Introduction
Essential hypertension (EH) is a risk factor for
cardiovascular diseases and organ damage, which has become a major
cause of morbidity and mortality worldwide (1). The World Health Organization (Geneva,
Switzerland) has reported that 29.2% of the global population will
develop hypertension by 2025, and the prevalence in China was
observed to be 26.7% in 2010 (2).
Although the etiology of EH remains unclear, previous studies have
indicated that low-grade chronic inflammation is a hallmark of EH
and contributes to target organ damage and the development of
atherosclerosis, which is a process mediated by circulating immune
cells, particularly leucocytes (3–5).
Toll-like receptors (TLRs), members of the
interleukin (IL) 1R superfamily, are transmembrane receptors with
extracellular leucine-rich repeats and an important intracellular
signaling domain. TLRs are expressed in monocytes, macrophages and
neutrophils, and recognize pathogen-associated molecular patterns
to initiate an innate immune response. TLR2 serves a role in
endothelial cell activation, macrophage recruitment and
pro-inflammatory cytokine production. In TLR2 signaling, TLR2
dimerizes with TLR1 or TLR6. The heterodimers recruit and activate
interleukin-1 receptor-associated kinase 4 via a myeloid
differentiation primary response protein MyD88 (MyD88)/MyD88
adaptor-like (Mal)-dependent mechanism, and therefore facilitate
the induction of cytokines (6–8).
Previously, elevated circulating pro-inflammatory cytokine markers
related to the TLR signaling pathway, including tumor necrosis
factor (TNF)-α, C-reactive protein (CRP) and IL-6, were
hypothesized to be important risk factors for EH (4,9).
TLRs have been suggested to serve a role in the pathogenesis of EH
(10,11) and atherosclerotic diseases
(12–14). However, the underlying mechanisms
that regulate this response remain unclear.
An important mechanism of epigenetic regulation, DNA
methylation is reversible and primarily occurs at cytosine residues
in cytosine-phosphate-guanine (CpG) dinucleotides, in mammalian
cells (15). Gene promoter
hypermethylation silences gene expression, while promoter
hypomethylation promotes active transcription (16). Previous studies into the etiology
of EH have focused on DNA methylation. ADD1, AGTR1
and GCK gene methylation have been demonstrated to be
associated with EH (17–19). Alexeeff et al (9) reported that the aberrant methylation
of TLR2, inducible nitric oxide synthase and interferon -γ
was associated with blood pressure.
However, the association of TLR2 methylation
with EH remains unclear. The present study aimed to investigate
whether TLR2 promoter methylation was associated with EH and
to assess the association of TLR2 promoter methylation with
age, blood pressure and other risk factors of EH.
Materials and methods
Sample collection
A total of 192 individuals, including 96 healthy
controls, and 96 newly-diagnosed patients with EH who had not
received anti-hypertensive therapy, were recruited at Ningbo
Seventh Hospital (Ningbo, China). Patients were defined as
hypertensive according to the ‘gold standard’ diagnostic criteria,
and exhibited ≥3 consecutive measurements of systolic blood
pressure (SBP) >140 mmHg and/or diastolic blood pressure (DBP)
>90 mmHg (20). Controls
exhibited SBP <120 mmHg and DBP <80 mmHg, and reported no
family history of hypertension in first degree relatives. All of
the participants were from Han Chinese families who had been
residing in Ningbo for ≥3 generations, with no history of secondary
hypertension, diabetes mellitus, stroke, renal failure, myocardial
infarction, drug abuse, or other serious diseases.
A calibrated mercury sphygmomanometer with an
adult-sized cuff was used to measure blood pressure based on the
standard protocols of the American Heart Association (21). Blood pressure was measured twice in
the supine position, ≥10 min apart by trained technicians. Blood
samples were drawn from the antecubital vein using vacutainer tubes
containing EDTA, and stored at −80°C for DNA extraction. The
protocol was approved by the ethics committee of Ningbo Seventh
Hospital, and written informed consent was obtained.
Biochemical analyses
Plasma levels of triglyceride, total cholesterol,
uric acid, high-density lipoprotein (HDL), low-density lipoprotein
(LDL), alanine aminotransferase, serum creatinine and leucocytes
were measured enzymatically using an AU2700 automatic analyzer
(Olympus Corporation, Tokyo, Japan). A Lab-Aid 820 nucleic acid
extraction analyzer (Xiamen Zeesan Biotech Co., Ltd., Xiamen,
China) was used to extract genomic DNA from peripheral blood
samples. DNA concentration was measured using a NanoDrop 2000
ultra-micro nucleic acid ultraviolet tester (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA).
The sequencing-by-synthesis technique of
pyrosequencing was used to measure methylation levels. DNA
sequences were reacted with sodium bisulfite (EpiTech Bisulfite
kit; Qiagen GmbH, Hilden, Germany) to convert unmethylated cytosine
residues to thymine, and subsequently amplified by polymerase chain
reaction (PCR) prior to being ‘sequenced by synthesis’ (Pyromark
Gold Q96, Qiagen GmbH) (22). The
CpG sites of target gene sequences and PCR primers were chosen
according to the scores automatically calculated by the PyroMark
Assay Design (version 2.0.1.15; Qiagen GmbH) using previously
established protocols for primer design (23). CpG island sequences were amplified
using a Mastercycler Nexus Gradient (Eppendorf, Hamburg, Germany)
in reactions containing 12 µl ZymoTaq™ Premix (Zymo
Research Corporation, Irvine, CA, USA), 8 µl DNase/RNase-free
H2O, 1.5 µl each of forward and reverse primer and 2 µl
bisulfite-converted DNA. Reactions were first denatured at 95°C for
10 min; amplified over 40 cycles at 95°C for 30 sec, 54.1°C for 40
sec and 72°C for 50 sec; and extended at 72°C for 7 min.
TLR2 CpG island sequences were amplified with the primers
presented in Table I.
 | Table I.Primers of the Toll-like receptor-2
cytosine-phosphate-guanine island sequence. |
Table I.
Primers of the Toll-like receptor-2
cytosine-phosphate-guanine island sequence.
| Primers | Sequence |
|---|
| Forward |
5′-Biotin-GGTAGTTGTAGGGGTAGGAT-3′ |
| Reverse |
5′-ACCCAAAAAAACTCTAAACCTC-3′ |
| Sequence |
5′-TTCCAAACAAATAACC-3′ |
Statistical analyses
Experimental data were analyzed using PASW
statistics software (version 19.0; IBM SPSS, Armonk, NY, USA).
Results are presented as the mean ± standard deviation or number
(percentage) of patients. Continuous variables, including DNA
methylation, age, body mass index (BMI), total cholesterol,
triglycerides, uric acid, HDL, LDL, serum creatinine and leucocyte
count, were compared by paired t-test or nonparametric test. The
Pearson χ2 or Fisher's exact test was used to analyze
the association between categorical variables (sex, smoking and
alcohol consumption) and essential hypertension. Pearson's
correlation analysis was used to investigate interactions among the
eight CpG sites in the TLR2 promoter sequence. Receiver
operating characteristic (ROC) curves were used to determine the
sensitivity of TLR2 promoter methylation as a predictor of
EH. Logistic regression was implemented to adjust for confounding
factors. Generalized multifactor dimensionality reduction (GMDR)
was applied to investigate underlying high-order interactions
between TLR2 promoter methylation and risk factors of EH.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of the 96 healthy controls and 96
patients with EH are presented in Table II. The age (±3 years) and sex
ratio was matched in the participants between the two groups. In
addition, as presented in Table
II, BMI (t=4.09; P=9.1×10−5), HDL (t=6.57;
P=2.6×10−9), triglyceride (t=2.33; P=0.022) and uric
acid (t=2.75; P=0.007) were significantly different between the two
groups.
 | Table II.Comparison of characteristics between
controls and EH group. |
Table II.
Comparison of characteristics between
controls and EH group.
|
Characteristics | Controls | EH | t(Z)/χ
2 | P-value |
|---|
| Age (mean ±
SD) | 56.3±8.2 | 56.7±8.7 | 0.32 | 0.747 |
| Sex,
male/female | 38/58 | 38/58 | n/a | n/a |
| Smoking,
yes/no | 79/17 | 69/27 | 2.95 | 0.086 |
| Alcohol
consumption, yes/no | 65/31 | 56/40 | 1.81 | 0.178 |
| BMI (mean ±
SD) | 22.16±2.30 | 23.6±3.09 | 4.09 |
9.1×10−5 |
| HDL, mg/dl (mean ±
SD) | 7.99±6.32 | 2.07±5.58 | 6.57 |
2.6×10−9 |
| LDL, mg/dl (mean ±
SD) | 3.21±0.87 | 3.31±0.68 | 0.90 | 0.370 |
| ALT, IU/l (mean ±
SD) | 26.41±16.1 | 28.27±12 | 0.89 | 0.370 |
| Triglyceride,
mmol/l (mean ± SD) | 1.21±0.68 | 1.43±0.72 | 2.33 | 0.022 |
| Total cholesterol,
mmol/l (mean ± SD) | 5.19±0.89 | 5.38±0.61 | 1.71 | 0.091 |
| Urea, mmol/l (mean
± SD) | 4.96±1.07 | 5.03±1.11 | 0.52 | 0.607 |
| Uric acid, µmol/l
(mean ± SD) | 300.32±73.15 | 325.75±82.63 | 2.75 | 0.007 |
| Serum creatinine,
µmol/l (mean ± SD) | 82.68±12.28 | 83.46±11.04 | 0.53 | 0.600 |
| WBC count (mean ±
SD) | 5.59±0.93 | 6.17±0.98 | 4.84 |
1.30×10−6 |
| Lymphocyte count
(mean ± SD) | 1.98±0.61 | 2.1±0.55 | 2.05 | 0.040 |
| Monocyte count
(mean ± SD) | 0.30±0.18 | 0.31±0.17 | 0.49 | 0.622 |
| Neutrophil
granulocyte count (mean ± SD) | 3.05±0.97 | 3.35±0.92 | 2.55 | 0.011 |
| Eosinophil
granulocyte count (mean ± SD) | 0.13±0.13 | 0.129±0.10 | 0.75 | 0.451 |
| Basophil
granulocyte count (mean ± SD) | 0.15±0.64 | 0.02±0.02 | 0.66 | 0.508 |
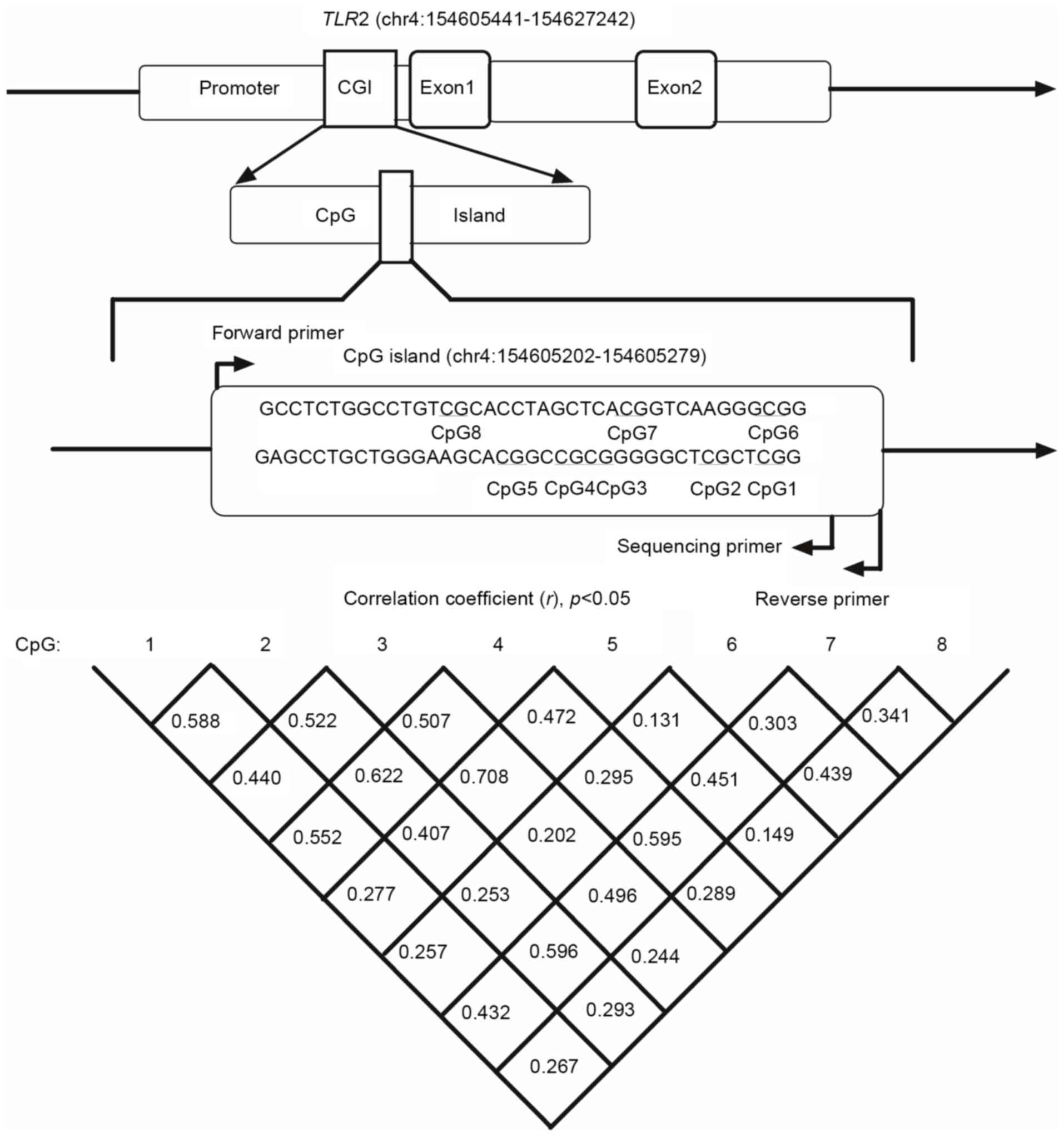
In the present study, eight CpG sites were selected
to investigate the association between methylation and EH in the
CpG island of the TLR2 gene promoter. Details of the eight
CpG sites are presented in Fig. 1
and Table III. As presented in
Table III and Fig. 2, eight CpG sites of the EH group
exhibited decreased methylation levels compared with healthy
controls; however, only CpG1 (2.83±1.34 vs. 3.44±1.75; P=0.009),
CpG6 (3.58±3.64 vs. 8.30±4.13; P<0.001) and CpG8 (8.91±5.32 vs.
11.33±3.87; P<0.001) were significantly different.
 | Table III.Logistic regression analysis of the
methylation levels of the eight CpG sites. |
Table III.
Logistic regression analysis of the
methylation levels of the eight CpG sites.
|
|
|
|
|
| Controls vs.
EH |
|---|
|
|
|
|
|
|
|
|---|
| Variables | Controls (mean ±
SD) | EH (mean ± SD) | t | P-value | OR (95% CI) |
P-valuea |
|---|
| CpG1 |
3.44±1.75 | 2.83±1.34 | 2.69 | 0.009 | 0.99
(0.838~1.173) | 0.921 |
| CpG2 |
1.93±1.84 | 1.71±1.57 | 0.97 | 0.336 | 0.91
(0.715~1.146) | 0.408 |
| CpG3 |
0.69±1.60 | 0.58±1.80 | 0.43 | 0.665 | 0.96
(0.743~1.248) | 0.776 |
| CpG4 |
1.65±2.08 | 1.65±2.06 | 0.00 | 1.000 | 1.07
(0.883~1.305) | 0.478 |
| CpG5 |
0.68±1.53 | 0.66±1.80 | 0.09 | 0.926 | 1.03
(0.805~1.327) | 0.798 |
| CpG6 |
8.30±4.13 | 3.58±3.64 | 8.31 |
6.5×10−13 | 1.10
(1.021~1.161) | 0.009 |
| CpG7 |
3.51±3.90 | 3.25±3.51 | 0.51 | 0.613 | 0.99
(0.893~1.103) | 0.889 |
| CpG8 | 11.33±3.87 | 8.91±5.32 | 3.82 |
2.4×10−4 | 0.98
(0.939~1.025) | 0.389 |
In order to adjust for confounding factors, logistic
regression was applied to obtain the odds ratio (OR) of CpG1-8. As
presented in Table III, when
adjusted for age, gender, smoking, alcohol consumption, uric acid,
serum creatinine, triglyceride, HDL and BMI, the results indicated
that the methylation level of CpG6 was an important risk factor for
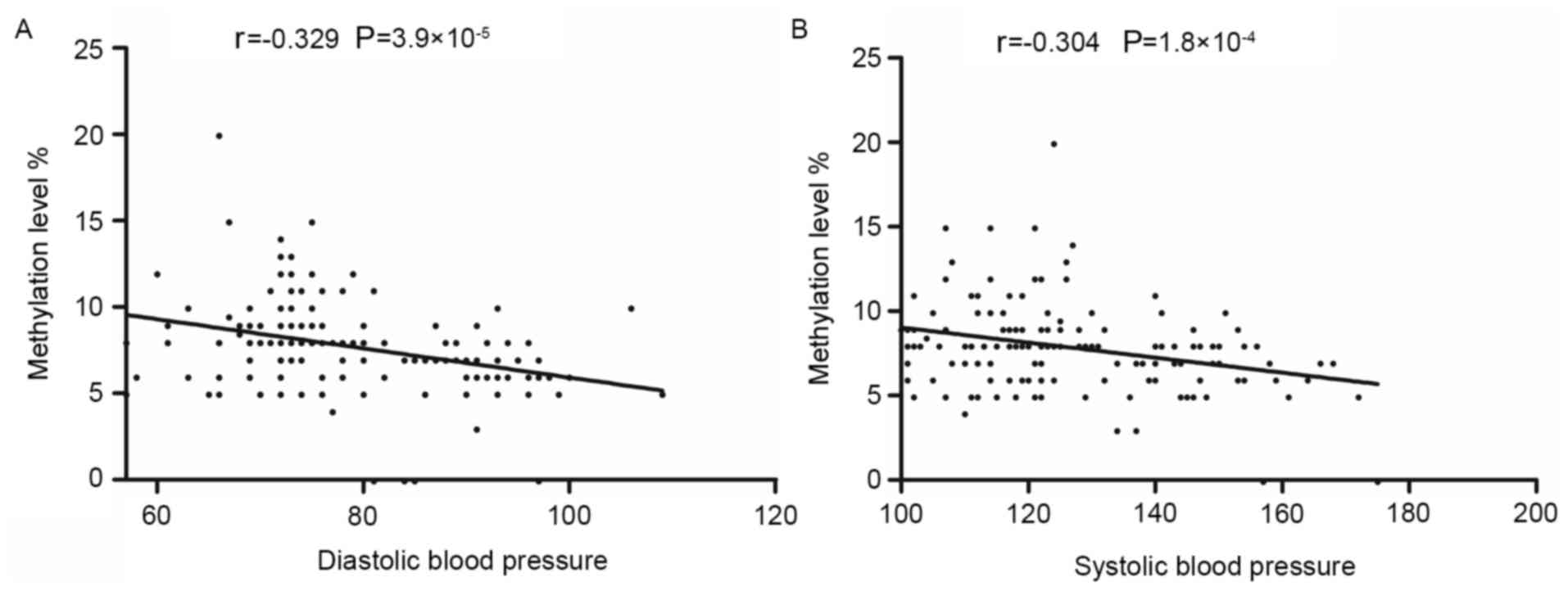
EH (OR=1.10; adjusted P=0.009). Pearson correlation analysis
demonstrated that the methylation level of CpG6 was negatively
correlated with SBP (r=−0.304; P<0.001) and DBP (r=−0.329;
P<0.001) (Fig. 3).
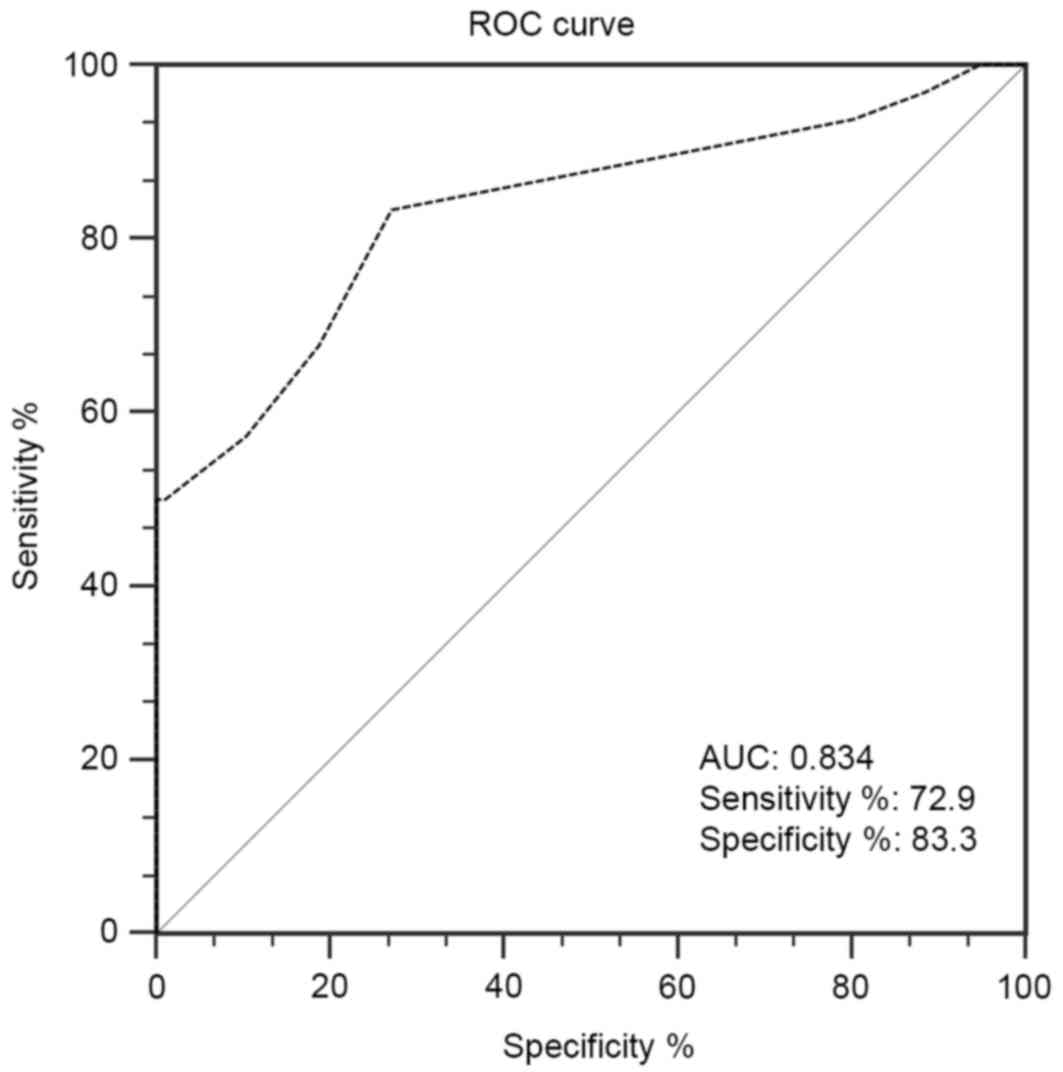
ROC curve analysis was used to analyze the
diagnostic value of CpG6 methylation to EH. The results presented
in Fig. 4 indicated that a
methylation level of 7.5% for CpG6 (area under the curve, 0.834;
P<0.001) was an appropriate threshold value to predict the risk
of EH.
GMDR was used to investigate high-order interactions
between the methylation of CpG sites of the TLR2 promoter
and other risk factors. The best models at various orders are
summarized in Table IV. The
two-order model between CpG6 and CpG8 is the best model of
gene-gene interaction (testing balanced accuracy, 0.874;
cross-validation consistency, 10/10; P=0.01), and the five-order
model among smoking, alcohol consumption, CpG6, CpG7 and CpG8 is
the best model of gene-environment interaction (testing balanced
accuracy, 0.985; cross-validation consistency, 8/10; P=0.01).
 | Table IV.Generalized multifactor
dimensionality reduction models of essential hypertension and
high-order interactions among CpG sites in the Toll-like receptor-2
promoter. |
Table IV.
Generalized multifactor
dimensionality reduction models of essential hypertension and
high-order interactions among CpG sites in the Toll-like receptor-2
promoter.
| Model | Testing balanced
accuracy | Cross-validation
consistency | Sign test
(P-value) |
|---|
| CpG8 | 0.762 | 10/10 | 10 (0.001) |
| CpG6-CpG8 | 0.874 | 10/10 | 10 (0.001) |
| CpG1-CpG6-CpG8 | 0.917 | 8/10 | 10 (0.001) |
| Alcohol
consumption-CpG6-CpG7-CpG8 | 0.945 | 7/10 | 9
(0.011) |
| Smoking-alcohol
consumption-CpG6-CpG7-CpG8 | 0.985 | 8/10 | 9
(0.011) |
Discussion
Previous studies have demonstrated that TLR2
activation may induce nicotinamide-adenine dinucleotide phosphate
oxidase to produce reactive oxygen species in monocytes and
macrophages (24–26), and endothelial TLR2 signaling may
result in inhibition of endothelial NO bioavailability (27). TLR2 dimers with TLR1 or TLR6 may
lead to the upregulation of cytokines by a MyD88/Mal and nuclear
factor (NF)-κB pathway-dependent mechanism. One such control may be
at the level of TLR expression itself. However, the underlying
molecular mechanisms remain unknown.
By investigating TLR2 gene promoter
methylation, the present study demonstrated that TLR2 gene
promoter methylation levels were decreased in patients with EH
compared with healthy controls, particularly the CpG1, CpG6 and
CpG8 sites. The hypomethylation of CpG6 hypomethylation was
demonstrated to be a risk factor of EH. Previous studies have
demonstrated that the methylation of gene promoters silences the
transcription of the genes (28–30),
and that an alteration in CpG methylation may influence gene
expression via directly interfering with transcription
factor-binding complexes or by histone modifications mediated by
methyl-CpG-binding proteins (31,32).
Therefore, the results of the present study suggest that
hypomethylation of the TLR2 gene promoter is likely to
increase the expression of the TLR2 gene and enhance
pro-inflammatory responses in EH. Shuto et al (33) have reported that promoter
hypomethylation of the TLR2 gene is associated with an
increased pro-inflammatory response and that TLR2 expression
is epigenetically upregulated in cystic fibrosis bronchial
epithelial cells. In a study of periodontitis, Benakanakere et
al (34) observed that
hypermethylation of the TLR2 promoter was able to diminish
TLR2 and pro-inflammatory cytokine expression in response to
infection with Porphyromonas gingivalis. Previous results
from DNA methylation profiles of patients with Keshan disease
compared with normal individuals demonstrated that selenium
deficiency led to decreased methylation of CpG islands in the
promoter region of TLR2, and upregulated mRNA and protein
levels of TLR2 (35). These
previous results demonstrated that hypomethylation of TLR2
promoter CpG islands was able to increase the expression of TLR2
mRNA and protein. The expression of the TLR2 gene will
impair vascular endothelial cell repair and release
pro-inflammatory cytokines via MyD88/Mal and NF-κB pathways,
including CRP, IL6 and TNF-α; these pro-inflammatory cytokines have
been reported to be associated with blood pressure (36–39).
Therefore, the hypomethylation of the TLR2 promoter may
serve a role in the development of EH by activating
pro-inflammatory responses.
In addition, Pearson correlation analysis in the
present study suggested that blood pressure was negatively
correlated with the methylation level of CpG6. The present results
further indicate that TLR2 gene promoter methylation serves
a role in the development of EH. However, the present results were
in contrast to those from a previous study (9), which reported a positive association
between TLR2 gene methylation and DBP. The disparity may be
due to different CpG sites being analyzed in the different studies.
In addition, different age ranges, race and inclusion criteria of
the samples may have led to the above discrepancy. DNA methylation
has been demonstrated to be a possible biomarker of cancer
(40,41), and the present study observed that
CpG6 methylation exhibited an appropriate threshold value to
predict the risk of EH according to the results of ROC curve
analysis. Therefore, the results of the present study may aid the
clinical diagnosis and prediction of EH.
EH is a multifactorial chronic disease; gene-gene
and gene-environment interactions contribute to its onset and
progression. GMDR is a nonparametric and genetic model-free
alternative to linear or logistic regression for detecting and
characterizing nonlinear interactions among discrete genetic and
environmental factors. GMDR is able to accommodate qualitative and
quantitative phenotypes, enhance prediction accuracy, and adjust
for discrete and continuous covariates (42); this increases the accuracy of the
analysis and means that a more meaningful conclusion may be drawn.
In the present study, a significant two-order gene-gene interaction
between CpG6 and CpG8 was observed, in addition to a significant
five-order gene-environment interaction among smoking, alcohol
consumption, CpG6, CpG7 and CpG8, which may contribute to the risk
of EH. However, the biological roles of these interactions are
unclear, and further investigation is required in future
studies.
There are certain limitations to the present study.
mRNA and protein expression were not investigated, therefore
transcriptomic regulation was not able to be demonstrated. In
addition, the analysis of eight CpG sites may not be representative
of the whole gene.
In conclusion, hypomethylation of the TLR2
gene promoter, particularly CpG6, is associated with the risk of
EH. In addition, the CpG6 site of TLR2 gene promoter
exhibits utility in the diagnosis of EH. The two-order interaction
between CpG6 and CpG8, and the five-order interaction among
smoking, drinking, CpG6, CpG7 and CpG8, may be associated with EH
risk. The present study may provide novel insights into the
pathogenesis of EH from an epigenetic aspect.
Acknowledgements
The present study was supported by the Zhejiang
Province Social Development Research Project (grant no.
2016C33178), the K.C. Wong Magna Fund in Ningbo University, Ningbo
Social Development Research Project (grant no. 2014C50051), the
Ningbo Scientific Innovation Team for Environment Hazardous Factor
Control and Prevention (grant no. 2016C51001), the Ningbo Medical
Science and Technology Plan Project (grant no. 2013A39), the
Outstanding (Postgraduate) Dissertation Growth Foundation of Ningbo
University (grant no. py2014015), and the Scientific Research
Innovation Foundation of Ningbo University (grant nos. G16097 and
G15070).
References
|
1
|
Oliveras A and de la Sierra A: Resistant
hypertension: Patient characteristics, risk factors, co-morbidities
and outcomes. J Human hypertens. 28:213–217. 2014. View Article : Google Scholar
|
|
2
|
Li D, Lv J, Liu F, Liu P, Yang X, Feng Y,
Chen G and Hao M: Hypertension burden and control in mainland
China: Analysis of nationwide data 2003–2012. Int J Cardiol.
184:637–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Virdis A, Dell'Agnello U and Taddei S:
Impact of inflammation on vascular disease in hypertension.
Maturitas. 78:179–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dinh QN, Drummond GR, Sobey CG and
Chrissobolis S: Roles of inflammation, oxidative stress, and
vascular dysfunction in hypertension. Biomed Res Int.
2014:4069602014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bautista LE, Vera LM, Arenas IA and
Gamarra G: Independent association between inflammatory markers
(C-reactive protein, interleukin-6, and TNF-alpha) and essential
hypertension. J Hum Hypertens. 19:149–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aliprantis AO, Yang RB, Weiss DS, Godowski
P and Zychlinsky A: The apoptotic signaling pathway activated by
Toll-like receptor-2. EMBO J. 19:3325–3336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Medzhitov R, Preston-Hurlburt P, Kopp E,
Stadlen A, Chen C, Ghosh S and Janeway CA Jr: MyD88 is an adaptor
protein in the hToll/IL-1 receptor family signaling pathways. Mol
Cell. 2:253–258. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Neill LA and Greene C: Signal
transduction pathways activated by the IL-1 receptor family:
Ancient signaling machinery in mammals, insects, and plants. J
Leukoc Biol. 63:650–657. 1998.PubMed/NCBI
|
|
9
|
Alexeeff SE, Baccarelli AA, Halonen J,
Coull BA, Wright RO, Tarantini L, Bollati V, Sparrow D, Vokonas P
and Schwartz J: Association between blood pressure and DNA
methylation of retrotransposons and pro-inflammatory genes. Int J
Epidemiol. 42:270–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marketou ME, Kontaraki JE, Zacharis EA,
Kochiadakis GE, Giaouzaki A, Chlouverakis G and Vardas PE: TLR2 and
TLR4 gene expression in peripheral monocytes in nondiabetic
hypertensive patients: The effect of intensive blood
pressure-lowering. J Clin Hypertens (Greenwich). 14:330–335. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dange RB, Agarwal D, Teruyama R and
Francis J: Toll-like receptor 4 inhibition within the
paraventricular nucleus attenuates blood pressure and inflammatory
response in a genetic model of hypertension. J Neuroinflammation.
12:312015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Favre J, Musette P, Douin-Echinard V,
Laude K, Henry JP, Arnal JF, Thuillez C and Richard V: Toll-like
receptors 2-deficient mice are protected against postischemic
coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol.
27:1064–1071. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mullick AE, Soldau K, Kiosses WB, Bell TA
III, Tobias PS and Curtiss LK: Increased endothelial expression of
Toll-like receptor 2 at sites of disturbed blood flow exacerbates
early atherogenic events. J Exp Med. 205:373–383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edfeldt K, Swedenborg J, Hansson GK and
Yan ZQ: Expression of toll-like receptors in human atherosclerotic
lesions: A possible pathway for plaque activation. Circulation.
105:1158–1161. 2002.PubMed/NCBI
|
|
15
|
Razin A, Webb C, Szyf M, Yisraeli J,
Rosenthal A, Naveh-Many T, Sciaky-Gallili N and Cedar H: Variations
in DNA methylation during mouse cell differentiation in vivo and in
vitro. Proc Natl Acad Sci USA. 81:2275–2279. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deaton AM and Bird A: CpG islands and the
regulation of transcription. Genes Dev. 25:1010–1022. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang LN, Liu PP, Wang L, Yuan F, Xu L,
Xin Y, Fei LJ, Zhong QL, Huang Y, Xu L, et al: Lower ADD1 gene
promoter DNA methylation increases the risk of essential
hypertension. PLoS One. 8:e634552013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan R, Mao S, Zhong F, Gong M, Yin F, Hao
L and Zhang L: Association of AGTR1 promoter methylation levels
with essential hypertension risk: A Matched Case-Control Study.
Cytogenet Genome Res. 147:95–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan R, Wang WJ, Zhong QL, Duan SW, Xu XT,
Hao LM, Zhao J and Zhang LN: Aberrant methylation of the GCK gene
body is associated with the risk of essential hypertension. Mol Med
Rep. 12:2390–2394. 2015.PubMed/NCBI
|
|
20
|
European Society of Hypertension-European
Society of Cardiology Guidelines Committee: 2003 European Society
of Hypertension-European Society of Cardiology guidelines for the
management of arterial hypertension. J Hypertens. 21:1011–1053.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perloff D, Grim C, Flack J, Frohlich ED,
Hill M, McDonald M and Morgenstern BZ: Human blood pressure
determination by sphygmomanometry. Circulation. 88:2460–2470. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bassil CF, Huang Z and Murphy SK:
Bisulfite pyrosequencing. Methods Mol Biol. 1049:95–107. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mikeska T, Felsberg J, Hewitt CA and
Dobrovic A: Analysing DNA methylation using bisulphite
pyrosequencing. Methods Mol Biol. 791:33–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beaulieu LM, Lin E, Morin KM, Tanriverdi K
and Freedman JE: Regulatory effects of TLR2 on megakaryocytic cell
function. Blood. 117:5963–5974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
West XZ, Malinin NL, Merkulova AA,
Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG and Byzova
TV: Oxidative stress induces angiogenesis by activating TLR2 with
novel endogenous ligands. Nature. 467:972–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
West AP, Brodsky IE, Rahner C, Woo DK,
Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS and
Ghosh S: TLR signalling augments macrophage bactericidal activity
through mitochondrial ROS. Nature. 472:476–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Speer T, Rohrer L, Blyszczuk P, Shroff R,
Kuschnerus K, Kränkel N, Kania G, Zewinger S, Akhmedov A, Shi Y, et
al: Abnormal high-density lipoprotein induces endothelial
dysfunction via activation of Toll-like receptor-2. Immunity.
38:754–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jones PL, Veenstra GJ, Wade PA, Vermaak D,
Kass SU, Landsberger N, Strouboulis J and Wolffe AP: Methylated DNA
and MeCP2 recruit histone deacetylase to repress transcription. Nat
Genet. 19:187–191. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morita S, Takahashi RU, Yamashita R,
Toyoda A, Horii T, Kimura M, Fujiyama A, Nakai K, Tajima S, Matoba
R, et al: Genome-wide analysis of DNA methylation and expression of
microRNAs in breast cancer cells. Int J Mol Sci. 13:8259–8272.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
31
|
Liu Y, Liu P, Yang C, Cowley AW Jr and
Liang M: Base-resolution maps of 5-methylcytosine and
5-hydroxymethylcytosine in Dahl S rats: Effect of salt and genomic
sequence. Hypertension. 63:827–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jones PA and Takai D: The role of DNA
methylation in mammalian epigenetics. Science. 293:1068–1070. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shuto T, Furuta T, Oba M, Xu H, Li JD,
Cheung J, Gruenert DC, Uehara A, Suico MA, Okiyoneda T and Kai H:
Promoter hypomethylation of Toll-like receptor-2 gene is associated
with increased proinflammatory response toward bacterial
peptidoglycan in cystic fibrosis bronchial epithelial cells. FASEB
J. 20:782–784. 2006.PubMed/NCBI
|
|
34
|
Benakanakere M, Abdolhosseini M, Hosur K,
Finoti LS and Kinane DF: TLR2 promoter hypermethylation creates
innate immune dysbiosis. J Dent Res. 94:183–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang G, Zhu Y, Dong X, Duan Z, Niu X and
Wei J: TLR2-ICAM1-Gadd45α axis mediates the epigenetic effect of
selenium on DNA methylation and gene expression in Keshan disease.
Biol Trace Elem Res. 159:69–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sung KC, Suh JY, Kim BS, Kang JH, Kim H,
Lee MH, Park JR and Kim SW: High sensitivity C-reactive protein as
an independent risk factor for essential hypertension. Am J
Hypertens. 16:429–433. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bautista LE, López-Jaramillo P, Vera LM,
Casas JP, Otero AP and Guaracao AI: Is C-reactive protein an
independent risk factor for essential hypertension? J Hypertens.
19:857–861. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chae CU, Lee RT, Rifai N and Ridker PM:
Blood pressure and inflammation in apparently healthy men.
Hypertension. 38:399–403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ito H, Ohshima A, Tsuzuki M, Ohto N, Takao
K, Hijii C, Yanagawa M, Ogasawara M and Nishioka K: Association of
serum tumour necrosis factor-alpha with serum low-density
lipoprotein-cholesterol and blood pressure in apparently healthy
Japanese women. Clin Exp Pharmacol Physiol. 28:188–192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dou CY, Fan YC, Cao CJ, Yang Y and Wang K:
Sera DNA Methylation of CDH1, DNMT3b and ESR1 Promoters as
Biomarker for the Early Diagnosis of Hepatitis B Virus-Related
Hepatocellular Carcinoma. Dig Dis Sci. 61:1130–1138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Konecny M, Markus J, Waczulikova I,
Dolesova L, Kozlova R, Repiska V, Novosadova H and Majer I: The
value of SHOX2 methylation test in peripheral blood samples used
for the differential diagnosis of lung cancer and other lung
disorders. Neoplasma. 63:246–253. 2016.PubMed/NCBI
|
|
42
|
Lou XY, Chen GB, Yan L, Ma JZ, Zhu J,
Elston RC and Li MD: A generalized combinatorial approach for
detecting gene-by-gene and gene-by-environment interactions with
application to nicotine dependence. Am J Hum Genet. 80:1125–1137.
2007. View
Article : Google Scholar : PubMed/NCBI
|