Introduction
Cervical cancer is the third most common type of
malignant tumor and the fourth leading cause of cancer-associated
mortality among women worldwide (1). Up to 35% of patients with locally
advanced cervical cancer previously treated with surgery or
radiation develop persistent, recurrent or metastatic disease,
whereas platinum-based chemotherapy represents the gold standard
for treatment (2). It is well
known that human papilloma virus (HPV) infection is essential in
cervical carcinogenesis (3), but
HPV infection alone is not sufficient to transform epithelial host
cells into cancer cells. Therefore, other factors, including the
upregulation of oncogenes and aberrant activation of associated
signaling pathways, may be involved in cervical carcinogenesis. A
number of previous studies have found that activation of the
canonical Wnt/β-catenin pathway is necessary and sufficient to
induce transformation, and is closely associated with the
tumorigenesis and progression of cervical cancer (1,2,4).
Pomegranate (Punica granatum), an ancient
fruit known to offer beneficial effects in medicine, is now being
recognized as a potential chemopreventive and anticancer agent.
Increasing evidence has confirmed the cancer preventive efficacy of
pomegranate in vitro and in in vivo animal models,
including breast, skin, prostate, lung and colon cancers (5–11).
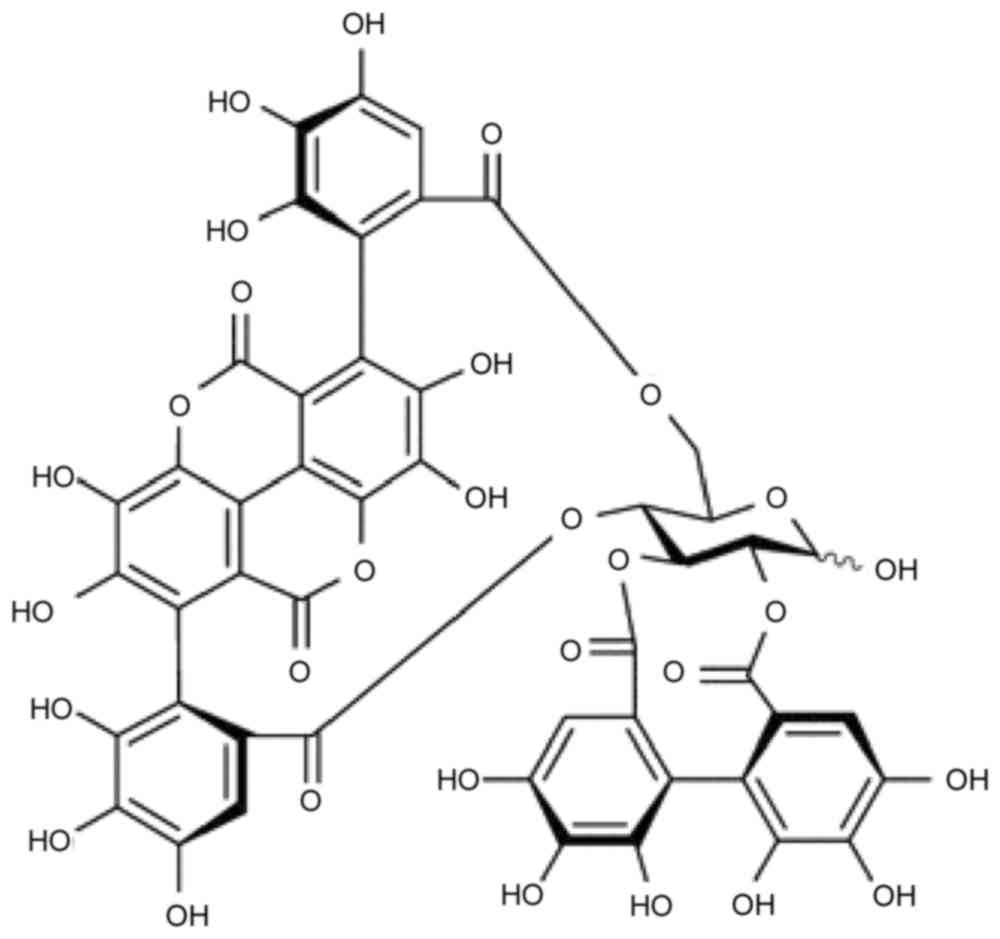
Punicalagin (2,3-hexahydroxydiphenoyl-gallagyl-D-glucose; PUN;
Fig. 1) is the major bioactive
component of pomegranate peel, and it has been shown to have
antioxidant, anti-inflammatory, antiviral, antiproliferation and
anticancer properties (6,12–18).
PUN has been shown to induce apoptosis in HL-60 human promyelocytic
leukemia cells, HT-29 and HCT116 colon cancer lines, Caco-2 colon
adenocarcinoma cells and U87MG glioma cells (8,18,19).
Previous investigations have revealed that PUN has effects on
various tumor cell lines, including upregulating the expression
levels of B-cell lymphoma-2 (Bcl-2)-associated X protein (Bax)
(7,10), Bcl-2-associated death promoter
(10), cleaved poly (ADP-ribose)
polymerase (PARP) (7,18,19)
and cytochrome c (8);
promoting the activation of caspase-3 (8) and caspase-9 (18); and downregulating the expression of
Bcl-2 (7,18), Bcl-extra large (XL) (7,8,10)
and cell cycle proteins, including cyclin A (8,18),
cyclin B1 (8,18), cyclin D1, cyclin D2 and cyclin E
(7), in addition to regulating the
proliferation and apoptosis of cancer cells.
In the present study, the effect of PUN on HeLa
cervical carcinoma cells was investigated. It was demonstrated that
PUN induced HeLa cell apoptosis, inhibited the Wnt/β-catenin
signaling pathway, caused G1/S phase transition arrest and altered
the expression of apoptosis-associated proteins. In addition, PUN
suppressed the invasion ability of HeLa cells through inhibiting
cell migration and altering the protein expression levels of tissue
inhibitor of metalloproteinase (TIMP)-2 and TIMP-3, and the
activities of matrix metalloproteinase (MMP)-2 and MMP-9.
Materials and methods
Chemical and reagents
The HeLa cells were obtained from the China Center
for Type Culture Collection (Wuhan, China). PUN (>98% HPLC
purity) was purchased from Must Bio-Tech Co., Ltd. (Chengdu,
China). A Cell Counting kit-8 (CCK)-8 was purchased from
MultiSciences Biotech Co., Ltd. (Hangzhou, China). Dulbecco's
modified Eagle's medium (DMEM) and phosphate-buffered saline (PBS)
were purchased from Jenom Biotech Co., Ltd. (Hangzhou, China).
Trypsin/EDTA solution and fetal bovine serum (FBS) were purchased
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
bicinchoninic acid (BCA) protein assay kit and cell cycle analysis
kit were purchased from Beyotime Institute of Biotechnology
(Suzhou, China). The MMP Gelatin Zymography Assay kit was purchased
from Applygen Technologies, Inc. (Beijing, China). Antibodies
against β-catenin (ab32572), c-myc (ab32072), cyclin D1 (ab134175),
Bcl-2 (ab59348), Bax (ab32503), β-actin (ab8227) and TIMP-3
(ab39184) were obtained from Abcam (Cambridge, UK). Antibodies
against TIMP-2 (sc21735) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
The HeLa cells were cultured in DMEM supplemented
with heat-inactivated 10% FBS and 1% antibiotics (100 IU penicillin
and 100 µg/ml streptomycin) in a humidified incubator at 37°C and
5% CO2. Logarithmically growing cells were used in all
the subsequent experiments.
Cell viability assay
The cells were seeded into 96-well plates at a
density of 5,000 cells/well and incubated for 24 h. The cells were
then treated with PUN (0, 12.5, 25, 50, 100 and 200 µM) for another
24, 36 and 48 h at 37°C with 5% CO2. Following
treatment, cell viability was measured using the CCK-8 method, in
which 10 µl of CCK-8 reagent was added and the cells were placed
into an incubator at 37°C with 5% CO2 for ~2 h. Finally,
the optical density at 450 nm was detected using a PerkinElmer
Victor3 1420 Multilabel Counter (PerkinElmer, Inc., Waltham, MA,
USA). Optical density values were employed to represent cell
viability.
Cell cycle analysis
The cells were seeded into 6-well plates at a
density of 2×105 cells/well for 24 h prior to cell cycle analysis.
Following synchronization and treatment for another 36 h with PUN
(0, 25, 50 and 100 µM), the cells were harvested and then fixed
with precooled 70% ethanol at 4°C overnight. The fixed cells were
washed with PBS and then stained using the cell cycle analysis kit
for 30 min at 4°C. The stained cells were determined using a
FACSCalibur system (BD Biosciences, Franklin Lakes, NJ, USA) to
examine cell cycle distribution, following which ModFit LT version
4.1 (Verity Software House, Inc., Topsham, ME, USA) was used for
data analysis.
Wound healing assay
The HeLa cells were seeded in a 2-cm Petri dish at a
concentration of 1×105 cells/ml and grown overnight. A wound was
then made in the cell culture by scratching on the cell layer with
a sharp tip, followed by incubation for a further 36 h with PUN (0
and 50 µM) in serum-free medium. The gap created by the wound in
the treated or untreated cells was then measured under a microscope
(CKX31; Olympus Corporation, Tokyo, Japan) to provide an indication
of the wound-healing capability of the cells.
MMP gelatin zymography
Following treatment with PUN (0, 25, 50 and 100 µM)
for 36 h, the culture medium was collected. Protein was extracted
with RIPA and analyzed using a BCA assay, then mixed with equal
volumes of 2X non-reduced loading buffer and 30 µg of total protein
was electrophoresed on 10% SDS-polyacrylamide gels containing 1
mg/ml gelatin as a protease substrate. The SDS was removed through
incubation in 2.5% Triton X-100 for 30 min, and the gels were
incubated in 20 mM glycine (pH 8.3), 10 mM CaCl2 and 1
µM ZnCl2 at 37°C overnight. The gels were stained with
Coomassie Blue to visualize zones of gelatinolytic activity with an
Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE,
USA). Gelatinase-dependent proteolysis was detected as a clear area
in a light-blue field. Following scanning of the experiment
results; Quantity One graphic analysis software version 4.6.2
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to perform
analysis of gray levels on the specific bands.
Western blot analysis
The cells were digested from the plates following
treatment with PUN (0, 25, 50 and 100 µM) for 36 h, following which
total protein was extracted from the HeLa cells using RIPA buffer
containing PMSF. To measure protein concentrations, a BCA assay kit
was used, according to the manufacturer's protocol. Following the
addition of protein loading buffer [200 mM of DTT, 40 mM of
Tris/HCl, 40% glycerol, 4% SDS (pH 6.8) and 0.032% bromophenol
blue] and denaturing at 95°C for 5 min, 30 µg of total protein was
separated from the samples by 10% SDS-polyacrylamide gel
electrophoresis and then transferred onto activated PVDF membranes.
Following blocking in 5% skimmed milk at 37°C for 1 h, the
membranes were blotted with appropriate primary antibodies at 4°C
overnight, followed by incubation with fluorescence-labeled
secondary antibodies (goat anti-mouse/rabbit IRDye700 and IRDye800;
C40109-04; 1:10,000; LI-COR Biosciences) for 1 h at 37°C. The
primary antibodies were as follows: Anti-β-catenin (1:5,000),
anti-c-myc (1:10,000), anti-cyclin D1 (1:10,000), anti-Bcl-2
(1:200), anti-Bax (1:1,000), anti-β-actin (1:1,000), anti-TIMP-2
(1:200) and anti-TIMP-3 (1:1,000). Signals were detected using an
Odyssey infrared imaging system (LI-COR Biosciences).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM SPSS, Armonk, NY, USA) and data are presented as the mean
± standard deviation. The data were subjected to one-way analysis
of variance. Differences between two groups were determined using
Dunnett test, and multiple means were compared using Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PUN decreases the viability of HeLa
cells
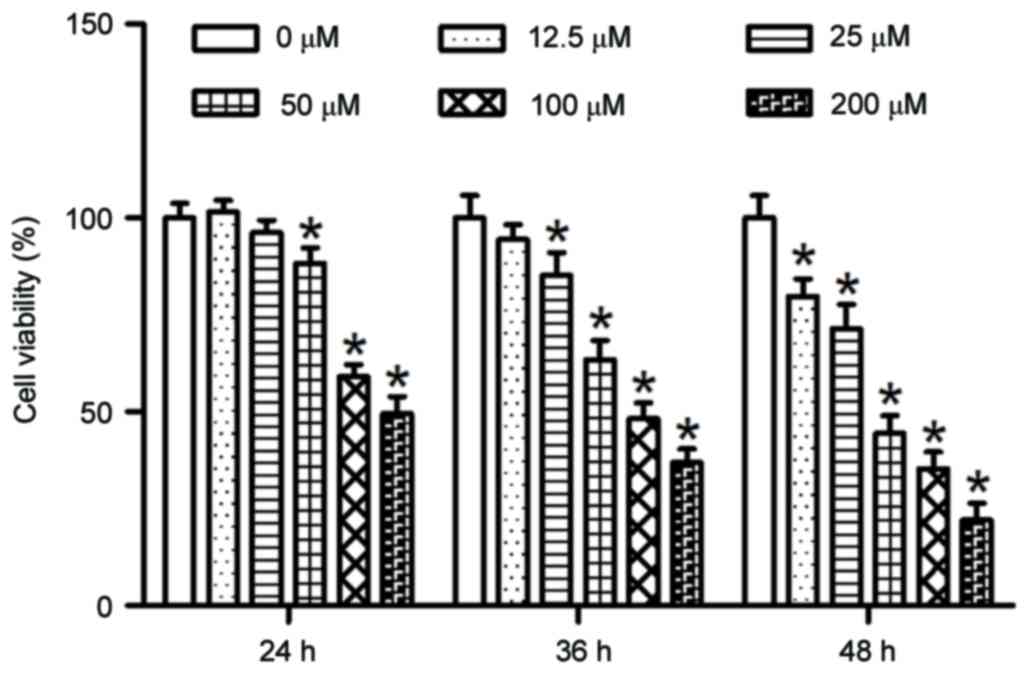
In order to evaluate the effects of PUN on cell
growth, HeLa cells were treated with increasing concentrations of
PUN (0, 12.5, 25, 50, 100 and 200 µM) for various durations (24, 36
and 48 h) and the viability of the cells was assessed using CCK-8
assay. As shown in Fig. 2,
following PUN treatment, the viability of the HeLa cells was
significantly decreased in a dose- and time-dependent manner.
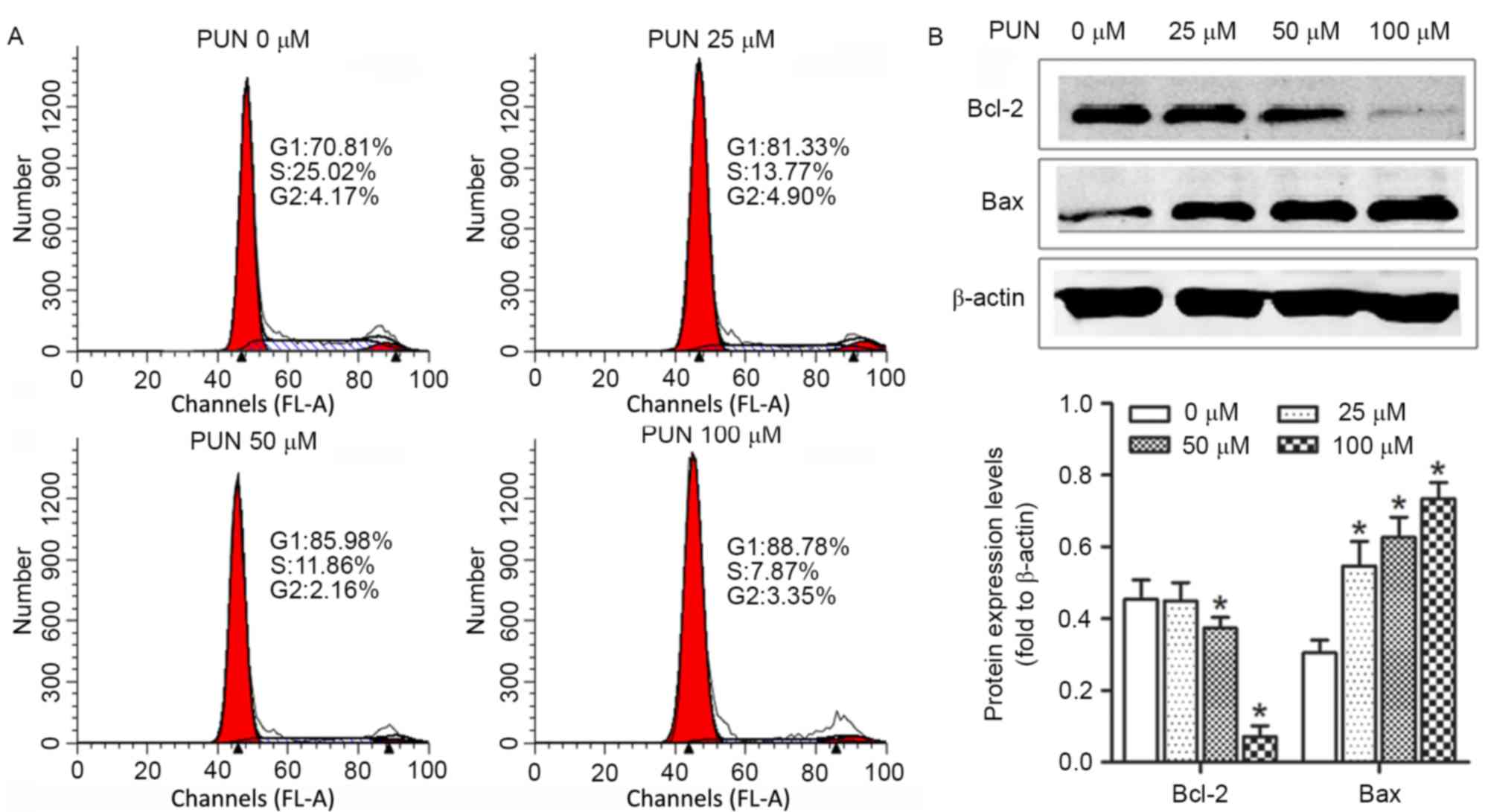
G1/S phase transition is arrested by
PUN in HeLa cells
To further investigate how PUN caused the growth
inhibition of HeLa cells, the cell cycle distribution was analyzed
with propidium iodide staining using FACS analysis following
treatment with increasing concentrations (0, 25, 50 and 100 µM) of
PUN. As demonstrated in Fig. 3A,
the number of cells in the G1 phase increased significantly
following PUN treatment for 36 h, compared with the control.
PUN alters the protein expression
levels of Bcl2 and Bax in HeLa cells
In order to elucidate the mechanisms underlying the
induction of apoptosis by PUN in HeLa cells, mitochondrial features
of the intrinsic apoptotic pathway were analyzed. Pro-apoptotic
members of the Bcl-2 family, including Bax, are required for the
induction of mitochondrial dysfunction during apoptosis. The
protein expression levels of Bax and Bcl-2 were assessed using
western blot analysis. The results indicated that treatment of the
HeLa cells with increasing doses (0, 25, 50 and 100 µM) of PUN for
36 h upregulated the expression of Bax and downregulated the
expression of anti-apoptotic Bcl-2 (Fig. 3B).
PUN inhibits the progression of HeLa
cell migration, and alters the expression of TIMP2 and TIMP-3 and
activities of MMP-2 and MMP-9
The effects of PUN on the progression of cell
migration were evaluated using a wound-healing assay. The size of
the region representing the wound site was significantly larger in
the cells treated with PUN (50 µM), compared with the control
(Fig. 4A). This indicated that the
untreated cells had higher wound-healing capacity, compared with
cells treated with PUN. In addition, the activities of MMP-2 and
MMP-9 were higher in the PUN-treated cells, compared with the
control group (Fig. 4B), as were
the activities of the MMP inhibitors, TIMP-2 and TIMP-3 (Fig. 4C). These results suggested that PUN
interfered with the invasion capabilities of the HeLa cells,
possibly by disrupting their migration, and altering the expression
of MMPs and TIMPs.
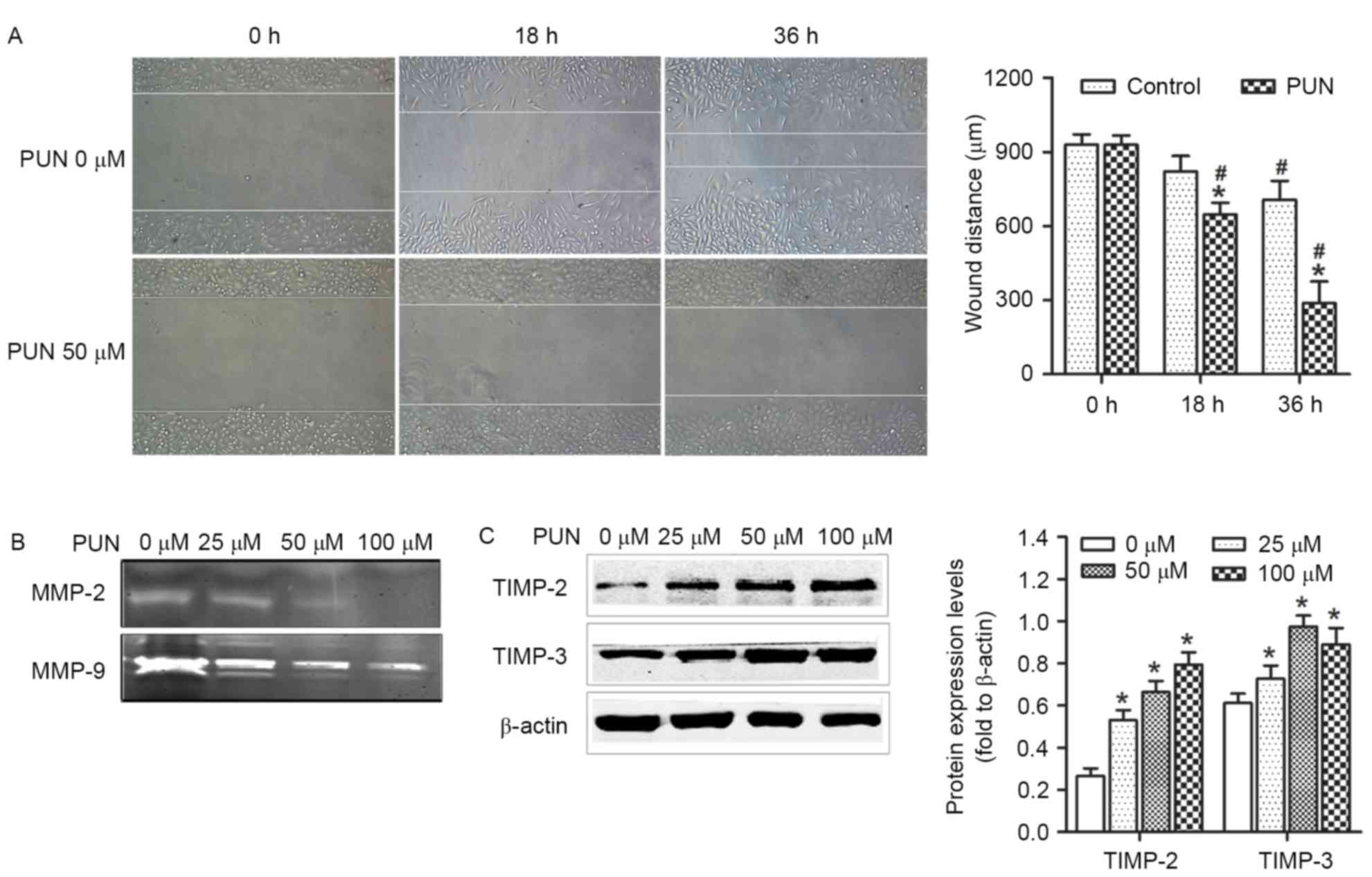 | Figure 4.Effect of PUN on cell migration,
expression levels of TIMP-2 and TIMP-3, and activities of MMP-2 and
MMP-9 in HeLa cells. (A) Wound-healing assay in which a uniform
scratch was made in cells cultured in serum-free Dulbecco's
modified Eagle's medium for 36 h. Images of the extent of closure
were captured at 0 and 36 h (original magnification, ×100). PUN
significantly inhibited the migration of HeLa cells. (B) MMP
gelatin zymography was used to detect the activities of MMP-2 and
MMP-9 in HeLa cells treated with increasing concentrations of PUN
(0, 25, 50 and 100 µM) for 36 h. The results showed that the
activities of MMP-2 and MMP-9 were decreased. (C) Expression levels
of TIMP-2 and TIMP-3 were analyzed using western blot analysis in
HeLa cells following treatment with PUN (0, 25, 50 and 100 µM).
TIMP-2 and TIMP-3 were downregulated following treatment with PUN.
Each experiment was performed three times and representative data
are shown. *P<0.05 vs. 0 µM PUN and #P<0.05 vs. 0
h. PUN, punicalagin; MMP, matrix metalloproteinase; TIMP, tissue
inhibitor of metalloproteinase. |
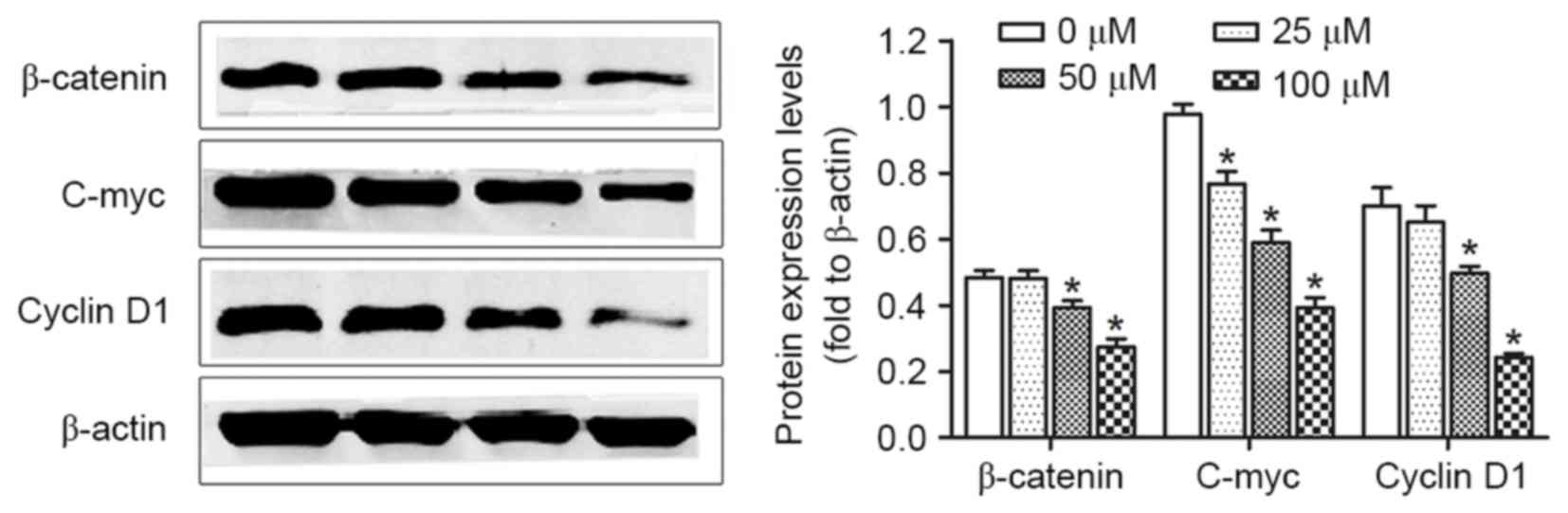
Downregulation of the β-catenin
signaling pathway by PUN
Activation of the β-catenin signaling pathway
constitutes an important event in the promotion of cell growth and
carcinogenesis of cervical cancer. The present study determined
whether PUN affects the expression of β-catenin in HeLa cells. The
HeLa cells were treated with 0, 25, 50 and 100 µM PUN for 36 h,
following which alterations in the levels of β-catenin and its
downstream factors, including cyclin D1 and c-myc, were examined
using western blot analysis. As demonstrated in Fig. 5, significant reductions in the
expression levels of β-catenin, cyclin D1 and c-myc were observed
in the PUN-treated cells, compared with the untreated cells.
Discussion
In the present study, it was shown that PUN, a major
bioactive component of pomegranate peel, effectively targeted HeLa
cervical cancer cells in vitro. Of note, PUN significantly
suppressed the proliferative and invasive properties of the HeLa
cells. The present study is the first, to the best of the authors'
knowledge, to demonstrate the possible molecular mechanisms
underlying the effect of PUN on the impairment of cervical cancer
cell growth through the β-catenin signaling pathway.
PUN inhibited the proliferation of HeLa cells in a
dose- and time-dependent manner in the present study. Previous
studies have reported the anti-proliferation and pro-apoptotic
effects of PUN in several cancer cell lines through alterations in
the expression of apoptosis-associated proteins and cell cycle
arrest (5,7–9,11,16,18,20).
Pomegranate fruit extract (PFE) has been reported to upregulate the
protein levels of pro-apoptotic Bax and Bcl2-antagonist/killer 1
(Bak), and downregulate the protein levels of anti-apoptotic Bcl-XL
and Bcl-2 in human prostate cancer PC3 cells (7,11).
PUN decreases the expression of Bcl-2, and increases the expression
levels of activated caspase-9 and PARP in human U87MG glioma cells
(18). Similarly, PUN induces
apoptosis in human colon cancer cells via the intrinsic pathway,
with release of cytochrome c into the cytosol, activation of
caspase-9 and caspase-3, and downregulation of Bcl-XL (8). In the present study, PUN
significantly decreased the protein expression of anti-apoptotic
Bax and increased the protein expression of pro-apoptotic Bcl-2 in
the HeLa cells. PFE has also been shown to decrease the expression
of cyclins D1, D2 and E, and cyclin-dependent kinase (cdk)2, cdk4
and cdk6 in PC3 human prostate cancer cells and A549 human lung
cancer cells, with cell cycle arrest at the G1 phase (7,9). By
contrast, PUN downregulate cyclin A and B1, and upregulates cyclin
E in Caco-2 human colon adenocarcinoma cells, with cell-cycle
arrest at the S phase (8). In
U87MG human glioma cells, PUN induces the upregulation of cyclin E
and the downregulation of cyclin A and B, with cell cycle arrest at
the G2/M phase (18). In the
present study, PUN inhibited the β-catenin signaling pathway and
decreased the expression of cyclin D1. Cyclin D1 is essential in
the phosphorylation of retinoblastoma and its release from E2
Transcription Factor, which results in progression of the cell
cycle and cellular proliferation. The cell cycle distribution assay
showed that the PUN-treated HeLa cells were arrested at the G1
phase. These contrasting results indicated that PUN may have
different effects on cell cycle progression in different cancer
cells.
Several epigenetic and genetic factors significantly
affect the genesis and development of cancer. Previous studies
(9,16) on A549 lung cancer cells revealed
the inhibition of mitogen-activated protein kinase,
phosphoinositide 3-kinase/AKT and nuclear factor (NF)-κB/p65
signaling, in addition to downregulation of the protein levels of
Ki-67 and proliferating cell nuclear antigen, by PUN. In addition,
PUN has been shown to increase the phosphorylation of 5′
AMP-activated protein kinase and p27T198, and then
induce autophagic cell death of U87MG cells (18). There is evidence that pomegranate
juice significantly suppresses the TNFα-induced protein expression
of cyclooxygenase 2, binding of NF-κB and activation of AKT in
several cancer cell lines (16).
In addition to these signaling pathways, the Wnt/β-catenin pathway
also contributes to tumorigenesis and progression. Substantial
investigation has revealed that the Wnt/β-catenin pathway is
associated with cervical cancer (1,2,4),
specifically in chemoresistance and possesses potential as a target
for chemosensitization. Wnt/β-catenin target genes, including
cyclin D1, c-myc and survivin, regulate cell proliferation and
apoptosis, thereby mediating cancer initiation and progression.
Wnt/β-catenin target genes can be divided into a
stemness/proliferation group, which is active early in tumor
progression, and an epithelial-mesenchymal transition/dissemination
group, which is expressed in late-stage tumors (21). The Wnt/β-catenin pathway has been
shown to be a therapeutic molecular target for cervical cancer. In
the present study, it was found that PUN decreased the expression
of β-catenin and its downstream proteins, including cyclin D1 and
c-myc, which are essential factors of cancer cell growth and
proliferation.
PFE has been reported to inhibit UV-mediated
expression of MMPs and decrease of TIMP-1, attenuate UV-induced
oxidative stress, and inhibit the stress-induced molecular pathways
associated with a high risk of carcinogenesis in EpiDerm™
(reconstituted human skin) (6,10,22).
Several studies have demonstrated the key involvement of MMPs in
tumor invasion and metastases (23–25).
Therefore, PUN may have functions in inhibiting tumor cell
invasion. In the present study, PUN significantly inhibited the
migration of HeLa cells when treated for 24 h. In addition, the
activities of MMP-2 and MMP-9 were decreased and the protein
expression levels of TIMP-2 and TIMP-3 were increased following
treatment with PUN.
In addition to the above roles, there have been a
number of studies on PUN and its antimicrobial activities against
bacteria, including Salmonella, Escherichia coli and
Vibrio cholerae, and viruses, including hepatitis C virus,
human immunodeficiency virus, H1N1 and human cytomegalovirus, with
mechanisms of actions including pH-independent bacterial and viral
growth inhibition, reductions in viral infectivity and binding to
host cell receptors, and structural damage to viruses (14,26).
Therefore, in addition to its antitumor effect, it was hypothesized
that PUN may have an anti-HPV effect, although this has not been
investigated.
In conclusion, the present study identified a novel
activity for PUN in HeLa human cervical cancer cells, namely, the
ability to induce the suppression of proliferation, cell cycle
arrest and inhibition of invasion. Therefore, PUN may be useful in
the development of adjuvant therapies to treat cervical cancer.
Acknowledgements
The authors would like to thanks all the teachers in
the Department of Gynecology and Obstetrics and Central Laboratory,
Renmin Hospital of Wuhan University, for their technical
assistance.
References
|
1
|
Liao CJ, Wu TI, Huang YH, Chang TC, Lai
CH, Jung SM, Hsueh C and Lin KH: Glucose-regulated protein 58
modulates β-catenin protein stability in a cervical adenocarcinoma
cell line. BMC Cancer. 14:5552014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li F, Wang T and Tang S: SOX14 promotes
proliferation and invasion of cervical cancer cells through
Wnt/β-catenin pathway. Int J Clin Exp Pathol. 8:1698–1704.
2015.PubMed/NCBI
|
|
3
|
Ljubojevic S and Skerlev M: HPV-associated
diseases. Clin Dermatol. 32:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Jiao S, Li X, Banu H, Hamal S and
Wang X: Therapeutic effects of antibiotic drug tigecycline against
cervical squamous cell carcinoma by inhibiting Wnt/β-catenin
signaling. Biochem Biophys Res Commun. 467:14–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehta R and Lansky EP: Breast cancer
chemopreventive properties of pomegranate (Punica granatum) fruit
extracts in a mouse mammary organ culture. Eur J Cancer Prev.
13:345–348. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aslam MN, Lansky EP and Varani J:
Pomegranate as a cosmeceutical source: Pomegranate fractions
promote proliferation and procollagen synthesis and inhibit matrix
metalloproteinase-1 production in human skin cells. J
Ethnopharmacol. 103:311–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malik A, Afaq F, Sarfaraz S, Adhami VM,
Syed DN and Mukhtar H: Pomegranate fruit juice for chemoprevention
and chemotherapy of prostate cancer. Proc Natl Acad Sci USA.
102:14813–14818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larrosa M, Tomás-Barberán FA and Espín JC:
The dietary hydrolysable tannin punicalagin releases ellagic acid
that induces apoptosis in human colon adenocarcinoma Caco-2 cells
by using the mitochondrial pathway. J Nutr Biochem. 17:611–625.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan N, Hadi N, Afaq F, Syed DN, Kweon MH
and Mukhtar H: Pomegranate fruit extract inhibits prosurvival
pathways in human A549 lung carcinoma cells and tumor growth in
athymic nude mice. Carcinogenesis. 28:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Syed DN, Malik A, Hadi N, Sarfaraz S, Afaq
F and Mukhtar H: Photochemopreventive effect of pomegranate fruil
extract on UVA-mediated activation of cellular pathways in normal
human epidermal keratinocytes. Photochem Photobiol. 82:398–405.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malik A and Mukhtar H: Prostate cancer
prevention through pomegranate fruit. Cell Cycle. 5:371–373. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aqil F, Munagala R, Vadhanam MV, Kausar H,
Jeyabalan J, Schultz DJ and Gupta RC: Anti-proliferative activity
and protection against oxidative DNA damage by punicalagin isolated
from pomegranate husk. Food Res Int. 49:345–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jean-Gilles D, Li L, Vaidyanathan VG, King
R, Cho B, Worthen DR, Chichester CO III and Seeram NP: Inhibitory
effects of polyphenol punicalagin on type-II collagen degradation
in vitro and inflammation in vivo. Chem Biol Interact. 205:90–99.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li G, Feng Y, Xu Y, Wu Q, Han Q, Liang X,
Yang B, Wang X and Xia X: The anti-infective activity of
punicalagin against Salmonella enterica subsp. enterica serovar
typhimurium in mice. Food Funct. 6:2357–2364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seeram NP, Adams LS, Henning SM, Niu Y,
Zhang Y, Nair MG and Heber D: In vitro antiproliferative, apoptotic
and antioxidant activities of punicalagin, ellagic acid and a total
pomegranate tannin extract are enhanced in combination with other
polyphenols as found in pomegranate juice. J Nutr Biochem.
16:360–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Syed DN, Afaq F and Mukhtar H: Pomegranate
derived products for cancer chemoprevention. Semin Cancer Biol.
17:377–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu X, Li H, Hou X, Li D, He S, Wan C, Yin
P, Liu M, Liu F and Xu J: Punicalagin Induces Nrf2/HO-1 Expression
via upregulation of PI3K/AKT pathway and inhibits LPS-induced
oxidative stress in RAW264.7 macrophages. Mediators Inflamm.
2015:3802182015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SG, Huang MH, Li JH, Lai FI, Lee HM
and Hsu YN: Punicalagin induces apoptotic and autophagic cell death
in human U87MG glioma cells. Acta Pharmacol Sin. 34:1411–1419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen LG, Huang WT, Lee LT and Wang CC:
Ellagitannins from Terminalia calamansanai induced apoptosis in
HL-60 cells. Toxicol In Vitro. 23:603–609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lansky EP and Newman RA: Punica granatum
(pomegranate) and its potential for prevention and treatment of
inflammation and cancer. J Ethnopharmacol. 109:177–206. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Afaq F, Zaid MA, Khan N, Dreher M and
Mukhtar H: Protective effect of pomegranate-derived products on
UVB-mediated damage in human reconstituted skin. Exp Dermatol.
18:553–561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aroui S, Najlaoui F, Chtourou Y, Meunier
AC, Laajimi A, Kenani A and Fetoui H: Naringin inhibits the
invasion and migration of human glioblastoma cell via
downregulation of MMP-2 and MMP-9 expression and inactivation of
p38 signaling pathway. Tumour Biol. 37:3831–3839. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hussain A, Harish G, Prabhu SA, Mohsin J,
Khan MA, Rizvi TA and Sharma C: Inhibitory effect of genistein on
the invasive potential of human cervical cancer cells via
modulation of matrix metalloproteinase-9 and tissue inhibitiors of
matrix metalloproteinase-1 expression. Cancer Epidemiol.
36:e387–e393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu H, Cao X, Zhang H, Sun G, Fan G, Chen L
and Wang S: Imbalance between MMP-2, 9 and TIMP-1 promote the
invasion and metastasis of renal cell carcinoma via SKP2 signaling
pathways. Tumour Biol. 35:9807–9813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Howell AB and D'Souza DH: The pomegranate:
Effects on bacteria and viruses that influence human health. Evid
Based Complement Alternat Med. 2013:6062122013. View Article : Google Scholar : PubMed/NCBI
|