Introduction
Lung cancer is one of the most common malignant
tumors and in 2012 accounted for ~1.82 million new cases and ~1.59
million mortalities worldwide (1,2).
Despite the advances in treatment methods that have been made
available in recent years, including minimally invasive surgical
approaches, chemotherapies, and targeted therapies, the 5-year
survival of patients with lung cancer is far from satisfying,
ranging between 10 and 20% for most geographic areas (3). There are two major pathological
subtypes that constitute the majority of lung cancers: lung
adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC),
which differ in a number of ways (4–7).
LUAD and LUSC originate from different cells and
have several major differences not only in biological patterns, but
also molecular characteristics and, most importantly, therapeutic
strategies (5,8). For example, activating mutations in
epidermal growth factor receptor and mutations in ALK fusion
proteins usually occur in LUAD, but not LUSC, rendering medications
targeted at these genes ineffective for LUSC (9). Therefore, comprehensive
investigations into the differences of molecular characteristics
and mechanisms of these two major subtypes of lung cancer are
required, which will lead to deeper understanding and
identification of novel molecular-targeted strategies for lung
cancer therapy.
A large amount of high-throughput data on multiple
types of cancer have recently been released by The Cancer Genome
Atlas (TCGA; http://cancergenome.nih.gov) database, including mRNA
and microRNA (miRNA) sequencing data from hundreds of LUAD and LUSC
samples. These data enabled the molecular differences between LUAD
and LUSC to be fully investigated. The present study explored
differences in gene expression, miRNA expression, Gene Ontology
(GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, and
molecular regulatory networks by bioinformatics analyses, and the
results may facilitate a better understanding of the different
molecular mechanisms of non-small cell lung cancer (NSCLC) and may
promote the discovery and development of new, accurate strategies
for lung cancer prevention, diagnosis, and treatment.
Materials and methods
mRNA and miRNA expression data
resources and preprocessing
Level 3 RNA sequencing data from 108 normal
pulmonary samples and 980 pulmonary carcinoma samples (that is, 490
LUAD with 58 normal control samples, and 490 LUSC with 50 normal
control samples), and level 3 miRNA sequencing data from 91 normal
pulmonary samples and 966 pulmonary carcinoma samples (that is, 499
LUAD with 46 normal control samples, and 467 LUSC with 45 normal
control samples) were released by TCGA prior to April 15, 2015, and
were obtained by the present study from the TCGA data portal
(https://portal.gdc.cancer.gov). Data
preprocessing was carried out as described in previous studies
(10).
Identification of differentially
expressed genes (DEGs) and differentially expressed miRNAs
(DEMs)
Genes and miRNAs that are differentially expressed
among normal, LUAD, and LUSC sample groups were identified as
previously reported (11). DEGs
with a fold change (tumor/normal) >2 or <0.5 and DEMs with a
fold change >2.5 or <0.4 were qualified for subsequent
analyses. A random variance model t-test was used to confirm the
DEGs and DEMs, as previously described (10). Following analysis of the
significance, fold change, and false discovery rate (FDR), mRNAs
and miRNAs that had both P<0.05 and FDR<0.05 were considered
to be significantly differentially expressed (10). Only the DEGs and DEMs that were
identified in both the LUAD and LUSC groups were included when
comparing the differences between gene and miRNA expressions.
GO and KEGG pathway analyses
To investigate the significantly enriched functions
and the significant pathways for these DEGs, GO term (http://geneontology.org) and KEGG pathway (http://www.genome.jp/kegg) analyses were conducted as
previously reported (12,13). Briefly, the two-tailed Fisher's
exact test and the χ2 test were used to classify the GO
categories or KEGG pathways, and the FDR was calculated for
multiple testing corrections. GO terms or KEGG pathways having both
P<0.05 and FDR<0.05 were considered to be significantly
different. Enrichment values were calculated to identify those
significant terms or pathways that provided the most concrete
functional descriptions in this analysis. GO-map and PathNet
analyses were conducted to further outline the functional links
among the related GO terms and the significant KEGG pathways.
TF-miRNA-gene network
The regulation networks among transcription factors
(TFs), DEMs and DEGs were established as previously described
(10,14). Briefly, the target DEGs of DEMs
were predicted using TargetScan (http://www.targetscan.org) and miRanda (http://www.microrna.org/microrna/home.do) (15,16).
Subsequently, the TFs that may regulate the expression of DEMs and
DEGs were identified using the TRANSFAC database (http://gene-regulation.com/pub/databases.html)
(17). Finally, TF-miRNA-gene
networks were created in LUAD and LUSC, as previously described
(10).
Statistical analysis
Statistical analysis was performed using the
software IBM SPSS version 20 (IBM SPSS, Armonk, NY, USA).
Results
Identification of DEGs and DEMs
Significant differences in expression levels were
detected for certain genes and miRNAs that may be used as
biomarkers for the early diagnosis, assessment, and monitoring of
lung cancer. As shown in Table I,
1,492 DEGs and 36 DEMs were identified as being significantly
different between LUAD and normal lung tissues; for LUSC vs. normal
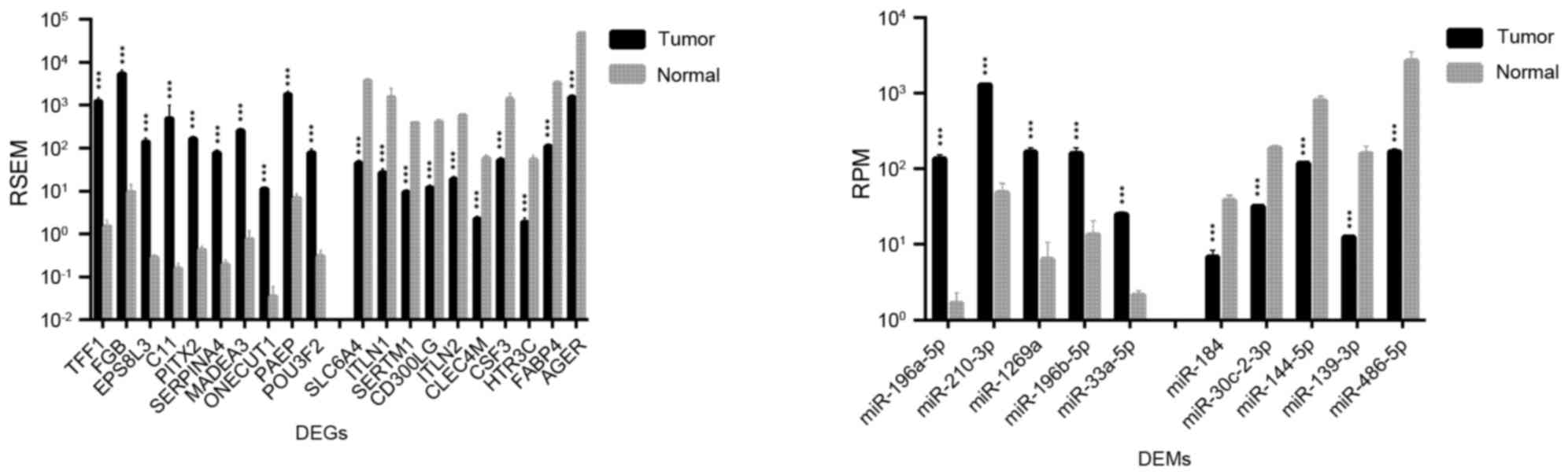
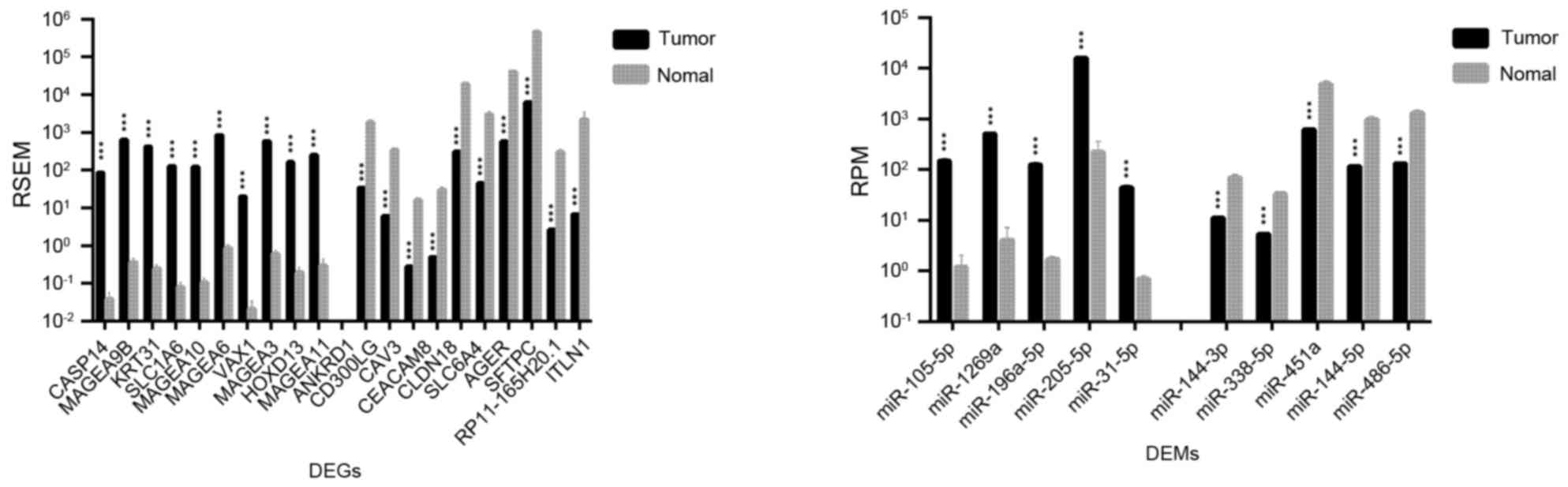
lung tissue, 2,726 DEGs and 45 DEMs were identified. The top 20
DEGs and the top 10 DEMs exhibiting the most significant
differential expressions in LUAD and LUSC are shown in Figs. 1 and 2, respectively.
 | Table I.DEGs and DEMs identified between
LUAD, LUSC and normal samples. |
Table I.
DEGs and DEMs identified between
LUAD, LUSC and normal samples.
| Comparison | DEGs | DEMs |
|---|
| LUAD vs.
Normal | 1,492 | 36 |
|
LUAD>Normal | 611 | 27 |
|
LUAD<Normal | 881 | 9 |
| LUSC vs.
Normal | 2,726 | 45 |
|
LUSC>Normal | 1,052 | 28 |
|
LUSC<Normal | 1,674 | 17 |
| LUAD vs. LUSC | 778 | 7 |
|
LUAD>LUSC | 340 | 1 |
|
LUAD<LUSC | 438 | 6 |
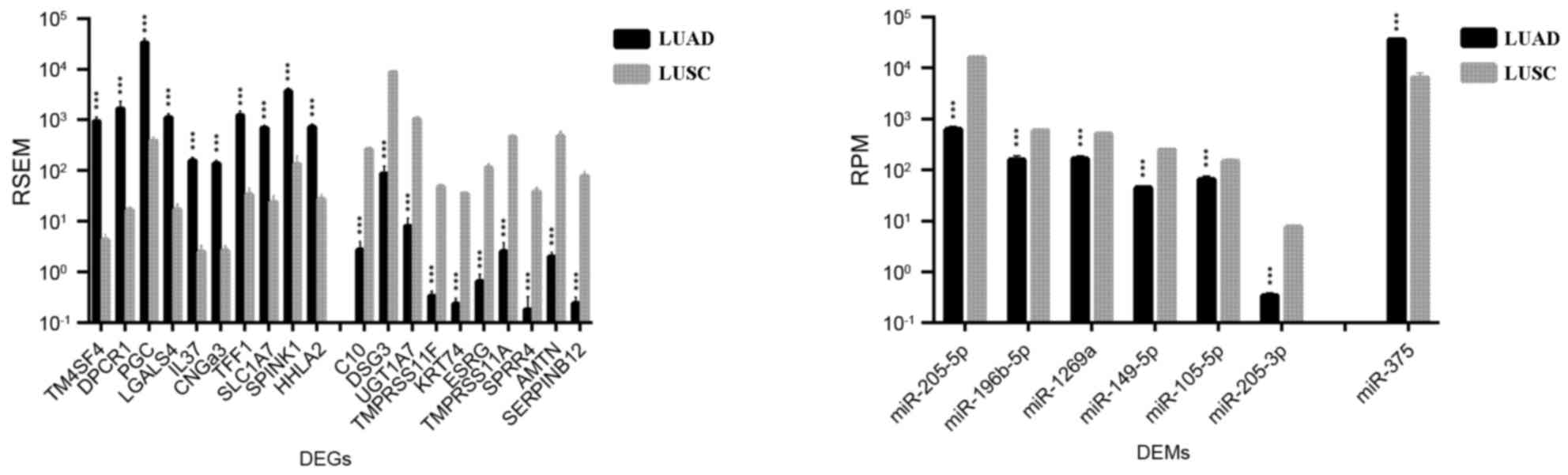
A total of 778 DEGs and 7 DEMs were identified in
both LUAD and LUSC (Table I). As
demonstrated in Fig. 3,
transmembrane 4 L six family member 4 (TM4SF4), diffuse
panbronchiolitis critical region 1 (DPCR1), prograstricsin
(PGC), galectin 4 (LGALS4), and interleukin 37
(IL37) were the top five DEGs that were more upregulated in
LUAD than in LUSC (TM4SF4, 214.5 fold change; IL37,
63.2 fold change, DPCR1, PGC and LGALS4, fold change
63.2–214.5); serpin family B member 12 (SERPINB12), amelotin
(AMTN), small proline-rich protein 4 (SPRR4),
transmembrane protease, serine 11A (TMPRSS11A), and
embryonic stem cell related (ESRG) were the top five DEGs
identified as being more upregulated in LUSC compared with LUAD
(fold change, 172.0–322.9). As expected, a number of
well-established biomarkers for LUAD and LUSC were also identified,
including transcription termination factor 1 (TTF1; fold
change LUAD/LUSC, 10.5), keratin 7 (KRT7; fold change,
3.63), SRY-box 2 (SOX2; fold change, 0.11), p63 (fold
change, 0.03), and KRT5 (fold change, 0.01).
miRNA miR-375 was the only DEM that was demonstrated
to be more upregulated in LUAD compared with LUSC (fold change,
5.62); six other DEMs were revealed to be upregulated in LUSC vs.
LUAD, including miR-205-5p, miR-205-3p, miR-149-5p, miR-196b-5p,
miR-1269a, and miR-105-5p (fold change, 2.3–25.2; Fig. 3).
Enriched GOs and pathways
GO and KEGG analyses were used in the present study
to provide a preliminarily perspective on the altered biological
functions and pathways in which the DEGs are enriched. In LUAD vs.
Normal lung tissue, DEGS were enriched in 806 GO terms and 84
pathways, whereas in LUSC vs. Normal, DEGs were enriched in 1266
GOs and 146 pathways (Table
II).
 | Table II.Basic information of the GOs and KEGG
pathways in which the DEGs and DEMs were enriched. |
Table II.
Basic information of the GOs and KEGG
pathways in which the DEGs and DEMs were enriched.
| Comparison | GOs | Pathways |
|---|
| LUAD vs.
Normal | 806 | 84 |
|
Upregulated | 237 | 24 |
|
Downregulated | 569 | 60 |
| LUSC vs.
Normal | 1266 | 146 |
|
Upregulated | 468 | 40 |
|
Downregulated | 798 | 106 |
| LUAD vs. LUSC | 409 | 47 |
|
LUAD>LUSC | 124 | 22 |
|
LUAD<LUSC | 285 | 25 |
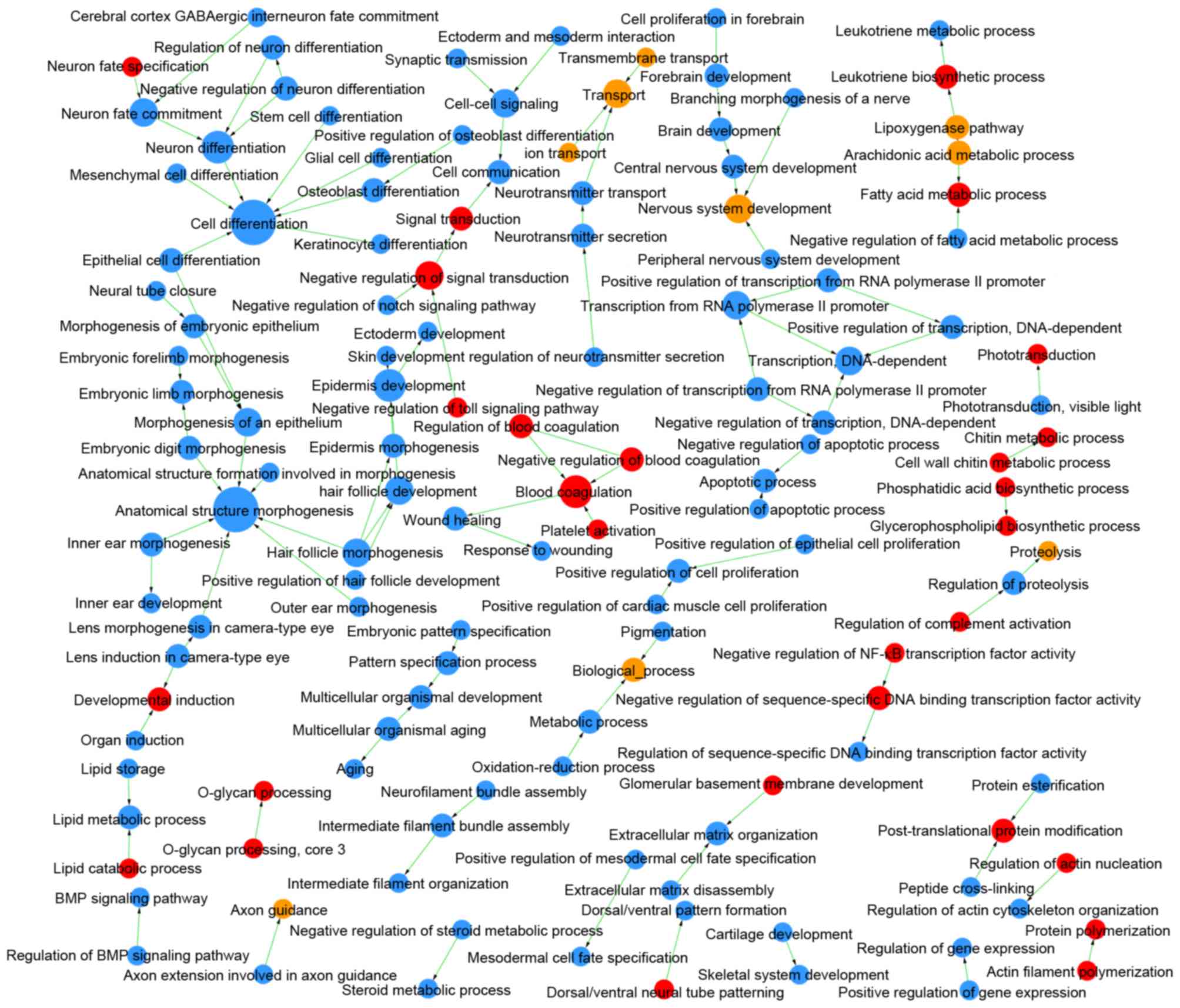
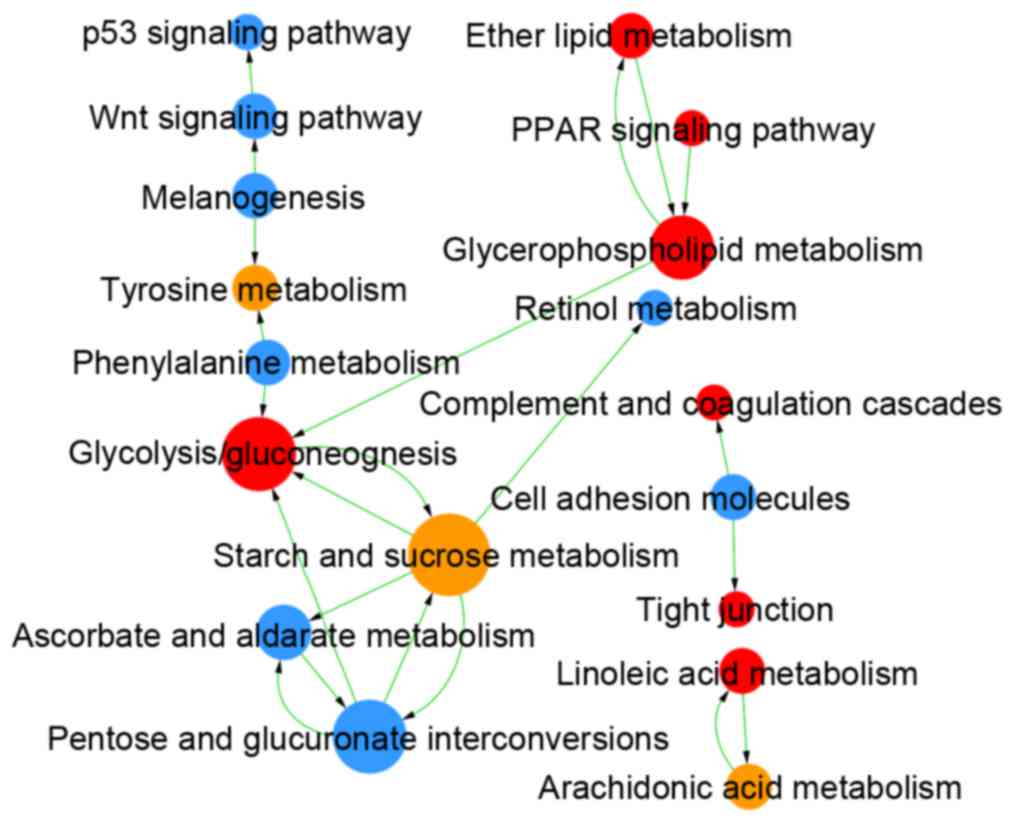
Comparing LUAD and LUSC, DEMs were enriched in 409
GOs and 47 pathways (Table I), and
the links among these GOs and pathways are integrated in Figs. 4 and 5, respectively. The DEGs identified as
upregulated in LUAD vs. LUSC were enriched in 124 GOs, such as
negative regulation of the toll signaling pathway (GO:0045751) and
negative regulation of nuclear factor (NF)-κB activity
(GO:0032088), and in 22 pathways, such as peroxisome
proliferator-activated receptor (PPAR) signaling pathway (id:03320)
and glycolysis/gluconeogenesis (id:00010). The upregulated DEGs in
LUSC vs. LUAD were enriched in 285 GOs, such as extracellular
matrix organization (GO:0030198) and cell differentiation
(GO:0030154), and in 25 pathways, such as cell adhesion molecules
(id:04514) and p53 signaling pathway (id:04115).
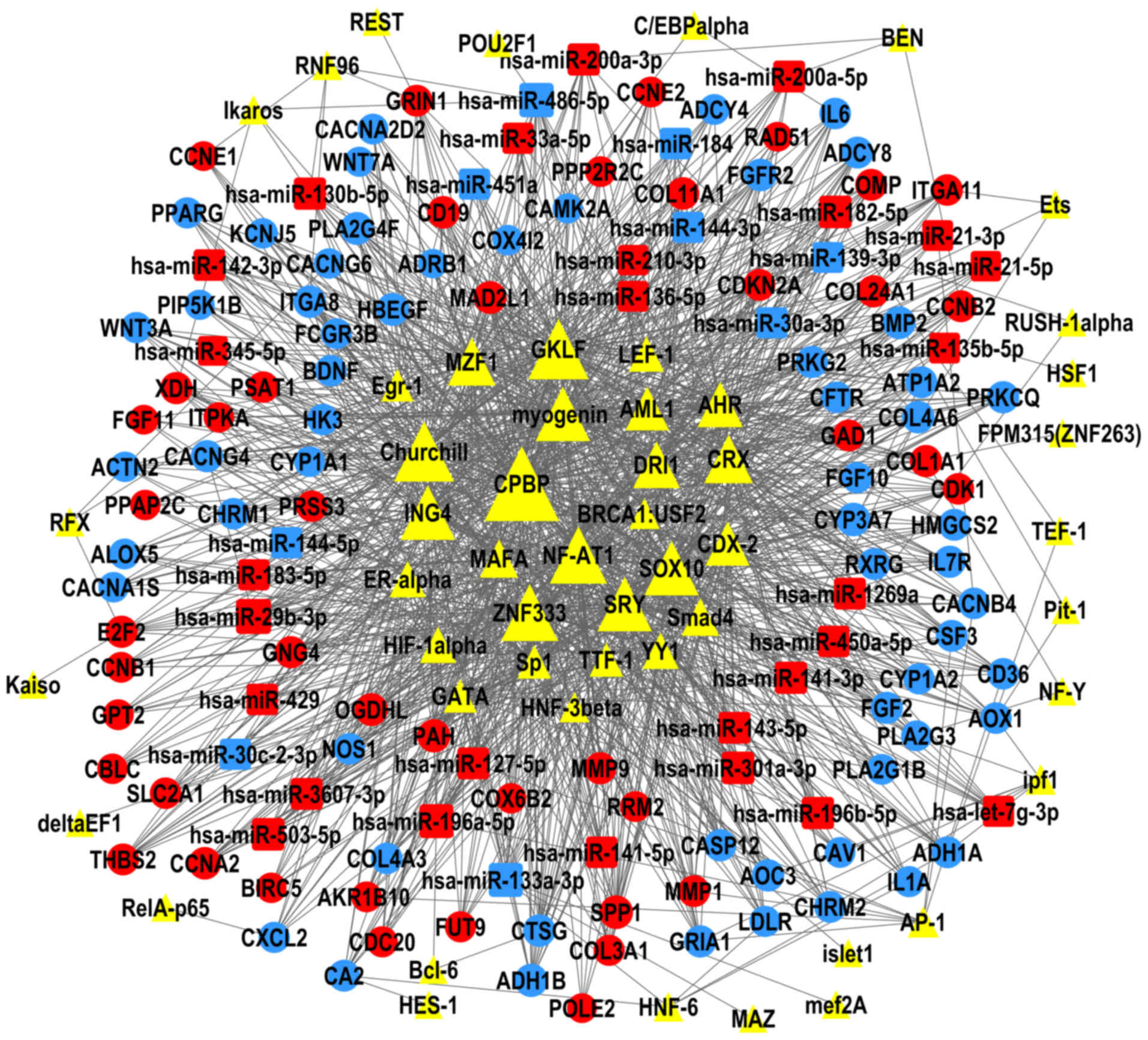
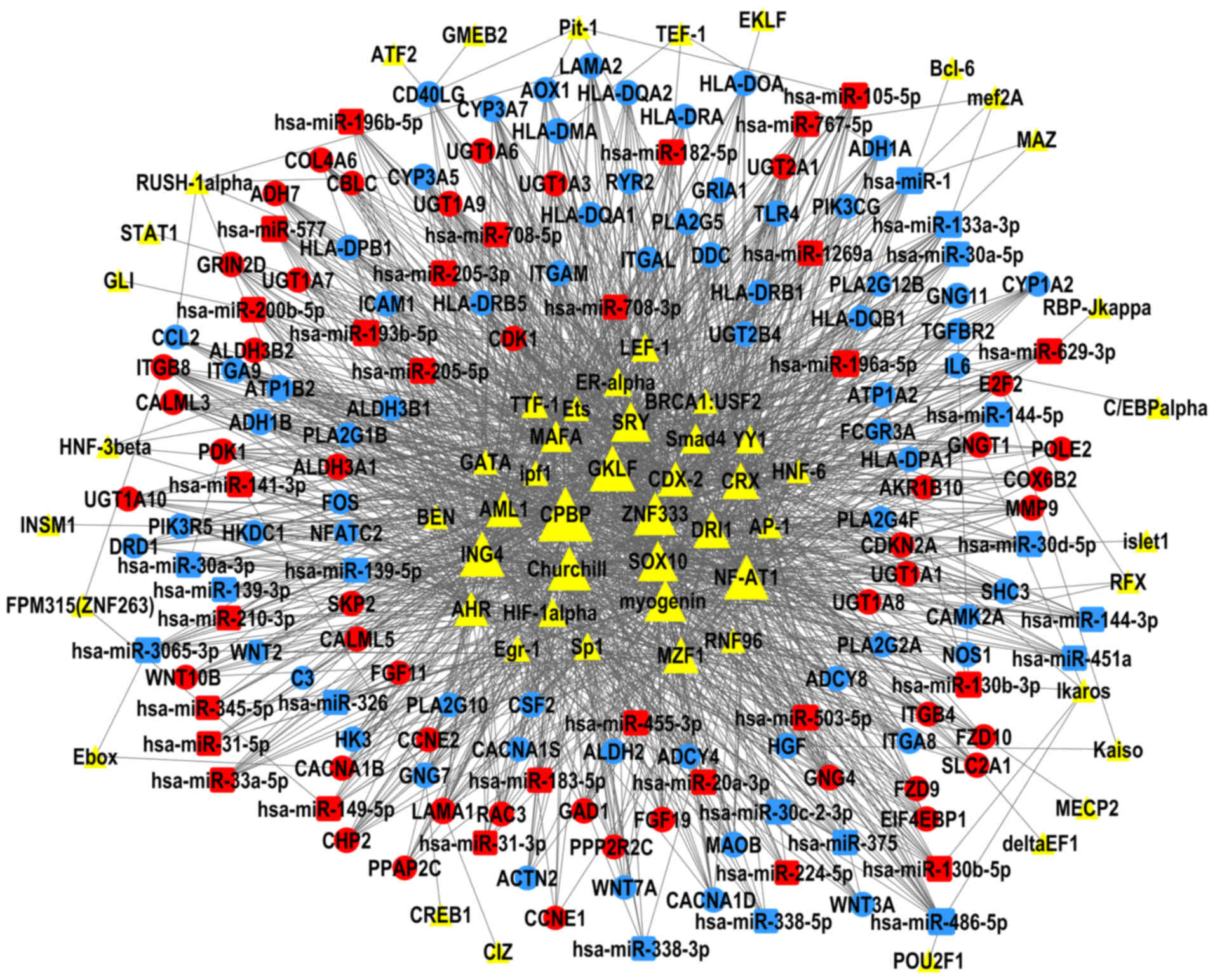
TF-miRNA-gene network
The present study constructed TF-miRNA-gene networks
of LUAD and LUSC (Figs. 6 and
7), using the large amount of
interrelated expression data of miRNAs and genes in the TCGA
database, to predict regulatory networks among the TFs, DEMs, and
DEGs. As the proposed networks demonstrated, the central TFs and
DEMs, i.e. the TFs and DEMs in the middle of the TF/DEM-miRNA-gene
network, in LUAD and LUSC were quite similar. The top six central
TFs were core promoter element-binding protein (CPBP), gut-enriched
Krüppel-like factor (GKLF), Churchill, nuclear factor of activated
T-cells 1 (NF-AT1), zinc finger protein 333 (ZNF333), and inhibitor
of growth protein 4 (ING4); these TFs were the same in LUAD and in
LUSC, indicating that there are still common regulatory mechanisms
shared between these two subtypes of lung cancer. LUAD and LUSC
shared 19 DEMs in common (data not shown), of which miR-486-5p,
miR-133a-3p, and miR-196a-5p were centrally positioned in the
predicted TF-miRNA-gene regulatory networks of both LUAD and LUSC.
LUAD and LUSC had different patterns of DEMs. miR-29b-3p was
upregulated and was predicted to regulate the most DEGs in the LUAD
network, but not in LUSC. miR-1, miR-105-5p, and miR-193b-5p were
only in the center of the LUSC network.
Discussion
The present study analyzed the differences in DEG
and DEM expressions in LUAD and LUSC compared with normal lung
tissue and, most importantly, the differences between LUAD and
LUSC. To further elucidate the functions of these DEGs and DEMs, GO
and KEGG pathway analyses were performed. In addition,
TF-miRNA-gene networks were constructed for LUAD and LUSC; however,
further independent validation with experimental data is still
required.
Cytology and pathology have traditionally been used
in the differential diagnosis of LUAD and LUSC; however, in some
cases, such as small biopsy samples or aspiration cytology samples,
additional tests of molecular characteristics were required
(18). Although several genes have
been used as biomarkers in lung cancer diagnosis and differential
diagnosis, more accurate and convenient biomarkers are still
needed. The present study identified 778 DEGs and 7 DEMs that were
differentially expressed between LUAD and LUSC. These DEGs and DEMs
may be possible candidates for differential diagnosis between LUAD
and LUSC, and several have already been used in clinical practice,
such as TTF-1, SOX2, p63, KRT5, and
KRT7 (6,19). Although a high fold change is not
the only criteria for biomarkers, those exhibiting significant
differences in expression, such as TM4SF4, DPCR1,
SERPINB12, and AMTN, may be worth further
investigation. In our previous study, several of the DEGs, such as
melanophilin (MLPH), transmembrane channel-like 5
(TMC5), surfactant associated 3 (SFTA3), desmoglein 3
(DSG3), desmocollin 3 (DSC3), and calmodulin-like 3
(CALML3), were confirmed to be differentially expressed in
LUAD and LUSC by immunohistochemical staining (7). miR-205-5p expression levels were
previously reported to be significantly higher in LUSC compared
with LUAD, both in serum and tissue (20); miR-375 was also demonstrated to be
highly expressed in LUAD (21),
which was consistent with the current data. The present study
offers a list of DEGs and DEMs in which better biomarkers may
exist.
Various gene functions and pathways that are greatly
altered in LUAD and LUSC have been identified in the present study,
suggesting that these GOs and pathways serve primary roles in lung
cancer pathogenesis, as do the DEGs that participate in these GOs
and pathways. Although LUAD and LUSC share a lot of common
activated GOs and pathways, they also display their own features.
According to the results, genes related to extracellular matrix
organization, such as matrix metalloproteinase (MMP)
3, MMP10 and MMP12, were upregulated in LUAD
and even significantly higher in LUSC compared with in normal
tissue. MMPs are key factors in the development of the tumor
microenvironment and drive cancer progression and metastasis, and
have been identified as prognostic factors for poor survival in
many types of cancer (22–24). In the present study, the PPAR
pathway was demonstrated to be activated in LUAD, but not in LUSC.
PPARs have been reported to be associated with breast, ovary,
prostate, bladder, gastric and colon adenocarcinoma carcinogenesis,
as well as in leukemia (25).
Tsubouchi et al (26)
proposed that a PPARγ agonist may be a useful therapeutic agent in
the treatment of human lung cancer. The p53 signaling pathway was
also identified as upregulated in LUSC; a critical role of the
p53 mutation in malignant transformation, histologic
progression, invasion, and metastasis has been previously
demonstrated in both in vitro and in vivo models of
lung cancer (27–29). Smoking was revealed to be closely
related to p53 mutation (4,30),
which may explain the prevalence of p53 alterations in
LUSC.
miR-29b acts as a tumor suppressor in breast cancer
and is a potential marker for recurrence and metastasis (31). miR-29b-3p in peripheral blood
mononuclear cells was reported to be a novel target for the
diagnosis of NSCLC (32). miR-1
was revealed to be downregulated in various types of cancers,
including LUSC, and could act as a tumor suppressor (33). Previous studies have suggested that
miR-1 functions through the regulation of oncogenic coronin 1C
(34), and the silencing miR-1
resulted in sensitization of LUSC to traditional chemotherapeutics
(35). miR-375 appears to serve
many different roles in carcinogenesis, and functions as an
oncogene or a tumor suppressor depending on the type of cancer
(36). miR-375 was previously
revealed to inhibit cell proliferation, invasion and motility in
several types of cancer, including NSCLC (37), whereas upregulated miR-375
expression may stimulate cell proliferation in thyroid carcinoma,
small-cell lung, breast and prostate cancers (38). Conversely, miR-375 was reported to
be downregulated in NSCLC, but the prognostic significance remains
unclear (39). Further research
into these TFs and miRNAs may lead to novel treatment of NSCLC.
In conclusion, the present study investigated the
differences between the gene and miRNA expression patterns in LUAD
and LUSC, and explored their different biological characteristics.
Further understanding of these differences may promote the
discovery and development of new, accurate strategies for the
prevention, diagnosis and treatment of lung cancer. Further
experiments are required to validate the results of the present
bioinformatics analysis.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81401875 and
81472225) and The Natural Science Foundation of Shanghai, China
(grant no. 14ZR1406000). The results are based upon data generated
by the TCGA Research Network. The authors would like to thank the
language-editing service, International Science Editing Co., for
editing this manuscript.
Glossary
Abbreviations
Abbreviations:
|
DEGs
|
differentially expressed genes
|
|
DEMs
|
differentially expressed microRNAs
|
|
FDR
|
false discovery rate
|
|
GO
|
gene ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
LUAD
|
lung adenocarcinoma
|
|
LUSC
|
lung squamous cell carcinoma
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance of cancer survival 1995–2009: Analysis
of individual data for 25,676,887 patients from 279
population-based registries in 67 countries (CONCORD-2). Lancet.
385:977–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/American thoracic society/European respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis WD, Rekhtman N, Riley GJ, Geisinger
KR, Asamura H, Brambilla E, Garg K, Hirsch FR, Noguchi M, Powell
CA, et al: Pathologic diagnosis of advanced lung cancer based on
small biopsies and cytology: A paradigm shift. J Thorac Oncol.
5:411–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan C, Yan L, Wang L, Sun Y, Wang X, Lin
Z, Zhang Y, Shi Y, Jiang W and Wang Q: Identification of
immunohistochemical markers for distinguishing lung adenocarcinoma
from squamous cell carcinoma. J Thorac Dis. 7:1398–1405.
2015.PubMed/NCBI
|
|
8
|
Cancer Genome Atlas Research Network:
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rekhtman N, Paik PK, Arcila ME, Tafe LJ,
Oxnard GR, Moreira AL, Travis WD, Zakowski MF, Kris MG and Ladanyi
M: Clarifying the spectrum of driver oncogene mutations in
biomarker-verified squamous carcinoma of lung: Lack of EGFR/KRAS
and presence of PIK3CA/AKT1 mutations. Clin Cancer Res.
18:1167–1176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan C, Yan L, Wang L, Jiang W, Zhang Y,
Xi J, Jin Y, Chen L, Shi Y, Lin Z and Wang Q: Landscape of
expression profiles in esophageal carcinoma by the cancer genome
atlas data. Dis Esophagus. 29:920–928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wright GW and Simon RM: A random variance
model for detection of differential gene expression in small
microarray experiments. Bioinformatics. 19:2448–2455. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanehisa M, Goto S, Kawashima S, Okuno Y
and Hattori M: The KEGG resource for deciphering the genome.
Nucleic Acids Res. 32:D277–D280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan L, Zhan C, Wu J and Wang S: Expression
profile analysis of head and neck squamous cell carcinomas using
data from the Cancer Genome Atlas. Mol Med Rep. 13:4259–4265.
2016.PubMed/NCBI
|
|
15
|
Garcia DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Betel D, Koppal A, Agius P, Sander C and
Leslie C: Comprehensive modeling of microRNA targets predicts
functional non-conserved and non-canonical sites. Genome Biol.
11:R902010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kel AE, Gössling E, Reuter I, Cheremushkin
E, Kel-Margoulis OV and Wingender E: MATCH: A tool for searching
transcription factor binding sites in DNA sequences. Nucleic Acids
Res. 31:3576–3579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD and Rekhtman N: Pathological
diagnosis and classification of lung cancer in small biopsies and
cytology: Strategic management of tissue for molecular testing.
Semin Respir Crit Care Med. 32:22–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwartz AM and Rezaei MK: Diagnostic
surgical pathology in lung cancer: Diagnosis and management of lung
cancer, 3rd ed: American college of chest physicians evidence-based
clinical practice guidelines. Chest. 143:(Suppl 5). e251S–e262S.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang M, Zhang P, Hu G, Xiao Z, Xu F,
Zhong T, Huang F, Kuang H and Zhang W: Relative expressions of
miR-205-5p, miR-205-3p, and miR-21 in tissues and serum of
non-small cell lung cancer patients. Mol Cell Biochem. 383:67–75.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu L, Todd NW, Xing L, Xie Y, Zhang H, Liu
Z, Fang H, Zhang J, Katz RL and Jiang F: Early detection of lung
adenocarcinoma in sputum by a panel of microRNA markers. Int J
Cancer. 127:2870–2878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehner C, Miller E, Nassar A, Bamlet WR,
Radisky ES and Radisky DC: Tumor cell expression of MMP3 as a
prognostic factor for poor survival in pancreatic, pulmonary, and
mammary carcinoma. Genes Cancer. 6:480–489. 2015.PubMed/NCBI
|
|
23
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol. 15:201–212. 2010. View Article : Google Scholar
|
|
25
|
Han S and Roman J: Peroxisome
proliferator-activated receptor gamma: A novel target for cancer
therapeutics? Anticancer Drug. 18:237–244. 2007. View Article : Google Scholar
|
|
26
|
Tsubouchi Y, Sano H, Kawahito Y, Mukai S,
Yamada R, Kohno M, Inoue K, Hla T and Kondo M: Inhibition of human
lung cancer cell growth by the peroxisome proliferator-activated
receptor-gamma agonists through induction of apoptosis. Biochem
Bioph Res Commun. 270:400–405. 2000. View Article : Google Scholar
|
|
27
|
Harvey M, Vogel H, Morris D, Bradley A,
Bernstein A and Donehower LA: A mutant p53 transgene accelerates
tumour development in heterozygous but not nullizygous
p53-deficient mice. Nat Genet. 9:305–311. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kemp CJ, Donehower LA, Bradley A and
Balmain A: Reduction of p53 gene dosage does not increase
initiation or promotion but enhances malignant progression of
chemically induced skin tumors. Cell. 74:813–822. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jackson EL, Olive KP, Tuveson DA, Bronson
R, Crowley D, Brown M and Jacks T: The differential effects of
mutant p53 alleles on advanced murine lung cancer. Cancer Res.
65:10280–10288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gibbons DL, Byers LA and Kurie JM:
Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 12:3–13.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shinden Y, Iguchi T, Akiyoshi S, Ueo H,
Ueda M, Hirata H, Sakimura S, Uchi R, Takano Y, Eguchi H, et al:
miR-29b is an indicator of prognosis in breast cancer patients. Mol
Clin Oncol. 3:919–923. 2015.PubMed/NCBI
|
|
32
|
Ma J, Lin Y, Zhan M, Mann DL, Stass SA and
Jiang F: Differential miRNA expressions in peripheral blood
mononuclear cells for diagnosis of lung cancer. Lab Invest.
95:1197–1206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nohata N, Hanazawa T, Enokida H and Seki
N: microRNA-1/133a and microRNA-206/133b clusters: Dysregulation
and functional roles in human cancers. Oncotarget. 3:9–21.
2012.PubMed/NCBI
|
|
34
|
Mataki H, Enokida H, Chiyomaru T, Mizuno
K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T,
Nakagawa M, et al: Downregulation of the microRNA-1/133a cluster
enhances cancer cell migration and invasion in lung-squamous cell
carcinoma via regulation of Coronin1C. J Hum Genet. 60:53–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nasser MW, Datta J, Nuovo G, Kutay H,
Motiwala T, Majumder S, Wang B, Suster S, Jacob ST and Ghoshal K:
Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression
of tumorigenic property of lung cancer cells and their
sensitization to doxorubicin-induced apoptosis by miR-1. J Biol
Chem. 283:33394–33405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jonsdottir K, Janssen SR, Da Rosa FC,
Gudlaugsson E, Skaland I, Baak JP and Janssen EA: Validation of
expression patterns for nine miRNAs in 204 lymph-node negative
breast cancers. PLoS One. 7:e486922012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shao X, Mei W, Weng W, Qin J, Zhou J, Liu
J and Cheng J: Mir-375 enhances ruthenium-derived compound Rawq01
induced cell death in human ovarian cancer. Int J Clin Exp Patho.
6:1095–1102. 2013.
|
|
38
|
Nishikawa E, Osada H, Okazaki Y, Arima C,
Tomida S, Tatematsu Y, Taguchi A, Shimada Y, Yanagisawa K, Yatabe
Y, et al: miR-375 is activated by ASH1 and inhibits YAP1 in a
lineage-dependent manner in lung cancer. Cancer Res. 71:6165–6173.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu H, Jiang L, Sun C, Li Guo L, Lin M,
Huang J and Zhu L: Decreased circulating miR-375: A potential
biomarker for patients with non-small-cell lung cancer. Gene.
534:60–65. 2014. View Article : Google Scholar : PubMed/NCBI
|