Introduction
Renal transplantation currently represents the best
treatment option for most patients with end-stage organ failure,
due to improved cardiovascular and mortality outcomes and quality
of life compared with dialysis (1,2).
Nevertheless, despite advances in immunosuppression, almost 35% of
renal transplant recipients have an episode of acute rejection (AR)
in the first year following transplantation (3). The presence of AR results in a 20%
reduction in the first year survival rate (3). The intensity of the rejection and the
therapeutic response may have a direct impact on the long-term
outcome of the graft. Early diagnosis is important in order to
allow appropriate treatment to be initiated (4). Histopathological examination of renal
biopsy tissue is regarded as the gold standard for diagnosing acute
rejection; however, due to an inability to repeatedly obtain
samples within short time frames to eliminate concurrent
complications, the development of a noninvasive method as a
substitute for histopathological examination is imperative
(5). A noninvasive test that could
be used for monitoring acute rejection would, therefore, be of
considerable value.
Approximately 70% of urinary proteins are generated
in the kidney, with the remainder generated in plasma (6,7),
therefore, small changes in urinary protein excretion may reflect
kidney state; previous studies have consistently revealed
significant differences between transplant recipients with acute
rejection and recipients with stable graft functions (8–10).
In addition, several urinary proteins and chemokines have been
identified as biomarkers of acute rejection following renal
transplantation, including increased levels of urinary fractalkine,
vascular endothelial growth factor, inducible protein-10 or
monokine induced by interferon γ (11,12).
Acute allograft rejection is predominantly a
Th1-driven cellular response, mediated by infiltrating lymphocytes
that produce specific cytokines and cytotoxic effector molecules,
culminating in tissue injury and ultimately graft dysfunction
(13). Recently, a new molecule
has been described as a hallmark of Th1-specific differentiated
cells, T-cell immunoglobulin and mucin domain-containing protein 3
(Tim-3; officially known as hepatitis A virus cellular receptor 2)
(14). Tim-3 is a type I membrane
protein that is preferentially expressed on terminal differentiated
Th1 cells (15). It exists as
either a membrane bound form (fTim-3) or in soluble form (sTim-3)
(16). Along with the other Tim
family members, Tim-3 is involved in several immune process,
including the development of autoimmune diseases, tolerance
induction and the regulation of Th1 immune responses (17,18).
To the best of our knowledge, there are currently no data
concerning levels of urinary sTim-3 in acute renal allograft
rejection.
In the present study, urinary sTim-3 excretion at
the time of acute rejection was evaluated and whether urinary
sTim-3 levels were associated with a response to anti-rejection
treatment. In addition, the value of this non-invasive method for
the assessment of renal allograft rejection was assessed.
Materials and methods
Patients and sample collection
Urine samples from 156 patients who received renal
transplants between June 2006 and December 2009 were examined in
our center. A total of 49 patients had biopsy-confirmed phenotypes
of acute rejection (AR) with an elevated serum creatinine level of
≥25% above baseline within 6 months of transplant, while 58
patients had stable grafts and no abnormal histological findings
(NO-AR) in protocol biopsies performed 2–3 months following
transplantation. Fresh first-morning mid-flow urine samples from
patients were collected every 2 weeks during the first 2 months
following transplantation. Urine samples were similarly collected
from the 10 patients with biopsy-proven acute tubular necrosis
(ATN), 29 patients with chronic allograft nephropathy (CAN) and 10
patients with subclinical rejection (SCR). Urine samples were not
collected on the day of biopsy. All subjects received primary
grafts from deceased donors. Detailed demographic characteristics
of these groups are summarized in Table I, with no significant differences
identified between groups (P>0.05; Table I). Patients with signs of infection
or malignant tumor were excluded from the study. Urine samples were
also collected from 40 healthy individuals as controls (average
age: 39.6±6.8 years; male/female: 24/16). Normal controls were
recruited from the medical examination center of our center. The
study was approved by the Ethics Committee of The First Affiliated
Hospital of College of Medicine of Zhejiang University (Zhejiang,
China) in accordance with the Declaration of Helsinki, and all of
the study participants provided written informed consent.
 | Table I.Demographic characteristics of
patients with renal transplants. |
Table I.
Demographic characteristics of
patients with renal transplants.
| Variable | AR (n=49) | ATN (n=10) | CAN (n=29) | SCR (n=10) | NO-AR (n=58) |
|---|
| Mean age (years) | 36.9±9.6 | 37.3±5.8 | 45.8±9.5 | 37.3±10 | 39.8±10.1 |
| Sex, [n(%)] |
|
|
|
|
|
| Male | 34 (69.4%) | 7 (70%) | 20 (68.9%) | 6 (66.7%) | 36 (62.1%) |
|
Female | 15 (30.6%) | 3 (30%) | 9 (31.1%) | 4 (33.3%) | 22 (37.9%) |
| Cause of ESRD
[n(%)] |
|
|
|
|
|
|
Glomerulonephritis | 38 (77.6%) | 8 (80%) | 24 (82.7%) | 8 (80%) | 44 (75.9%) |
|
Hypertension | 2 (4.1%) | 0 | 1 (3.4%) | 0 | 2 (3.4%) |
|
Obstructive uropathy | 1 (2.1%) | 0 | 0 | 0 | 1 (1.7%) |
|
Diabetes | 4 (8.1%) | 1 (10%) | 2 (6.9%) | 1 (10%) | 4 (6.8%) |
|
Others | 4 (8.1%) | 1 (10%) | 2 (7%) | 1 (10%) | 7 (12.1%) |
| Dialysis
time (months) | 6.5±4.1 | 5.3±2.8 | 6.3±4.7 | 5.5±6.2 | 6.9±7.1 |
| HLA
mismatch | 3.6±1.3 | 3.5±1.2 | 3.9±1.5 | 3.3±1.4 | 3.2±1.3 |
| Cold
ischaemia (h) | 8.3±1.9 | 8.6±2.0 | 8.5±2.1 | 8.4±1.8 | 8.1±1.6 |
| Panel reactive
antibody [n(%)] |
|
|
|
|
|
|
<10% | 44 (89.8%) | 9 (90%) | 26 (89.7%) | 9 (90%) | 54 (93.1%) |
|
>10% | 5 (10.2%) | 1 (10%) | 3 (10.3%) | 1 (10%) | 4 (6.9%) |
Details of immune suppressive protocols used in
Chinese renal allograft recipients have been previously reported by
this group (19). All subjects who
were recruited to participate in the present study were receiving a
combination of three immunosuppressive drugs at the time of
transplantation, composed of a calcineurin inhibitor (tacrolimus or
cyclosporine, tacrolimus: trough level 5–10 ng/ml, or cyclosporin:
Trough level 200–300 ng/ml for six months following the
transplant), prednisone (in tapering doses from 80–10 mg/day within
the first month after transplant), and azathioprine or
mycophenolatemofetil. A3 day course of intravenous
methylprednisolone (6 mg/kg) was used as anti-rejection therapy
following clinical and biopsy-proven diagnosis of acute rejection.
Steroid-resistant acute rejection (SRAR) was defined as lack of
response to steroid treatment (graft function had no improvement or
worsened) and was treated with OKT3 (5 mg/day) for 5–7 days. In
addition, plasma exchange therapy was performed in patients
diagnosed with humoral rejection. A single experienced renal
pathologist who was unaware of the results of the study used Banff
97 classification (20,21) to evaluate all biopsy specimens.
Fresh urine samples were immediately centrifuged for
10 min at 1,600 × g at 4°C, then the supernatant was aliquoted and
stored at −80°C until required. Urinary creatinine and protein were
measured for the purpose of calibration; sTim-3 levels determined
by ELISA were expressed per millimole of urinary creatinine to
correct for differences in urine concentration.
Determination of sTim-3 in urine by
ELISA
sTim-3 was measured in urine samples using a
commercial human sTim-3 ELISA kit (catalog no. T133-90; Groundwork
Biotechnology Diagnosticate, Ltd., San Diego, CA, USA). Undiluted
urine samples were tested in duplicate according to the
manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). Parameters between
groups were compared using the Mann-Whitney or Kruskal-Wallis test
and post hoc tests for nonparametric continuous data. To measure
the sensitivity and specificity of urinary sTim-3 in distinguishing
between AR and NO-AR patients, a conventional receiver operating
characteristic (ROC) curve was generated. ROC curves were also used
to determine the sensitivities and specificities for sTim-3
measurements to diagnose SCR and predict the outcome following
acute rejection. The diagnostic and predictive value of this model
was investigated by the area under the ROC curve. Youden's index,
defined as sensitivity + specificity-1, was used for the
computation of the diagnostic threshold. Results were expressed in
the text as mean ± standard error of the mean unless otherwise
stated. P<0.05 was considered to indicate a statistically
significant difference.
Results
A total of 156 patients, enrolled between January
2006 and December 2009 in our single-center, were studied,
including 49 with biopsy-proven AR. Among the 49 patients with AR,
37 were diagnosed as cellular rejection and 12 were humoral
rejection according to antibody-mediated rejection criteria. For 37
patients with cellular rejection, 20, 14 and 3 were diagnosed as
grade I, grade II and grade III, respectively. Of the remaining
patients, 58 patients had NO-AR, 10 patients had SCR in protocol
biopsy, 10 patients had biopsy-proven ATN and 29 patients had
biopsy-proven CAN. Additionally, urine samples from 40 healthy
individuals were collected as controls.
Urinary sTIM-3 in patients with stable
renal function
In the 58NO-AR patients, the level of urinary sTim-3
did not change significantly during the first 8 weeks following
transplantation, with a value of 1,732±182 ng/mmol creatinine at 2
weeks, 1,504±154.1 ng/mmol creatinine at 4 weeks, 1,515±87.2
ng/mmol creatinine at 6 weeks and 1,415±144.4 ng/mmol creatinine at
8 weeks (Fig. 1).
Urinary sTIM-3 in patients with acute
renal allograft rejection
All patients diagnosed with AR (n=49) were treated
with intravenous methylprednisolone. Among them, 18 patients with
reversible creatinine increases were classified as SSAR, while 31
patients showing no improvement following steroids were classified
as SRAR. Patients with SRAR were further treated with OKT3, plasma
exchange or both. A total of 47 patients were successfully
controlled. Anti-rejection treatment appeared to be unsuccessful in
2 patients. In these cases, graft function deteriorated rapidly,
with grafts failing within 3 months of the transplant
procedure.
A total of 31 of the 49 patients with AR also had
urine samples taken before or after the occurrence of AR. The
concentration of urinary sTim-3 in patients during the period of AR
(4,221±529.6, 95%CI: 3,123–5,266 ng/mmol creatinine) was
significantly higher than during stable renal function, both before
the occurrence of AR (1,507±229.3, 95%CI: 1,239–2,065 ng/mmol
creatinine; P<0.001) and after AR reversion (1,493±210, 95%CI:
1,267–1,960 ng/mmol creatinine; P<0.001).
Urinary sTim-3 is an indicator of
acute renal allograft rejection
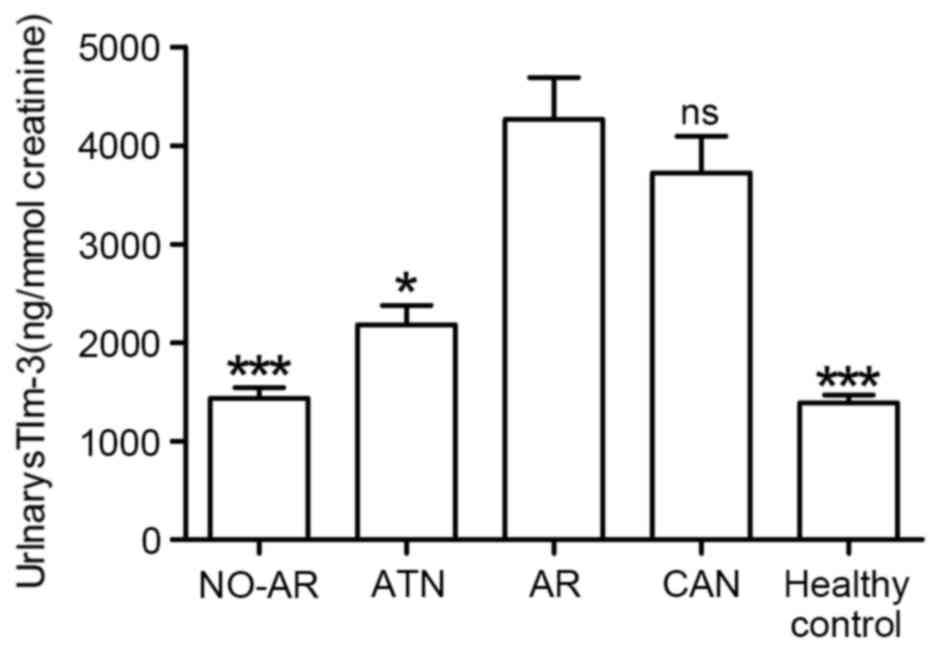
Compared with those without rejection (patients with
NO-AR, ATN or healthy controls), patients with AR had significantly
higher levels of urinary sTim-3 (Fig.
2): 4,356±440.4, 95%CI: 3,473–5,242 ng/mmol creatinine in
patients with AR; 1,430±106.2, 95%CI: 1,118–1,548 ng/mmol
creatinine in patients with NO-AR (P<0.001 vs. AR); 2,060±217,
95% CI: 1,679–2,680 ng/mmol creatinine in patients with ATN (P=0.02
vs. AR); and 1,269±99.3, 95% CI: 1,068–1,469 ng/mmol creatinine in
healthy controls (P<0.001 vs. AR).Urinary sTim-3levels in
patients with AR were also higher than in patients with CAN
(3,920±543.5, 95%CI: 3,473–5,242 ng/mmol creatinine), however, the
difference was not statistically significant (P=0.058 vs. AR;
Fig. 2). Urinary sTim-3
concentrations in patients with NO-AR and in the 40 healthy
controls were extremely similar, both remaining at relatively low
levels, as depicted in Fig. 2.
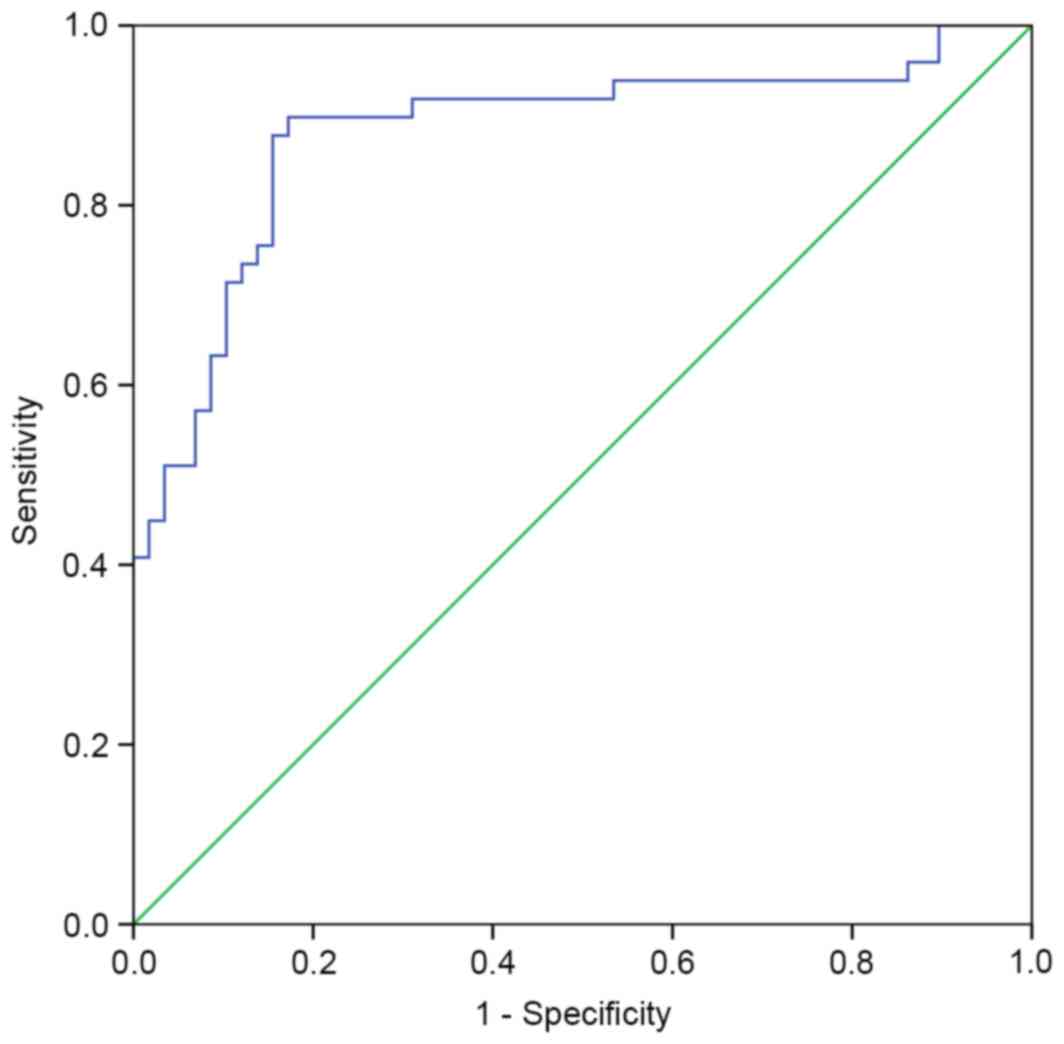
A ROC curve was performed to determine the
discriminatory power of sTim-3 levels for diagnosis of acute
rejection (Fig. 3). The area under
ROC curve was 0.88 (95% CI: 0.809–0.951). The optimal cutoff level
for sTim-3 was 1,836 ng/mmol creatinine, with a sensitivity and
specificity of 89.8 and 82.8%, respectively (P<0.001). When the
cutoff level was set at 2,764 ng/mmol creatinine, the corresponding
specificity was 91.4%.
Levels of urinary sTim-3 in cellular
and humoral rejection
Among the 49 patients with AR, 37 were diagnosed as
cellular rejection and 12 were humoral rejection according to
antibody-mediated rejection criteria. Patients with cellular
rejection excreted sTim-3 (4,010±490.7, 95% CI: 3,010–4,985 ng/mmol
creatinine) less than those with humoral rejection (5,091±961.5
ng/mmol creatinine, 95% CI: 3,012–7,210 ng/mmol creatinine), but
this effect was not statistically significant (P=0.15).
High levels of urinary-3 are
predictive of SRAR and graft loss
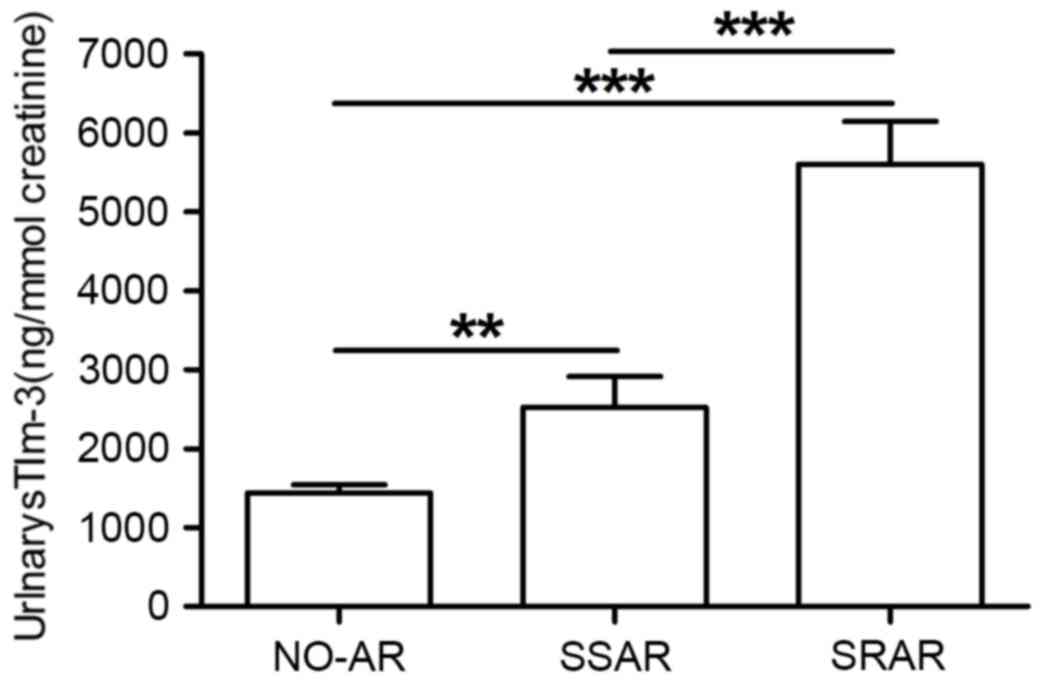
The 31 patients with SRAR had significantly greater
urinary sTim-3 concentrations (5,660±616.5, 95% CI: 4,387–6,890
ng/mmol creatinine) than the 18 patients with SSAR (2,753±386.7,
95% CI: 1,930–3,576 ng/mmol creatinine) and 58 patients with NO-AR
(P<0.001; Fig. 4). Patients
with SSAR excreted significantly more urinarys TIM-3 than NO-AR
patients (P=0.009; Fig. 4). The
ROC curve demonstrated the sensitivity and specificity of various
cutoff levels for urinarys Tim-3 to predict the incidence of SRAR
(Fig. 5). The area under the ROC
curve was 0.86 (95% CI: 0.744–0.976, P<0.001). The cutoff point
that optimized the combined sensitivity and specificity for sTim-3
was 3,242.9 ng/mmol creatinine. At this threshold, the sensitivity
was 83.9% and the specificity was 88.9% (P<0.001).
The 2 patients with graft loss appeared to have
higher urinary sTim-3 concentrations than the 47 patients with
reversible acute rejection (6,496±1,488, 95% CI: 2,981–10,065
ng/mmol creatinine vs. 4,252±445.1, 95% CI: 3,356–5,249 ng/mmol
creatinine), but the difference between these two groups was not
statistically significant (P=0.17). A negative association between
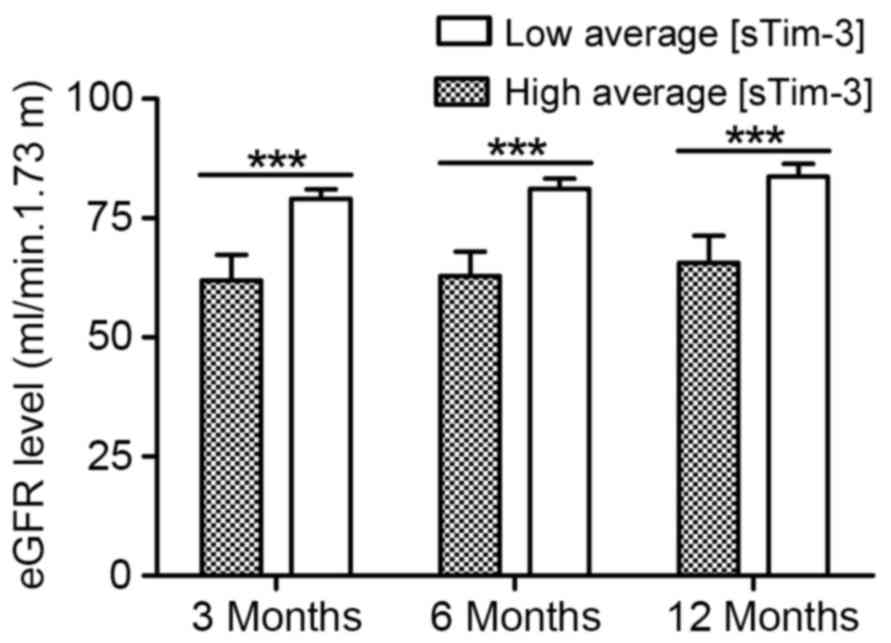
urinary sTim-3 concentration and estimated glomerular filtration
rate (eGFR) was also identified (Fig.
6). As demonstrated in Fig. 6,
patients with a high average urinary sTim-3 concentration within 2
months of transplantation (>4,000 ng/mmol creatinine; n=99) had
a significantly lower eGFR than the group with low average sTim-3
levels (<2,000 ng/mmol creatinine; n=57): 3 months after
transplantation, eGFR in the group with lower average sTim-3 was
80.2±2.2 ml/min 1.73 m2 vs. 59.4±5.9 ml/min 1.73
m2 in the group with higher sTim-3 levels (P<0.001);
85.2±2.6 ml/min 1.73 m2 in the group with lower average
sTim-3 vs. 60.9±8.6 ml/min 1.73 m2 in the group with
higher sTim-3 levels 6 months after transplantation (P<0.001);
and 86.6±3.6 ml/min 1.73 m2in the group with lower
average sTim-3 vs. 60.9±8.6 ml/min 1.73 m2 in the group
with higher sTim-3 levels 12 months after transplantation
(P<0.001).
Urinary sTim-3 is an indicator of
subclinical rejection
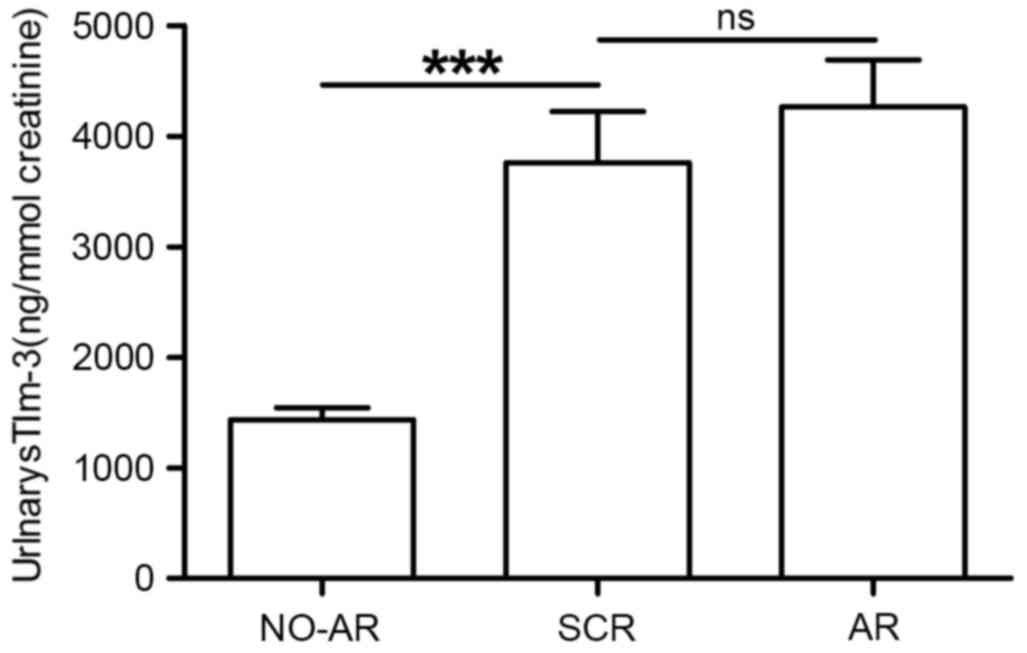
A total of 10 patients were diagnosed with
subclinical rejection (SCR) in protocol biopsy according to Banff
97 classification; urinary sTim-3 levels were determined to be
suitable as an indicator in these patients too. Patients with SCR
excreted urinary sTim-3 at a significantly higher level
(3,783±760.9, 95% CI: 2,087–5,478 ng/mmol creatinine) than patients
with NO-AR (1,430±106.2, 95% CI: 1,118–1,548 ng/mmol creatinine;
P<0.001; Fig. 7). However,
there was no significant difference in urinary sTim-3
concentrations between patients with SCR and AR (P=0.72; Fig. 7).
A ROC curve was used to present the sensitivity and
specificity of urinary sTim-3 levels as an indicator of SCR
(Fig. 8). The area under the ROC
curve was 0.934 (95% CI: 0.87–0.99; P<0.001). The cutoff point
that maximized the combined sensitivity and specificity for sTim-3
was 1,857 ng/mmol creatinine. At this threshold, the corresponding
sensitivity and specificity was 90 and 82.8%, respectively
(P<0.001).
Discussion
To the best of our knowledge, this is the first time
it has been demonstrated that the level of sTim-3 in urine may
predict the incidence of AR and the response to anti-rejection
therapy. Despite progress in immune suppression, acute allograft
rejection remains one of the biggest obstacles in renal
transplantation. At present, core needle biopsy represents the most
reliable method to diagnose renal allograft rejection, although it
is an invasive procedure with potential associated complications
for the graft and the patient. Furthermore, once AR has been
confirmed by biopsy, it is still difficult to predict therapeutic
response accurately (12). It
would be desirable to use a noninvasive technique tominimize the
frequency of using biopsy for the diagnosis of acute rejection and
to predict therapeutic response as much as possible. Some studies
have identified some cytokines, such as adhesion molecules and
chemokines (8), and reported that
utilizing these cytokines is of great value in the treatment of
renal allograft recipients (11,12).
Tim-3 is exclusively expressed on the cell surface
of fully differentiated CD4+ Th1 cells (14). The membrane bound form of Tim-3
includes an N-terminal immunoglobulin V domain, a mucin domain
followed by a transmembrane domain and a short cytoplasmic tail.
Although the soluble form (a splice variant) of Tim-3 lacks both
the mucin and transmembrane domains, it still possesses the binding
specificity of the membrane-bound form. Galectin-9 has been
identified as a ligand for Tim-3; specific binding cross-talk
between galectin-9 and Tim-3 causes an inhibitory signal, resulting
in apoptosis of Th1 cells and negatively regulated Th1 type
immunity (16). Previous studies
have also revealed that Tim-3 may participate in tolerance
induction, and blockade of this molecule exacerbates experimental
autoimmune encephalomyelitis as well as disease in the non-obese
diabetic model of Type I diabetes (16,17).
In studies using galectin-9 to modulate Tim-3 activity, the
rejection of fully allogeneic skin grafts was remarkably delayed,
by up to 6 days, in mice receiving galectin-9 (22). Furthermore, in another experimental
model, Tim-3 blockage resulted in abrogation of tolerance induction
by costimulation blockage (17). A
preliminary study also suggested that urinary Tim-3 mRNA is highly
expressed in renal acute rejection (23). However, to the best of our
knowledge, there are no data concerning the urinary sTim-3 in acute
renal allograft rejection to date.
In the present study, a significant difference was
discovered in the urinary excretion of sTim-3 between patients with
NO-AR and those with AR or ATN. Patients with NO-AR displayed a
relatively steady level of sTim-3, which generated no significant
difference in the urine at during the early period (8 weeks)
following transplantation. In view of the phenomenon that the level
of urinary sTim-3 changed along with the occurrence of AR, sTim-3
might be a promisingly suitable marker to monitor renal function
following transplantation. By non-invasive measurement of urinary
sTim-3 levels, AR could be easily differentiated from ATN, and
would be a preferred method compared with total urinary protein
(24–27). Furthermore, high levels of urinary
sTim-3 might effectively predict SRAR and graft loss. The urinary
sTim-3 levels in patients with SRAR had significantly higher
urinary sTim-3 concentrations than those with SSAR. While a cutoff
point of urinary sTim-3 concentration was set at 3,242.9 ng/mmol
creatinine, the sensitivity and specificity for the diagnosis of
SRAR reached 83.9 and 88.9%, respectively. Achieving a more
appropriate therapy by utilizing these results at an early stage
following AR may effectively helped to prevent the further
deterioration of immune injury, as well as to avoid the
side-effects of high-dose steroid treatment. Furthermore, patients
with subclinical rejection (SCR) were also demonstrated to excrete
a significantly higher level of urinary sTim-3 than patients with
NO-AR. At a urinary sTim-3 concentration of 1,857 ng/mmol
creatinine, the sensitivity and specificity for the diagnosis of
SCR was 90 and 82.8%, respectively. Therefore, by means of
measuring the level of urinary sTim-3, the probable immune state of
the patients following renal transplantation can potentially be
easily assessed and predicted. These results, of higher levels of
the potential Th1-regulatory molecule sTim-3 in patients with AR or
SCR, indicate that sTim-3 might competitively bind to the ligand of
Tim-3 with fTim-3, which may reduce the binding of fTim-3 with the
ligand of Tim-3, resulting in reduced Th1 cell apoptosis and
positive regulation of the general immune status toward Th1 type.
However, the diagnostic and predictive value of urinary Tim-3 needs
to be verified in prospective multicenter studies. Further studies
are warranted to confirm this association and to investigate the
underlying mechanism.
In conclusion, the monitoring of sTim-3 in urine may
be a novel and promising non-invasive approach for the detection of
AR. Furthermore, measurement of sTim-3 in urine may contribute to
predict the response to anti-rejection therapy and a poor outcome
following AR. Likewise, other common causes of renal dysfunction,
including polyoma virus nephritis, urinary infections and
nephrotoxicity, should also be investigated prior to it becoming a
non-invasive diagnostic test.
Acknowledgements
The study was supported by research grants from the
Projects of Medical and Health Technology Development Program in
Zhejiang province, (grant no. 2014KYA057) and the Foundation of
Zhejiang Provincial Natural Science Foundation of China (grant no.
LQ16H050003).
References
|
1
|
Curtis JJ: End-stage renal disease
patients: Referral for transplantation. J Am Soc Nephrol. 9:(12
Suppl). S137–S140. 1998.PubMed/NCBI
|
|
2
|
Karam VH, Gasquet I, Delvart V, Hiesse C,
Dorent R, Danet C, Samuel D, Charpentier B, Gandjbakhch I, Bismuth
H and Castaing D: Quality of life in adult survivors beyond 10
years after liver, kidney and heart transplantation.
Transplantation. 76:1699–1704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hariharan S, Johnson CP, Bresnahan BA,
Taranto SE, McIntosh MJ and Stablein D: Improved graft survival
after renal transplantation in the United States, 1988 to 1996. N
Engl J Med. 342:605–612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meier-Kriesche HU, Schold JD, Srinivas TR
and Kaplan B: Lack of improvement in renal allograft survival
despite a marked decrease in acute rejection rates over the most
recent era. Am J Transplant. 4:378–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beckingham IJ, Nicholson ML and Bell PR:
Analysis of factors associated with complications following renal
transplant needle core biopsy. Br Urol. 73:13–15. 1994. View Article : Google Scholar
|
|
6
|
Thongboonkerd V, Mcleish KR, Arthur JM and
Klein JB: Proteomic analysis of normal human urinary proteins
isolated by acetone precipitation or ultracentrifugation. Kidney
Int. 62:1461–1469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pieper R, Gatlin CL, Mcgrath AM, Makusky
AJ, Mondal M, Seonarain M, Field E, Schatz CR, Estock MA, Ahmed N,
et al: Characterization of the human urinary proteome: A method for
high-resolution display of urinary proteins on two-dimensional
electrophoresis gels with a yield of nearly 1400 distinct protein
spots. Proteomics. 4:1159–1174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hauser IA, Spiegler S, Kiss E, Gauer S,
Sichler O, Scheuermann EH, Ackermann H, Pfeilschifter JM, Geiger H,
Gröne HJ and Radeke HH: Prediction of acute renal allograft
rejection by urinary monokine induced by IFN-gamma (MIG). J Am Soc
Nephrol. 16:1849–1858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muthukumar T, Dadhania D, Ding R,
Snopkowski C, Naqvi R, Lee JB, Hartono C, Li B, Sharma VK, Seshan
SV, et al: Messenger RNA for FOXP3 in the urine of renal-allograft
recipients. N Engl J Med. 353:2342–2351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Riordan E, Orlova TN, Mei JJ, Butt K,
Chander PM, Rahman S, Mya M, Hu R, Momin J, Eng EW, et al:
Bioinformatic analysis of the urine proteome of acute allograft
rejection. J Am Soc Nephrol. 15:3240–3248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng W, Chen J, Jiang Y, Wu J, Shou Z, He
Q, Wang Y, Chen Y and Wang H: Urinary fractalkine is a marker of
acute rejection. Kidney Int. 74:1454–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng W, Chen J, Jiang Y, Shou Z, Chen Y
and Wang H: Acute renal allograft rejection is associated with
increased levels of vascular endothelial growth factor in the
urine. Nephrology (Carlton). 13:73–79. 2008.PubMed/NCBI
|
|
13
|
D'elios MM, Josien R, Manghetti M, Amedei
A, de Carli M, Cuturi MC, Blancho G, Buzelin F, del Prete G and
Soulillou JP: Predominant Th1 cell infiltration in acute rejection
episodes of human kidney grafts. Kidney Int. 51:1876–1884. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, et al: Th-1 specific cell surface protein TIM-3 regulates
macrophage activation and severity of an autoimmune disease.
Nature. 415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuchroo VK, Umetsu DT, DeKruyff RH and
Freeman GJ: The TIM gene family: Emerging roles in immunity and
disease. Nat Rev Immunol. 3:454–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sánchez-Fueyo A, Tian J, Picarella D,
Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T,
Kuchroo VK, et al: TIM-3 inhibits T helper type 1-mediated auto-
and alloimmune responses and promotes immunological tolerance. Nat
Immunol. 4:1093–1101. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khademi M, Illés Z, Gielen AW, Marta M,
Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris
RA, et al: T cell Ig- and mucin-domain-containing molecule-3
(TIM-3) and tim-1 molecules are differentially expressed on human
Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear
cells in multiple screrosis. J Immunol. 172:7169–7176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu JY, Chen JH, Wang YM, He Q and Wu DB:
Improved clinical outcomes in Chinese renal allograft recipients
receiving lower dose immunosuppressants. Transplantation.
78:713–718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Racusen LC, Solez K, Colvin RB, Bonsib SM,
Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo
AB, et al: The Banff 97 working classification of renal allograft
pathology. Kidney Int. 55:713–723. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Racusen LC, Colvin RB, Solez K, Mihatsch
MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ,
Fishbein MC, et al: Antibody-mediated rejection criteria-an
addition to the Banff 97 classification of renal allograft
rejection. Am J Transplant. 3:708–714. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, He W, Yuan J, Wu K, Zhou H, Zhang
W and Chen ZK: Activation of Tim-3-Galectin-9 pathway improves
survival of fully allogeneic skin grafts. Transpl Immunol.
19:12–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Renesto PG, Ponciano VC, Cenedeze MA,
Saraiva Câmara NO and Pacheco-Silva A: High expression of Tim-3
mRNA in urinary cells from kidney transplant recipients with acute
rejection. Am J Transplant. 7:1661–1665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Escobedo-Villarreal MM, Mercado-Moreira
AB, Muñoz-Espinosa LE, Gamboa-Esparza M, Pérez-Rodríguez E and
Cordero-Pérez P: Urinary protein detection by iTRAQÒ associated
with renal transplant complications and its modification with
therapy. Cir Cir. 83:393–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghys LF, Paepe D, Taffin ER, Vandermeulen
E, Duchateau L, Smets PM, Delanghe J and Daminet S: Serum and
urinary cystatin C in cats with feline immunodeficiency virus
infection and cats with hyperthyroidism. J Feline Med Surg.
18:658–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada T, Arakawa Y, Mii A, Kashiwagi T,
Kaneko T, Utsumi K, Masuda Y, Shimizu A, Iino Y and Katayama Y: A
case of monoclonal immunoglobulin G1-lambda deposition associated
with membranous feature in a patient with hepatitis C viral
infection. Clin Exp Nephrol. 16:468–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mäkelä S, Mustonen J, Ala-Houhala I, Hurme
M, Koivisto AM, Vaheri A and Pasternack A: Urinary excretion of
interleukin-6 correlates with proteinuria in acute Puumala
hantavirus-induced nephritis. Am J Kidney Dis. 43:809–816. 2004.
View Article : Google Scholar : PubMed/NCBI
|