirashima are board
members of GalPharma Co., Ltd (Takamatsu, Japan). These two authors
hold the following patent associated with material pertinent to
this article: ‘Novel modified galectin-9 proteins and use thereof’,
which was applied for by GalPharma Co., Ltd. and was issued in
Japan (4792390), the USA (8268324), European Patent Office (EPO;
1736541), Canada (2561696), India (239130) and Korea (10-1222281)
as of 2 December 2013. These two authors have the following
products associated with material pertinent to this article:
stable-form Gal-9. The authors thank Ms. Kayo Endo, Ms. Fuyuko
Kokado, Ms. Keiko Fujikawa, Ms. Kayo Hirose, Dr Miwako Watanabe,
Ms. Noriko Murao and Dr Kayo Ogawa, Department of Gastroenterology
and Neurology, Faculty of Medicine, Kagawa University, for
providing technical assistance.
Glossary
Abbreviations
Abbreviations:
|
Gal-9
|
galectin-9
|
|
miRNAs
|
microRNAs
|
|
ConA
|
concanavalin A
|
References
|
1
|
Fujiwara K, Mochida S, Matsui A, Nakayama
N, Nagoshi S and Toda G: Intractable Liver Diseases Study Group of
Japan: Fulminant hepatitis and late onset hepatic failure in Japan.
Hepatol Res. 38:646–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kern M, Popov A, Scholz K, Schumak B,
Djandji D, Limmer A, Eggle D, Sacher T, Zawatzky R, Holtappels R,
et al: Virally infected mouse liver endothelial cells trigger CD8+
T-cell immunity. Gastroenterology. 138:336–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujiwara K, Yasui S, Yonemitsu Y, Arai M,
Kanda T, Nakano M, Oda S and Yokosuka O: Fixed point observation of
etiology of acute liver failure according to the novel Japanese
diagnostic criteria. J Hepatobiliary Pancreat Sci. 22:225–229.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikegami T, Taketomi A, Soejima Y,
Yoshizumi T, Sanefuji K, Kayashima H, Shimada M and Maehara Y:
Living donor liver transplantation for acute liver failure: A
10-year experience in a single center. J Am Coll Surg. 206:412–418.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saita N, Goto E, Yamamoto T, Cho I,
Tsumori K, Kohrogi H, Maruo K, Ono T, Takeya M, Kashio Y, et al:
Association of galectin-9 with eosinophil apoptosis. Int Arch
Allergy Immunol. 128:42–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oomizu S, Arikawa T, Niki T, Kadowaki T,
Ueno M, Nishi N, Yamauchi A and Hirashima M: Galectin-9 suppresses
Th17 cell development in an IL-2-dependent but Tim-3-independent
manner. Clin Immunol. 143:51–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seki M, Oomizu S, Sakata KM, Sakata A,
Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N, et al:
Galectin-9 suppresses the generation of Th17, promotes the
induction of regulatory T cells, and regulates experimental
autoimmune arthritis. Clin Immunol. 127:78–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niki T, Tsutsui S, Hirose S, Aradono S,
Sugimoto Y, Takeshita K, Nishi N and Hirashima M: Galectin-9 is a
high affinity IgE-binding lectin with anti-allergic effect by
blocking IgE-antigen complex formation. J Biol Chem.
284:32344–32352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kageshita T, Kashio Y, Yamauchi A, Seki M,
Abedin MJ, Nishi N, Shoji H, Nakamura T, Ono T and Hirashima M:
Possible role of galectin-9 in cell aggregation and apoptosis of
human melanoma cell lines and its clinical significance. Int J
Cancer. 99:809–816. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JF, Mandel EM, Thomson JM, Wu Q,
Callis TE, Hammond SM, Conlon FL and Wang DZ: The role of
microRNA-1 and microRNA-133 in skeletal muscle proliferation and
differentiation. Nat Genet. 38:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bala S, Marcos M and Szabo G: Emerging
role of microRNAs in liver diseases. World J Gastroenterol.
15:5633–5640. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyoshi H, Kato K, Iwama H, Maeda E,
Sakamoto T, Fujita K, Toyota Y, Tani J, Nomura T, Mimura S, et al:
Effect of the anti-diabetic drug metformin in hepatocellular
carcinoma in vitro and in vivo. Int J Oncol. 45:322–332.
2014.PubMed/NCBI
|
|
15
|
Morishita A and Masaki T: miRNA in
hepatocellular carcinoma. Hepatol Res. 45:128–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Japan Society of Clinical Chemistry, .
Recommendation for measuring enzyme activity in human serum.
Lactate dehydrogenase (1989–08-30). Japanese Journal of Clinical
Chemistry. 19:228–246. 1990.
|
|
17
|
Cortez KJ and Kottilil S: Beyond
interferon: Rationale and prospects for newer treatment paradigms
for chronic hepatitis C. Ther Adv Chronic Dis. 6:4–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
European Association For The Study Of The
Liver: EASL clinical practice guidelines: Management of chronic
hepatitis B virus infection. J Hepatol. 57:167–185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
European Association for Study of Liver:
EASL clinical practice guidelines: Management of hepatitis C virus
infection. J Hepatol. 60:392–420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bockmann JH, Dandri M, Lüth S, Pannicke N
and Lohse AW: Combined glucocorticoid and antiviral therapy of
hepatitis B virus-related liver failure. World J Gastroenterol.
21:2214–2219. 2015.PubMed/NCBI
|
|
21
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv K, Zhang Y, Zhang M, Zhong M and Suo Q:
Galectin-9 ameliorates Con A-induced hepatitis by inducing
CD4(+)CD25(low/int) effector T-Cell apoptosis and increasing
regulatory T cell number. PLoS One. 7:e483792012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uchida Y, Ke B, Freitas MC, Yagita H,
Akiba H, Busuttil RW, Najafian N and Kupiec-Weglinski JW: T-cell
immunoglobulin mucin-3 determines severity of liver
ischemia/reperfusion injury in mice in a TLR4-dependent manner.
Gastroenterology. 139:2195–2206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirao H, Uchida Y, Kadono K, Tanaka H,
Niki T, Yamauchi A, Hata K, Watanabe T, Terajima H and Uemoto S:
The protective function of galectin-9 in liver ischemia and
reperfusion injury in mice. Liver Transpl. 21:969–981. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mita E, Hayashi N, Iio S, Takehara T,
Hijioka T, Kasahara A, Fusamoto H and Kamada T: Role of Fas ligand
in apoptosis induced by hepatitis C virus infection. Biochem
Biophys Res Commun. 204:468–474. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feldstein AE, Canbay A, Angulo P, Taniai
M, Burgart LJ, Lindor KD and Gores GJ: Hepatocyte apoptosis and fas
expression are prominent features of human nonalcoholic
steatohepatitis. Gastroenterology. 125:437–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujita K, Iwama H, Sakamoto T, Okura R,
Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et
al: Galectin-9 suppresses the growth of hepatocellular carcinoma
via apoptosis in vitro and in vivo. Int J Oncol. 46:2419–2430.
2015.PubMed/NCBI
|
|
28
|
Kobayashi K, Morishita A, Iwama H, Fujita
K, Okura R, Fujihara S, Yamashita T, Fujimori T, Kato K, Kamada H,
et al: Galectin-9 suppresses cholangiocarcinoma cell proliferation
by inducing apoptosis but not cell cycle arrest. Oncol Rep.
34:1761–1770. 2015.PubMed/NCBI
|
|
29
|
Kanitz A, Imig J, Dziunycz PJ, Primorac A,
Galgano A, Hofbauer GF, Gerber AP and Detmar M: The expression
levels of microRNA-361-5p and its target VEGFA are inversely
correlated in human cutaneous squamous cell carcinoma. PLoS One.
7:e495682012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HW, Lo HH, Chiu YL, Chang SJ, Huang
PH, Liao KH, Tasi CF, Wu CH, Tsai TN, Cheng CC and Cheng SM:
Dysregulated miR-361-5p/VEGF axis in the plasma and endothelial
progenitor cells of patients with coronary artery disease. PLoS
One. 9:e980702014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haywood L, McWilliams DF, Pearson CI, Gill
SE, Ganesan A, Wilson D and Walsh DA: Inflammation and angiogenesis
in osteoarthritis. Arthritis Rheum. 48:2173–2177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H,
Xiao J, Shan H, Wang Z and Yang B: The muscle-specific microRNAs
miR-1 and miR-133 produce opposing effects on apoptosis by
targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci.
120:3045–3052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li QJ, Chau J, Ebert PJ, Sylvester G, Min
H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, et al:
miR-181a is an intrinsic modulator of T cell sensitivity and
selection. Cell. 129:147–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raisch J, Darfeuille-Michaud A and Nguyen
HT: Role of microRNAs in the immune system, inflammation and
cancer. World J Gastroenterol. 19:2985–2996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li G, Yu M, Lee WW, Tsang M, Krishnan E,
Weyand CM and Goronzy JJ: Decline in miR-181a expression with age
impairs T cell receptor sensitivity by increasing DUSP6 activity.
Nat Med. 18:1518–1524. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan C, Chen H, Wang L, Yang S, Fu H, Zheng
Y, Miao M and Jiao B: Down-regulation of MiR-127 facilitates
hepatocyte proliferation during rat liver regeneration. PLoS One.
7:e391512012. View Article : Google Scholar : PubMed/NCBI
|