Introduction
An animal model of ovariectomy (OVX) previously
demonstrated that OVX induced blockade of estrogen (1). The resulting estrogen deficiency has
been reported to be closely associated with brain dysfunction in
the hippocampus (2). After binding
to estrogen receptor α (ERα) or β (ERβ), estrogen promotes
mitogen-activated protein-kinase (MAPK), phosphatidylinositol
3-kinase (PI3K) and phospholipase C γ (PLCγ) pathways (3), which influence synaptic formation,
neuronal plasticity, cognition and neuroprotection in
neurodegenerative diseases (4–7). In
addition, estrogen has an important role in the synthesis and
expression of brain-derived neurotrophic factor (BDNF), which
regulates neuronal function in the hippocampus (8). BDNF exists as two forms in tissues:
Precursor BDNF (proBDNF) and mature BDNF (mBDNF). mBDNF is
synthesized from proBDNF following cleavage by tissue plasminogen
activator (tPA) (9). In general,
mBDNF acts by binding to the tropomyosin-related kinase B (TrkB)
receptor in the MAPK and PI3K-Akt pathways (10–14),
which has positive effects for neuronal proliferation,
differentiation and the development of synapses associated with
hippocampal function (15,16). ProBDNF also acts positively for
neuronal function by binding to the p75 neurotrophin receptor
(p75NTR). However, the proBDNF-p75NTR pathway also includes
pro-apoptotic activation (10–14).
BDNF expression is reduced by OVX, which induces an estrogen block
(17,18). In an animal model of OVX, BDNF was
reduced markedly and hippocampal function declined (2). Previous studies investigating
hippocampal function in OVX focused on mBDNF pathways alone
(17,18). Contrary to the previous OVX
results, regular exercise has been previously demonstrated to have
positive effects on brain function (19,20),
and affects the proliferation, differentiation and survival of
neuronal cells, and aids neuronal plasticity by promoting
neurotrophins (21). Exercise
enhances BDNF expression by positively affecting tPA, which is
associated with the synthesis of mBDNF in the hippocampus (22) and the TrkB pathway (16,23,24).
Chronic exercise has neuroprotective effects on hippocampal
dysfunction, dementia and neurodegenerative diseases (25–28).
In addition, exercise improves cognitive decline induced by OVX
(1). The majority of previous
studies regarding the effects of exercise on brain function by OVX
focused on BDNF expression and cognition in the hippocampus
(29–31). A limited number of studies have
sought to determine the underlying mechanism of proBDNF and mBDNF
pathways associated with hippocampal function in OVX. The present
study investigated whether regular exercise increased mBDNF
synthesis from proBDNF in the OVX rat model, and determined the
underlying molecular mechanisms of proBDNF and mBDNF signaling
pathways.
Materials and methods
Animals
Female, 6-week-old Sprague-Dawley rats (n=50;
160–200 g; Samtako Bio Korea Co., Ltd., Republic of Korea) were
adapted to the laboratory environment (temperature, 22±1°C;
relative humidity, 55±3%; 12-h light/12-h dark photoperiod) for 2
weeks. All rats were housed in pairs, given free access to water
and fed a standard chow diet (protein, 21%; fat, 5%; nitrogen-free
extract, 55%; fiber, 4%; adequate mineral and vitamin content).
Studies were performed in accordance with Chung-Nam University
standards for the Care and Use of Laboratory Animals (publication
no. CNU-00203). Rats were allocated to the following groups: Sham
control group (SC; n=10); OVX control group (OC; n=10); OVX
low-exercise group (OLE; n=10); and OVX moderate-exercise group
(OME; n=10).
Ovariectomy
Following anesthetization using ketamine/xylazine (8
mg/kg body weight), the dorsal mid-lumbar area between the first
and third lumbar was shaved and the midline was incised. A single
5.5–10 mm long incision was made in the muscle wall on the right
and left sides approximately one-third of the distance between the
spinal cord and the ventral midline. The ovary was exteriorized
through the muscle wall and removed. In the SC group, the ovary was
exteriorized but not removed.
Exercise protocol
After a 1-week recovery from surgery, rats in the
OLE and OME groups were subjected to treadmill exercise for 8
weeks, 5 days a week. The speed of treadmill exercise was 8 m/min
(grade 0%) in weeks 1–4 and 10 m/min (grade 0%) in weeks 5–8 for
the OLE group, and 12 m/min in weeks 1–4 and 18 m/min (grade 0%) in
weeks 5–8 for the OME group (32).
The duration was gradually increased from 30 to 60 min. OLE and OME
groups were subjected to 30 min for in weeks 1 and 2, 40 min in
weeks 3 and 4, 50 min in weeks 5 and 6, and 60 min in weeks 7 and
8. The non-exercise groups, including SC and OC, were exposed to
environmental stress similar to that experienced with treadmill
use, including noise and vibration, and restricted food and water
during treadmill exercise. To minimize the stress of exercise,
treadmill exercise was performed without external stimuli and
electronic shock.
Tissue preparation
Upon completion of the 8-week exercise program, the
rats were anesthetized at 48 h after the final exercise session by
intraperitoneal injection of xylazine (8 mg/kg) and ketamine (40
mg/kg). For protein analyses, brains of rats from each group were
extracted and the hippocampus was dissected and stored at
−80°C.
Western blotting
To prepare protein for western blotting, each
hippocampus was crushed in a solution containing 150 mM NaCl, 5 mM
EDTA, 50 mM Tris-HCl (pH 8.0), 1% NP-40, 1 mM aprotinin, 0.1 mM
leupeptin and 1 mM pepstatin, and centrifuged at 15,294 × g for 15
min at 4°C. Proteins were quantified by a Bradford assay and 30 µg
was loaded onto a 10% gel, subjected to SDS-PAGE and transferred to
a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked in TBS containing 0.001% Tween-20
(TBS-T) and 5% bovine serum albumin (Bovogen Biologicals Ltd.,
Victoria, Australia) at 4°C for 90 min. After washing, the membrane
was incubated overnight at 4°C with the following primary
antibodies: Rabbit anti-GAPDH (1:1,000; catalog no. ABS16; EMD
Millipore, Billerica, MA, USA), rabbit anti-proBDNF (1:1,000;
catalog no. sc-546; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), rabbit anti-mBDNF (1:1,000; catalog no. sc-546; Santa Cruz
Biotechnology, Inc.), rabbit anti-tPA (1:1,000; catalog no.
sc-15346; Santa Cruz Biotechnology, Inc.), rabbit anti-TrkB
(1:1,000; catalog no. sc-119; Santa Cruz Biotechnology, Inc.),
rabbit anti-p75NTR (1:1,000; catalog no. 4201S; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-total (t)-JNK
(1:1,000; catalog no. sc-571; Cell Signaling Technology, Inc.),
rabbit anti-phosphorylated (p)-JNK (1:1,000; catalog no. sc-135642;
Cell Signaling Technology, Inc.) and rabbit anti-nuclear factor-κB
(NF-κB; 1:1,000; catalog no. 3031S; Cell Signaling Technology,
Inc.). Subsequently, membranes were washed 3 times with TBS-T for
10 min and incubated with a goat anti-rabbit IgG secondary antibody
conjugated to alkaline phosphatase (1:2,000; catalog no. sc-2007;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The
membrane was washed 3 times with TBS-T for 10 min. Membranes were
exposed to Luminata™ (EMD Millipore, Billerica, MA, USA) and
protein bands were imaged using a Kodak Image Station 440CF (Kodak,
Rochester, NY, USA) and were quantified using Kodak ID version 3.5
densitometry software (Kodak). Membranes were stripped with
stripping buffer [20 ml 10% SDS, 12.5 ml 0.5 M Tris HCL (pH 6.8),
67.5 ml ultra pure water, 0.8 ml beta-mercaptoethanol] for 45 min
at 50°C and washed 5 times with TBS-T for 10 min. Following
stripping, membranes were blocked and re-probed with the
appropriate primary antibody 3–4 times. This was performed at least
twice, and the results demonstrate blots from one representative
experiment.
Statistical analysis
All data was analyzed using SPSS software version
16.0 (SPSS, Inc., Chicago, IL, USA) by one-way analysis of variance
followed by Tukey's post hoc test to compare among the experimental
groups. Results are presented as the mean + standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of exercise on mBDNF, TrkB and
tPA in the hippocampus
The OC group exhibited reduced mBDNF, TrkB and tPA
protein expression in the hippocampus compared with the SC group
(Fig. 1). By contrast, in the OLE
and OME groups, significantly increased mBDNF, TrkB and tPA protein
expression was observed in the hippocampus compared with the OC
group (Fig. 1). Exercise intensity
differences were also apparent as expression levels were
significantly different between the OLE and OME groups (Fig. 1).
 | Figure 1.Expression of (A) mBDNF, (B) TrkB and
(C) tPA protein in the hippocampus of control and OVX rats. Results
are presented as the mean + standard deviation. Different letters
represent significant differences compared with the other groups
(P<0.05). mBDNF, mature brain-derived neurotrophic factor; TrkB,
tropomyosin-related kinase B; tPA, tissue plasminogen activator;
OVX, ovariectomy; SC, sham control; OC, OVX control; OLE, OVX +
low-exercise; OME, OVX + moderate-exercise; O.D., optical
density. |
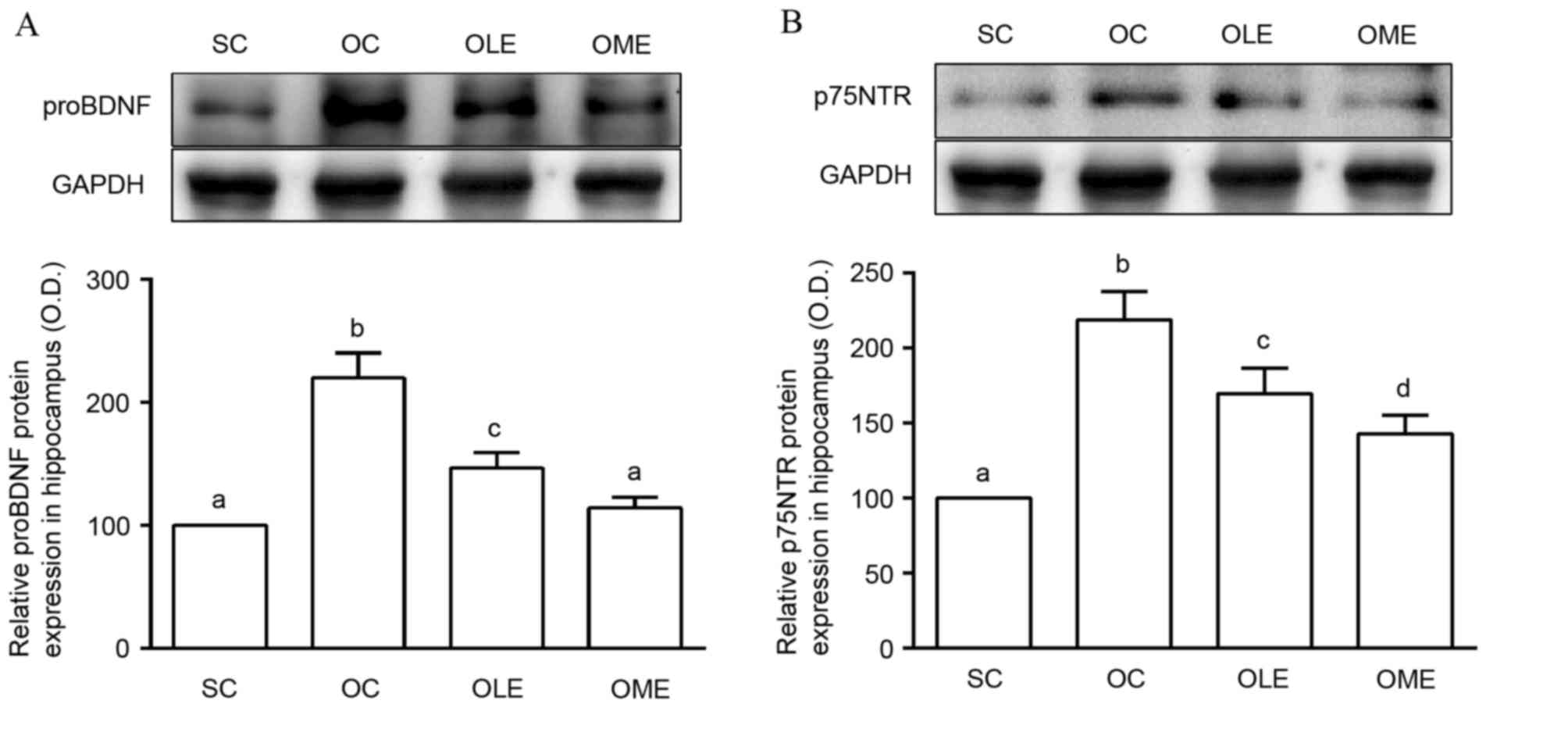
Effects of exercise on proBDNF and
p75NTR in hippocampus
The OC group exhibited significantly increased
proBDNF and p75NTR protein expression in the hippocampus compared
with the SC group (Fig. 2).
Furthermore, the OLE and OME groups had reduced proBDNF and p75NTR
protein expression in the hippocampus compared with the OC group
(Fig. 2). There were significant
differences in exercise intensity between the OLE and OME groups
(P<0.05; Fig. 2).
Effects of exercise on t-JNK, p-JNK,
and NF-κB in the hippocampus
A significantly increased p-JNK/t-JNK ratio and
reduced NF-κB protein expression was observed in the hippocampus of
OC rats compared with the SC group (Fig. 3). In addition, a reduced
p-JNK/t-JNK ratio and increased NF-κB protein expression was
observed in the hippocampus of OLE and OME groups, compared with
the OC group (Fig. 3). There were
significant differences in NF-κB expression between the OLE and OME
groups (P<0.05; Fig. 3).
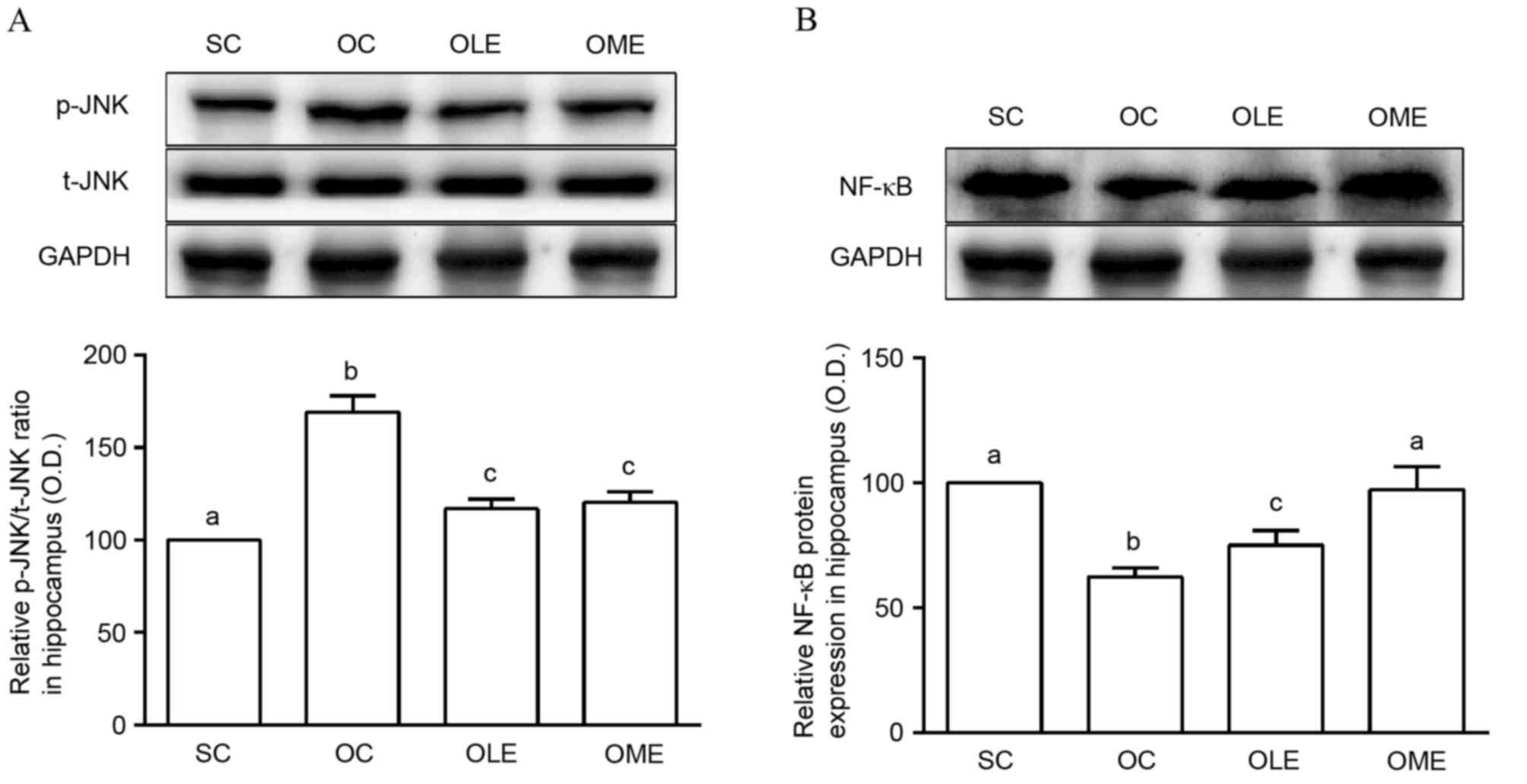 | Figure 3.Expression of (A) p-JNK/t-JNK and (B)
NF-κB protein in the hippocampus of control and OVX rats. Results
are presented as the mean + standard deviation. Different letters
represent significant differences compared with the other groups
(P<0.05). JNK, c-Jun N-terminal protein kinase; p-JNK,
phosphorylated-JNK; t-JNK, total-JNK; NF-κB, nuclear factor-κB;
OVX, ovariectomy; SC, sham control; OC, OVX control; OLE, OVX +
low-exercise; OME, OVX + moderate-exercise; O.D., optical
density. |
Discussion
The present study investigated the effects of
exercise on BDNF pathways in the hippocampus of ovariectomized rats
and demonstrated that tPA, mBDNF and NF-κB protein expression was
reduced, and the proBDNF, p75NTR and p-JNK level was increased by
OVX compared with sham rats. The observed alterations in protein
expression were reversed by regular exercise.
As a sex hormone, estrogen regulates diverse
processes, including bone mineral density and calcium intake.
Estrogen also influences intracellular pathways, synaptic structure
and physiological functions of neurons in several brain regions
(4–6), and regulates neurotrophin expression
and has neuroprotective effects against diseases (2). In the hippocampus, estrogen is
particularly important in enhanced cognitive functions by
supporting neurogenesis and synaptic plasticity, and protects
hippocampal function from neurodegenerative disease (2,7).
Estrogen has direct effects in various pathways through binding to
estrogen receptors (17) or
indirect neuronal functions by regulating extrinsic factors, such
as neurotrophins (33). In
particular, estrogen is associated with BDNF synthesis and
expression in the hippocampus (8).
The interaction between estrogen and BDNF is crucial for
hippocampal functions (3,34).
BDNF released from neuronal cells exists in
precursor and mature forms, and mBDNF is synthesized from proBDNF
(9,35,36).
proBDNF is proteolytically cleaved intracellularly by enzymes,
including furin and pro-protein convertase (37–39),
or secreted as proBDNF and subsequently cleaved to create mBDNF by
extracellular proteases, such as metalloproteases and plasmin
(40). Plasmin is converted from
plasminogen by tPA as a key regulator of BDNF synthesis, and has a
crucial role in BDNF-dependent, late-phase, long-term potential
associated with hippocampal plasticity (40). Various studies investigating the
interaction between estrogen and BDNF in the hippocampus have
demonstrated that BDNF expression is reduced by an estrogen deficit
in ovariectomized rats (17,18).
In the present study, hippocampal mBDNF was significantly reduced
in the OVX treatment group compared with SC rats, and tPA also was
reduced by OVX. The results indicate that tPA may be involved in
the reduced expression of mBDNF induced by OVX. mBDNF exerts its
actions on neuronal structure, function and synaptic plasticity
underlying memory and cognition (41–43)
by activation of MAPK, PI3K-Akt, PLCγ,
Ca2+/calmodulin-dependent protein kinase and cAMP
response element-binding protein pathways following interactions
with TrkB (23,44).
While proBDNF was originally considered to be a
precursor with no inherent biological function, multiple reports
have indicated that proBDNF is secreted from neurons and modulates
synaptic functions through certain pathways by binding p75NTR
(45,46). The interaction between proBDNF and
p75NTR aids neuronal functions through underlying pathways
(46). proBDNF-p75NTR functions in
prosurvival responses via the NF-κB cascade (13), and is also associated with
long-term hippocampal depression (45) and induces pro-apoptotic responses
(10–14) via the JNK cascade (47–49).
In particular, apoptosis induced by proBDNF-p75NTR has been
previously demonstrated in neurodegenerative diseases; Sierksma
et al (50) noted that
hippocampal proBDNF was increased significantly in an animal model
of Alzheimer's disease.
In the present study, the OVX group exhibited
significantly higher proBDNF, p75NTR and JNK, associated with
pro-apoptotic responses, compared with the SC group. Conversely,
NF-κB was reduced by OVX. As a result, we hypothesize that the
pro-apoptotic response of JNK underlying the proBDNF pathway
functions in hippocampal dysfunctions induced by OVX. Estrogen
deficit-associated hippocampal dysfunction may be associated with
inhibition of the mBDNF pathway and the pro-apoptotic action of
proBDNF. Exercise is beneficial for hippocampal functions (19,20),
and it is associated with increased mBDNF expression and signaling
(22). In addition, exercise has a
role in the improvement and protection of the hippocampus in
neurodegenerative diseases (51).
Jin et al (1) reported that
exercise improved cognitive functions in a rat model of OVX. To
investigate the effects of exercise on OVX, rats were subjected to
treadmill exercise following OVX and it was observed that regular
exercise improved BDNF pathways in the hippocampus, increasing the
levels of mBDNF, TrkB and tPA, and reducing levels of proBDNF,
p75NTR and JNK. These results suggested that exercise may suppress
the pro-apoptotic response of the proBDNF-p75NTR pathway by
increasing synthesis of mBDNF and activating tPA. Thus, exercise
may enhance neuronal functions in the OVX rat brain.
The present study has several important
implications. First, hippocampal dysfunction induced by OVX was
caused by dysfunction of the mBDNF pathway and the pro-apoptotic
response associated with the proBDNF pathway. In addition, it was
demonstrated that pro-apoptotic action through the proBDNF-p75NTR
cascade involved the JNK pathway. Furthermore, dysfunction of BDNF
signaling was improved by exercise. Therefore, regular exercise may
improve BDNF pathways in the hippocampus of OVX rats. These results
may aid future studies investigating the effects of exercise on
proBDNF and mBDNF pathways, in addition to hippocampal function,
estrogen deficiency and menopause.
Acknowledgements
This work was supported by an Incheon National
University Research Grant in 2013 (Incheon, Republic of South
Korea).
References
|
1
|
Jin J, Jing H, Choi G, Oh MS, Ryu JH,
Jeong JW, Huh Y and Park C: Voluntary exercise increases the new
cell formation in the hippocampus of ovariectomized mice. Neurosci
Lett. 439:260–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kiss A, Delattre AM, Pereira SI, Carolino
RG, Szawka RE, Anselmo-Franci JA, Zanata SM and Ferraz AC:
17β-estradiol replacement in young, adult and middle-aged female
ovariectomized rats promotes improvement of spatial reference
memory and an antidepressant effect and alters monoamines and BDNF
levels in memory- and depression-related brain areas. Behav Brain
Res. 227:100–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scharfman HE and MacLusky NJ: Similarities
between actions of estrogen and BDNF in the hippocampus:
Coincidence or clue? Trends Neurosci. 28:79–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woolley CS: Acute effects of estrogen on
neuronal physiology. Annu Rev Pharmacol Toxicol. 47:657–680. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Srivastava DP: Two-step wiring
plasticity-a mechanism for estrogen-induced rewiring of cortical
circuits. J Steroid Biochem Mol Biol. 131:17–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srivastava DP, Waters EM, Mermelstein PG,
Kramár EA, Shors TJ and Liu F: Rapid estrogen signaling in the
brain: Implications for the fine-tuning of neuronal circuitry. J
Neurosci. 31:16056–16063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Henderson VW: Alzheimer's disease: Review
of hormone therapy trials and implications for treatment and
prevention after menopause. J Steroid Biochem Mol Biol. 142:99–106.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solum DT and Handa RJ: Estrogen regulates
the development of brain-derived neurotrophic factor mRNA and
protein in the rat hippocampus. J Neurosci. 22:2650–2659.
2002.PubMed/NCBI
|
|
9
|
Teng KK, Felice S, Kim T and Hempstead BL:
Understanding proneurotrophin actions: Recent advances and
challenges. Dev Neurobiol. 70:350–359. 2010.PubMed/NCBI
|
|
10
|
Boyd JG and Gordon T: A dose-dependent
facilitation and inhibition of peripheral nerve regeneration by
brain-derived neurotrophic factor. Eur J Neurosci. 15:613–626.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Troy CM, Friedman JE and Friedman WJ:
Mechanisms of p75-mediated death of hippocampal neurons. Role of
caspases. J Biol Chem. 277:34295–34302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teng HK, Teng KK, Lee R, Wright S, Tevar
S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, et al: ProBDNF
induces neuronal apoptosis via activation of a receptor complex of
p75NTR and sortilin. J Neurosci. 25:5455–5463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reichardt LF: Neurotrophin-regulated
signalling pathways. Philos Trans R Soc Lond B Biol Sci.
361:1545–1564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cunha C, Brambilla R and Thomas KL: A
simple role for BDNF in learning and memory? Front Mol Neurosci.
3:12010.PubMed/NCBI
|
|
15
|
Lu Y, Christian K and Lu B: BDNF: A key
regulator for protein synthesis-dependent LTP and long-term memory?
Neurobiol Learn Mem. 89:312–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshii A and Constantine-Paton M:
Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity,
and disease. Dev Neurobiol. 70:304–322. 2010.PubMed/NCBI
|
|
17
|
Scharfman HE and MacLusky NJ: Estrogen and
brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity
of steroid hormone-growth factor interactions in the adult CNS.
Front Neuroendocrinol. 27:415–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luine V and Frankfurt M: Interactions
between estradiol, BDNF and dendritic spines in promoting memory.
Neuroscience. 239:34–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cotman CW and Berchtold NC: Exercise: A
behavioral intervention to enhance brain health and plasticity.
Trends Neurosci. 25:295–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fabel K and Kempermann G: Physical
activity and the regulation of neurogenesis in the adult and aging
brain. Neuromolecular Med. 10:59–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vivar C, Potter MC and van Praag H: All
about running: Synaptic plasticity, growth factors and adult
hippocampal neurogenesis. Curr Top Behav Neurosci. 15:189–210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sartori CR, Vieira AS, Ferrari EM, Langone
F, Tongiorgi E and Parada CA: The antidepressive effect of the
physical exercise correlates with increased levels of mature BDNF,
and proBDNF proteolytic cleavage-related genes, p11 and tPA.
Neuroscience. 180:9–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chao MV, Rajagopal R and Lee FS:
Neurotrophin signalling in health and disease. Clin Sci (Lond).
110:167–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Minichiello L: TrkB signalling pathways in
LTP and learning. Nat Rev Neurosci. 10:850–860. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colcombe SJ, Erickson KI, Raz N, Webb AG,
Cohen NJ, McAuley E and Kramer AF: Aerobic fitness reduces brain
tissue loss in aging humans. J Gerontol A Biol Sci Med Sci.
58:176–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kramer AF, Colcombe SJ, McAuley E, Scalf
PE and Erickson KI: Fitness, aging and neurocognitive function.
Neurobiol Aging. 26:(Suppl 1). S124–S127. 2005. View Article : Google Scholar
|
|
27
|
Erickson KI, Colcombe SJ, Elavsky S,
McAuley E, Korol DL, Scalf PE and Kramer AF: Interactive effects of
fitness and hormone treatment on brain health in postmenopausal
women. Neurobiol Aging. 28:179–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rodrigues MA, Verdile G, Foster JK,
Hogervorst E, Joesbury K, Dhaliwal S, Corder EH, Laws SM, Hone E,
Prince R, et al: Gonadotropins and cognition in older women. J
Alzheimers Dis. 13:267–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee MC, Okamoto M, Liu YF, Inoue K, Matsui
T, Nogami H and Soya H: Voluntary resistance running with short
distance enhances spatial memory related to hippocampal BDNF
signaling. J Appl Physiol (1985). 113:1260–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quirié A, Hervieu M, Garnier P, Demougeot
C, Mossiat C, Bertrand N, Martin A, Marie C and Prigent-Tessier A:
Comparative effect of treadmill exercise on mature BDNF production
in control versus stroke rats. PLoS One. 7:e442182012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gomez-Pinilla F and Hillman C: The
influence of exercise on cognitive abilities. Compr Physiol.
3:403–428. 2013.PubMed/NCBI
|
|
32
|
Schefer V and Talan MI: Oxygen consumption
in adult and AGED C57BL/6J mice during acute treadmill exercise of
different intensity. Exp Gerontol. 31:387–392. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kritzer MF and Kohama SG: Ovarian hormones
differentially influence immunoreactivity for dopamine
beta-hydroxylase, choline acetyltransferase, and serotonin in the
dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp
Neurol. 409:438–451. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Numakawa T, Yokomaku D, Richards M, Hori
H, Adachi N and Kunugi H: Functional interactions between steroid
hormones and neurotrophin BDNF. World J Biol Chem. 1:133–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seidah NG, Benjannet S, Pareek S, Chrétien
M and Murphy RA: Cellular processing of the neurotrophin precursors
of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett.
379:247–250. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu B, Pang PT and Woo NH: The yin and yang
of neurotrophin action. Nat Rev Neurosci. 6:603–614. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lessmann V, Gottmann K and Malcangio M:
Neurotrophin secretion: Current facts and future prospects. Prog
Neurobiol. 69:341–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mowla SJ, Pareek S, Farhadi HF, Petrecca
K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS and Murphy RA:
Differential sorting of nerve growth factor and brain-derived
neurotrophic factor in hippocampal neurons. J Neurosci.
19:2069–2080. 1999.PubMed/NCBI
|
|
39
|
Greenberg ME, Xu B, Lu B and Hempstead BL:
New insights in the biology of BDNF synthesis and release:
Implications in CNS function. J Neurosci. 29:12764–12767. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pang PT, Teng HK, Zaitsev E, Woo NT,
Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL and Lu B: Cleavage
of proBDNF by tPA/plasmin is essential for long-term hippocampal
plasticity. Science. 306:487–491. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Waterhouse EG and Xu B: New insights into
the role of brain-derived neurotrophic factor in synaptic
plasticity. Mol Cell Neurosci. 42:81–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cohen-Cory S, Kidane AH, Shirkey NJ and
Marshak S: Brain-derived neurotrophic factor and the development of
structural neuronal connectivity. Dev Neurobiol. 70:271–288.
2010.PubMed/NCBI
|
|
43
|
Cowansage KK, LeDoux JE and Monfils MH:
Brain-derived neurotrophic factor: A dynamic gatekeeper of neural
plasticity. Curr Mol Pharmacol. 3:12–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Binder DK and Scharfman HE: Brain-derived
neurotrophic factor. Growth Factors. 22:123–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Woo NH, Teng HK, Siao CJ, Chiaruttini C,
Pang PT, Milner TA, Hempstead BL and Lu B: Activation of p75NTR by
proBDNF facilitates hippocampal long-term depression. Nat Neurosci.
8:1069–1077. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Srivastava DP, Woolfrey KM and Evans PD:
Mechanisms underlying the interactions between rapid estrogenic and
BDNF control of synaptic connectivity. Neuroscience. 239:17–33.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Harrington AW, Kim JY and Yoon SO:
Activation of Rac GTPase by p75 is necessary for c-jun N-terminal
kinase-mediated apoptosis. J Neurosci. 22:156–166. 2002.PubMed/NCBI
|
|
48
|
Becker EB, Howell J, Kodama Y, Barker PA
and Bonni A: Characterization of the c-Jun N-terminal kinase-BimEL
signaling pathway in neuronal apoptosis. J Neurosci. 24:8762–8770.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bhakar AL, Howell JL, Paul CE, Salehi AH,
Becker EB, Said F, Bonni A and Barker PA: Apoptosis induced by
p75NTR overexpression requires Jun kinase-dependent phosphorylation
of Bad. J Neuroscience. 23:11373–11381. 2003.PubMed/NCBI
|
|
50
|
Sierksma AS, Vanmierlo T, De Vry J,
Raijmakers ME, Steinbusch HW, van den Hove DL and Prickaerts J:
Effects of prenatal stress exposure on soluble Aβ and brain-derived
neurotrophic factor signaling in male and female APPswe/PS1dE9
mice. Neurochem Int. 61:697–701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Johansson BB and Ohlsson AL: Environment,
social interaction, and physical activity as determinants of
functional outcome after cerebral infarction in the rat. Exp
Neurol. 139:322–327. 1996. View Article : Google Scholar : PubMed/NCBI
|