Introduction
Colorectal cancer (CRC) is a major public health
problem accounting for >1 million cases of new cancer cases
worldwide annually (1,2). Although treatment strategies have
improved in recent years, ~half a million patients still succumb to
this disease every year (3). As a
commonly used chemotherapeutic agent for CRC, oxaliplatin improves
the response rate of patients and prolongs progression-free
survival (4–6). However, oxaliplatin resistance is
still a serious problem, the underlying mechanisms of which remain
largely unknown.
Connexins (Cxs) are a large family of transmembrane
proteins that exist in 21 isoforms expressed on all human organs
and tissues. A total of six connexins compose a hemi-channel. Two
hemi-channels dock together to form an integral gap junction (GJ)
that regulates direct molecular transfer between the neighboring
cells, including calcium, glutathione, cyclic adenosine
monophosphate and cyclic guanosine monophosphate (molecules
weighing <1 kDa) (7). Molecules
transferred via GJs are essential for many physiological and
pathological events (8,9). Cx43 is the most important of the Cx
gene family, and has been reported to be associated with tumor
progression and resistance to chemotherapeutic agents; for example,
Cx43 GJ depression resulted in the resistance of temozolomide and
cisplatin to glioblastoma and lung adenocarcinoma (10,11).
Therefore, the present study investigated the effects of Cx43 on
the cytotoxicity of oxaliplatin in colon cancer cells, in order to
confer a novel basis for therapies combating drug resistance.
Comprehensive strategies in the treatment of CRC
have been developed for many years. One of the most important
components is pain relief; cancer patients are often treated with
analgesics and antineoplastic drugs concurrently to eliminate the
pain resulted by cancers or the antineoplastic therapies (12,13).
However, the influence of analgesic agents on the antitumor
activity of antineoplastic drugs has rarely been reported. The
present study investigated the influence of commonly used analgesic
agents, such as fentanyl, remifentanil and sufentanil, on the
cytotoxicity of oxaliplatin, and the underlying mechanisms.
Materials and methods
Cell lines and cell culture
The Lovo, Colo320, HCT116 and HT29 human CRC cell
lines (14) were obtained from the
American Type Culture Collection (Manassas, VA, USA). Lovo was
cultured in F-12K medium; Colo320 was cultured in RPMI-1640 medium;
and HCT116 and HT29 were cultured in McCoy's 5a medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). HeLa and
HeLa-Cx43 cells (Sun Yat-sen University, Guangzhou, China) were
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc.). All mediums were supplemented with 10%
fetal bovine serum (FBS) and 100 U/ml penicillin-streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) in a 5% CO2
incubator with 90% humidity at 37°C (Thermo Fisher Scientific,
Inc.).
Colony-forming assay
A colony-forming assay was performed to determine
cytotoxicity mediated by GJs. The cells were cultured at high- and
low-density. At high density cell culture, cells were seeded at
100,000 cells/cm2. When cells were exposed to drugs, the
cultures were confluent 80–100% and GJs were formed. At low density
cell culture, cells were seeded at 10,000 cells/cm2.
When cells were exposed to drugs, the cultures were not in contact
with each other and GJs were not formed. Cells were treated with 0,
25, 50, 75, 100 or 125 µM oxaliplatin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 24 h, and then washed with medium without
FBS, harvested by trypsinization (Invitrogen; Thermo Fisher
Scientific, Inc.), counted with a cell counting plate, diluted in
medium containing FBS and penicillin-streptomycin, and seeded into
6-well plates at a density of 100 cells/cm2. After 7
days, colony formation was assessed by staining with crystal violet
(Sigma-Aldrich; Merck KGaA). Colonies containing more than 50 cells
were scored (6,15).
Chemical treatment
Cells were pretreated with a connexin mimetic
peptide, gap26, for 1 h (300 µM; Sigma-Aldrich; Merck KGaA) prior
to other assays to inhibit Cx43 GJ function. Dimethyl sulfoxide (1
µl/ml) served as the vehicle control (Sigma-Aldrich; Merck KGaA).
All cell lines, including Lovo, Colo320, HCT116 and HT29, were
treated for 24 h with 10 nM fentanyl, 10 µM remifentanil and 0.5 nM
sufentanil, which were all obtained from Yichang Humanwell
Pharmaceutical Co., Ltd. (Yichang, China).
Parachute dye-coupling assay
Cx43 GJ function was detected by parachute
dye-coupling assay in 24-well plates. When cells grew into 80–100%
confluent, cells were randomly selected in one well for use as
donor cells. Donor cells were labeled with two different
fluorescent dyes, CM-DiI (5 µM; Invitrogen; Thermo Fisher
Scientific, Inc.) and calcein-acetoxymethyl ester (5 µM,
Invitrogen; Thermo Fisher Scientific, Inc.). CM-DiI did not spread
to the neighboring cells, but calcein-acetoxymethyl ester stained
coupled cells through Cx43 GJ. The cells were washed with medium
without FBS and penicillin-streptomycin, harvested by
trypsinization (Invitrogen; Thermo Fisher Scientific, Inc.),
counted with cell counting plate, diluted with medium containing
FBS and penicillin-streptomycin and seeded onto the receiver cells
(80–100% confluent, GJs formed) at a 1:150 donor/receiver ratio. A
total of 4 h later, GJ function was examined under a fluorescence
microscope (EclipseE800; Nikon Corporation, Tokyo, Japan). The mean
number of receiver cells containing dye around the donor cell was
counted and normalized to that of control cultures without any
treatments (7).
Western blotting
Western blotting was performed as described
previously (16,17). Cells were washed three times with
wash buffer [0.01 mol/l PBS, 0.138 mol/l NaCl, 0.02% NaN3 (pH 7.4)]
and then lysed with 0.05 ml/cm2 lysis buffer for 2 h
(Nanjing Keygen Biotech Co., Ltd., Nanjing, China) at 4°C. Samples
were centrifuged at 12,000 × g for 10 min at 4°C. Protein
concentrations were determined using the Bicinchoninic Acid method
(Nanjing Keygen Biotech Co., Ltd.). Cell lysates (25 µg) were
separated by 10% SDS-PAGE (Invitrogen; Thermo Fisher Scientific,
Inc.) and transferred onto a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were
blocked with 5% non-fat dry milk (Sigma-Aldrich; Merck KGaA) at
room temperature for 30 min. Following this, the membranes were
incubated with mouse monoclonal anti-human Cx43 (1:4,000; cat. no.
C8093; Sigma-Aldrich; Merck KGaA) and anti-β-actin (1:10,000; cat.
no. A1978; Sigma-Aldrich; Merck KGaA) antibodies overnight at 4°C.
After several washes with TBST (150 mM NaCL, 20 mM Tris-HCL, 0.05%
Tween-20), the membranes were incubated for 1 h at room temperature
with a goat polyclonal anti-mouse IgG horseradish peroxidase
(HRP)-conjugated secondary antibody (1:4,000; cat. no. M6898;
Sigma-Aldrich; Merck KGaA). Protein bands were detected with an
Enhanced Chemiluminescence system (KGP1125; Nanjing KeyGen Biotech.
Co., Ltd.) and quantified using Alpha View software version
2.2.14407 (ProteinSimple; Bio-Techne, Minneapolis, MN, USA)
(16,17).
Statistical analysis
Statistical analysis was performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). Multiple comparisons were
analyzed using one-way analysis of variance, followed by Tukey's
post hoc comparisons. P<0.05 was considered to indicate a
statistically significant difference. Data are presented as the
mean ± standard deviation.
Results
Oxaliplatin cytotoxicity varies in CRC
cells with or without Cx43 channels
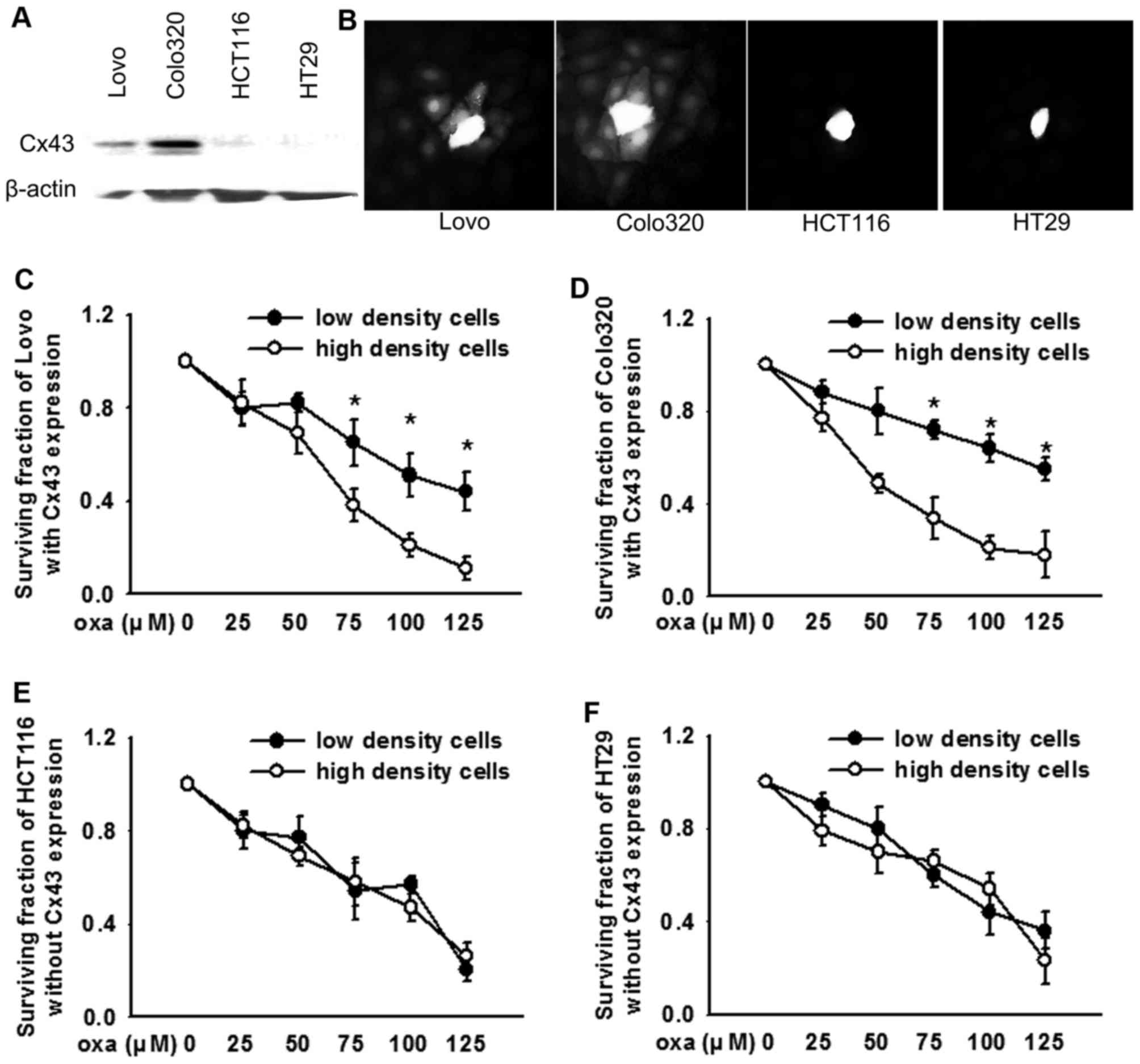
Lovo, Colo320, HCT116, HT29 CRC cells with or
without Cx43 expression were used to investigate the effects of
Cx43 channels on the cytotoxicity of oxaliplatin. The results of
the present study were consistent with a previous study (14), in that Cx43 was expressed in the
Lovo and Colo320 cell lines, but not in HCT116 or HT29 cells
(Fig. 1A). A dye coupling assay
demonstrated that Cx43 expressed on Lovo and Colo320 cells formed
functional GJs (Fig. 1B). The four
types of human CRC cell lines were cultured at low or high density
and exposed to various concentrations of oxaliplatin. The
cytotoxicity of oxaliplatin on Lovo (Fig. 1C) and Colo320 (Fig. 1D) cells at high density cell
cultures (Cx43 expressed and GJs formed) was greater compared with
low density cell cultures (Cx43 expressed, but no GJs formed). In
contrast, this density-dependent cell cytotoxicity was not
identified in HCT116 (Fig. 1E) or
HT29 (Fig. 1F) cells (Cx43 not
expressed and no GJs formed), which indicates that clonogenic
survival had no difference at high or low density cell
cultures.
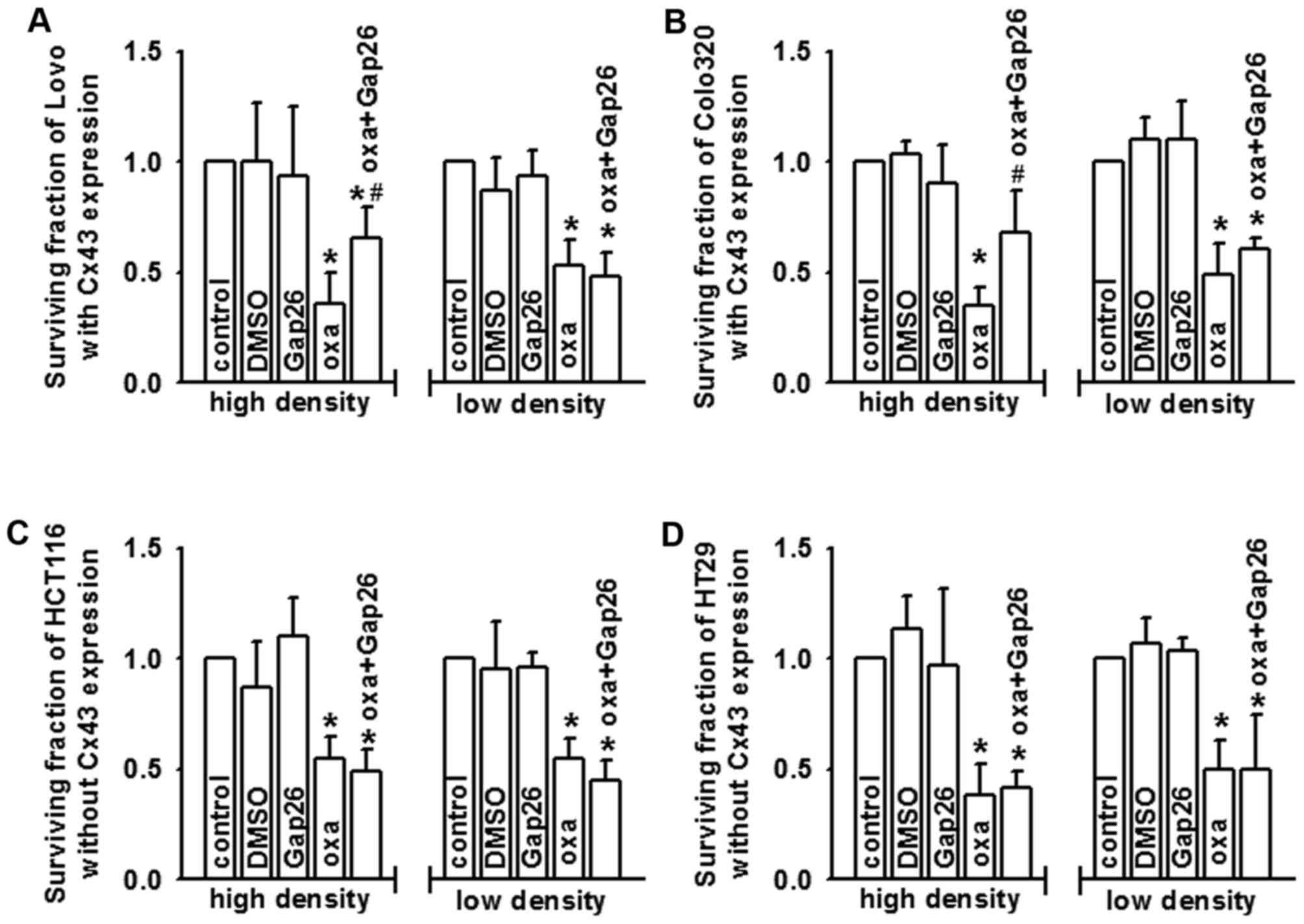
Oxaliplatin cytotoxicity is mediated
by Cx43 channels
Oxaliplatin cytotoxicity (100 µM, 24 h) was
attenuated in Lovo (Fig. 2A) and
Colo320 (Fig. 2B) cells at high
density cell culture (Cx43 expressed and GJs formed), as Cx43
channel function was suppresed by gap26, a specific inhibitor of
Cx43 channels. Although there was Cx43 expressed on Lovo and
Colo320 cells, GJs did not form at low density cell cultures
because cells made no contact with each other. Pre-treatment with
gap26 did not alter the cytotoxicity of oxaliplatin. In HCT116
(Fig. 2C) and HT29 (Fig. 2D) cells, gap26 pre-treatment had no
effects on the cytotoxicity of oxaliplatin in high or low density
cell cultures, because there was no Cx43 expressed on the two cell
lines. Therefore, Cx43 GJ function may contribute to the
cytotoxicity of oxaliplatin.
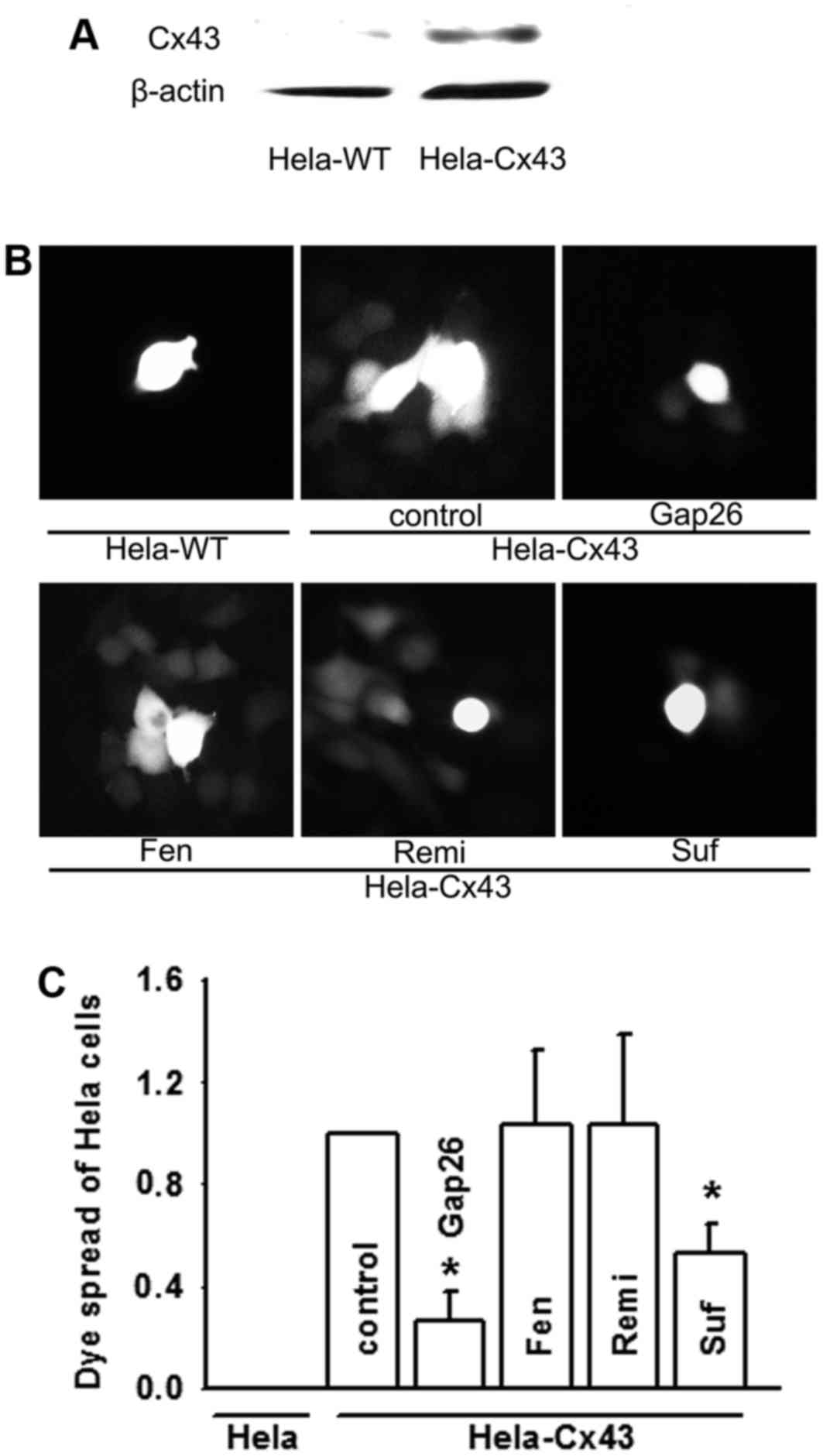
Cx43 channel function may be
attenuated by sufentanil, but not affected by fentanyl or
remifentanil
HeLa cells, without any connexin protein expression,
are frequently used to investigate connexin channel function by
transfection with connexin proteins (6). The present study observed the effects
of fentanyl, sufentanil and remifentanil on Cx43 GJ function in
HeLa cells with or without Cx43 expression (Hela-Cx43, Cx43
expressed stably; Hela-WT, no Cx43 expressed; Fig. 3A). Dye transfer between Hela-Cx43
cells was clearer compared with Hela-WT cells, which indicates that
Hela-Cx43 cells formed functional GJs (Fig. 3B and C). The dye coupling assay
also revealed that sufentanil suppressed Cx43 GJ function, and the
inhibition rate was consistent with that of the Cx43 channel
specific inhibitor, gap26. Fentanyl and remifentanil had no effects
on dye transfer mediated by GJs composed of Cx43 (Fig. 3B and C).
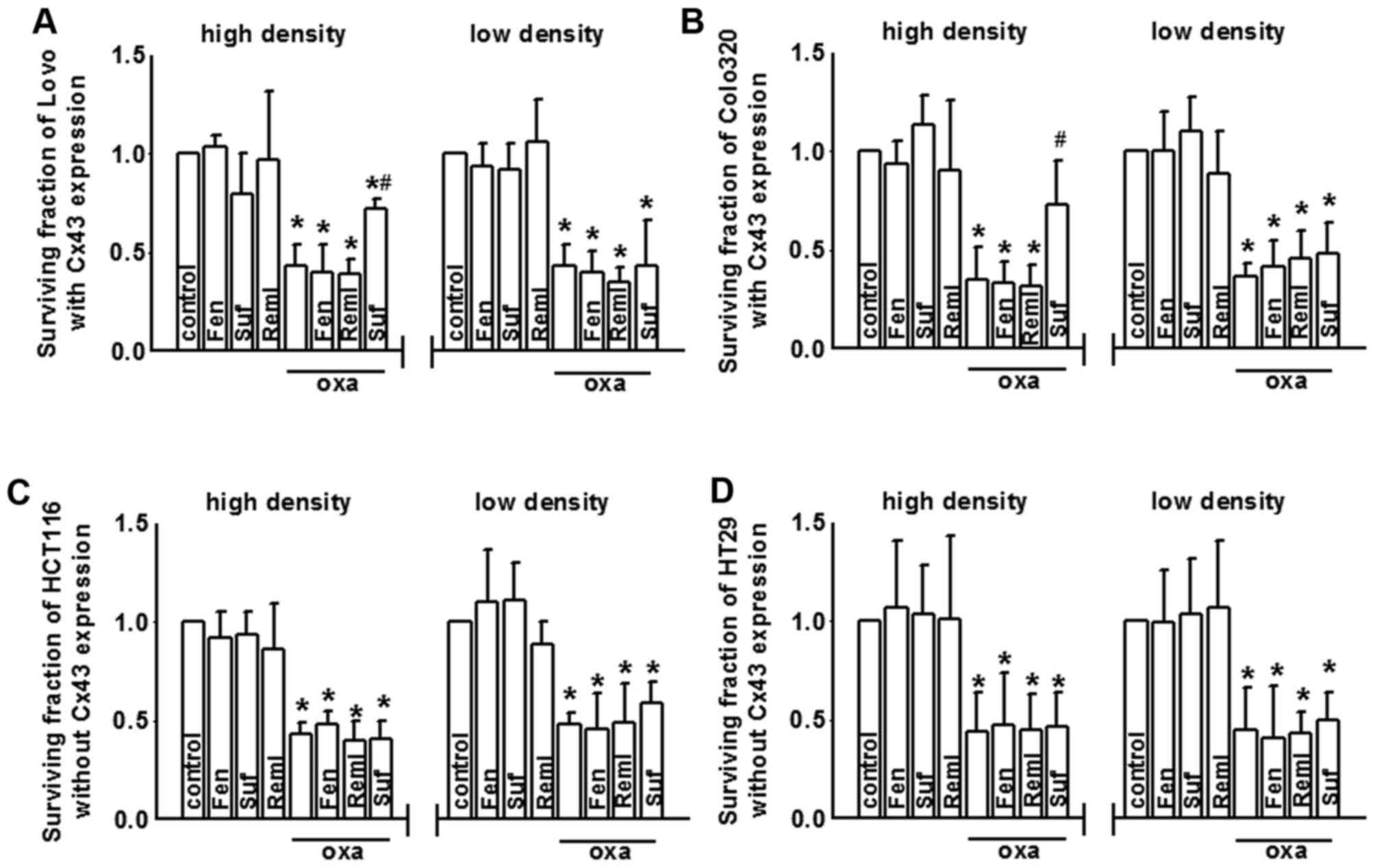
Sufentanil attenuates oxaliplatin
cytotoxicity via inhibiting Cx43 GJ function
As oxaliplatin cytotoxicity was demonstrated to be
regulated by Cx43 channels in CRC cells (Fig. 2), and as sufentanil inhibited Cx43
GJ function (Fig. 3), it was
hypothesized that sufentanil may affect oxaliplatin cytotoxicity in
colon cancer cells via altering Cx43 GJ function. Therefore, the
present study assessed oxaliplatin cytotoxicity in CRC cell lines
with or without Cx43 expression when pretreated with fentanyl,
sufentanil and remifentanil. After oxaliplatin exposure, cell
growth of the four colon cancer cell lines Lovo, Colo320, HCT116
and HT29 were all reduced at both low density and high density cell
cultures (Fig. 4A-D,
respectively). However, the effects of fentanyl, sufentanil and
remifentanil on oxaliplatin cytotoxicity in CRC cells with or
without Cx43 expression were markedly different. Fig. 4A and B indicate that sufentanil
pre-treatment attenuated oxaliplatin cytotoxicity in Lovo and
Colo320 cells at high density cell cultures (Cx43 expressed and GJs
formed), as the survival fraction increase, but had no effects at
low density cell cultures (Cx43 expressed, but functional GJs not
formed). Fentanyl and remifentanil pre-treatment did not alter the
cytotoxicity of oxaliplatin, at high density cell culture and low
density cell cultures. However, cell density-dependent cytotoxicity
was not observed in HCT116 (Fig.
4C) and HT29 (Fig. 4D) cells
without Cx43 expression. No significant differences in oxaliplatin
cytotoxicity were observed in high or low density cell cultures
when pretreated with sufentanil (Fig.
4C and D).
Discussion
The present study investigated the influence of
three analgesics, fentanyl, remifentanil and sufentanil, on
oxaliplatin cytotoxicity in various CRC cell lines with or without
Cx43 expression. The results demonstrated that in CRC cell lines
with Cx43 expression (Lovo and Colo320), oxaliplatin exerted its
effects in a cell density-dependent manner, as the survival
fraction was much lower in high density cell cultures compared with
in low density cell cultures. More importantly, sufentanil
attenuated oxaliplatin cytotoxicity by inhibiting Cx43 channel
function, but fentanyl and remifentanil had no effect. In contrast,
in CRC cell lines without Cx43 expression (HCT116 and HT29),
fentanyl, remifentanil and sufentanil had no any influence on
oxaliplatin cytotoxicity in high and low density cell cultures.
This investigation lead to the hypothesis that some analgesics
commonly used concurrently with oxaliplatin or other antineoplastic
agents in clinical settings, inhibited Cx43 GJ function and thereby
attenuated the antineoplastic efficiency of oxaliplatin for tumors
with Cx43 expression. Therefore, the choice of analgesic for
different cancer cells may impact the treatment effects of
chemotherapeutic drugs, which should be considered by
clinicians.
Until recently, CRC was one of the most important
causes of cancer-associated mortality worldwide (1,18).
Although comprehensive strategies in CRC treatment have been
improved for many years, the 5-year survival rate remains only 10%
in patients with metastases (19,20),
the most important reason for which was the development of drug
resistance during therapy (21,22).
However, the mechanisms of drug resistance remain largely unknown.
Oxaliplatin, a third-generation platinum-based antineoplastic
agent, is commonly used for CRC treatment. Its application improves
the response rate and prolongs progression-free survival of
patients with metastases. However, ~40% patients develop resistance
(23,24). The present study identified a
potential mechanism of oxaliplatin resistance, in that inhibition
of Cx43 GJ function attenuated the cytotoxicity of oxaliplatin.
Loss of Cx43 is common in the development of cancers; its
deficiency contributes to the drug resistance (25,26).
It has previously been reported that Cx43 suppression results in
temozolomide and cisplatin resistance in the treatment of
glioblastoma or lung adenocarcinoma (10). The ‘bystander effect’ mediated by
GJ is used to explain the mechanisms of drug resistance.
Chemotherapy drugs attack cancer cells directly and lead to cell
death. Notably, the attacked cells generate various toxic products,
termed ‘death signals’, which are transferred between neighboring
cells through GJs. ‘Death signals’ not only attack neighboring
cells directly, but also activate different signal pathways,
relative with cytotoxicity or apoptosis (15,27).
This effect amplifies the cytotoxicity of chemotherapy drugs. The
results of the present study supported this conclusion that
inhibition of Cx43 GJ function attenuates the cytotoxicity of
oxaliplatin. Therefore, Cx43 expression recovery may represent an
effective way to resolve drug resistance.
Notably, in the present study, a commonly used
anesthetic in clinical anesthesia and intensive care unit sedation,
sufentanil, inhibited GJ function and attenuated the cytotoxicity
of oxaliplatin in CRC lines with Cx43 expression, but fentanyl and
remifentanil had no effect. This issue should be considered by
clinicians, because all of the three analgesics are currently
extensively used for the management of pain; cancer patients are
often treated concurrently with antineoplastic drugs and
analgesics. Fentanyl, remifentanil and sufentanil interact with
opioid receptors, and remifentanil and sufentanil selectively
target the µ opioid receptor belonging to the G protein-coupled
receptor family, which is considered to be one of the most
significant protein families due to their importance as therapeutic
targets (28). G protein-coupled
receptors are involved in ligand recognition and subsequent
activation or inactivation, because of their most essential
characteristic, conformational flexibility (28,29).
Compared with fentanyl and remifentanil, sufentanil is highest
affinity agonist targeting the µ opioid receptor (30). This suggests that sufentanil and µ
opioid receptors may activate downstream signaling pathways of G
proteins, resulting in Cx43 GJ function alternation. However, this
hypothesis should be clarified in the future studies.
In conclusion, the present study demonstrated that
in CRC cells, especially with Cx43 expression, such as Lovo and
Colo320, sufentanil treatment decreased the cytotoxicity of
oxaliplatin via inhibiting GJs composed of Cx43. These results may
be beneficial for the treatment of CRC and reduction of treatment
resistance.
References
|
1
|
Lee W, Belkhiri A, Lockhart AC, Merchant
N, Glaeser H, Harris EI, Washington MK, Brunt EM, Zaika A, Kim RB
and El-Rifai W: Overexpression of OATP1B3 confers apoptotic
resistance in colon cancer. Cancer Res. 68:10315–10323. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirschi B, Gallmeier E, Ziesch A,
Marschall M and Kolligs FT: Genetic targeting of B-RafV600E affects
survival and proliferation and identifies selective agents against
BRAF-mutant colorectal cancer cells. Mol Cancer. 13:1222014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan S, Peng X, Peng W, Zhao Y and Wei Y:
Enhancement of oxaliplatin-induced cell apoptosis and tumor
suppression by 3-methyladenine in colon cancer. Oncol Lett.
9:2056–2062. 2015.PubMed/NCBI
|
|
4
|
Choi JH, Won YW, Kim HS, Oh YH, Lim S and
Kim HJ: Oxaliplatin-induced sinusoidal obstruction syndrome
mimicking metastatic colon cancer in the liver. Oncol Lett.
11:2861–2864. 2016.PubMed/NCBI
|
|
5
|
Fan F, Gray MJ, Dallas NA, Yang AD, Van
Buren G II, Camp ER and Ellis LM: Effect of chemotherapeutic stress
on induction of vascular endothelial growth factor family members
and receptors in human colorectal cancer cells. Mol Cancer Ther.
7:3064–3070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, You T, Yuan D, Han X, Hong X, He
B, Wang L, Tong X, Tao L and Harris AL: Cisplatin and oxaliplatin
inhibit gap junctional communication by direct action and by
reduction of connexin expression, thereby counteracting cytotoxic
efficacy. J Pharmacol Exp Ther. 333:903–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo C, Yuan D, Li X, Yao W, Luo G, Chi X,
Li H, Irwin MG, Xia Z and Hei Z: Propofol attenuated acute kidney
injury after orthotopic liver transplantation via inhibiting gap
junction composed of connexin 32. Anesthesiology. 122:72–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Graziano AC, Parenti R, Avola R and
Cardile V: Krabbe disease: Involvement of connexin43 in the
apoptotic effects of sphingolipid psychosine on mouse
oligodendrocyte precursors. Apoptosis. 21:25–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spagnol G, Kieken F, Kopanic JL, Li H,
Zach S, Stauch KL, Grosely R and Sorgen PL: Structural studies of
the Nedd4 WW domains and their selectivity for the connexin43
(Cx43) carboxyl terminus. J Biol Chem. 291:7637–7650. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gielen PR, Aftab Q, Ma N, Chen VC, Hong X,
Lozinsky S, Naus CC and Sin WC: Connexin43 confers Temozolomide
resistance in human glioma cells by modulating the mitochondrial
apoptosis pathway. Neuropharmacology. 75:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le HT, Sin WC, Lozinsky S, Bechberger J,
Vega JL, Guo XQ, Sáez JC and Naus CC: Gap junction intercellular
communication mediated by connexin43 in astrocytes is essential for
their resistance to oxidative stress. J Biol Chem. 289:1345–1354.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He B, Tong X, Wang L, Wang Q, Ye H, Liu B,
Hong X, Tao L and Harris AL: Tramadol and flurbiprofen depress the
cytotoxicity of cisplatin via their effects on gap junctions. Clin
Cancer Res. 15:5803–5810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myers J and Shetty N: Going beyond
efficacy: Strategies for cancer pain management. Curr Oncol.
15:(Suppl 1). S41–S49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sirnes S, Bruun J, Kolberg M, Kjenseth A,
Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E and
Rivedal E: Connexin43 acts as a colorectal cancer tumor suppressor
and predicts disease outcome. Int J Cancer. 131:570–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Liu B, Wang Q, Yuan D, Yang Y,
Hong X, Wang X and Tao L: Propofol depresses the cytotoxicity of
X-ray irradiation through inhibition of gap junctions. Anesth
Analg. 112:1088–1095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan DD, Chi XJ, Jin Y, Li X, Ge M, Gao
WL, Guan JQ, Zhang AL and Hei ZQ: Intestinal injury following liver
transplantation was mediated by TLR4/NF-κB activation-induced cell
apoptosis. Mol Med Rep. 13:1525–1532. 2016.PubMed/NCBI
|
|
17
|
Yuan D, Sun G, Zhang R, Luo C, Ge M, Luo G
and Hei Z: Connexin 43 expressed in endothelial cells modulates
monocyte-endothelial adhesion by regulating cell adhesion proteins.
Mol Med Rep. 12:7146–7152. 2015.PubMed/NCBI
|
|
18
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alcindor T and Beauger N: Oxaliplatin: A
review in the era of molecularly targeted therapy. Curr Oncol.
18:18–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Howells LM, Sale S, Sriramareddy SN,
Irving GR, Jones DJ, Ottley CJ, Pearson DG, Mann CD, Manson MM,
Berry DP, et al: Curcumin ameliorates oxaliplatin-induced
chemoresistance in HCT116 colorectal cancer cells in vitro and in
vivo. Int J Cancer. 129:476–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ekblad L, Kjellström J and Johnsson A:
Reduced drug accumulation is more important in acquired resistance
against oxaliplatin than against cisplatin in isogenic colon cancer
cells. Anticancer Drugs. 21:523–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Huang XF, Qiao L and Katsifis A:
Insulin caused drug resistance to oxaliplatin in colon cancer cell
line HT29. J Gastrointest Oncol. 2:27–33. 2011.PubMed/NCBI
|
|
23
|
Peng L, Zhu H, Wang J, Sui H, Zhang H, Jin
C, Li L, Xu T and Miao R: MiR-492 is functionally involved in
Oxaliplatin resistance in colon cancer cells LS174T via its
regulating the expression of CD147. Mol Cell Biochem. 405:73–79.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
To KK, Poon DC, Wei Y, Wang F, Lin G and
Fu LW: Data showing the circumvention of oxaliplatin resistance by
vatalanib in colon cancer. Data Brief. 7:437–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Segretain D, Decrouy X, Dompierre J,
Escalier D, Rahman N, Fiorini C, Mograbi B, Siffroi JP, Huhtaniemi
I, Fenichel P and Pointis G: Sequestration of connexin43 in the
early endosomes: An early event of Leydig cell tumor progression.
Mol Carcinog. 38:179–187. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leithe E, Sirnes S, Omori Y and Rivedal E:
Downregulation of gap junctions in cancer cells. Crit Rev Oncog.
12:225–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanson M, Marcaud V, Robin E, Valery C,
Sturtz F and Zalc B: Connexin 43-mediated bystander effect in two
rat glioma cell models. Cancer Gene Ther. 9:149–155. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fossepre M, Leherte L, Laaksonen A and
Vercauteren DP: On the modularity of the intrinsic flexibility of
the µ opioid receptor: A computational study. PloS One.
9:e1158562014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katritch V, Cherezov V and Stevens RC:
Structure-function of the G protein-coupled receptor superfamily.
Annu Rev Pharmacol Toxicol. 53:531–556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu W, Wei N, Jiang CN, Cui S and Yuan J:
Effects of sufentanil on human gastric cancer cell line SGC-7901 in
vitro. Cent Eur J Immunol. 39:299–305. 2014. View Article : Google Scholar : PubMed/NCBI
|