Introduction
Cervical cancer is one of the most common malignant
tumors in the female reproductive system (1). Cervical cancer is a complicated
process involving many factors, from being a precancerous lesion,
before becoming an in situ carcinoma and then an
infiltrating carcinoma. Human papillomavirus (HPV) infection is one
of the primary causes of cervical cancer tumorigenesis. HPV DNA can
integrate into the cervical cells, and its encoded oncoprotein E6
will continue to activate the extracellular signal-regulated kinase
(ERK1/2) signaling pathway, thus promoting cancerous processes and
inhibiting apoptosis (2,3).
It was reported that ERK1/2 phosphorylation may be
used as a marker of cervical cancer differentiation (4). The ERK1/2 signaling pathway serves a
critical role in the carcinogenesis process of cervical cancer.
ERK1/2 expresses in the cytoplasm in normal tissue and keeps
inactivated in normal cervical epithelium proliferation.
Conversely, ERK1/2 is expressed both in cytoplasm and nuclei in
cervical cancer tissues, and maintains the state of activation.
ERK1/2 is a tyrosine kinase involved in the signal cascade
Ras/Raf/MEK/ERK. Phosphorylation is the method for activation of
ERK, leading to the transmission of extracellular signals to the
nucleus. It further promotes the target gene expression, such as
c-Jun and c-Fos. The protein products of the later can bind with
transcription factor activator proten-1 in the form of a homodimer
or heterodimer, thus activating myc gene expression (5–7). Myc
can activate a series of cell proliferation signals and induce
cyclin D1 expression, leading to cancer cells abnormal
proliferation, differentiation, apoptosis and metastasis (8,9).
Therefore, ERK activation can inhibit cancer cell apoptosis
(10,11). Furthermore, there are numerous
methods applied in clinic to treat cervical cancer by interfering
in the ERK1/2 signaling pathway, such as cisplatin administration
(12,13).
Baicalein belong to the group of flavonoid drugs,
and is the active ingredient extracted from the root of Chinese
traditional medicine Scutellaria baicalensis. It has
multiple effects, including antibacterial, antiviral,
anti-inflammatory, anti-allergic, anti-oxidative and
anti-tumorigenic. Recent studies reported that baicalein may
inhibit bladder cancer, prostate cancer, and liver cancer cell
growth (14–16), while its role in cervical cancer
cells has not been fully elucidated. The possible mechanism of
baicalein includes: 1) Affecting the arachidonic acid metabolic
pathway; 2) inhibiting cell proliferation; 3) promoting cell
apoptosis; and 4) suppressing tumor angiogenesis formation
(17,18). U0126 is the highly specific
inhibitor of mitogen-activated protein kinase (MAPK) kinases, MEK1
and MEK2. It can obviously inhibit c-Fos and c-Jun mRNA and protein
upregulation through blocking MAPK signal transduction, resulting
in AP-1 transcriptional activity suppression (19–21).
The present study jointly used baicalein and U0126
to stimulate the cervical cancer cell line HeLa, and observe their
impacts on cell apoptosis and migration.
Materials and methods
Cell line and reagents
Human cervical cancer cell line HeLa was purchased
from Shanghai Bioleaf Biotechnology Co., Ltd (Shanghai, China). The
cells were maintained in Dulbecco's Modified Eagle's medium
containing 20% fetal calf serum and cultured at 37°C and 5%
CO2. Baicalein purchased from National Institutes for
Food and Drug Control (Beijing, China) was dissolved in dimethyl
sulfoxide (Beyotime Institute of Biotechnology, Jiangsu, China) at
50 mol/l. U0126 was purchased from Shanghai Haoran Biological
Technology Co., Ltd. (Shanghai, China) www.haoranbio.com.
Cell Counting Kit (CCK) −8 assay
HeLa cells (2×103 cells/ml) were seeded
in a 96-well plate in 100 µl medium. Different concentrations of
baicalein (1, 2, 5, 10, 20, 50, 100, 200 and 300 µM) or U0126 (1,
2, 5, 10, 20 and 30 µM) were used to treat cells for 4 h with three
replicates. Then, 10 µl CCK-8 (Beyotime Institute of Biotechnology)
was added to the well for 4 h to form formazan. Finally, the plate
was read at 450 nm to draw the curve.
Cell cycle detection
HeLa cells (2×105 cells/ml) were seeded
in a 12-well plate and treated with baicalein or U0126. The cells
were collected and washed by PBS twice. Next, they were added to 1
ml 70% precooled ethanol at 4°C overnight. Then the cells were
washed with PBS and added with 100 mg/l RNase (Beyotime Institute
of Biotechnology) at 37°C for 30 min. Following staining with 50
mg/l propidium iodide at 4°C in the absence of light for 30 min,
the cells were detected using a FACSCalibur instrument (BD
Biosciences, Franklin Lakes, NJ, USA) with the excitation
wavelength at 488 nm. All the experiments were repeated three
times. Data were analyzed using SPSS software version 19.0 (IBM
SPSS, Armonk, NY, USA).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
HeLa cells were washed by PBS twice following
treatment and then fixed in 4% PFA at room temperature for 10 min.
The apoptotic cells were detected using a TUNEL assay kit (Promega,
Madison, WI, USA) according to the manufacturer's protocol. Next,
the cells were treated by 3% hydrogen peroxide for 10 min to
inhibit endogenous catalase. Following incubation with protease at
room temperature for 30 min, the cells were washed by PBS twice.
Subsequently, 50 µl TUNEL detection solution (containing terminal
deoxynucleotidyl transferase and digoxigenin-labelled nucleotides)
was added and incubated at room temperature for 60 min. Cells were
washed with PBS three times and observed under a fluorescence
microscope.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) assay
Following treating with 20 µM baicalein or 10 µM
U0126, HeLa cells were collected and washed by PBS twice. Then the
cells were resuspended in 400 µl 1X binding buffer and 5 µl Annexin
V-FITC was added in the absence of light for 15 min. A total of 10
µl PI was added to the cells and incubated in darkness for 5 min.
The cells were then tested using flow cytometry. The results were
analyzed by BD CellQuest Pro software version 5.1 (BD Biosciences).
Experiments were repeated three times.
Wound healing assay
A total of 6×106 HeLa cells were seeded
in a 24-well plate for 24 h to form a monolayer cell. Then the
cells were scratched using a sterile toothpick to form a straight
line. Following washing with PBS, the cells were treated with
baicalein (20 µM). Finally, the plate was observed under a light
microscope at different time points. The wound areas were imaged
and automatically calculated using TScratch software version: 1.0
(Computational Science and Engineering Laboratory, ETH Zurich,
Zurich, Switzerland) (22).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Beyotime
Institute of Biotechnology) and reverse transcribed to cDNA using
the RevertAid First Strand cDNA Synthesis kit (cat no. K1622;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The primers used
were designed by Primer 6.0 Premier (Premier Biosoft International,
CA, USA). RT-qPCR was applied to test target gene expression.
Reaction conditions: 55°C for 1 min, followed by 35 cycles of 92°C
for 30 sec, 58°C for 45 sec and 72°C for 35 sec. GAPDH was used as
internal reference. 2−∆∆Ct was applied to calculate
relative expression level (23).
The primers sequences were as follows: ERK1/2 forward,
5′-AATCACACGGTAGACACTGAAATGCC-3′ and reverse,
5′-CATCATCCCATCTAAAATGTCCCCTG-3′; Bcl-2-associated X protein (Bax)
forward, 5′-CATATAACCCCGTCAACGCAG-3′ and reverse,
5′-CCAGCCCATGATGGTTCTGAT-3′; B cell lymphoma 2 (Bcl-2) forward,
5′-GGTGGGGTCATGTGTGTGG-3′ and reverse,
5′-CGGTTCAGGTACTCAGTCATCC-3′; CyclinD1 forward,
5′-GCTGCGAAGTGGAAACCATC-3′ and reverse,
5′-CCTCCTTCTGCACACATTTGAA-3′; MMP2 forward,
5′-TACAGGATCATTGGCTACACACC-3′ and reverse,
5′-GGTCACATCGCTCCAGACT-3′; MMP9 forward,
5′-TGTACCGCTATGGTTACACTCG-3′ and reverse,
5′-GGCAGGGACAGTTGCTTCT-3′; GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′.
Western blot analysis
HeLa cells were incubated on ice for 20 min and
protease inhibitor and radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology) was added to extract protein.
Following centrifugation at 9,000 g for 10 min at 4°C, the
supernatant was moved to a new Eppendorf tube. Protein
concentration was determined using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The protein was separated using 10%
SDS-PAGE and transferred to polyvinylidene difluoride membrane.
Following blocking with 5% skim milk for 1.5 h, the membrane was
incubated with primary antibodies, cyclin D1 (catalog no.
ab137875), ERK1/2 (catalog no. ab36991), p-ERK1/2 (catalog no.
ab151279), MMP2 (catalog no. ab37150), MMP9 (catalog no. ab137867),
bax (catalog no. ab182733), bcl-2 (catalog no. ab32124), and GAPDH
(catalog no. ab8245) (dilution 1:1,500; Abcam, Cambridge, UK) at
4°C for 8 h. Then the membrane was incubated with secondary
antibody conjugated to horse radish peroxidase (catalog no.
ab131368, dilution 1:3,000; Abcam) at 37°C for 30 min and washed
with PBS containing 20% Tween 20. Finally, the membrane was
visualized using 3,3′-Diaminobenzidine substrate and images were
captured. Protein bands were quantified using Quantity One 1-D
Analysis software version 4.6.9 (Bio-Rad, Hercules, CA, USA).
Experiments were repeated for three times.
Statistical analysis
Data was presented as mean ± standard deviation and
analyzed using SPSS software version 19.0 (IBM SPSS, Armonk, NY,
USA). Student's t-test or one-way analysis of variance followed by
the Boferroni post hoc test) was applied for data comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baicalein and U0126 suppressed HeLa
induced cell apoptosis
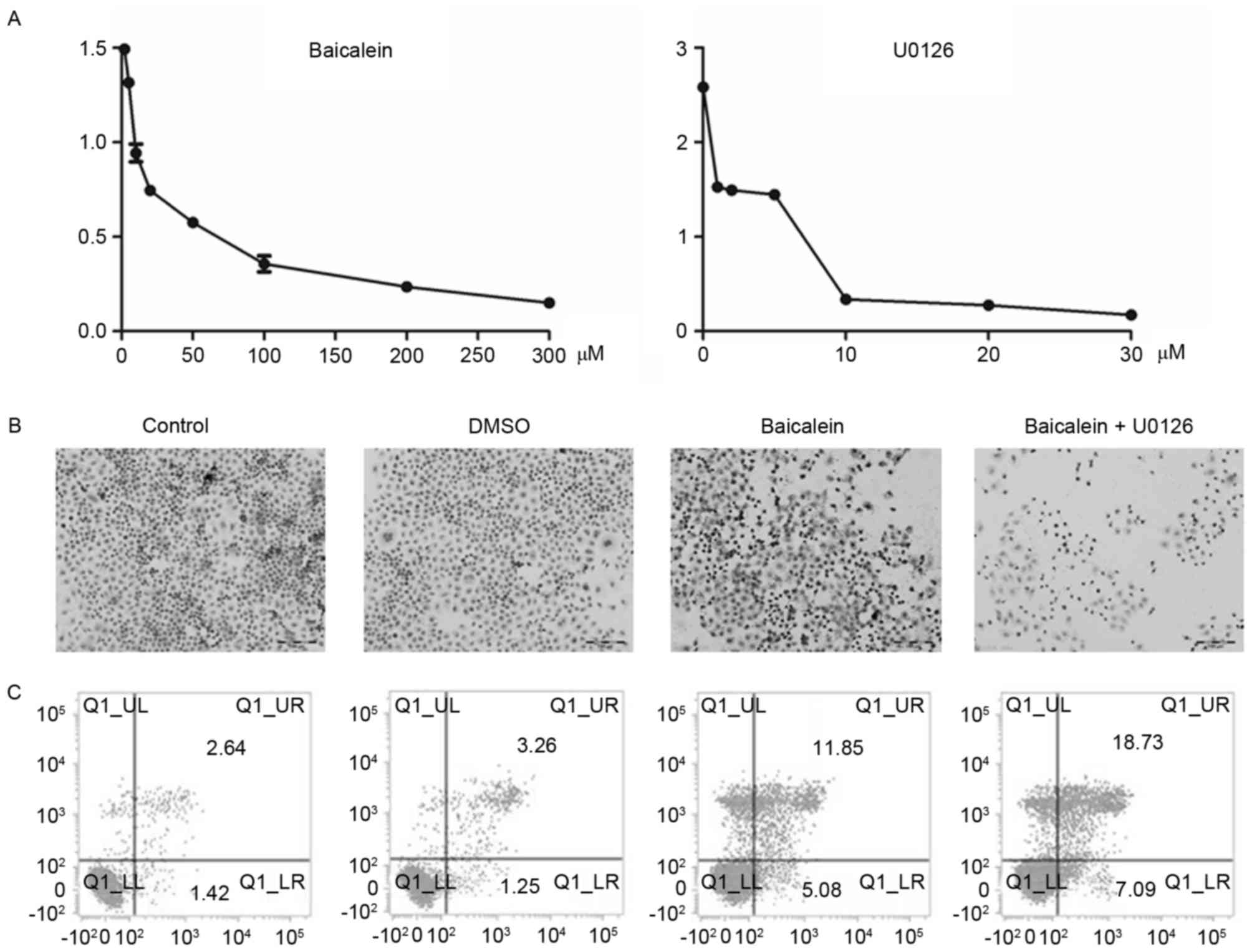
To explore the inhibitory effect of baicalein and
U0126 on HeLa cells, different concentrations of baicalein (1, 2,
5, 10, 20, 50, 100, 200 and 300 µM) or U0126 (1, 2, 5, 10, 20 and
30 µM) were used to treat HeLa cells for 24 h. CCK-8 results
demonstrated that HeLa cell viability significantly decreased by
administration of baicalein or U0126 with dose dependence
(P<0.05; Fig. 1A). To
investigate baicalein and U0126 impact on HeLa cell apoptosis. HeLa
cells were treated with 20 µM baicalein and 10 µM U0126. A TUNEL
assay revealed that both baicalein and U0126 induced HeLa cell
apoptosis synergistically (Fig.
1B). To further examine which phase of apoptosis was baicalein
and U0126 mainly act on. Annexin V/PI staining indicated that
baicalein and U0126 increased both early and late phases of
apoptosis with synergistic effect (Fig. 1C).
Baicalein and U0126 restrained the
MCF-7 cell cycle
As ERK can regulate cell cycle, the authors intended
to clarify the impact of baicalein and U0126 on the HeLa cell
cycle. Baicalein (20 µM) or U0126 (10 µM) was added to HeLa cells
for 24 h and flow cytometry demonstrated that, compared with
control group, cell content increased in G0/G1 phase, reduced in S
phase, and had no significant change in G2/M phase in the baicalein
group (Fig. 2A). U0126 addition
markedly declined cell content in S phase, when compared with the
single baicalein group (Fig. 2A).
In addition, cyclin D1 expression was investigated in HeLa cells
treated by baicalein or U0126. Both RT-qPCR and western blot
analysis demonstrated that cyclin D1 mRNA and protein levels
reduced following 20 µM baicalein treatment for 24 h. U0126 further
declined cyclin D1 expression (Figs.
2B and C).
Baicalein inhibited HeLa
migration
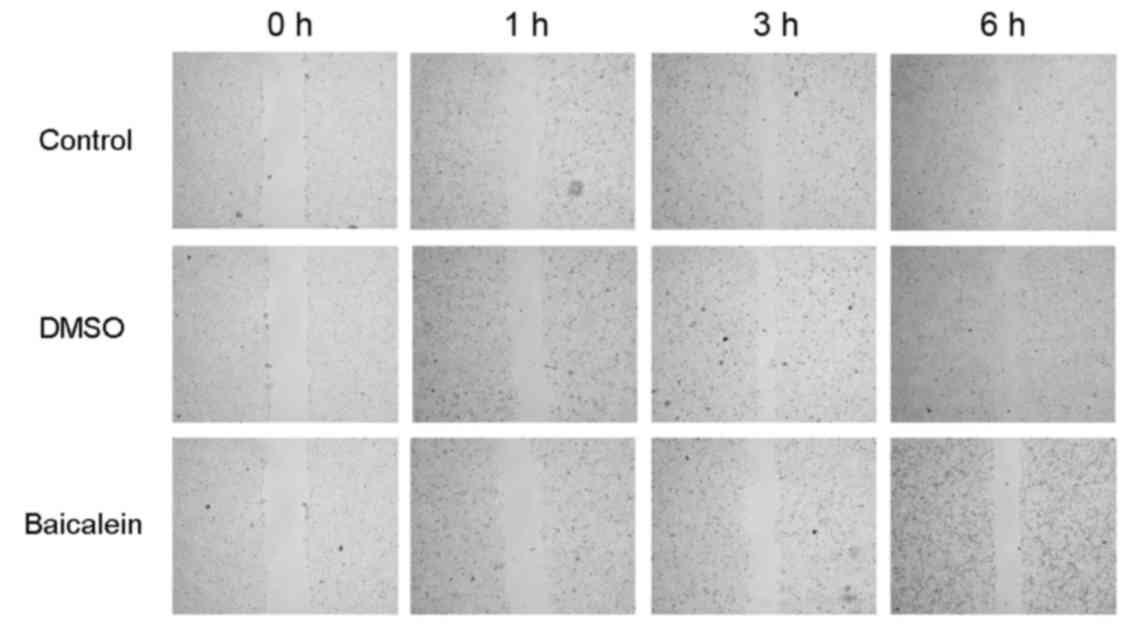
To study the effect of baicalein on HeLa cell
migration, the authors treated HeLa cells with 20 µM baicalein for
1, 3 and 6 h. A wound healing assay demonstrated that the scratch
width in the baicalein group was significantly larger than that in
control, indicating that baicalein can restrain HeLa cell migration
(Fig. 3).
Baicalein and U0126 affected ERK
signaling pathway and apoptosis related molecule expression in HeLa
cells
As a previous study reported that baicalein may
regulate the ERK signaling pathway (24), the present study tested the effect
of baicalein on the ERK signaling pathway and related proteins in
HeLa cells. RT-qPCR results demonstrated that baicalein obviously
declined ERK1/2, MMP2 and MMP9 levels in HeLa cells. Moreover, it
significantly elevated levels of pro-apoptotic factor Bax mRNA
expression and downregulated apoptosis suppression gene Bcl-2 mRNA
level (Fig. 4A). Furthermore, the
expression of proteins of the ERK signaling pathway was analyzed.
Western blotting revealed that ERK1/2 phosphorylation, MMP2 and
MMP9 levels were markedly declined in HeLa cells treated by
baicalein. In addition, Bax and Bcl-2 proteins presented a similar
trend with mRNA expression. Baicalein, together with U0126, more
obviously suppressed ERK signaling pathway related proteins
expression, when compared with single baicalein treatment (Fig. 4B).
 | Figure 4.Baicalein and U0126 affected ERK
signaling pathway expression in HeLa cells. (A) ERK1/2, MMP2, MMP9,
Bax and Bcl-2 mRNA expression tested by real time PCR. (B) ERK1/2,
MMP2, MMP9, Bax and Bcl-2 protein expression detected by western
blot analysis. Data are presented as the mean ± standard deviation.
Lanes: 1, control; 2, control + DMSO; 3, baicalein (20 µM). 4,
U0126 (10 µM); 5, baicalein (20 mM) + U0126 (10 µM). ERK,
extracellular signal-regulated kinase; MMP, matrix
metalloproteinase; Bax, Bcl-2-associated X protein; Bcl-2, B-cell
lymphoma 2; DMSO, dimethyl sulfoxide. |
Discussion
Cervical cancer is a common malignant tumor in
females (1). At present,
radiotherapy, surgery and chemotherapy are the main treatment
methods. All of these methods improve patient's survival through
suppressing cancer cell proliferation and metastasis, and promoting
cell apoptosis. However, there are numerous adverse reactions of
current chemotherapy drugs (25,26).
Thus, searching for effective drugs for cervical cancer treatment
is of great significance.
Baicalein has been demonstrated to exhibit antitumor
effects in numerous types of cancer (27–29).
However, the antitumor effect and the associated mechanisms in
breast cancer cells remain unclear. The present study revealed that
baicalein significantly inhibited the proliferative and metastatic
ability of breast cancer cell line, HeLa, by regulating the
expression levels of MMP2 and MMP9 via inhibition of the ERK
signaling pathway. To the best of the authors' knowledge, the
present study is the first to investigate the antitumor effect of
baicalein on breast cancer cell line, HeLa.
The ERK signaling pathway is important for tumor
cell proliferation through regulating the cell cycle (30). ERK can facilitate cancer cell
growth by promoting cell movement from the G1 phase to the S phase
(31). The current results
exhibited that baicalein blocked HeLa cells in G0/G1 phase, and
U0126 also decreased cell content in S phase. In addition, cyclin
D1 level declined following baicalein and U0126 treatment,
indicating that both baicalein and U0126 may restrain breast cancer
proliferation through blocking the cell cycle.
Abnormal apoptosis is an important reason for
carcinogenesis. Apoptosis is a specific process with featured
biochemical processes and cell morphology changes, including the
death receptor and mitochondrial pathways. They can activate
caspases through a series of signal transduction, thus leading to
the degradation of the nucleus and various substrates in the
cytoplasm (32,33). Caspase-3 is the most common
executor of the apoptosis pathway (34,35).
The Bcl-2 family serves a critical role in regulating apoptosis,
while ERK1/2 phosphorylation can achieve anti-apoptosis and promote
proliferation through phosphorylating Bcl-2, activating
transcriptional factors, and interfering TNF related apoptosis
inducing ligand (36,37). Bax and Bcl-2 are important members
in Bcl-2 family, and the ratio of Bax and Bcl-2 determines whether
cell apoptosis occurs or not (38,39).
These results indicated that baicalein and U0126 treatment both
restrained HeLa cell viability and induced cell apoptosis.
Furthermore, they significantly declined Bcl-2 expression and
enhanced Bax level. All of these results suggested that baicalein
and ERK signaling pathway inhibitor may suppress cervical cancer
cell proliferation and induce apoptosis.
Tumor invasive metastasis is a complicated
pathological process affected by multiple factors, genes and
processes. Extracellular matrix (ECM) degradation and basement
membrane integrity damage are the prerequisites of tumor cell
metastasis (40,41). The role of MMPs in tumor metastasis
mainly includes: 1) Destructing local tissue structure and
promoting tumor growth; 2) destructing the basement membrane
barrier, which facilitates tumor metastasis; 3) promoting tumor
neovascularization by improving the ECM. The degradation of ECM
mainly depends on proteolytic enzymes; MMPs are an extremely
important type of proteolytic enzyme (42,43).
MMPs are Zn2+- and Ca2+-dependent proteases
that may be classified by four types according to specific
substrates: 1) Collagenase (MMP-1, -8 and -13); 2) gelatinase
(MMP-2 and -9); 3) stromelysin (MMP-3, -7, -10 and -11); 4)
Membrane type metal protein enzymes (MMP-14, -15, -16 and -17)
(44,45). MMP2 and MMP9 can specifically
degrade the main component of basement membrane, collagen type IV
(46,47). MMP2 and MMP9 serve an important
role in cervical cancer invasion and metastasis. It was reported
that MMP2 and MMP9 is significantly overexpressed in cervical
cancer tissue, thus leading to ECM and vascular basement membrane
acceleration of degradation. Therefore, it facilitates cancer cells
emigrating from the primary lesion and enhances tumor cell
metastatic ability (48).
Moreover, MMP2 is an early marker of tumor, and its high expression
is one of the important characteristics of tumor metastasis
(49). These results revealed that
baicalein and U026 markedly decreased MMP2 and MMP9 levels in HeLa
cells, and their combination further declined MMP2 and MMP9 mRNA
and protein expression.
Taken together, baicalein and U0126 can induce HeLa
cell apoptosis and restrain migration through the ERK signaling
pathway. Their combination can further suppress breast cancer cell
proliferation and metastasis, providing great significance for
breast cancer treatment.
Acknowledgements
This work supported by the Fund of the Sichuan
Provincial Department of Science and Technology (grant no.
14JC01353-LH67), the Mutual Fund of Science&Technology
Department of Sichuan province [grant no. 2015LZCYD-S02 (1/11)],
the Fund of Department of Education of Sichuan Province (grant no.
16ZB0195) and the Fund of Science and Technology of Luzhou City
(grant no. 2014-S-35).
References
|
1
|
Chan DN and So WK: A systematic review of
randomised controlled trials examining the effectiveness of breast
andcervical cancer screening interventions for ethnic minority
women. Eur J Oncol Nurs. 19:536–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maurer K, Luo H, Shen Z, Wang G, Du H,
Wang C, Liu X, Wang X, Qu X, Wu R and Belinson J: Evaluation of a
new solid media specimen transport card for high risk HPV detection
and cervical cancer prevention. J Clin Virol. 76:14–19. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qureshi R, Arora H, Biswas S, Perwez A,
Naseem A, Wajid S, Gandhi G and Rizvi MA: Mutation analysis of EGFR
and its correlation with the HPV in Indian cervical cancer
patients. Tumour Biol. 37:9089–9098. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang H, Shi Y, Tuokan T, Chen R and Wang
X: Expression of aquaporin 8 and phosphorylation of Erk1/2 in
cervical epithelial carcinogenesis: Correlation with
clinicopathological parameters. Int J Clin Exp Pathol. 7:3928–3937.
2014.PubMed/NCBI
|
|
5
|
Asati V, Mahapatra DK and Bharti SK:
PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as
anticancer agents: Structural and pharmacological perspectives. Eur
J Med Chem. 109:314–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kake S, Usui T, Ohama T, Yamawaki H and
Sato K: Death-associated protein kinase 3 controls the tumor
progression of A549 cells through ERKMAPK/c-Myc signaling. Oncol
Rep. 37:1100–1106. 2017.PubMed/NCBI
|
|
7
|
Davis RJ: Transcriptional regulation by
MAP kinases. Mol Reprod Dev. 42:459–467. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sipos F, Firneisz G and Műzes G:
Therapeutic aspects of c-MYC signaling in inflammatory and
cancerous colonic diseases. World J Gastroenterol. 22:7938–7950.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Tai Y, Lisanti MP and Liao DJ:
c-Myc induction of programmed cell death may contribute to
carcinogenesis: A perspective inspired by several concepts of
chemical carcinogenesis. Cancer Biol Ther. 11:615–626. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Branca M, Ciotti M, Santini D, Bonito LD,
Benedetto A, Giorgi C, Paba P, Favalli C, Costa S, Agarossi A, et
al: Activation of the ERK/MAP kinase pathway in cervical
intraepithelial neoplasia is related to grade of the lesion but not
to high-risk human papillomavirus, virus clearance, or prognosis in
cervical cancer. Am J Clin Pathol. 122:902–911. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Zhang Y, Liu S, Zhang Q, Wang Y,
Tong L, Chen X, Ji Y, Shang Q, Xu B, et al: Metadherin confers
chemoresistance of cervical cancer cells by inducing autophagy and
activating ERK/NF-κB pathway. Tumour Biol. 34:2433–2440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narayan G, Xie D, Ishdorj G, Scotto L,
Mansukhani M, Pothuri B, Wright JD, Kaufmann AM, Schneider A,
Arias-Pulido H and Murty VV: Epigenetic inactivation of TRAIL decoy
receptors at 8p12-21.3 commonly deleted region confers sensitivity
to Apo2L/trail-Cisplatin combination therapy in cervical cancer.
Genes Chromosomes Cancer. 55:177–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Umayahara K, Takekuma M, Hirashima Y, Noda
SE, Ohno T, Miyagi E, Hirahara F, Hirata E, Kondo E, Tabata T, et
al: Phase II study of concurrent chemoradiotherapy with weekly
cisplatin and paclitaxel in patients with locally advanced uterine
cervical cancer: The JACCRO GY-01 trial. Gynecol Oncol.
140:253–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng
P, Chen A and Huang H: The Fascinating Effects of Baicalein on
Cancer: A Review. Int J Mol Sci. 17:pii: E1681. 2016. View Article : Google Scholar
|
|
15
|
Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng
X, Su J, Zhou Z, Xu Z, Nilsson S and Liu Z: Baicalein inhibits
prostate cancer cell growth and metastasis via the
caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 406:111–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Z, Zhu S, Han X, Wang Z, Wu S and
Zheng R: Baicalein inhibits hepatocellular carcinoma cells through
suppressing the expression of CD24. Int Immunopharmacol.
29:416–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng Y, Guo C, Yang Y, Li F, Zhang Y,
Jiang B and Li Q: Baicalein induces apoptosis of human cervical
cancer HeLa cells in vitro. Mol Med Rep. 11:2129–2134.
2015.PubMed/NCBI
|
|
18
|
Aryal P, Kim K, Park PH, Ham S, Cho J and
Song K: Baicalein induces autophagic cell death through AMPK/ULK1
activation and downregulation of mTORC1 complex components in human
cancer cells. FEBS J. 281:4644–4658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rose HM, Stuiver M, Thongwichian R,
Theillet FX, Feller SM and Selenko P: Quantitative NMR analysis of
Erk activity and inhibition by U0126 in a panel of patient-derived
colorectal cancer cell lines. Biochim Biophys Acta. 1834:1396–1401.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo M, Wei H, Hu J, Sun S, Long J and Wang
X: U0126 inhibits pancreatic cancer progression via the KRAS
signaling pathway in a zebrafish xenotransplantation model. Oncol
Rep. 34:699–706. 2015.PubMed/NCBI
|
|
21
|
Lodi A, Woods SM and Ronen SM: Treatment
with the MEK inhibitor U0126 induces decreased hyperpolarized
pyruvate to lactate conversion in breast, but not prostate, cancer
cells. NMR Biomed. 26:299–306. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gebäck T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang YF, Xu YL, Tang ZH, Li T, Zhang LL,
Chen X, Lu JH, Leung CH, Ma DL, Qiang WA, et al: Baicalein induces
beclin 1- and extracellular signal-regulated kinase-dependent
autophagy in ovarian cancer cells. Am J Chin Med. 45:123–136. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herfs M, Yamamoto Y, Laury A, Wang X,
Nucci MR, McLaughlin-Drubin ME, Münger K, Feldman S, McKeon FD,
Xian W and Crum CP: A discrete population of squamocolumnar
junction cells implicated in the pathogenesis of cervical cancer.
Proc Natl Acad Sci USA. 109:10516–10521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Trunk MJ, Wentzensen N and von Knebel
Doeberitz M: Molecular pathogenesis of cervical cancer and its
first steps. Pathologe. 26:283–290. 2005.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim DH, Hossain MA, Kang YJ, Jang JY, Lee
YJ, Im E, Yoon JH, Kim HS, Chung HY and Kim ND: Baicalein, an
active component of Scutellaria baicalensis Georgi, induces
apoptosis in human colon cancer cells and prevents AOM/DSS-induced
colon cancer in mice. Int J Oncol. 43:1652–1658. 2013.PubMed/NCBI
|
|
28
|
Li HL, Zhang S, Wang Y, Liang RR, Li J, An
P, Wang ZM, Yang J and Li ZF: Baicalein induces apoptosis via a
mitochondrial-dependent caspase activation pathway in T24 bladder
cancer cells. Mol Med Rep. 7:266–270. 2013.PubMed/NCBI
|
|
29
|
Lin YT, Yang JS, Lin HJ, Tan TW, Tang NY,
Chaing JH, Chang YH, Lu HF and Chung JG: Baicalein induces
apoptosis in SCC-4 human tongue cancer cells via a Ca2+-dependent
mitochondrial pathway. In Vivo. 21:1053–1058. 2007.PubMed/NCBI
|
|
30
|
Liu Z, Ren L, Liu C, Xia T, Zha X and Wang
S: Phenformin induces cell cycle change, apoptosis, and
mesenchymal-epithelial transition and regulates the
AMPK/mTOR/p70s6k and MAPK/ERK pathways in breast cancer cells. PLoS
One. 10:e01312072015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Tomaso Portaz AC, Caimi GR, Sánchez M,
Chiappini F, Randi AS, de Kleiman Pisarev DL and Alvarez L:
Hexachlorobenzene induces cell proliferation, and aryl hydrocarbon
receptor expression (AhR) in rat liver preneoplastic foci and in
the human hepatoma cell line HepG2. AhR is a mediator of ERK1/2
signaling, and cell cycle regulation in HCB-treated HepG2 cells.
Toxicology. 336:36–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Green DR and Llambi F: Cell Death
Signaling. Cold Spring Harb Perspect Biol. 7:pii: a006080. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Savitskaya MA and Onishchenko GE:
Mechanisms of apoptosis. Biochemistry (Mosc). 80:1393–1405. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bortner CD, Scoltock AB, Cain DW and
Cidlowski JA: T-cell development of resistance to apoptosis is
driven by a metabolic shift in carbon source and altered activation
of death pathways. Cell Death Differ. 23:889–902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iranpour M, Moghadam AR, Yazdi M, Ande SR,
Alizadeh J, Wiechec E, Lindsay R, Drebot M, Coombs KM and Ghavami
S: Apoptosis, autophagy and unfolded protein response pathways in
Arbovirus replication and pathogenesis. Expert Rev Mol Med.
18:e12016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Casagrande N, De Paoli M, Celegato M,
Borghese C, Mongiat M, Colombatti A and Aldinucci D: Preclinical
evaluation of a new liposomal formulation of cisplatin, lipoplatin,
to treat cisplatin-resistant cervical cancer. Gynecol Oncol.
131:744–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cetina L, González-Enciso A, Cantú D,
Coronel J, Pérez-Montiel D, Hinojosa J, Serrano A, Rivera L,
Poitevin A, Mota A, et al: Brachytherapy versus radical
hysterectomy after external beam chemoradiation with gemcitabine
plus cisplatin: A randomized, phase III study in IB2-IIB cervical
cancer patients. Ann Oncol. 24:2043–2047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tjalma WA, Weyler JJ, Bogers JJ,
Pollefliet C, Baay M, Goovaerts GC, Vermorken JB, van Dam PA, van
Marck EA and Buytaert PM: The importance of biological factors
(bcl-2, bax, p53, PCNA, MI, HPV and angiogenesis) in invasive
cervical cancer. Eur J Obstet Gynecol Reprod Biol. 97:223–230.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao LW, Zhong XH, Yang SY, Zhang YZ and
Yang NJ: Inotodiol inhabits proliferation and induces apoptosis
through modulating expression of cyclinE, p27, bcl-2, and bax in
human cervical cancer HeLa cells. Asian Pac J Cancer Prev.
15:3195–3199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alečković M and Kang Y: Welcoming Treat:
Astrocyte-derived exosomes induce pten suppression to foster brain
metastasis. Cancer Cell. 28:554–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
von Karstedt S, Conti A, Nobis M,
Montinaro A, Hartwig T, Lemke J, Legler K, Annewanter F, Campbell
AD and Taraborrelli L: Cancer cell-autonomous TRAIL-R signaling
promotes KRAS-driven cancer progression, invasion, and metastasis.
Cancer Cell. 27:561–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Che YL, Luo SJ, Li G, Cheng M, Gao YM, Li
XM, Dai JM, He H, Wang J, Peng HJ, et al: The C3G/Rap1 pathway
promotes secretion of MMP-2 and MMP-9 and is involved in serous
ovarian cancer metastasis. Cancer Lett. 359:241–249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang C, Ma HX, Jin MS, Zou YB, Teng YL,
Tian Z, Wang HY, Wang YP and Duan XM: Association of matrix
metalloproteinase (MMP)-2 and -9 expression with
extra-gastrointestinal stromal tumor metastasis. Asian Pac J Cancer
Prev. 15:4187–4192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Avolio C, Ruggieri M, Giuliani F, Liuzzi
GM, Leante R, Riccio P, Livrea P and Trojano M: Serum MMP-2 and
MMP-9 are elevated in different multiple sclerosis subtypes. J
Neuroimmunol. 136:46–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fatar M, Stroick M, Steffens M, Senn E,
Reuter B, Bukow S, Griebe M, Alonso A, Lichtner P, Bugert P, et al:
Single-nucleotide polymorphisms of MMP-2 gene in stroke subtypes.
Cerebrovasc Dis. 26:113–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alves AC, Albertini R, dos Santos SA,
Leal-Junior EC, Santana E, Serra AJ, Silva JA Jr and de Carvalho
Pde T: Effect of low-level laser therapy on metalloproteinase MMP-2
and MMP-9 production and percentage of collagen types I and III in
a papain cartilage injury model. Lasers Med Sci. 29:911–919.
2014.PubMed/NCBI
|
|
47
|
Tunik S, Ayaz E, Akpolat V, Nergiz Y, Isen
K, Celik MS and Seker U: Effects of pulsed and sinusoidal
electromagnetic fields on MMP-2, MMP-9, collagen type IV and
E-cadherin expression levels in the rat kidney: An
immunohistochemical study. Anal Quant Cytopathol Histpathol.
35:253–260. 2013.PubMed/NCBI
|
|
48
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9 in
human cervical and ovarian cancer cell lines by cytokines, inducers
and inhibitors. Oncol Rep. 23:605–614. 2010.PubMed/NCBI
|
|
49
|
Fernandes T, de Angelo-Andrade LA, Morais
SS, Pinto GA, Chagas CA, Maria-Engler SS and Zeferino LC: Stromal
cells play a role in cervical cancer progression mediated by MMP-2
protein. Eur J Gynaecol Oncol. 29:341–344. 2008.PubMed/NCBI
|