Introduction
Inflammatory myopathies, mainly polymyositis (PM)
and dermatomyositis (DM), are a group of autoimmune diseases.
Immunohistochemical studies on PM/DM muscle biopsies have
demonstrated that T lymphocytes often infiltrate muscle fibres
(1,2). This abnormal behavior of T
lymphocytes is a characteristic of the pathogenesis of these
diseases, although their underlying mechanism remains unclear; in
particular, how circulating T lymphocytes affect inflammatory
myopathy.
Our previous study (3) demonstrated that T-cell subsets in
peripheral blood were significantly fewer in PM/DM patients.
Numerous other studies have additionally indicated that PM/DM
patients demonstrate significant decreases in their cluster of
differentiation (CD) 3+CD4+ T cell counts
(4–8). However, the exact function and
mechanism of peripheral blood T lymphocyte subsets was not
investigated in these studies.
T-cell homeostasis is disrupted in autoimmune
diseases, including systemic lupus erythmatosus (SLE) and
rheumatoid arthritis (RA) (1,9–11).
There is growing evidence that T-cell homeostasis is, in part,
regulated by the balance between apoptosis and autophagy (12,13).
Previous studies have demonstrated that the lymphocytes of patients
with autoimmune diseases undergo significantly higher rates of
apoptosis compared with healthy controls. It is also widely
accepted that increased rates of apoptosis in circulating
lymphocytes can trigger an autoimmune reaction due to the release
of autoantigens (14).
Autophagy is a highly conserved process by which
subcellular components are sequestered and degraded via a lysosomal
signalling pathway (15). It has
been reported (16) that autophagy
serves various functions in the immune system, depending on its
cellular contexts or stimuli. On one hand, autophagy serves as a
pro-survival mechanism by clearing away intracellular pathogens and
antigen presentation, in addition to contributing to lymphocyte
homeostasis, thus preventing apoptotic cell death (16). In the absence of the
autophagy-related gene (Atg)-5 or Atg-7, CD4+ and
CD8+ T lymphocytes rapidly undergo apoptosis (17,18).
On the other hand, autophagy may additionally mediate cell death
(17,18). When stress conditions are too harsh
or prolonged, the autophagic machinery is overwhelmed, thus
triggering cell death (19).
Previous studies (20–24)
have indicated that Atg5 and Atg16/1 polymorphisms are associated
with susceptibility SLE, RA and Crohn's disease. Although numerous
studies have focused on the role of autophagy and apoptosis in
autoimmune diseases, very little information is available
specifically about autophagy in PM/DM. Previous examinations of
inclusion-body myositis (IBM) and PM tissues with mitochondrial
pathology have revealed a marked increase in levels of
microtubule-associated protein 1A/1B-light chain 3 (LC3)-II, a
marker for mature autophagosomes (25). The accumulation of autophagosomes
was also a confirmed trend in IBM muscle biopsies (26). Alger et al (27) demonstrated that autophagy markers
were upregulated in the muscle fibers of PM/DM patients and in
murine myositis. All these studies focused on the role of autophagy
in local muscle tissues and recognized autophagy as a non-immune
mechanism in idiopathic inflammatory myopathies. However, autophagy
is not only a lysosome-mediated catabolic process; it additionally
serves a complex function in T cell development, activation,
survival and proliferation. Specifically, autophagy helps to
regulate cell death and survival in T cells (28). Increased induction of cell death
has been reported in Beclin-1-deficient T cells, which correlates
with the reduced size of the peripheral T cell compartment in mice
bearing those cells (28).
A thorough understanding of the potential underlying
mechanisms of autophagy in T-cells remains to be elucidated.
Therefore, the present study aimed to investigate the occurrence of
autophagy in T cells and its potential role in the development of
PM/DM to find a novel perspective on this immune mechanism.
Materials and methods
Subjects
Peripheral blood was obtained from 24 patients with
PM/DM (6 PM, 18 DM; 8 male, 16 female; mean age, 37±13 years;
range, 19–74 years) who were inpatients of the Rheumatology
Department of China-Japan Friendship Hospital (Beijing, China). The
diagnoses of PM and DM were determined by combining the Bohan and
Peter criteria with the European Neuromuscular Centre pathology
diagnosis criteria (29–31). The muscle specimens were stained
with hematoxylin-eosin, modified Gomori trichrome and a number of
enzyme stains, including nicotinamide adenine
dinucleotide-tetrazolium reductase, succinic dehydrogenase,
adenosine triphosphatase, CD3, CD4, CD8, CD20, CD45RO and major
histocompatibility complex class I. All subjects additionally had
PM and DM histologically proven by their muscle biopsy results. At
the time the serum samples were taken, none of the patients had
received immunosuppressive agents during the previous month. These
agents included prednisolone, hydroxychloroquine, cyclophosphamide,
azathioprine, mycophenolate mofetil and methotrexate. A total of 21
age- and sex-matched healthy individuals were selected to be
healthy controls. Complete medical histories were taken and
physical examinations were conducted for all patients during their
first visit. Clinical and laboratory data, including serum muscular
enzyme levels and auto-antibody levels, were obtained at the time
serum samples were taken.
Disease activity at the time of diagnosis was
assessed using the Myositis Disease Activity Assessment Visual
Analogue Scales (MYOACT) established by the International Myositis
Assessment and Clinical Studies Group. The study was approved by
the ethics committee of the China-Japan Friendship Hospital and all
subjects gave written informed consent to participate in the
study.
Flow cytometry for analyzing T cell
subgroup counts
The amount of CD3+CD4+ T cells
and CD3+CD8+ T cells in peripheral blood was
determined by flow cytometry using the following monoclonal
antibodies: Anti-CD4-fluorescein isothiocyanate (FITC; catalog co.
557705, BD Biosciences, Franklin Lakes, NJ, USA),
anti-CD8-phycoerythrin (PE; catalog no. 555745, BD Biosciences) and
anti-CD3-PE-cyanine (catalog no. 555749, BD Biosciences). Serum
samples were processed within 6 h of being obtained. Isotype
control-stained cells were also prepared. Data were analyzed using
a Cytomics FC500 system (Beckman Coulter, Inc., Brea, CA, USA). In
brief, after incubation 1×106 cells were suspended with
2.0 ml of a 1:10 dilution of FACS Lysing solution (BD Biosciences)
and incubated with anti-CD4-FITC, anti-CD8-PE. and
anti-CD3-PE-cyanine antibodies in the dark for 10 min at room
temperature. Cells were centrifuged at 540 × g for 5 min at 4°C,
the supernatant was discarded using Pasteur pipettes and the cell
pellet was suspended in 50 µl buffer solution. Cells were then
washed with 2.0 ml PBS containing 0.5% bovine serum albumin (BSA;
Hyclone, GE Healthcare Life Sciences, Chalfont, UK) and 0.09%
sodium azide, vortexed, and centrifuged at 540 × g for 5 min at
4°C. Finally, the supernatant was discarded and cells were
suspended in 200 µl PBS containing 0.5% BSA. Cells were kept at 4°C
prior to analysis. After staining, samples were incubated for 15
min in the dark. Flow cytometric analysis was performed after 2 h
fixation. The data were analyzed with CXP analysis software version
2.1 (Beckman Coulter, Inc., Brea, CA. USA).
T cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were
isolated by Ficoll-Hypaque (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) density-gradient centrifugation at 400 × g for 30 min at
37°C, of samples from the PM/DM patients and the healthy controls.
Separation of fresh CD3+ T cells from PBMCs was carried
out by performing immunomagnetic-based depletion of non-T cells
using the Pan T-Cell Isolation kit II (Miltenyi Biotec,
Bergisch-Gladbach, Germany). The cell purity was >95%.
The cells were subsequently stimulated with 10 µg/ml
phytohemagglutinin (Sigma-Aldrich; Merck KGaA) and maintained in
Roswell Park Memorial Institute 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 5% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 2 mM
L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA). They were subsequently treated with 50
nM rapamycin (Sigma-Aldrich; Merck KGaA) for 48 h.
Transmission electron microscopy
Freshly isolated CD3+ T cells were
prepared as described above. Briefly, CD3+ T cells were
fixed in 3% glutaraldehyde, stained with 1% osmium tetroxide,
embedded in SeaPlaque agarose (Cambrex Bio Science Rockland, Inc.,
ME, USA), dehydrated with ethanol and embedded in Epon/Araldite
resin. Thin sections were cut to 70 nm, placed on Butvar-coated 200
mesh copper grids, post-stained with 3% aqueous uranyl acetate and
Reynolds lead citrate and subsequently observed under a
transmission electron microscope (TEM) using 200 kV power and
×15,000 magnification. Autophagic cells were defined as cells with
≥5 autophagosomes. The percentage of autophagic cells was
quantified by examining >100 randomly selected TEM fields by two
investigators.
RNA isolation, reverse
transcription-polymerase chain reaction (RT-PCR) and
RT-quantitative (q)PCR
RNA was isolated from the harvested CD3+
T cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. All the
RNA was reverse-transcribed into cDNA (Promega Corporation,
Madison, WI, USA). RT-PCR was performed in 96-well plate format
using the ABI 7000 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primers used were as follows:
Forward, 5′-GGCTGAGAGACTGGATCAGG-3′ and reverse,
5′-CTGCGTCTGGGATAACG-3′ for Beclin-1; forward,
5′-GAGAAGCAGCTTCCTGTTCTGG-3′ and reverse,
5′-GTGTCCGTTCACCAACAGGAAG-3′ for LC3-II; and forward,
5′-GGACTTCGAGCAAGAGATGG-3′ and reverse, 5′-TGTGTTGGCGTACAGGTCTTT-3′
for β-actin. mRNA expression levels of β-actin were measured as an
internal reference. The amplification program comprised three
stages: An initial 95°C Taq activation stage for 10 min, followed
by 45 cycles of 95°C denaturation for 5 sec, and 60°C annealing for
35 sec. Data were exported into SPSS version 17.0 software (SPSS
Inc, Chicago, IL, USA) for further statistical analysis. Gene
expression was quantified relative to the expression of endogenous
reference genes by calculating the differences in cycle threshold
and relative values determined by the 2−ΔΔCq method
(32).
Western blot analysis
Purified CD3+ T lymphocytes were lysed in
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing 100 mM Tris-HCl, pH 8; 150 mM NaCl; 1% Triton
X-100; 1 mM MgCl2; and 25 mM
Na3VO4 and a mixture of protease inhibitors
(Thermo Fisher Scientific, Inc.). Protein content was determined by
performing a Bradford assay (Thermo Fisher Scientific, Inc.).
Lysates were centrifuged at ~14,000 × g for 15 min to pellet the
cell debris. Equal amounts of protein (50 ng) was separated by 10%
SDS-PAGE and transferred onto nitrocellulose membranes. Membranes
were blocked at room temperature for 1 h in a blocking solution
containing 5% skimmed milk in Tris-buffered solution (50 mM
Tris-HCl, 150 mM NaCl, pH=7.5) and 0.1% v/v Tween-20. Membranes
were incubated at 4°C overnight with various primary antibodies,
including: rabbit anti-human Beclin polyclonal antibody (1:200;
catalog no. PD017; Medical & Biological Laboratories Co., Ltd.,
Nagoya, Japan); mouse anti-human LC3 monoclonal antibody (1:200;
catalog no: SAB1305552; Sigma-Aldrich; Merck KGaA) and anti β-actin
polyclonal antibody (1:500; catalog no. PM053; Medical &
Biological Laboratories Co., Ltd). Membranes were washed and
subsequently incubated with peroxidase-conjugated goat anti-mouse
IgG (1:500, catalog no. ZB-2305, ZSGB-Bio, Beijing, China), and
peroxidase-conjugated goat anti-rabbit IgG (1:500, catalog no.
ZB-2301, ZSGB-Bio) for 1 h at room temperature. Membranes were
developed with an Enhanced Chemiluminescence substrate (catalog no.
WP20005; Thermo Fisher Scientific, Inc.), and protein bands were
captured using a UVP gel imaging system (UVP, Upland, CA, USA).
Protein bands were digitized and subjected to densitometry using
ImageJ software version 1.48 (National Institutes of Health,
Bethesda, MD, USA), on a GS-700 Imaging Densitometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). β-actin was used as a
loading control to normalize the density of protein bands.
T cell apoptosis analysis
Apoptotic CD3+ T cells were assayed using
the Annexin V-FITC kit and propidium iodide (PI) staining according
to the manufacturer's protocol (BD Biosciences, Franklin Lakes, NJ,
USA). Apoptosis in cells was assessed using the FITC Annexin V
Apoptosis Detection kit (BD Biosciences; Annexin V-FITC, PI
solution and annexin V binding buffer). This assay involves
staining cells with Annexin V-FITC (a phospholipid-binding protein
that binds to disrupted cell membranes) in combination with PI (a
vital dye that binds to DNA that penetrates into apoptotic cells).
Flow cytometric analysis was performed to determine the percentage
of cells that were undergoing apoptosis (Annexin V+/PI- and Annexin
V+/PI+).
Statistical analysis
For group comparisons associated with binary data,
either the χ2 test or Fisher's exact test was used.
Comparisons of continuous data were made using the Student's
t-tests or the Mann-Whitney U test. Data are expressed as the mean
± standard deviation and all statistical calculations were
performed using SPSS version 17.0 software (SPSS Inc, Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical, serological features and
peripheral blood T cell subsets in PM/DM patients
Muscle biopsies were performed on specimens from the
24 PM/DM patients who were enrolled in the study. Among them, 6
patients had PM and 18 had DM. The clinical characteristics of the
patients recruited for this study are summarized in Table I. The male to female ratio was 1:2
among the study subjects. The mean age of onset of PM and DM in
these patients was 42.4 and 44.6 years, respectively. In addition,
the mean disease duration that the PM and DM patients experienced
was 31.5 and 15.2 months, respectively. Disease activity was
further assessed using MYOACT tools. The results demonstrated that
all PM and DM patients were at the disease activation stage. PM
patients had mean MYOACT scores of 6.3±1.2, while DM patients had
mean scores of 6.7±1.6.
 | Table I.Clinical features of PM/DM patients
and healthy controls. |
Table I.
Clinical features of PM/DM patients
and healthy controls.
| Clinical
feature | PM | DM | HC | P-value |
|---|
| Patients and
healthy controls, no (%) | 6 (25) | 18 (75) | 21 | NA |
| Male:female
ratio | 1:5 | 1:1.6 | 1:2.2 | >0.05 |
| Age of onset, mean
± standard deviation (range), yrs | 42.2±14.9
(23–58) | 44.6±15.1
(18–70) | 43.9±17.2
(20–62) | >0.05 |
| Duration of
disease, mean ± standard deviation (range), months | 31.5±41.1
(3–108) | 15.2±18.5
(1–72) | NA | NA |
| Clinical
characteristics, n (%) |
|
Fever | 3 (50) | 6 (33) | NA |
|
|
Cutaneous manifestations | 0 (0) | 18 (100) | NA |
|
| Muscle
weakness | 6 (100) | 18 (100) | NA |
|
|
Arthritis | 1 (16.7) | 5 (27.8) | NA |
|
|
Dysphagia | 2 (33.3) | 5 (27.8) | NA |
|
|
Interstitial lung disease | 3 (50) | 10 (55.6) | NA |
|
| Cardiac
involvement | 1 (16.7) | 0 (0) | NA |
|
|
Mechanic's hands | 1 (16.7) | 3 (16.7) | NA |
|
| Levels of CK at the
time of blood sampling, mean ± standard deviation (IU/l) | 1873.8±4364.5 | 4151.8±5304.7 | NA |
>0.05a |
| Anti-Jo-1
positive | 2 (33.3) | 4 (22.2) | NA |
>0.05a |
| ANA positive | 2 (33.3) | 8 (44.4) | NA |
>0.05a |
| MYOACT total
disease activity score at the time of blood sampling, mean ±
standard deviation | 6.3±1.2 | 6.7± 1.6 | NA |
>0.05a |
| White blood cell
counts (x109/l) | 6.1±0.7 | 6.5±1.1 | 5.3± 0.8 |
>0.05a |
| CD3+ T
cell count (cell/mm3) | 489.2±357.8 | 534.6±309.2 | 1532±1092.4 |
<0.05a |
|
CD3+CD4+ T cell
count (cell/mm3) | 273.6±157.1 | 256.2±210.0 | 681.7±262.5 | <0.05 |
|
CD3+CD8+ T cell
count (cell/mm3) | 210.3±182.2 | 229.6±207.8 | 437.5±275.4 | <0.05 |
Counts of various peripheral blood T-lymphocyte
subpopulations were evaluated by flow cytometry. T-cell lymphopenia
was identified in PM and DM patients. As demonstrated in Table I, counts of CD3+ T,
CD3+CD4+ T and CD3+CD8+
T cells in PM/DM patients were all significantly reduced compared
with healthy controls (all P<0.05). Although there were fewer T
lymphocytes in PM and DM patients, neutrophils levels were normal
in PM and DM patients.
Peripheral blood T cells increased
apoptosis rates in PM/DM patients
Considering that peripheral blood T lymphocytopenia
was identified in PM/DM, the first goal of the present study was to
investigate whether T lymphocytopenia in peripheral blood from
PM/DM patients was caused by T-cell apoptosis. T-cell apoptosis was
assessed by flow cytometry. Freshly isolated circulating
CD3+ T lymphocytes were analyzed for apoptosis using
FITC-conjugated Annexin V and PI by flow cytometry (Fig. 1A). As demonstrated in Fig. 1B, the median percentage of
apoptotic CD3+ T cells in patients with PM was
9.95±0.83%, whereas it was 14.3±0.88% in patients with DM. These
were significantly increased compared with the healthy controls
(5.83±0.45%; P<0.001). In addition, the percentage of apoptotic
CD3+ T cells in DM patients was significantly increased
compared with PM patients (P<0.05). Following this, the
correlation between apoptotic rates and CD3+ T
lymphocytes was examined. A significant negative correlation
between the higher rates of CD3+ T lymphocyte apoptosis
and counts of CD3+ T cells in PM/DM patients was
identified (P<0.05; Fig. 1C and
D). As was expected, increased rates of apoptosis led to
reduced T-cell counts.
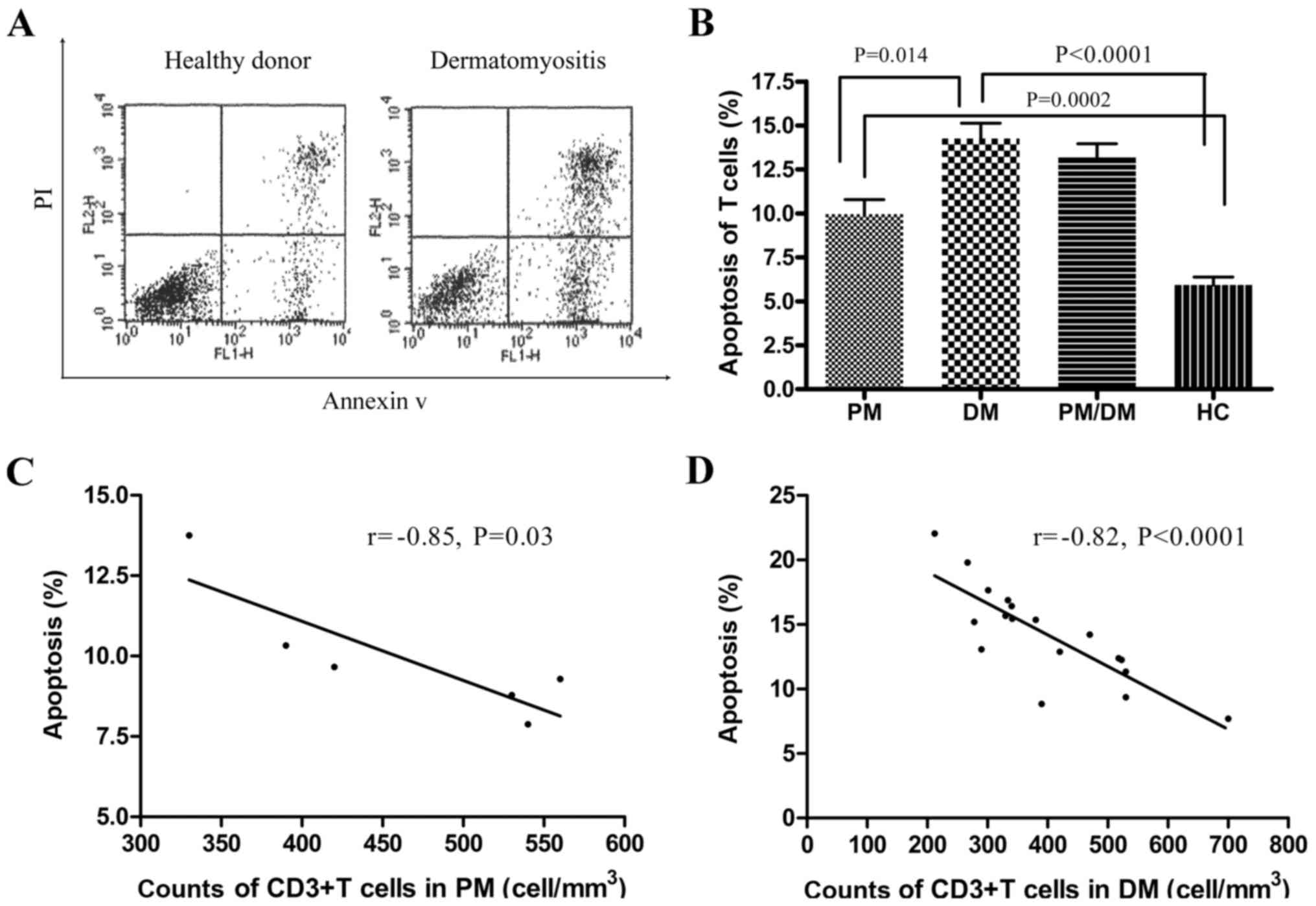 | Figure 1.Apoptosis detection in freshly
isolated peripheral blood T cells from patients with PM/DM and from
healthy controls. (A) Flow cytometry analysis of lymphocyte
apoptosis. Representative dot plots of flow cytometry analysis (PI
on y-axis vs. AV on x-axis). Numbers reported for AV
single-positive cells and for AV/PI double-positive cells represent
percentages of apoptotic lymphocytes in the bottom-right quadrants.
(B) The median percentage of apoptotic CD3+ T
lymphocytes in peripheral blood was obtained from 9 patients with
PM, 18 patients with DM and 21 healthy controls. Percentages of
apoptotic T cells in PM (9.95±0.83%) and DM (14.3±0.88%) were
significantly increased compared with HCs (5.83±0.45%). Data are
presented as the mean ± standard deviation. There was a significant
negative correlation between increased apoptosis rates of
CD3+ T lymphocytes and counts of CD3+ T cells
in (C) PM and (D) DM patients (r=−0.85, P=0.03; r=−0.82,
P<0.0001, respectively). PM, polymyositis; DM, dermatomyositis;
PI, propidium iodide; AV, Annexin V; HC, healthy controls; CD,
cluster of differentiation. |
Decreased autophagy levels in freshly
isolated T cells from PM/DM patients
According to data from a previous study (28), autophagy additionally contributes
to the maintenance of lymphocyte homeostasis. Therefore, to study
how the autophagy machinery in peripheral CD3+ T
lymphocytes determines cell survival in PM/DM patients, these cells
were examined for the presence of autophagosomes using TEM, and for
the presence of the autophagosomal markers LC3-II and Beclin-1 by
western blot analysis and RT-qPCR. Autophagosomes were imaged in
healthy control (Fig. 2A) and DM
(Fig. 2B) patients. In addition,
TEM revealed fewer numbers of autophagosomes in PM/DM patients than
in healthy controls (P<0.05; Fig.
2C). To examine autophagic activity in PM/DM patients, Beclin-1
mRNA and protein expression levels were examined in addition to LC3
mRNA and protein expression, using RT-qPCR and western blot
analysis. LC3 mRNA (Fig. 2D) and
protein (Fig. 2E) expression
levels were significantly reduced in PM/DM patients compared with
healthy controls (P<0.01 and P<0.001, respectively). Beclin-1
mRNA (Fig. 2F) and protein
(Fig. 2G) expression levels were
additionally reduced in PM/DM patients compared with healthy
controls (P<0.01 and P<0.001, respectively). Representative
images of mRNA and protein expression levels of LC3 and Beclin-1
are presented in Fig. 2H and I,
respectively. Together, these results indicated that autophagy
rates are reduced in PM/DM and that autophagy may be involved in
the pathogenesis of PM/DM.
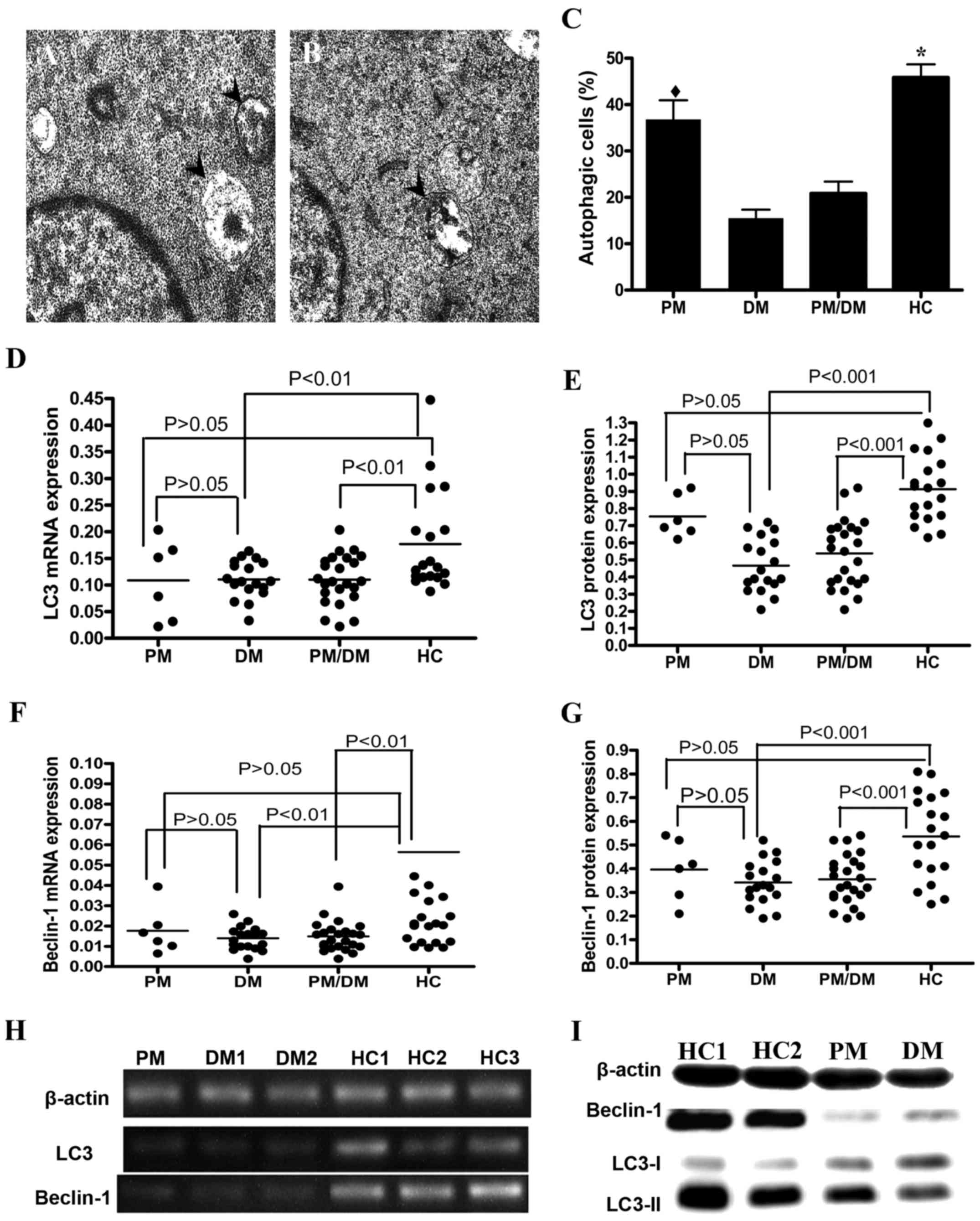 | Figure 2.Autophagy levels in freshly isolated
CD3+ T cells from PM/DM patients. TEM images are
representative samples from (A) healthy control and (B) DM
patients. A total of 10 fields in each sample were randomly
examined. Arrows denote autophagosomes. (C) Quantitative analysis
of number of autophagosomes detected by TEM with 200 kV power and
×15,000 magnification. Data are expressed as the mean ± standard
deviation. *P<0.0001 vs. DM andPM/DM groups;
♦P>0.05 vs. PM and HC patients. RT-qPCR and western
blot analysis were performed to evaluate the expression of
autophagy-specific markers LC3-II and Beclin-1. Dot plots are
representative of independent experiments performed on the
CD3+ T cells of 21 healthy donors, of 6 PM patients and
of 18 DM patients. LC3 (D) mRNA and (E) protein expression levels.
Beclin-1 (F) mRNA and (G) protein expression levels. Representative
(H) RT-qPCR and (I) western blot images of Beclin-1 and LC3mRNA and
protein expression levels, respectively. β-actin served as an
internal control. PM, polymyositis; DM, dermatomyositis; TEM,
transmission electron microscope; HC, healthy controls; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; LC3,
microtubule-associated protein 1A/1B-light chain 3; CD, cluster of
differentiation. |
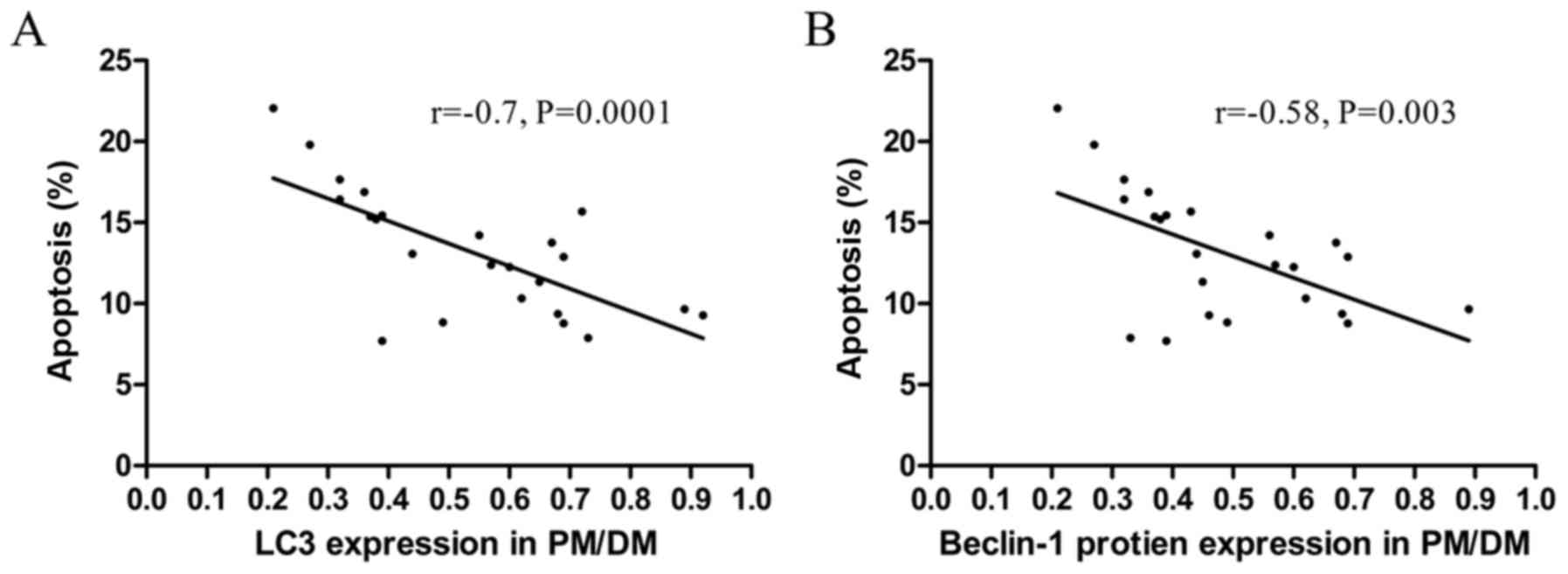
To understand the role of autophagy in peripheral
CD3+ T cell survival, the correlation between LC3-II or
Beclin-1 levels and apoptosis rates in peripheral CD3+ T
cells in PM/DM patients was analyzed. As presented in Fig. 3A, there was a significant negative
correlation between LC3-II protein levels and apoptosis rates in
circulating CD3+ T cells in PM/DM patients (r=−0.7,
P=0.0001). There was additionally a significant negative
correlation between apoptosis rates and Beclin-1 protein levels in
peripheral blood CD3+ T cells in PM/DM patients
(r=−0.58, P=0.003; Fig. 3B). This
result may indirectly suggest that decreased autophagy activity
contributes to the apoptosis of peripheral blood T cells.
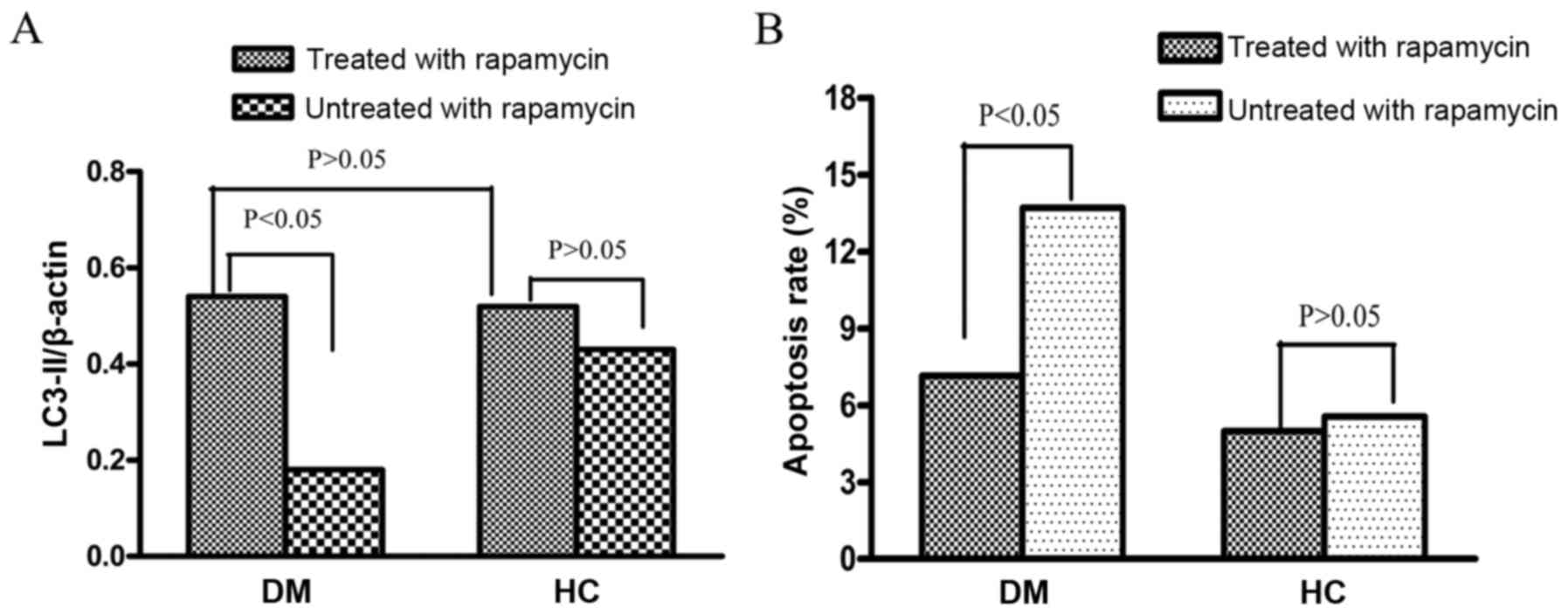
Autophagy prevents CD3+ T
cell apoptosis in PM/DM patients
To explore whether autophagy has a cytoprotective
effect on peripheral blood T cells in PM/DM patients, the effect of
autophagy on T-cell survival in vitro was next examined.
First, CD3+ T cells from 10 DM patients were treated
with the autophagy inducer rapamycin for 48 h. Following this
treatment, LC3-II protein levels in CD3+ T cells of DM
patients were significantly increased compared with untreated
CD3+ T cells (0.54±0.17 vs. 0.18±0.08 P<0.05,
Fig. 4A). Secondly, LC3-II protein
levels in the CD3+ T cells of healthy controls were
detected. No significant difference was observed in LC3-II protein
expression levels between CD3+ T cells treated with
rapamycin and those not treated with rapamycin (0.52±0.28 vs.
0.43±0.23; P>0.05). Furthermore, no significant difference was
observed in LC3-II levels between the rapamycin-treated T cells of
DM patients and those of healthy controls (Fig. 4A; P>0.05).
The apoptosis rates of CD3+ T cells from
PM/DM patients and healthy controls following rapamycin treatment
were additionally assessed. The results demonstrated that all
CD3+ T cells had decreased apoptosis rates following
treatment by rapamycin, regardless of donors. Apoptosis rates in
PM/DM patients treated with rapamycin were significantly reduced
compared with untreated patients (7.15±0.21% vs. 13.7±0.33%;
P<0.05; Fig. 4B). Apoptosis
rates in rapamycin-treated healthy controls were slightly reduced
compared with untreated healthy controls; however, this was not
significant (4.98±0.14% vs. 5.56±0.27%; P>0.05; Fig. 4B).
Similar results were observed in PM patients (data
not shown). All these results implied that autophagy activation
could lead to cytoprotection in PM/DM patients.
Discussion
To the best of our knowledge, the present study
addressed for the first time the autophagic behaviour of peripheral
blood T lymphocytes in PM/DM patients. The results suggested that
decreased autophagy and increased apoptosis in peripheral blood T
cells from PM/DM patients may be involved in the pathogenesis of
inflammatory myopathies. The results from the present study also
suggested that decreased autophagy may serve a role in the
increased apoptosis of peripheral blood T cells. Taken together,
these findings clarify the potential role of autophagy in
preventing inflammatory myopathies by regulating peripheral T-cell
survival.
The pathogenesis of inflammatory myopathy is
complex. Previous studies have mainly focused on the muscular
microenvironment (33–35); research on peripheral blood immune
cells is lacking. T-cell homeostasis is disrupted in autoimmune
diseases, including SLE and RA (36,37).
Recent immunohistochemical studies on muscle biopsies from our
laboratory and others have also demonstrated that T lymphocytes
often infiltrate muscle fiber in PM/DM (38,39).
T-cell abnormalities have additionally been observed in murine
models (40). However, only a
small number of studies have investigated the characteristics and
pathogenesis of PM/DM in peripheral blood T lymphocytes. Data from
our previous study (3) and the
present study demonstrated that circulating T lymphopenia occurs in
PM/DM patients. The findings of the present study are consistent
with a recent study that additionally identified a high frequency
of lymphopenia in PM/DM (41).
A potential reason for this lymphopenia in PM/DM is
increased apoptosis of peripheral T cells. The results of the
present study demonstrated that apoptosis rates were significantly
increased in PM/DM patients compared with healthy controls.
Furthermore, PM/DM patients possessed lymphopenia that inversely
correlated with T-cell apoptosis, suggesting a cause and effect
association. Similar findings were observed in SLE patients: Dhir
et al (42) confirmed an
increased rate of apoptosis in the T-lymphocytes of SLE patients,
and identified that peripheral blood T lymphopenia had a direct
negative correlation with T-cell apoptosis rates.
Numerous mechanisms have been proposed to explain
the increased apoptosis rates of T cells in autoimmune diseases.
Among these, the importance of autophagy in the maintenance of
T-lymphocytes homeostasis has been recognized (15,16).
Autophagy has been identified to be deregulated in CD3+
T cells from lupus-prone mice (43). T lymphocytes from SLE patients were
resistant to autophagic induction and exhibited an upregulation of
genes that inhibit autophagy (44). This resistance and inhibition may
explain the increased apoptosis rates detected in SLE patients
(44,45). In agreement with these results, the
present study demonstrated that autophagy decreased and apoptosis
increased in circulating CD3+ T cells. Increased
induction of cell death has been reported (28) in Beclin-1-deficient T cells, which
correlated with the reduced size of the peripheral T- cell
compartment in mice bearing those cells. The present study
demonstrated that circulating CD3+ T-cell counts in
PM/DM patients were reduced compared with healthy controls, which
may result from decreased autophagy. CD3+ T cells from
PM/DM patients treated with rapamycin had even further reduced
apoptosis rates compared with similar but untreated T cells
(P<0.05), while apoptosis rates were similarly decreased in
treated and untreated CD3+ T cells from healthy controls
(P>0.05). These findings indicated that abnormal autophagy may
result in increased apoptosis of peripheral blood T cells in
PM/DM.
Links between autophagy and apoptosis have been
observed in various eukaryotic cells (46). Atg7−/− T lymphocytes
upregulate the expression of Bcl-2 (47). Beclin-1-deficient T lymphocytes
exhibit markedly increased levels of pro-apoptotic pro-caspase-8,
pro-caspase-3 and Bcl-2-like protein 11 (48). However, the exact mechanism by
which autophagy regulates apoptosis in T lymphocytes remains to be
elucidated. Numerous mechanisms have been proposed to explain the
involvement of autophagy in the regulation of T cell survival
(49). Altered degradation of
mitochondria with increased production of reactive oxygen species
(ROS) has been observed in autophagy-deficient T cells (47). An uncontrolled burst in the
generation of ROS following T-cell receptor engagement may account
for the increased rates of cell death observed in activated
autophagy-deficient T cells. Endoplasmic reticulum stability is
additionally dependent on autophagy in T cells, and
autophagy-deficient T cells demonstrate functional defects in
calcium signaling, a potential consequence of altered mobilization
from the intracellular calcium stores of the endoplasmic reticulum
(28).
In conclusion, the present study indicated that
autophagy may serve a potential cytoprotective role in PM/DM,
potentially via inhibition of apoptosis in circulating
CD3+ T cells. These data suggested that autophagy may
not only be cytoprotective in preventing inflammatory myopathies,
but additionally serve a pivotal role in apoptosis regulation.
Further studies are required to investigate the underlying
molecular mechanisms and interactions between abnormal autophagy
and apoptosis in the development of PM/DM.
Acknowledgements
The authors thank all the patients and healthy
participants in the present study, which was funded by the General
Program of the National Natural Science Foundation of China (grant
nos. 81172860 and 81571603), the Major Research Plan of the
National Natural Science of China (grant no. 91542121), the Youth
Program of the National Natural Science Foundation of China (grant
no. 81401363) and the Capital Health Research and Development of
Special Program (grant no. 2014-4-4062).
References
|
1
|
Malmström V, Venalis P and Albrecht I: T
cells in myositis. Arthritis Res Ther. 14:2302012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venalis P and Lundberg IE: Immune
mechanisms in polymyositis and dermatomyositis and potential
targets for therapy. Rheumatology (Oxford). 53:397–405. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang DX, Lu X, Zu N, Lin B, Wang LY, Shu
XM, Ma L and Wang GC: Clinical significance of peripheral blood
lymphocyte subsets in patients with polymyositis and
dermatomyositis. Clin Rheumatol. 31:1691–1697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishii W, Matsuda M, Shimojima Y, Itoh S,
Sumida T and Ikeda S: Flow cytometric analysis of lymphocyte
subpopulations and TH1/TH2 balance in patients with polymyositis
and dermatomyositis. Intern Med. 47:1593–1599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aleksza M, Szegedi A, Antal-Szalmás P,
Irinyi B, Gergely L, Ponyi A, Hunyadi J, Sipka S, Zeher M, Szegedi
G and Dankó K: Altered cytokine expression of peripheral blood
lymphocytes in polymyositis and dermatomyositis. Ann Rheum Dis.
64:1485–1489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Viguier M, Fouéré S, de la Salmonière P,
Rabian C, Lebbe C, Dubertret L, Morel P and Bachelez H: Peripheral
blood lymphocyte subset counts in patients with dermatomyositis:
Clinical correlations and changes following therapy. Medicine
(Baltimore). 82:82–86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eisenstein DM, O'Gorman MR and Pachman LM:
Correlations between change in disease activity and changes in
peripheral blood lymphocyte subsets in patients with juvenile
dermatomyositis. J Rheumatol. 24:1830–1832. 1997.PubMed/NCBI
|
|
8
|
Ishida T, Matsumoto Y, Ohashi M and Sasaki
R: Analysis of lymphocyte subpopulations in peripheral blood in
adult and juvenile cases of dermatomyositis. J Dermatol. 20:30–34.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cope AP, Schulze-Koops H and Aringer M:
The central role of T cells in rheumatoid arthritis. Clin Exp
Rhuematol. 25 5 Suppl 46:S4–S11. 2007.
|
|
10
|
La Cava A: Lupus and T cells. Lupus.
18:196–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wehrens EJ, Prakken BJ and van Wijk F: T
cells out of control-impaired immune regulation in the inflame
joint. Nat Rev Rheumatol. 9:34–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pope RM: Apoptosis as a therapeutic tool
in rheumatoid arthritis. Nat Rev Immunol. 2:527–535. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Citro A, Barnaba V and Martini H: From T
cell apoptosis to chronic immune activation in inflammatory
diseases. Int Arch Allergy Immunol. 164:140–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lleo A, Invernizzi P, Selmi C, Coppel RL,
Alpini G, Podda M, Mackay IR and Gershwin ME: Autophagy:
Highlighting a novel player in the autoimmunity scenario. J
Autoimmun. 29:61–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhattacharya A and Eissa NT: Autophagy and
autoimmunity crosstalks. Front Immunol. 4:882013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuballa P, Nolte WM, Castoreno AB and
Xavier RJ: Autophagy and the immune system. Annu Rev Immunol.
30:611–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Y, Galluzzi L, Zitvogel L and Kroemer
G: Autohagy and cellular immune responses. Immunity. 39:211–227.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu
Z, Xu JH, Cai ZM, Huang W, Zhao GP, et al: Genome-wide association
study in a Chinese Han population identifies nine new
susceptibility loci for systemic lupus erythematosus. Nat Genet.
41:1234–1237. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
International Consortium for Systemic
Lupus Erythematosus Genetics (SLEGEN), ; Harley JB,
Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL,
Tsao BP, Vyse TJ, Langefeld CD, et al: Genome-wide association scan
in women with systemic lupus erythematosus identifies
susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat
Genet. 40:204–210. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou XJ, Lu XL, Lv JC, Yang HZ, Qin LX,
Zhao MH, Su Y, Li ZG and Zhang H: Genetic association of PRDM1-ATG5
intergenic region and autophagy with systemic lupus erythematosus
in a Chinese population. Ann Rheum Dis. 70:1330–1337. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raychaudhuri S, Thomson BP, Remmers EF,
Eyre S, Hinks A, Guiducci C, Catanese JJ, Xie G, Stahl EA, Chen R,
et al: Genetic variants at CD28, PRDM1 and CD2/CD58 are associated
with rheumatoid arthritis risk. Nat Genet. 41:1313–1318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parkes M, Barrett JC, Prescott NJ,
Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER,
Cummings FR, Soars D, et al: Sequence variants in the autophagy
gene IRGM and multiple other replicating loci contribute to Crohn's
disease susceptibility. Nat Genet. 39:830–832. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Temiz P, Weihl CC and Pestronk A:
Inflammatory myopathies with mitochondrial pathology and protein
aggregates. J Neurol Sci. 278:25–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nogalska A, D'Agostino C, Terracciano C,
Engel WK and Askanas V: Impaired autophagy in sporadic
inclusion-body myositis and in endoplasmic reticulum
stress-provoked cultured human muscle fibers. Am J Pathol.
177:1377–1387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alger HM, Raben N, Pistilli E, Francia DL,
Rawat R, Getnet D, Ghimbovschi S, Chen YW, Lundberg IE and Nagaraju
K: The role of TRAIL in mediating autophagy in myositis skeletal
muscle: A potential nonimmune mechanism of muscle damage. Arthritis
Rheum. 63:3448–3457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia W and He YW: Temporal regulation of
intracellular organelle homeostasis in T lymphocytes by autophagy.
J Immunol. 186:5313–5322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bohan A and Peter JB: Polymyositis and
dermatomyositis (first of two parts). N Engl J Med. 292:344–347.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bohan A and Peter JB: Polymyositis and
dermatomyositis (second of two parts). N Engl J Med. 292:403–407.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoogendijk JE, Amato AA, Lecky BR, Choy
EH, Lundberg IE, Rose MR, Vencovsky J, de Visser M and Hughes RA:
119th ENMC international workshop: Trial design in adult idiopathic
inflammatory myopathies, with the exception of inclusion body
myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul
Disord. 14:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Findlay AR, Goyal NA and Mozaffar T: An
overview of polymyositis and dermatomyositis. Muscle Nerve.
51:638–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mammen AL: Autoimmune myopathies:
Autoantibodies, phenotypes and pathogenesis. Nat Rev Neurol.
7:343–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amato AA and Greenberg SA: Inflammatory
myopathies. Continuum (Minneap Minn). 19(6 Muscle Disease):
1615–1633. 2013.PubMed/NCBI
|
|
36
|
Suárez-Fueyo A, Bradley SJ and Tsokos GC:
T cells in systemic lupus erythematosus. Curr Opin Immunol.
43:32–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu DJ and Adamopoulos IE: Autophagy and
autoimmunity. Clin Immunol. 176:55–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng QL, Zhang YL, Shu XM, Yang HB, Zhang
L, Chen F, Lu X and Wang GC: Elevated serum levels of soluble CD163
in polymyositis and dermatomyositis: Associated with macrophage
infiltration in muscle tissue. J Rheumatol. 42:979–987. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fasth AE, Dastmalchi M, Rahbar A,
Salomonsson S, Pandya JM, Lindroos E, Nennesmo I, Malmberg KJ,
Söderberg-Nauclér C, Trollmo C, et al: T cell infiltrates in the
muscles of patients with dermatomyositis and polymyositis are
dominated by CD28null T cells. J Immunol. 183:4792–4799. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugihara T, Sekine C, Nakae T, Kohyama K,
Harigai M, Iwakura Y, Matsumoto Y, Miyasaka N and Kohsaka H: A new
murine model to define the critical pathologic and therapeutic
mediators of polymyositis. Arthritis Rheum. 56:1304–1314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Espinosa-Ortega F, Gómez-Martin D,
Santana-de Anda K, Romo-Tena J, Villaseñor-Ovies P and
Alcocer-Varela J: Quantitative T cell subsets profile in peripheral
blood from patients with idiopathic inflammatory myopathies:
Tilting the balance towards proinflammatory and pro-apoptotic
subsets. Clin Exp Immunol. 179:520–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dhir V, Singh AP, Aggarwal A, Naik S and
Misra R: Increased T-lymphocyte apoptosis in lupus correlates with
disease activity and may be responsible for reduced T-cell
frequency: A cross-sectional and longitudinal study. Lupus.
18:785–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gros F, Arnold J, Page N, Décossas M,
Korganow AS, Martin T and Muller S: Macroautophagy is deregulated
in murine and human lupus T lymphocytes. Autophagy. 8:1113–1123.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alessandri C, Barbati C, Vacirca D,
Piscopo P, Confaloni A, Sanchez M, Maselli A, Colasanti T, Conti F,
Truglia S, et al: T lymphocytes from patients with systemic lupus
eythematosus are resistant to induction of autophagy. FASEB J.
26:4722–4732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Emlen W, Niebur J and Kadera R:
Accelerated in vitro apoptosis of lymphocytes from patients with
systemic lupus erythematosus. J Immunol. 152:3685–3692.
1994.PubMed/NCBI
|
|
46
|
Thorburn A: Apoptosis and autophagy:
regulatory connections between two supposedly different processes.
Apoptosis. 13:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pua HH, Guo J, Komatsu M and He YW:
Autophagy is essential for mitochondrial clearance in mature T
lymphocytes. J Immunol. 182:4046–4055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kovacs JR, Li C, Yang Q, Li G, Garcia IG,
Ju S, Roodman DG, Windle JJ, Zhang X and Lu B: Autophagy promotes
T-cell survival through degradation of proteins of the cell death
machinery. Cell Death Differ. 19:144–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Valdor R and Macian F: Autophagy and the
regulation of the immune response. Pharmacol Res. 66:475–483. 2012.
View Article : Google Scholar : PubMed/NCBI
|