Introduction
Ischemic cardiomyopathy (ICM) involving damage to
cardiac structure and function induced by irreversible myocardial
cell necrosis is a leading cause of morbidity and mortality
worldwide as a result of modern lifestyle (1). The currently available primary
treatments include drug interventions, percutaneous coronary
intervention (PCI), coronary artery bypass grafting (CABG) and
heart transplantation (2).
Although these methods lower the fatality rates and improve the
quality of life of patients, a number of limitations and defects
still exist. For example, CABG has been observed to lower the rates
of mortality and myocardial infarction, however, it increased the
incidence of stroke. In addition, the differences in
quality-of-life were smaller than expected as many of the patients
who were initially treated with PCI required a repeat
revascularization procedure (3).
However, regenerative medicine is continuously developing, and
transplant therapy using muscle tissue has become a primary focus
within the field. The aim of this approach is to implant
appropriate seed stem cells into specific tissue engineering
materials in vitro. Integrated mechanical, chemical and
biological signals would then be applied to stimulate and construct
functional myocardial tissue. Finally, the tissue would be
implanted into the patient to repair or replace the damaged cardiac
muscle.
Selecting the appropriate seed cells is the most
important step during the construction of myocardial tissues.
Adipose-derived mesenchymal stem cells (ASCs) have been
successfully isolated from human adipose tissue by Zuk et al
(4) using widely available
materials. The culture period is long, however, the cells produced
exhibit strong proliferation abilities. In addition, this method is
ethically approved, and the stem cells have the potential to
differentiate into multiple germ layers, which can be induced to
differentiate into cardiomyocytes directly (5,6). In
addition, ASCs exhibit the same immunosuppressive effects and
paracrine signaling abilities as bone mesenchymal stem cells
(7–9).
Stent materials and the culture microenvironment are
equally important in myocardial tissue engineering. Previous
studies have demonstrated that the spatial microstructure of stent
materials has a significant impact on the proliferation and
differentiation of seed stem cells (10,11).
The ideal stent material for tissue engineering is a natural
tissue, and the cultivation environment for cell stent planting
should be similar to the microenvironment of human myocardial
tissues in order to enhance stem cell adhesion and proliferation,
as well as their differentiation into myocardial cells (6,7).
Therefore, the concept of using extracellular matrix (ECM) in
myocardial tissue engineering has been proposed, and associated
studies have gained a great deal of attention (12–14).
Consequently, the present study compared the effect of two types of
poly-β-hydroxyethyl methacrylate (PHEMA) stents (transparent and
white PHEMA), on ASC proliferation, adhesion and their
differentiation into cardiomyocyte-like cells.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) was
purchased from Hyclone; GE Healthcare Life Sciences (Logan, UT,
USA); fetal bovine serum (FBS) was purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA); cell counting kit
(CCK)-8 solution was purchased from Yeasen Biotech (Hong Kong) Co.,
Ltd., (Hong Kong, China); the type I collagen enzyme, decitabine
(5-aza-2′-deoxycytidine) and laminin (LN) were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany); 2-hydroxyethyl
methacrylate (HEMA) was purchased from Rohm & Haas Company
(Philadelphia, PA, USA); ethylene glycol dimethacrylate (EGDMA) was
purchased from Tokyo Kasei Kogyo Co., Ltd., (Tokyo, Japan);
ammonium persulfate (APS) was purchased from Ajax Finechem; Thermo
Fisher Scientific, Inc.; N,N,N',N'-tetramethylethylenediamine
(TEMED) was purchased from Sigma-Aldrich; Merck KGaA; the GATA
binding protein 4 (Gata4; cat. no. GTX113194), NK2 homeobox 5
(Nkx2.5; cat. no. GTX133155), cardiac troponin T (cTnT; cat. no.
GTX28295), connexin-43 (Cx43; cat. no. GTX11369), myogenic
differentiation (MyoD; cat. no. GTX100885), α-smooth muscle actin
(α-SMA), desmin (cat. no. GTX103557) and β-actin (cat. no.
GTX110564) antibodies were purchased from GeneTex, Inc. (Irvine,
CA, USA); horseradish peroxidase (HRP)-conjugated goat anti-rabbit
IgG and HRP-conjugated goat anti-mouse IgG secondary antibodies
(heavy and light chain; cat. no. 106003) were purchased from
Neobioscience Technology Company (Shenzhen, China).
Preparation of the PHEMA porous
hydrogel stent and morphological analysis
As described by Lou et al (15), 1.5 ml of HEMA monomer (Rohm &
Haas Company) was injected into a small cylindrical polystyrene
mold with a diameter of 15 mm. The monomer was polymerized at 50°C
for 20 h, before the mixture was poured into a Soxhlet extractor.
Ionized water was used to elute residual monomers and oligomers for
48 h at room temperature (18–20°C). The crosslinking agent EGDMA
(Tokyo Kasegi Kogyo Co., Ltd.), the APS initiator (Ajax Finechem;
Thermo Fisher Scientific, Inc.), TEMED (Sigma-Aldrich; Merck KGaA)
and deionized water were added to the two polymer types, which were
prepared in a HEMA sponge, to conduct polymerization. To prepare
transparent PHEMA, 101.5 µl EGDMA, 80 µl APS, 40 µl TEMED and 6 g
of deionized water were added. For white PHEMA, 36.5 µl EGDMA, 80
µl APS, 40 µl TEMED and 15 g of deionized water were added. The
percentage of water in the transparent PHEMA was 29.9 and 74.8% in
white PHEMA. Morphological analysis was performed on the surface
and on cross-sections of the two polymer types using scanning
electron microscopy (magnification, ×1,000).
Advanced ASC separation and
cultivation methodology
A total of 5 female patients (age, 27±2 years) from
The First Affiliated Hospital, Sun Yat-sen University (Guangdong,
China) were enrolled in January 2012. Using the syringe negative
pressure method as described by Panfilov et al (16), 50 ml human abdominal subcutaneous
suction fat fluid was collected from the excess tissues excised
during plastic and reconstructive surgery (excessive inflation
fluid, auxiliary ultrasonic emulsification or resonance technology
were not required). A total of 50 ml phosphate-buffered saline
(PBS) was added, followed by thorough mixing and centrifugation at
1,200 × g for 10 min at 37°C. The supernatant was removed and
transferred into a fresh centrifuge tube (50 ml) with 5-fold the
volume of Collagenase I (0.075%; concentration of working liquid;
Sigma; Merck KGaA). The adipose tissue was then cut into small
pieces. The tube was subsequently sealed at 37°C and mixed at 200 ×
g for 30 min at 37°C. Digestion was terminated by adding the same
volume of DMEM containing 10% FBS and the solution was centrifuged
at 1,200 × g for 10 min at 37°C. The supernatant was removed for
incubation at room temperature for 5 min, followed by
centrifugation 1,200 × g for 5 min at 37°C. The supernatant was
removed and 10X volumes of medium was added. A nylon cell strainer
(Corning Life Sciences, Corning, NY, USA) with a pore diameter of
100 mm was used to filter the tissue block. Cells were incubated in
10-cm culture dishes (inoculation density of 30–50%) at 37°C and
95% relative humidity in culture medium (DMEM containing 10% FBS)
to a final volume of 10 ml. The medium was refreshed following 24
h, then once every 2.5 days. Cells were passaged when the cell
density reached 80–90%. When the cell density of the passage (P) 1
generation reached 80–90%, cells were cryopreserved for induction
of differentiation. P2 generation cells were used immediately for
the following experiments. All procedures were conformed to the
principles outlined in The Declaration of Helsinki. The study
protocol was approved by the Human Ethics Committee of The First
Affiliated Hospital, Sun Yat-sen University (Guangzhou, China).
Written informed consent was obtained for the collection and
utilization of tissue samples from all subjects included in the
present study.
Cell culture
Human LN (1.2 mg/ml) and decitabine (10 mmol/ml)
were added to the pores of the white and transparent PHEMA to
induce differentiation of the ASCs into myocardial cells. Following
incubation for 2 h at 37°C, PBS was used to wash the PHEMA stents.
Stent materials were not added to the remaining two pores. To each
hole, 5×104 ASCs were applied. DMEM (2 ml) containing
10% FBS were added to the blank control pores. For the inducer
group, DMEM (2 ml) containing 10 µmol/ml 5-Aza-2′-deoxycytidine and
LN (1.2 µg/ml) + 10% FBS was added the following day and incubated
for 24 h at 37°C with 90% humidity. For the stent+inducer group,
DMEM (2 ml) containing 10 µmol/ml 5-Aza-2′-deoxycytidine and LN
(1.2 µg/ml) on white PHEMA or transparent PHEMA + 10% FBS was
added. The concentration of LN and 5-Aza-2′-deoxycytidine added to
the cells was described by van Dijk et al (17).
Cell proliferation analysis
Digestive enzymes were added into the 4 sample
holes. A total of 100 µl cell solution (~3,000 cells) was added to
each hole. Following 24 h, 10 µl CCK-8 solution and 90 ml complete
medium were applied. The cells were then incubated for a further 1
h, and the absorbance was measured at 450 nm.
Test for cell adhesion
To test cell adhesion capabilities, 10 g/l bovine
serum albumin (BSA), 50 mg/l Matrigel (dilution, 1:8) or 10 mg/l
fibronectin (FN) were added into 96-well plates, with 50 µl in each
well. The cells were incubated at 4°C with 90% humidity overnight.
BSA was used as the control base. Excess liquid in the culture
plate was removed. A total of 50 µl serum-free culture medium
containing 10 g/l BSA was added into each well and incubated in a
water bath at 37°C for 30 min. A total of 4 ml of 0.25% digestive
enzymes were added into the 4 sample holes and the cell density was
adjusted to 1×105 cells/ml. The cell suspension (100 µl)
was inoculated in the coated 96-well plate; 3 parallel samples were
used for each group. A total of 10 g/l medium containing BSA was
used for control culture at 37°C for 1 h and the nutrient solution
was removed. The CCK-8 method was used to determine the absorbance
at 450 nm. With the absorbance value of adherent cells in the BSA
group as the reference, the adherence rates of the Matrigel group
and FN group were calculated. Adhesion rate was calculated using
the following formula: Adhesion rate (%) = [(ODMatrigel
group or ODFN group /ODBSA group) −1]
×100%.
Western blot analysis to determine the
direction of ASC differentiation
ASCs from the 4 sample groups were cultured for 2
weeks at 37°C with 90% humidity. The cells were lysed in lysis
buffer [150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 0.1% SDS, 1% Triton
X-100] containing protease and phosphatase inhibitors (Roche
Diagnostics, Basel, Switzerland). Cell lysate protein content was
determined using a bicinchoninic acid protein assay kit
(Sigma-Aldrich; Merck KGaA). An equal amount of whole cell
extracted protein (10 µg) was subjected to 12% SDS-PAGE gel,
transferred to PVDF membranes and blocked by non-fat milk in an
incubator (26°C, 40 × g, 2 to 4 h). The blocking mixture was then
discarded, and a hybrid solution containing primary antibodies
(GATA4, Nkx2.5, cTnT, desmin, Cx43, MyoD, α-SMA) was added and
incubated at 4°C overnight. A secondary antibody hybrid solution
(1:10,000) was added the following day, and membranes were
incubated at 26°C for 1 h (40 × g). An electrochemiluminescence kit
(Thermo Fisher Scientific, Inc.) and Kodak gel imaging system 2200
(Kodak, Rochester, NY, USA) were used to collect and analyze
images.
Statistical analysis
SPSS software (version, 13.0; SPSS, Inc., Chicago,
IL, USA) was used for data processing. Data are presented as the
mean ± standard deviation. Student's t-test was used for
comparisons between groups, and a one-way analysis of variance with
the Bonferroni post hoc test were used to compare differences among
>3 groups. P<0.05 and P<0.01 were considered to indicate
statistically significant differences.
Results
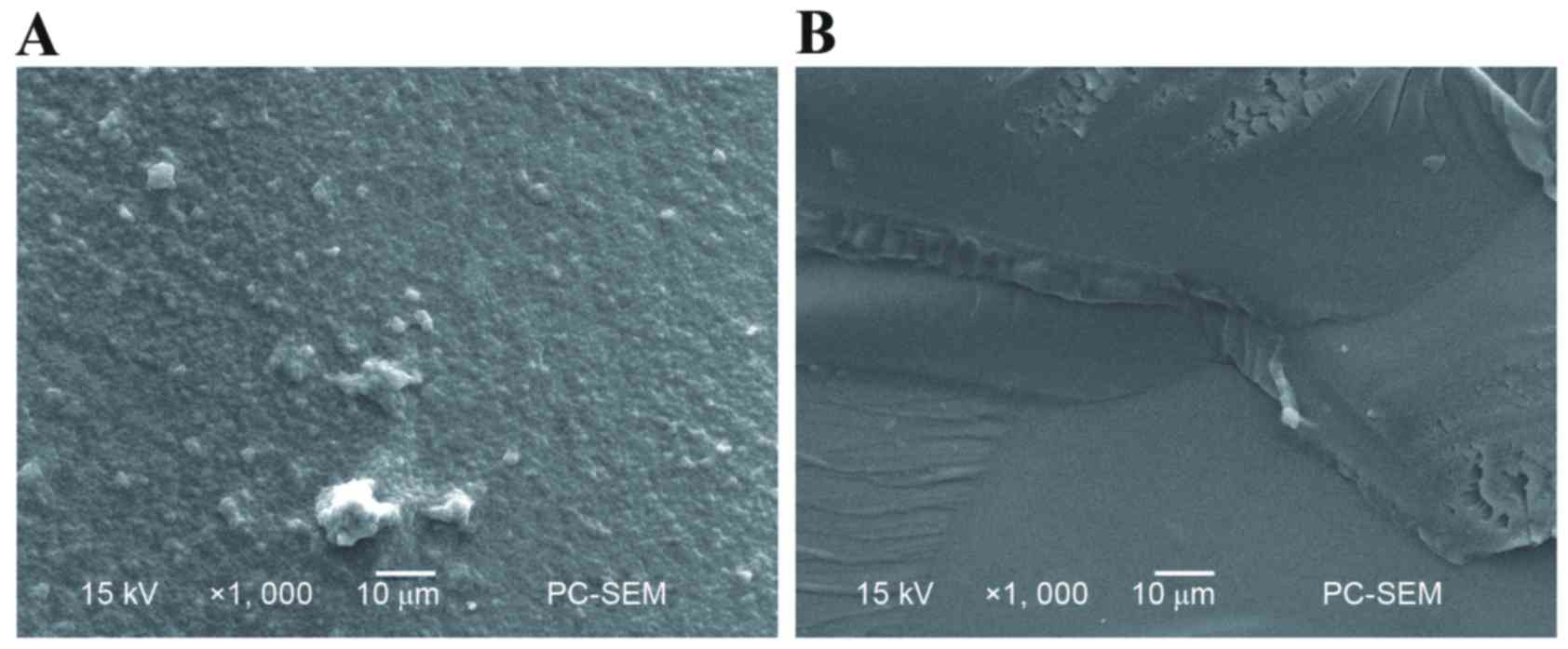
Morphological analysis of the PHEMA
polymer
The two types of polymers exhibited different
surface and cross-sectional morphological characteristics.
Differences in pore structure between white and transparent PHEMA
polymers are shown in Figs. 1 and
2. The white PHEMA is a milky
white polymer with noticeable porous structures. By contrast, the
transparent PHEMA is a little translucent and is similar to
homogeneous, non-porous hydrogels.
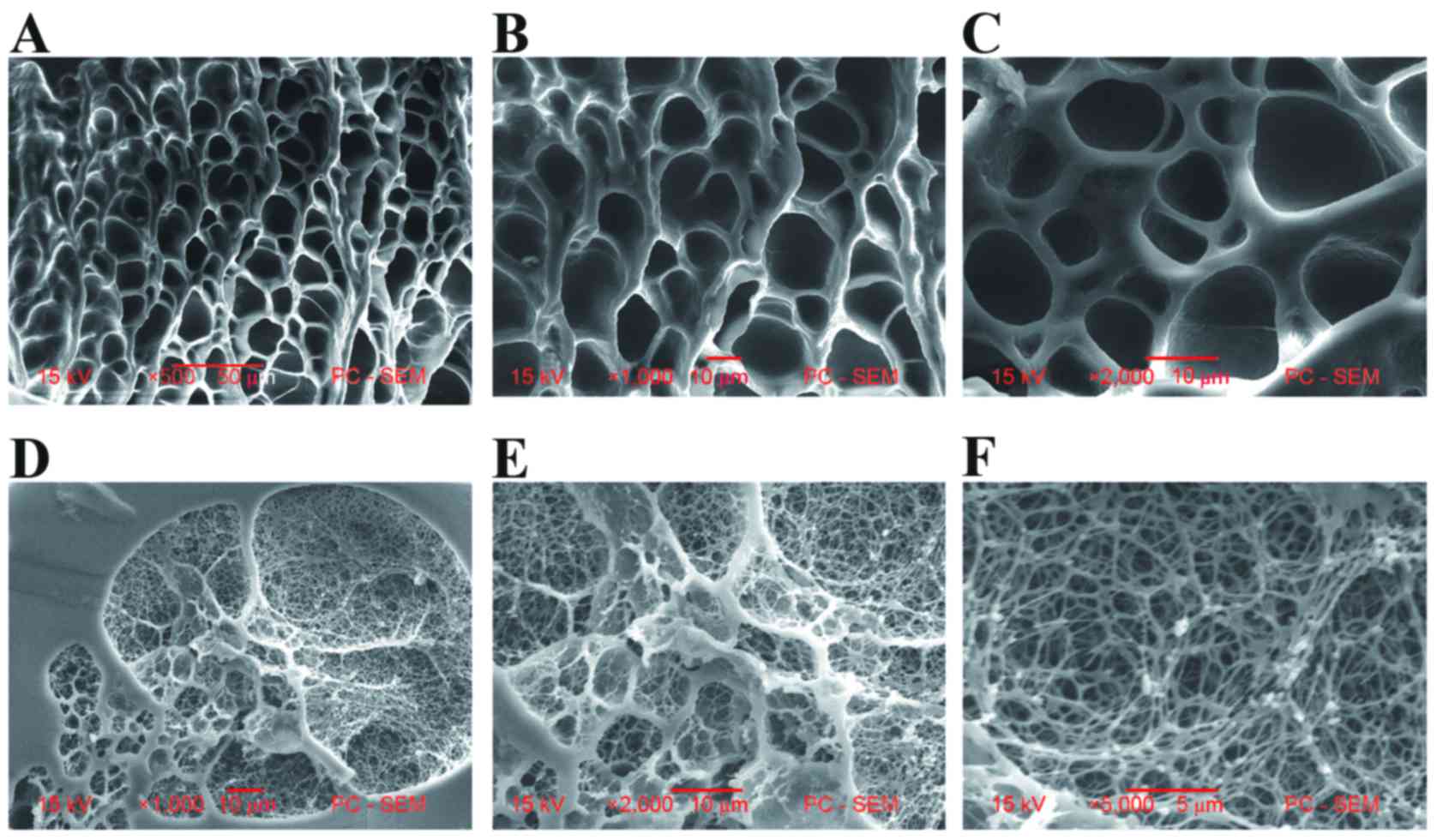 | Figure 2.Comparative electron microscope
images of the cross-sectional morphologies of white and transparent
PHEMA polymers. Images of the porous network structure of white
PHEMA polymer samples (Monomers, 25.2%; Water, 74.8%) at (A) ×500,
(B) ×1,000 and (C) ×2,000 magnifications. The porous network
structure containing nanofibers of transparent PHEMA polymer
samples (Monomers, 70.1%; Water, 29.9%) at (D) ×1,000, (E) ×2,000,
(F) ×5,000 magnifications. PHEMA, poly-β-hydroxyethyl methacrylate;
PC-SEM, personal computer-scanning electron microscopy. |
Cell proliferation analysis
Cell proliferation increased to varying extents
among all experimental groups, as determined using the CCK-8 assay
(Fig. 3). The absorbance of each
experimental group increased in a time-dependent manner (Fig. 3). When compared with the control
group, the white and transparent stent treated groups demonstrated
significantly increased proliferation rates at 48, 72 and 96 h
(P<0.05 and P<0.01; Fig. 3).
The transparent PHEMA treated group exhibited higher rates of
proliferation when compared with the white PHEMA treated group at
72 and 96 h (P<0.05). Therefore, the highest proliferation rate
was observed in the transparent PHEMA polymer group at 96 h. The
results demonstrated that, under the identical culture conditions,
inducers and material microstructures effectively promote the
proliferation and growth of ASCs. In addition, the transparent
PHEMA polymer microstructure demonstrated the greatest
proliferation promotion ability.
Cell adhesion analysis
Cells cultured on Matrigel and FN-coated surfaces
demonstrated marked differences in adherence capabilities (Table I). The transparent material group
demonstrated the greatest cell adhesion ability, which was
significantly greater when compared with the white material group
(P<0.01; Table I). The results
demonstrated that the inducers and material microstructures
effectively promoted the adhesion of ASCs, when compared with the
controls. In addition, the microstructure composed of transparent
material may present the most suitable candidate for vaccinations
of ASCs.
 | Table I.Adhesion rate of adipose stem cells
following different treatments. |
Table I.
Adhesion rate of adipose stem cells
following different treatments.
|
| Adhesion rate
(%) |
|---|
|
|
|
|---|
| Treatment
group | Matrigel | FN |
|---|
| Control group | 28.06±0.35 | 33.74±1.24 |
|
5-aza-2-deoxycytidin & LN |
36.17±1.50a |
48.36±1.35c |
|
5-aza-3-deoxycytidin & LN on white
PHEMA |
58.39±2.26a |
65.99±2.30c |
|
5-aza-4-deoxycytidin & LN on
transparent PHEMA |
72.88±1.64a,b |
78.95±1.53c,d |
Western blot analysis to determine the
direction of differentiation of the ASCs
Western blotting was used for the semi-quantitative
detection of specific proteins expressed in myocardial stem cells
and myocardial cells. Fig. 4
demonstrates that differentiated ASCs expressed the myocardial
proteins cTnT, Cx43, desmin, GATA-4 and Nkx-2.5. The results
demonstrated that a limited number of ASCs in the blank control
group, which were treated without inducers and stent materials,
appeared to have differentiated into myocardial stem cells and
myocardial cells (Fig. 4). When
compared with the blank control group, a statistically significant
increase in the expression of myocardial-specific proteins in the
inducer-treated control group was observed (P<0.05; Fig. 4). In addition, when compared to the
inducer-treated control group, a statistically significant
difference in the rate of differentiation between the two stent
material structures under the same experimental conditions was
observed (P<0.05; Fig. 4). The
results suggest that the PHEMA stent structure effectively promoted
ASCs to differentiate into myocardial cells. Compared with the
other groups, the differentiation rate was highest in the
transparent PHEMA group (Mon 70.1%, Wat 29.9%), and was
significantly different to the white PHEMA group (Mon 25.2%, Wat
74.8%; P<0.05) and the blank control group (P<0.01; Fig. 4). The percentage increase in the
expression of specific myocardial proteins relative to the controls
were as follows: GATA-4, 5.23%; Nkx-2.5, 5.66%; cTnT, 36.35%;
desmin, 42.57%; and Cx43, 5.78%. The percentage increase in the
expression of the myocyte-specific protein, MyoD and the smooth
muscle-specific protein, α-SMA were 1.03 and 1.07%, respectively,
relative to the controls. These results suggest that PHEMA stent
structures with a high number of matrixes and a low water content
may promote the differentiation of ASCs to myocardial cells.
 | Figure 4.Level of cardiomyocyte-like cell
differentiation across the 4 groups. (A) Western blotting analysis
of protein expression levels and (B) quantification of the results.
The percentage increase in the protein expression levels of
differentiation markers relative to control in the adipose-derived
stem cells in the transparent group were as follows: GATA-4, 5.23%;
Nkx-2.5, 5.66%; cTnT, 36.35%; desmin, 42.57%; Cx43, 5.78%. There
were no differences observed in the expression of MyoD and α-SMA
among groups. *P<0.05, **P<0.01 and ***P<0.001 vs. control
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. 5-aza-2′-deoxycytidine and laminin
group; $P<0.05 and $$P<0.01 vs. white
PHEMA group. Gata4, GATA binding protein 4; Nkx2.5, NK2 homeobox 5;
cTnT, cardiac troponin T; Cx43, connexin-43; MyoD, myogenic
differentiation; α-SMA, α-smooth muscle actin; PHEMA,
poly-β-hydroxyethyl methacrylate. |
Discussion
The development and application of myocardial tissue
engineering may provide a novel approach for the clinical treatment
of ICM; however, there are currently limitations with regard to the
low survival rate of stem cells and the low differentiation rate of
cardiomyocyte-like cells following transplantation of seed stem
cells (18,19). ASCs are known to be one of the most
appropriate type of seed cells for myocardial tissue engineering
(20). In order to identify
appropriate seed cells, the focus of myocardial tissue engineering
research has altered to focus on the construction of a bionic model
of myocardial ECM (21,22). This primarily uses technology to
integrate stent materials and biologically active substances, as
well as stimulate mechanical or chemical signals to create a
suitable environment for the survival of stem cells and the
differentiation of myocardial cells (23). HEMA is an artificial polymer
material used widely in the field of clinical medicine (24). It demonstrates effective
biocompatibility, degradability, and resistance to high
temperature, acid and alkali hydrolysis. In addition, HEMA
possesses a certain level of mechanical strength, elasticity and
plasticity (22,25–28).
Furthermore, the hydrogel form, comprised of the hydrophilic
polymer, serves an important role in clinical applications
including tissue regeneration, heart transplantation and skin
grafts (22,29–31).
Its three-dimensional rubber structure and high-level of water
retention are very similar to that observed in human tissues. The
present study used HEMA substrates to form PHEMA hydrogel stents.
On the one hand, the hydroxyl and carboxyl groups increase the
hydrophilic properties of the polymer, however, the hydrophobic
methyl groups and the main stem maintain the hydrolytic stability
of the polymer and support a certain degree of mechanical strength
in the matrix (32,33). In addition, the PHEMA hydrogel form
possesses an ideal porous structure. The characteristics of the
induction of photopolymerization to phase-separation may be
generated by a one-step polymerization reaction.
Previous studies have demonstrated that
5-azathioprine (5-Aza) promotes the differentiation of ASCs to
cardiomyocyte-like cells during cultivation (34,35).
Planat-Bénard et al (6) and
Rangappa et al (36)
successfully induced ASC differentiation into active myocardial
cells using 5-Aza. Decitabine is an analogue of 2′-deoxycytidine,
and demonstrates a 30-fold higher level of inhibitory activity on
DNA methylation when compared with 5-Aza (37,38).
Previous studies have indicated that two natural ECM components, LN
and FN, serve an important role in the growth and differentiation
of ectomesenchymal stem cells in vitro (39–41).
LN and FN are highly expressed in the normal myocardium following
myocardial infarction (42). Van
Dijk et al (17) confirmed
that FN enhanced the cell adhesion rate of ASCs, while LN improved
the differentiation rate of ASCs to cardiomyocyte-like cells.
Notably, the data demonstrated that under the co-induction of
decitabine and LN, the differentiation rate of ASCs to myocardial
cells was as high as 61%. A previous study has revealed that stem
cell proliferation decreases following exposure to the inducer
5-Aza (43). The present study
utilized a novel type of decitabine and LN to induce and
effectively promote the proliferation of ASCs.
The specific myocardial transcription factors,
Nkx2.5 and GATA-4, serve an important role in the early embryonic
development of the heart (44).
Bai et al (35) and
Gassanov et al (45)
revealed that Nkx2.5 induces the transcription of a series of genes
in a downstream signaling pathway, by combining the zinc finger
structure at the end of GATA-4c, thereby inducing the expression of
a large number of myocardial transcription factors (46,47).
Therefore, Nkx2.5 is able to control the original myocardial tube
formation and cyclization, as well as myocardial cell
differentiation. In addition, the expression of Nkx2.5 is one of
the earliest characteristics of the differentiation of cardiac
precursor cells. In the present study, ASCs may have differentiated
into cardiomyocyte-like cells following induction, as an
overexpression of desmin, cTnT, Cx43, Nkx2.5 and GATA4, which are
markers of cardiomyocyte-like cells, was observed. The blank
control group exhibited increased transcription factor and protein
expression of Nkx2.5 and GATA4, demonstrating that free
differentiation of ASCs to cardiomyocyte-like cells had occurred
without induction. When compared with the blank control group, the
level of differentiation in the inducer control group was
significantly different, suggesting that the inducer effectively
promotes ASC differentiation into cardiomyocyte-like cells.
Positive expression of the transcription factors, Nkx2.5 and
GATA-4, in the PHEMA groups were higher than that observed in the
control group. The results indicated that under the same
experimental conditions plus exposure to inducers and chemical
factors produced via ASC paracrine signaling mechanisms, the PHEMA
stent microstructure promotes the differentiation of ASCs into
cardiomyocyte-like cells, and the expression of cardiac
differentiation-associated transcription factors. The protein
expression levels of cardiac transcription factors were highest in
the transparent PHEMA group (Mon 70.1%, Wat 29.9%). In addition,
the expression levels of the specific myocardial proteins, cTnT,
desmin and Cx43 were highest in the transparent PHEMA group, and
the expression levels of the specific proteins MyoD and α-SMA in
myocardial stem cells were lower, suggesting that it may be easier
to produce the cardiomyocyte-like phenotype in the transparent
PHEMA microstructure. Although the western botting data was not
consistent with the high differentiation rate observed by van Dijk
et al (17), it was higher
than the positive rate observed by Gaustad et al (48).
Cx43 is one of the most important proteins present
in the gap junction channels located between mammalian ventricular
muscle cells (49). It serves an
important role in cardiac development, normal cardiac electrical
activity, heart diastolic movement and the differentiation of stem
cells to myocardial cells (50).
In addition, Cx43 has been observed to be a functional marker of
stem cell differentiation to cardiomyocyte-like cells (51). Shulz and Heusch (52) noted that the positive and negative
expression of Cx43 greatly influenced the pathophysiological
processes associated with ischemic heart disease and
atherosclerosis. Thomas et al (53) demonstrated that when the content of
Cx43 was reduced by 50%, ventricular conduction velocity was
subsequently reduced by 38%, which induces intraventricular blocks
and leads to arrhythmia and sudden death. This demonstrated that
the microstructure of transparent PHEMA may be advantageous to the
formation of Cx43 between cardiomyocyte-like cells and further
formation of electrical coupling. However, the present study failed
to observe the synchronous pulse of cardiomyocyte-like cells under
the microscope. If regular mechanical traction and electrical
stimulation that simulate diastole are used to increase ASC
mechanical signaling, it is possible that the differentiation rate
of cardiomyocyte-like cells may be greatly improved.
The present study only investigated two forms of
PHEMA stents. The most suitable proportion of matrix-to-water in
the PHEMA stent necessary for the differentiation of
cardiomyocyte-like cells requires further investigation, and will
be explored in future experiments.
In conclusion, inducers and material stent
microstructures effectively promote the proliferation, growth and
adhesion of ASCs. In addition, the transparent material
microstructure was revealed to be a more suitable candidate for ASC
vaccinations. The experiments provide additional evidence to
suggest that in PHEMA stents, a structure with a high number of
matrixes and a low water content, increases the rate of ASC
differentiation to myocardial cells.
References
|
1
|
LaPar DJ, Kron IL and Yang Z: Stem cell
therapy for ischemic heart disease: Where are we? Curr Opin Organ
Transplant. 14:79–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang TI, Shilane D, Kazi DS, Montez-Rath
ME, Hlatky MA and Winkelmayer WC: Multivessel coronary artery
bypass grafting versus percutaneous coronary intervention in ESRD.
J Am Soc Nephrol. 23:2042–2049. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdallah MS, Wang K, Magnuson EA, Spertus
JA, Farkouh ME, Fuster V and Cohen DJ: FREEDOM Trial Investigators:
Quality of life after PCI vs CABG among patients with diabetes and
multivessel coronary artery disease: A randomized clinical trial.
JAMA. 310:1581–1590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyahara Y, Nagaya N, Kataoka M, Yanagawa
B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, et
al: Monolayered mesenchymal stem cells repair scarred myocardium
after myocardial infarction. Nat Med. 12:459–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Planat-Bénard V, Menard C, André M, Puceat
M, Perez A, Garcia-Verdugo JM, Pénicaud L and Casteilla L:
Spontaneous cardiomyocyte differentiation from adipose tissue
stroma cells. Circ Res. 94:223–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McIntosh K, Zvonic S, Garrett S, Mitchell
JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms
RW, et al: The immunogenicity of human adipose-derived cells:
Temporal changes in vitro. Stem Cells. 24:1246–1253. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puissant B, Barreau C, Bourin P, Clavel C,
Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, et
al: Immunomodulatory effect of human adipose tissue-derived adult
stem cells: Comparison with bone marrow mesenchymal stem cells. Br
J Haematol. 129:118–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laflamme MA and Murry CE: Heart
regeneration. Nature. 473:326–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh D, Nayak V and Kumar A:
Proliferation of myoblast skeletal cells on three-dimensional
supermacroporous cryogels. Int J Biol Sci. 6:371–381. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin J, Jeong SI, Shin YM, Lim KS, Shin HS,
Lee YM, Koh HC and Kim KS: Transplantation of mesenchymal stem
cells within a poly(lactide-co-epsilon-caprolactone) scaffold
improves cardiac function in a rat myocardial infarction model. Eur
J Heart Fail. 11:147–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akhyari P, Kamiya H, Haverich A, Karck M
and Lichtenberg A: Myocardial tissue engineering: The extracellular
matrix. Eur J Cardiothorac Surg. 34:229–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Novakovic Vunjak G, Eschenhagen T and
Mummery C: Myocardial tissue engineering: In vitro models. Cold
Spring Harb Perspect Med. 4:pii: a014076. 2014.
|
|
14
|
Kim Y, Ko H, Kwon IK and Shin K:
Extracellular matrix revisited: Roles in tissue engineering. Int
Neurourol J. 20:(Suppl 1). S23–S29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lou X, Munro S and Wang S: Drug release
characteristics of phase separation pHEMA sponge materials.
Biomaterials. 25:5071–5080. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panfilov IA, de Jong R, Takashima S and
Duckers HJ: Clinical study using adipose-derived mesenchymal-like
stem cells in acute myocardial infarction and heart failure.
Methods Mol Biol. 1036:207–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Dijk A, Niessen HW, Ursem W, Twisk JW,
Visser FC and van Milligen FJ: Accumulation of fibronectin in the
heart after myocardial infarction: A putative stimulator of
adhesion and proliferation of adipose-derived stem cells. Cell
Tissue Res. 332:289–298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tandon N, Cannizzaro C, Chao PH, Maidhof
R, Marsano A, Au HT, Radisic M and Vunjak-Novakovic G: Electrical
stimulation systems for cardiac tissue engineering. Nat Protoc.
4:155–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann WH: Remuscularizing failing
hearts with tissue engineered myocardium. Antioxid Redox Signal.
11:2011–2023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai R, Wang Z, Samanipour R, Koo KI and
Kim K: Adipose-derived stem cells for tissue engineering and
regenerative medicine applications. Stem Cells Int.
2016:67373452016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harrison BS and Atala A: Carbon nanotube
applications for tissue engineering. Biomaterials. 28:344–353.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horák D, Hlídková H, Hradil J, Lapčíková M
and Šlouf M: Superporous poly(2-hydroxyethyl methacrylate) based
scaffolds: Preparation and characterization. Polymer. 49:2046–2054.
2008. View Article : Google Scholar
|
|
23
|
Lutolf MP and Hubbell JA: Synthetic
biomaterials as instructive extracellular microenvironments for
morphogenesis in tissue engineering. Nat Biotechnol. 23:47–55.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vijayasekaran S, Hicks CR, Chirila TV,
Fitton JH, Clayton AB, Lou X, Platten S, Crawford GJ and Constable
IJ: Histologic evaluation during healing of hydrogel core-and-skirt
keratoprostheses in the rabbit eye. Cornea. 16:352–359. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atzet S, Curtin S, Trinh P, Bryant S and
Ratner B: Degradable poly(2-hydroxyethyl
methacrylate)-co-polycaprolactone hydrogels for tissue engineering
scaffolds. Biomacromolecules. 9:3370–3377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kopeček J: Hydrogels from soft contact
lenses and implants to self-assembled nanomaterials. J Polym Sci A
Polym Chem. 47:5929–5946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chirila TV: An overview of the development
of artificial corneas with porous skirts and the use of PHEMA for
such an application. Biomaterials. 22:3311–3317. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castner DG and Ratner BD: Biomedical
surface science: Foundations to frontiers. Surface Sci. 500:28–60.
2002. View Article : Google Scholar
|
|
29
|
Lee KY and Mooney DJ: Hydrogels for tissue
engineering. Chem Rev. 101:1869–1879. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Refojo MF: Hydrophobic interaction in
poly(2-hydroxyethyl methacrylate) homogeneous hydrogel. J Polym Sci
A1. 5:3103–3113. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosiak JM and Yoshii F: Hydrogels and
their medical applications. Nuclear Instruments Methods Physics Res
Section B: Beam Interactions Materials Atoms. 151:56–64. 1999.
View Article : Google Scholar
|
|
32
|
Poncin-Epaillard F, Vrlinic T, Debarnot D,
Mozetic M, Coudreuse A, Legeay G, El Moualij B and Zorzi W: Surface
treatment of polymeric materials controlling the adhesion of
biomolecules. J Funct Biomater. 3:528–543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thevenot P, Hu W and Tang L: Surface
chemistry influence implant biocompatibility. Curr Top Med Chem.
8:270–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schäffler A and Büchler C: Concise review:
Adipose tissue-derived stromal cells-basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bai X, Pinkernell K, Song YH, Nabzdyk C,
Reiser J and Alt E: Genetically selected stem cells from human
adipose tissue express cardiac markers. Biochem Biophys Res Commun.
353:665–671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rangappa S, Fen C, Lee EH, Bongso A and
Sim EK: Transformation of adult mesenchymal stem cells isolated
from the fatty tissue into cardiomyocytes. Ann Thorac Surg.
75:775–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang DZ, Gai LY, Liu HW, Jin QH, Huang JH
and Zhu XY: Transplantation of autologous adipose-derived stem
cells ameliorates cardiac function in rabbits with myocardial
infarction. Chin Med J (Engl). 120:300–307. 2007.PubMed/NCBI
|
|
38
|
Burlacu A: Can 5-azacytidine convert the
adult stem cells into cardiomyocytes? A brief overview. Arch
Physiol Biochem. 112:260–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Christman KL, Fok HH, Sievers RE, Fang Q
and Lee RJ: Fibrin glue alone and skeletal myoblasts in a fibrin
scaffold preserve cardiac function after myocardial infarction.
Tissue Eng. 10:403–409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chastain SR, Kundu AK, Dhar S, Calvert JW
and Putnam AJ: Adhesion of mesenchymal stem cells to polymer
scaffolds occurs via distinct ECM ligands and controls their
osteogenic differentiation. J Biomed Mater Res A. 78:73–85. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Malek S, Kaplan E, Wang JF, Ke Q, Rana JS,
Chen Y, Rahim BG, Li M, Huang Q, Xiao YF, et al: Successful
implantation of intravenously administered stem cells correlates
with severity of inflammation in murine myocarditis. Pflugers Arch.
452:268–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
French KM, Maxwell JT, Bhutani S,
Ghosh-Choudhary S, Fierro MJ, Johnson TD, Christman KL, Taylor WR
and Davis ME: Fibronectin and cyclic strain improve cardiac
progenitor cell regenerative potential in vitro. Stem Cells Int.
2016:83643822016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi S, Wu X, Wang X, Hao W, Miao H, Zhen L
and Nie S: Differentiation of bone marrow mesenchymal stem cells to
cardiomyocyte-like cells is regulated by the combined low dose
treatment of transforming growth factor-β1 and 5-azacytidine. Stem
Cells Int. 2016:38162562016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kado M, Lee JK, Hidaka K, Miwa K, Murohara
T, Kasai K, Saga S, Morisaki T, Ueda Y and Kodama I: Paracrine
factors of vascular endothelial cells facilitate cardiomyocyte
differentiation of mouse embryonic stem cells. Biochem Biophys Res
Commun. 377:413–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gassanov N, Devost D, Danalache B, Noiseux
N, Jankowski M, Zingg HH and Gutkowska J: Functional activity of
the carboxyl-terminally extended oxytocin precursor Peptide during
cardiac differentiation of embryonic stem cells. Stem Cells.
26:45–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kasahara H, Bartunkova S, Schinke M,
Tanaka M and Izumo S: Cardiac and extracardiac expression of
Csx/Nkx2.5 homeodomain protein. Circ Res. 82:936–946. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu C, Police S, Rao N and Carpenter MK:
Characterization and enrichment of cardiomyocytes derived from
human embryonic stem cells. Circ Res. 91:501–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gaustad KG, Boquest AC, Anderson BE,
Gerdes AM and Collas P: Differentiation of human adipose tissue
stem cells using extracts of rat cardiomyocytes. Biochem Biophys
Res Commun. 314:420–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bloor DJ, Wilson Y, Kibschull M, Traub O,
Leese HJ, Winterhager E and Kimber SJ: Expression of connexins in
human preimplantation embryos in vitro. Reprod Biol Endocrinol.
2:252004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Souders CA, Bowers SL and Baudino TA:
Cardiac fibroblast: The renaissance cell. Circ Res. 105:1164–1176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Szaraz P, Librach M, Maghen L, Iqbal F,
Barretto TA, Kenigsberg S, Gauthier-Fisher A and Librach CL: In
vitro differentiation of first trimester human umbilical cord
perivascular cells into contracting cardiomyocyte-like cells. Stem
Cells Int. 2016:75132522016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schulz R and Heusch G: Connexin 43 and
ischemic preconditioning. Cardiovasc Res. 62:335–344. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thomas SA, Schuessler RB, Berul CI,
Beardslee MA, Beyer EC, Mendelsohn ME and Saffitz JE: Disparate
effects of deficient expression of connexin43 on atrial and
ventricular conduction: Evidence for chamber-specific molecular
determinants of conduction. Circulation. 97:686–691. 1998.
View Article : Google Scholar : PubMed/NCBI
|