Introduction
Osteoarthritis (OA) is a common disease of the
elderly worldwide, which is characterized by articular cartilage
destruction and local inflammation, resulting in pain, disability
and a significantly reduced quality of life for the affected
individuals (1). Accumulating
evidence (2,3) has indicated that low-grade systemic
and local inflammation is involved in the progression of OA
(4). Several inflammatory
mediators, such as interleukin (IL)-1β, nitric oxide (NO),
prostaglandin E2 (PGE2) and fragments of
cartilage proteins including matrix metalloproteinases (MMPs), have
been reported to be elevated in patients with OA (5). IL-1β is an important proinflammatory
cytokine during the inflammatory response, which has been reported
to serve critical roles in joint inflammation and cartilage
destruction processes. For example, IL-1β elevates NO production,
improves cyclooxygenase-2 (COX-2) activity and upregulates the
expression of MMPs, including MMP-13, which is associated with
extracellular matrix (ECM) degradation in cartilage (6,7).
Based on the significant role of inflammation in OA,
anti-inflammatory treatment may have significant value in the
treatment of OA.
Previous research has also indicated that IL-1β, or
other certain stimuli including tumor necrosis factor (TNF)-α or
lipopolysaccharide (LPS), may activate nuclear factor (NF)-κB
(8), which regulates the
expression of several other proinflammatory cytokines and
proteases, and mediates critical events in the inflammatory
responses of chondrocytes (9).
Under normal physiological circumstances, NF-κB is sequestered and
bound to its inhibitory protein, inhibitor-κBα (IκBα). When
inflammation is induced, IκBα undergoes proteasomal degradation.
This event is followed by translocation of cytoplasmic NF-κB into
the nucleus, where it activates transcription of numerous
proinflammatory cytokines, adhesion molecules and inflammatory
mediators, such as inducible nitric oxide synthase (iNOS), TNF-α
and MMPs.
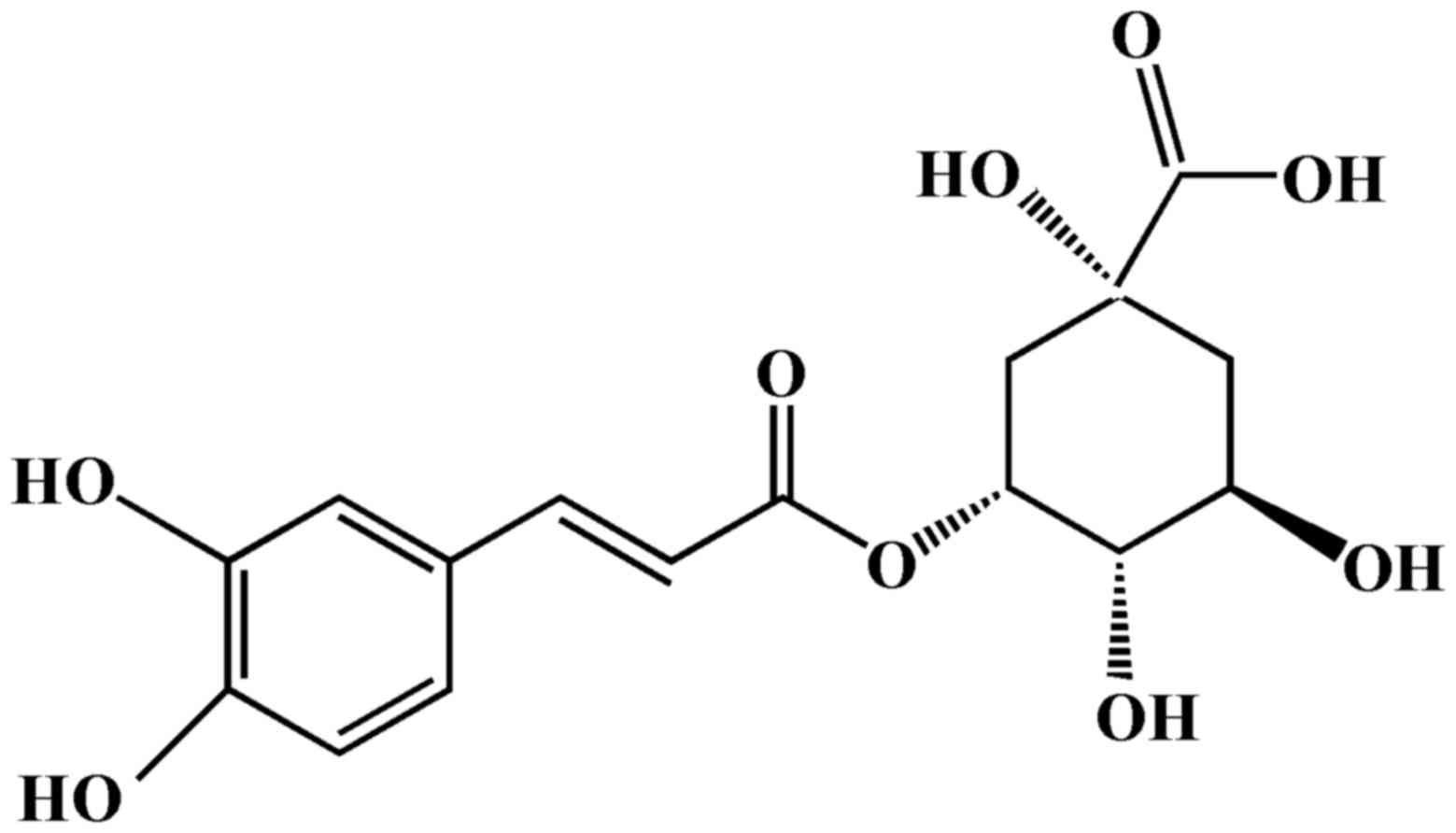
Chlorogenic acid (CGA; Fig. 1) is formed from the esterification
of caffeic acid and quinic acid, and is a polyphenolic compound
isolated from numerous plants, including coffee beans (10), Lonicerae japonicae (11) and Eucommiae cortex (12). Previous studies based on in
vivo and in vitro systems have revealed that CGA exerts
antioxidant (13), antibacterial
(14) and antitumor activities
(15). Furthermore, CGA has
demonstrated protective effects against several experimental
disorders, including mastitis (16), gastroesophageal reflux disease
(17) and LPS-induced acute lung
injury (18) via downregulation of
the inflammatory response. These findings suggested that CGA may
serve as a novel therapeutic agent for the treatment of
inflammatory diseases. Notably, in vitro studies have
demonstrated that CGA attenuates the production of several
inflammatory mediators, including IL-1β, IL-6 and TNF-α in
LPS-stimulated RAW264.7 cells (19). In addition, Lee et al
demonstrated the anticandidal and anti-arthritic effects of CGA by
inhibiting the synthesis of NO in LPS-activated macrophages
(20). CGA may also decrease
osteoclast differentiation and bone resorption in vivo and
may have therapeutic potential in treating diseases associated with
inflammatory bone destruction (21). Studies have addressed the
therapeutic potential of CGA on arthritis; however, its ability to
protect against IL-1β-induced OA remains poorly understood.
Human SW-1353 chondrosarcoma cells stimulated with
IL-1β possess similar properties to the articular chondrocytes of
OA (6). The present study employed
this OA-like chondrocyte cellular model as an in vitro tool,
in order to investigate the effects of CGA on the expression levels
of iNOS/NO, IL-6, COX-2/PGE2, collagen II/MMP-13 and
NF-κB signaling pathways. The results indicated that CGA may be of
clinical value in the treatment of OA.
Materials and methods
Chemicals and reagents
CGA (purity >98%) was purchased from Beijing
Dewei Sodium Biotechnology Co., Ltd. (Beijing, China) and confirmed
by high-performance liquid chromatography by the Research and
Engineering Center for Natural Medicine, Xi'an Jiaotong University
(Xi'an, China). L-15 medium was obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Fetal bovine serum was
purchased from Hyclone (GE Healthcare Life Sciences, Logan, UT,
USA). Trypsin was purchased from Amresco, LLC (Solon, OH, USA).
IL-1β was purchased from PeproTech, Inc. (Rocky Hill, NJ, USA).
Ethylenediamine tetra-acetic acid (EDTA) and
1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Griess reagent was purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). The enzyme-linked immunosorbent assay
(ELISA) kit for human IL-6 (cat. no. D6050) was purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). Antibodies against
COX-2 (cat. no. 12375-1-AP), MMP-13 (cat. no. 18165-1-AP), collagen
II (cat. no. 15943-1-AP), NF-κB (cat. no. 10745-1-AP) and IκB-α
(cat. no. 10268-1-AP) were purchased from ProteinTech, Inc.
(Rosemont, IL, USA) Antibodies against phosphorylated (p)-NF-κB
(cat. no. 3033) and p-IκB-α (cat. no. 2859) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). PGE2
rabbit polyclonal antibody (cat. no. ab2318) and iNOS rabbit
monoclonal antibody (cat. no. ab178945) were purchased from Abcam
(Cambridge, MA, USA). Rabbit anti-GAPDH (cat. no. PA1-987),
bicinchoninic acid (BCA) protein assay reagent kit and Pierce
enhanced chemiluminescent (ECL) Plus Substrate were purchased from
Pierce (Thermo Fisher Scientific, Inc.).
Cell culture
Human SW-1353 chondrocytes were purchased from the
Shanghai Institute of Cell Biology, the Chinese Academy of Sciences
(Shanghai, China) and cultured in L-15 supplemented with 10% fetal
bovine serum, 100 U/ml penicillin and, 100 µg/ml streptomycin.
Cells were incubated in a humidified incubator in an atmosphere
containing 5% CO2 at 37°C. The culture medium was
replaced every 3 days.
Cell viability assay
To evaluate the cytotoxic effects of CGA, cell
viability was assessed using the MTT assay. Exponentially growing
SW-1353 cells were plated into 96-well plates (2×106 cells/ml) and
cultivated overnight. The following day, CGA (31.25, 62.5, 125,
250, 500 or 1,000 µg/ml), or various concentrations of CGA along
with IL-1β (10 ng/ml), was added to the cells, which were incubated
for 48 h at 37°C. The control cells received an equal amount of
dimethyl sulfoxide (DMSO). Following removal of the medium, 180 µl
serum-free medium and 20 µl MTT (5 mg/ml) was added to each well,
and the plates were incubated at 37°C for 4 h. The supernatants
were replaced with 150 µl DMSO to dissolve the crystals, the plates
were agitated for 10 min, and the absorbance was measured at 490 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Survival was calculated according to the formula:
Survival=ODtest /ODcontrol, where OD
indicates optical density.
NO assay
NO production was assessed spectrophotometrically
via the determination of nitrite concentrations in the cell culture
supernatant using Griess reagent. SW-1353 chondrocytes (2×106
cell/ml) were pretreated with various concentrations of CGA (50,
100, 200 or 500 µg/ml) for 24 h, and were subsequently exposed to
IL-1β (10 ng/ml) for 24 h at 37°C. After 24 h the supernatants were
collected. Each supernatant was mixed with an equal volume of
Griess reagent (150 µl) and incubated for 5 min at room
temperature. Absorbance was subsequently determined at 540 nm.
Sodium nitrite was used to prepare the standard curve.
ELISA detection of IL-6
production
SW-1353 chondrocytes (2×106 cell/ml) were pretreated
with various concentrations of CGA (50, 100, 200 or 500 µg/ml) for
24 h, after which the cells were stimulated with IL-1β (10 ng/ml)
for 24 h at 37°C. The amount of IL-6 secreted into the culture
medium was measured using a commercially available IL-6-specific
ELISA kit according to the manufacturer's protocol.
Western blot analysis
SW-1353 chondrocytes (2×106 cells/ml) were seeded
and cultured in 6-well plates. The cells were pretreated with CGA
(50 or 200 µg/ml) for 24 h at 37°C, after which the cells were
exposed to IL-1β (10 ng/m) for 30 min. Total proteins were isolated
using lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM
EDTA, 20 mM NaF, 0.5% NP-40, 1% Triton, 1% protease inhibitor and
phosphatase inhibitor cocktail]. Concentrations were determined
using a BCA Protein Quantification kit. The proteins (30 µg/sample)
were then separated on different percentages of SDS-polyacrylamide
gel (6–10%) and were transferred onto polyvinylidene difluoride
membranes. Nonspecific sites were blocked with Tris-buffered saline
0.1% Tween-20 (TBST) plus 5% nonfat dry milk for 2 h at room
temperature, and the membranes were then incubated with primary
antibodies (1:1,000 dilution) in 5% skim milk overnight at 4°C. The
blots were subsequently washed three times with TBST, and incubated
with horseradish peroxidase-linked secondary antibodies (1:15,000
dilution; cat. no. 31463; Pierce; Thermo Fisher Scientific, Inc.)
for 2 h at room temperature, and washed three further times with
TBST. Protein bands were detected using a Pierce ECL Plus
Substrate. GAPDH band intensity was used as internal control to
normalize protein expression levels. The optical density was
measured using Quantity One 1-D Analysis software (version 4.4;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All results were presented as the mean ± standard
error of the mean of ≥3 independent experiments for each test.
Differences among the various groups were assessed by one-way
analysis of variance followed by Dunnett's test. Statistical
analyses were performed using SPSS v. 12.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of CGA and/or IL-1β on human
SW-1353 chondrocyte cell viability
An MTT assay was used to determine the effects of
CGA on the cell viability of IL-1β-induced human SW-1353
chondrocytes. The results indicated that CGA was cytotoxic to human
SW-1353 chondrocytes at a concentration of 1,000 µg/ml; however,
cell viability in cells treated with up to 500 µg/ml CGA was not
significantly different from the control cells, with or without
IL-1β stimulation (Fig. 2). Based
on these results, the following experiments were conducted using
CGA at concentrations ≤500 µg/ml.
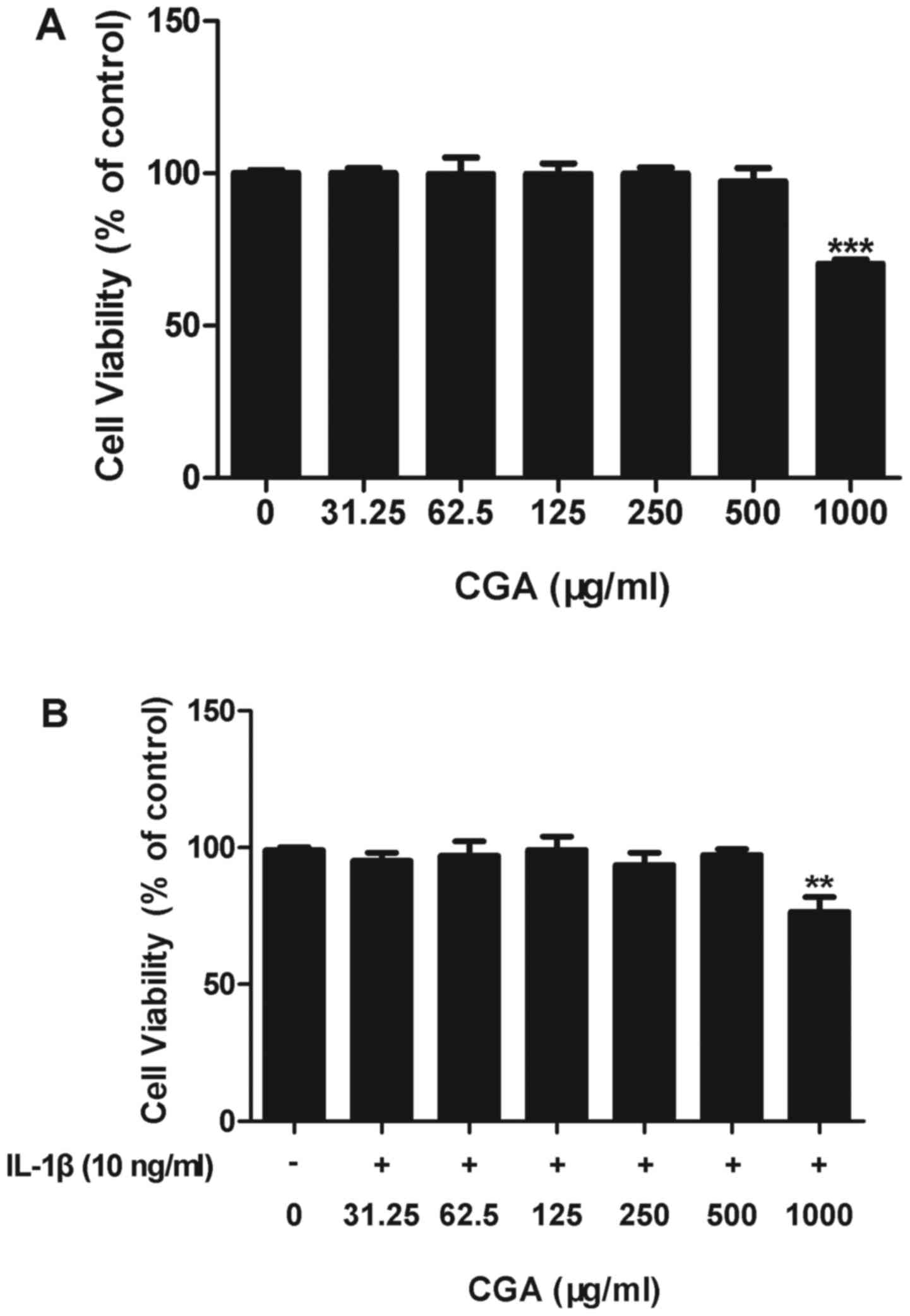 | Figure 2.Effects of CGA and/or IL-1β on the
viability of human SW-1353 chondrocytes. (A) Chondrocytes were
treated with CGA (31.25, 62.5, 125, 250, 500, or 1,000 µg/ml) alone
for 48 h. (B) Chondrocytes were treated with CGA (31.25, 62.5, 125,
250, 500 or 1,000 µg/ml) and IL-1β (10 ng/ml) for 48 h. Values are
expressed as the mean ± standard error of the mean (n=3).
**P<0.01; ***P<0.001 vs. the control group. CGA, chlorogenic
acid; IL, interleukin. |
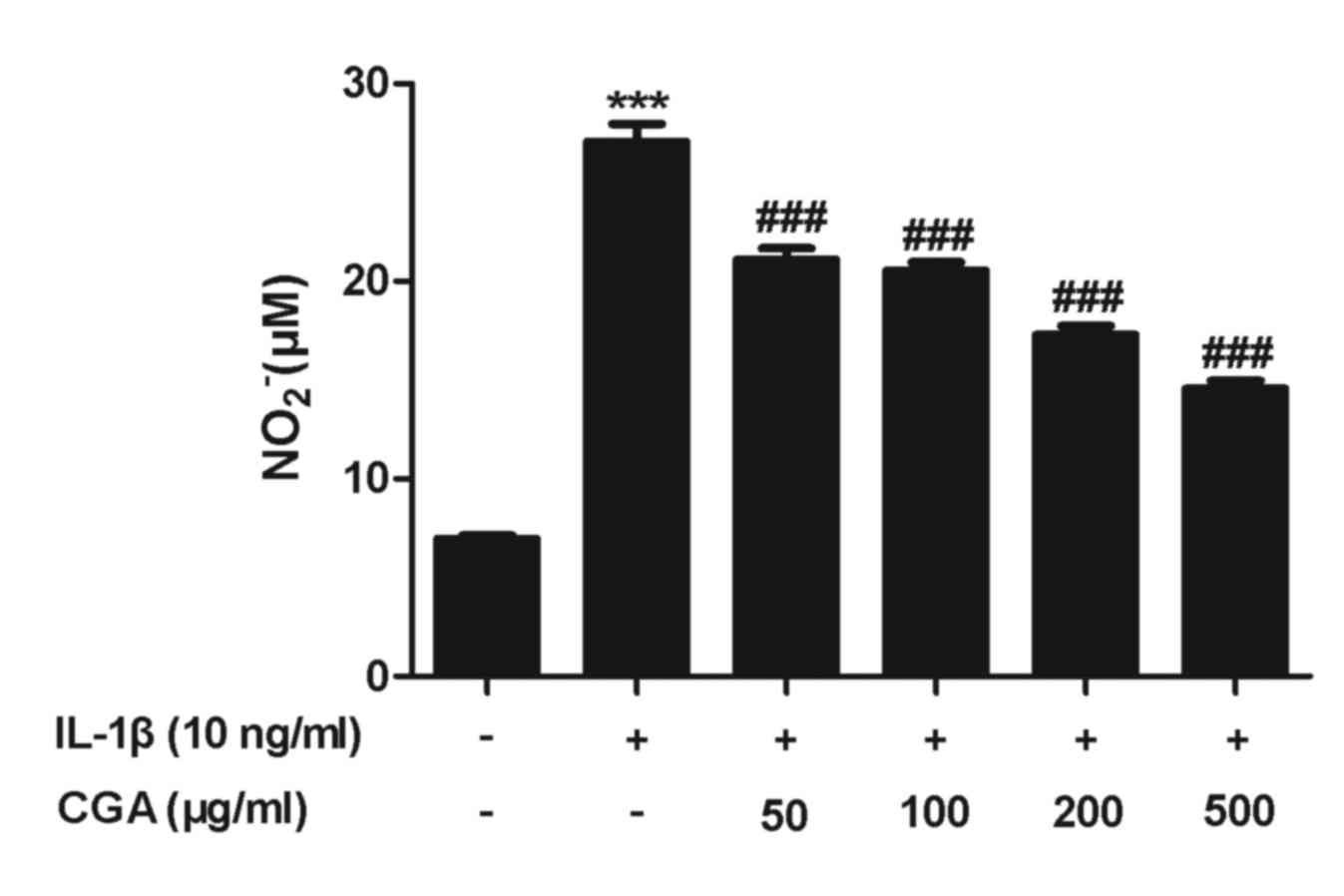
Effects of CGA on IL-1β-stimulated NO
production and iNOS expression in human SW-1353 chondrocytes
NO serves a pivotal role in OA progression. The
results of the viability assay indicated that treatment with 500
µg/ml CGA for 48 h was not cytotoxic; therefore, the inhibitory
effects of CGA (50, 100, 200 and 500 µg/ml) on NO release in
stimulated SW-1353 chondrocytes were examined. NO production was
significantly enhanced following IL-1β activation for 24 h, and
pretreatment with CGA significantly diminished the IL-1β-induced NO
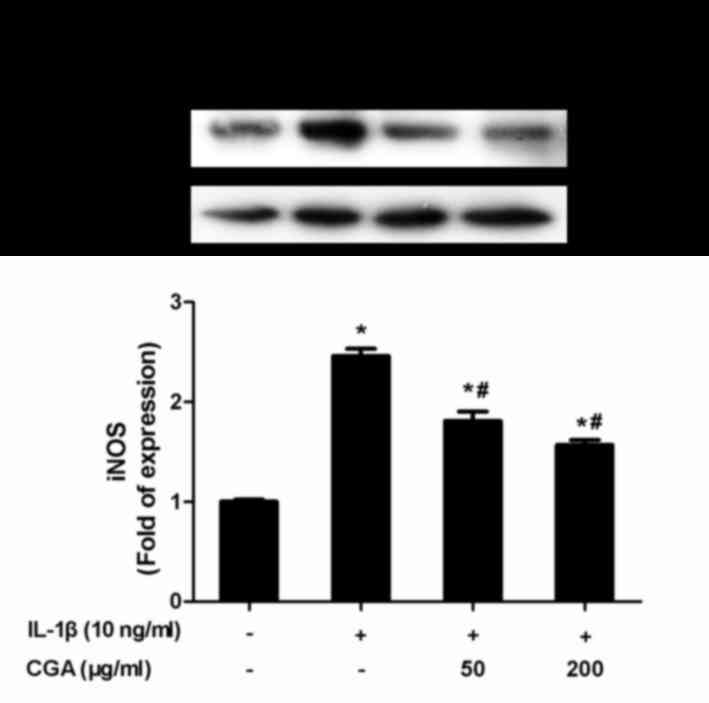
release in a concentration-dependent manner (Fig. 3). NO synthesis is predominantly
catalyzed by iNOS; therefore, iNOS expression in SW-1353
chondrocytes was investigated. Consistent with the aforementioned
results, CGA was able to inhibit IL-1β-induced iNOS protein
expression (Fig. 4).
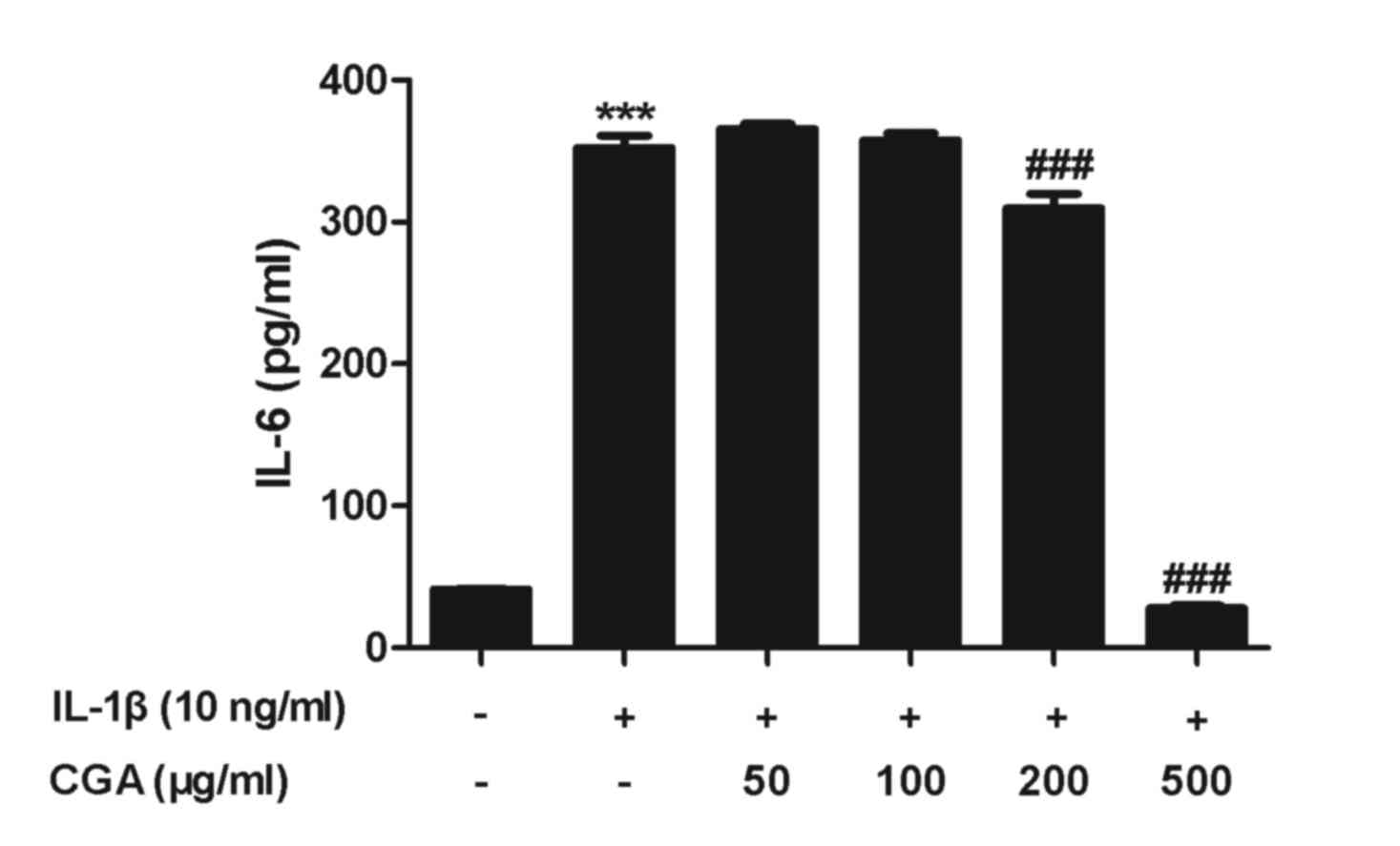
Effects of CGA on the expression of
IL-6 in IL-1β-stimulated SW-1353 chondrocytes
To evaluate the effects of CGA on IL-6 production in
human SW-1353 chondrocytes, IL-6 secretion was examined by ELISA.
Human SW-1353 chondrocytes were pretreated with CGA for 24 h and
then stimulated with IL-1β for 24 h. IL-6 secretion from human
SW-1353 chondrocytes was stimulated by IL-1β treatment, and this
was reduced by treatment with CGA (Fig. 5). The observed alterations occurred
in a concentration-dependent manner.
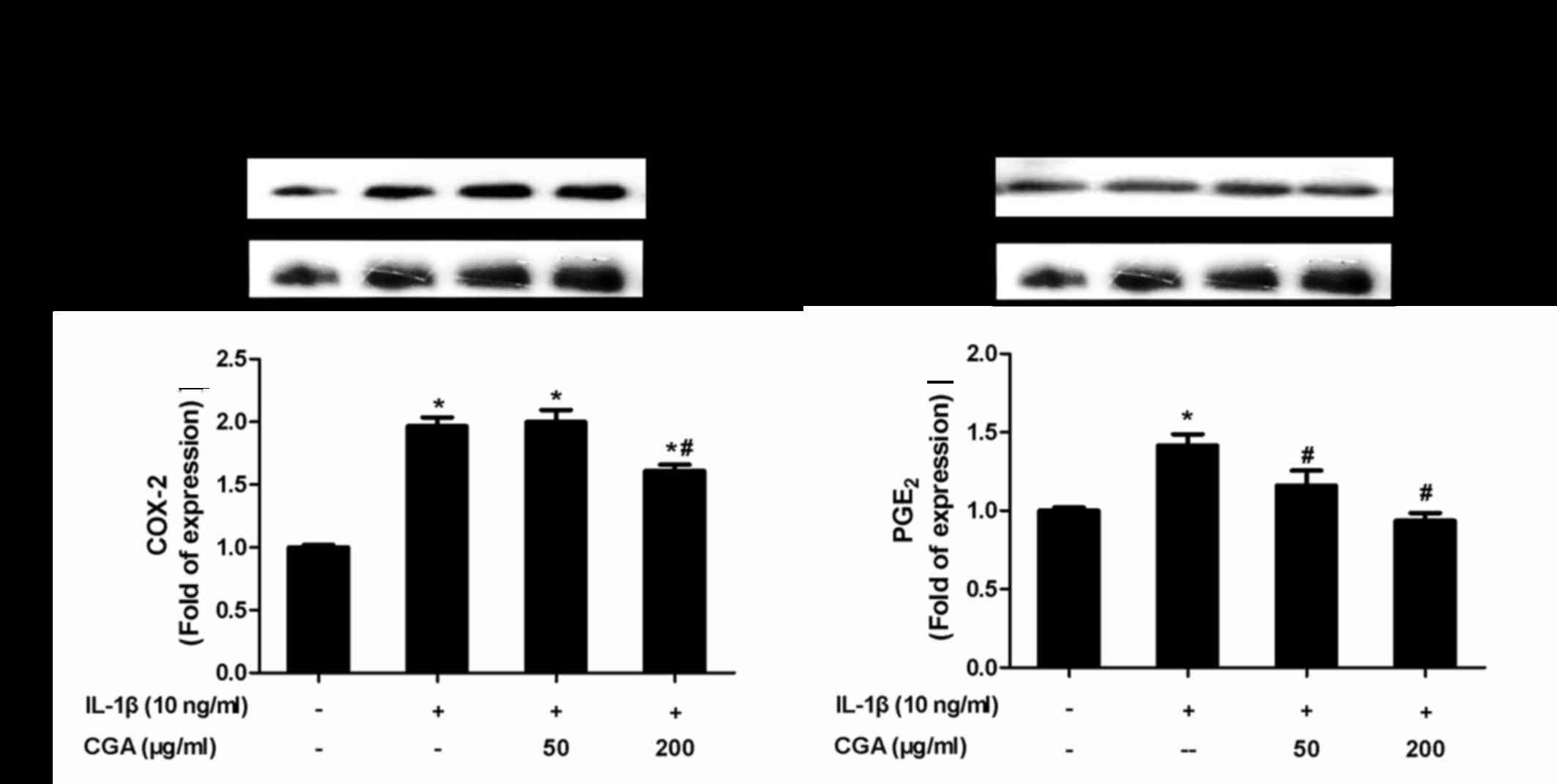
Inhibitory effects of CGA on COX-2 and
PGE2 expression in IL-1β-stimulated SW-1353
chondrocytes
To evaluate whether CGA affected the levels of COX-2
and PGE2 in IL-1β-induced SW-1353 chondrocytes, the
protein expression levels of COX-2 and PGE2 were
investigated by western blot analysis (Fig. 6). The results indicated that CGA
significantly diminished IL-1β-stimulated expression of COX-2 and
PGE2, thus suggesting that CGA may inhibit the synthesis
of PGE2 by influencing the enzymatic activity of
COX-2.
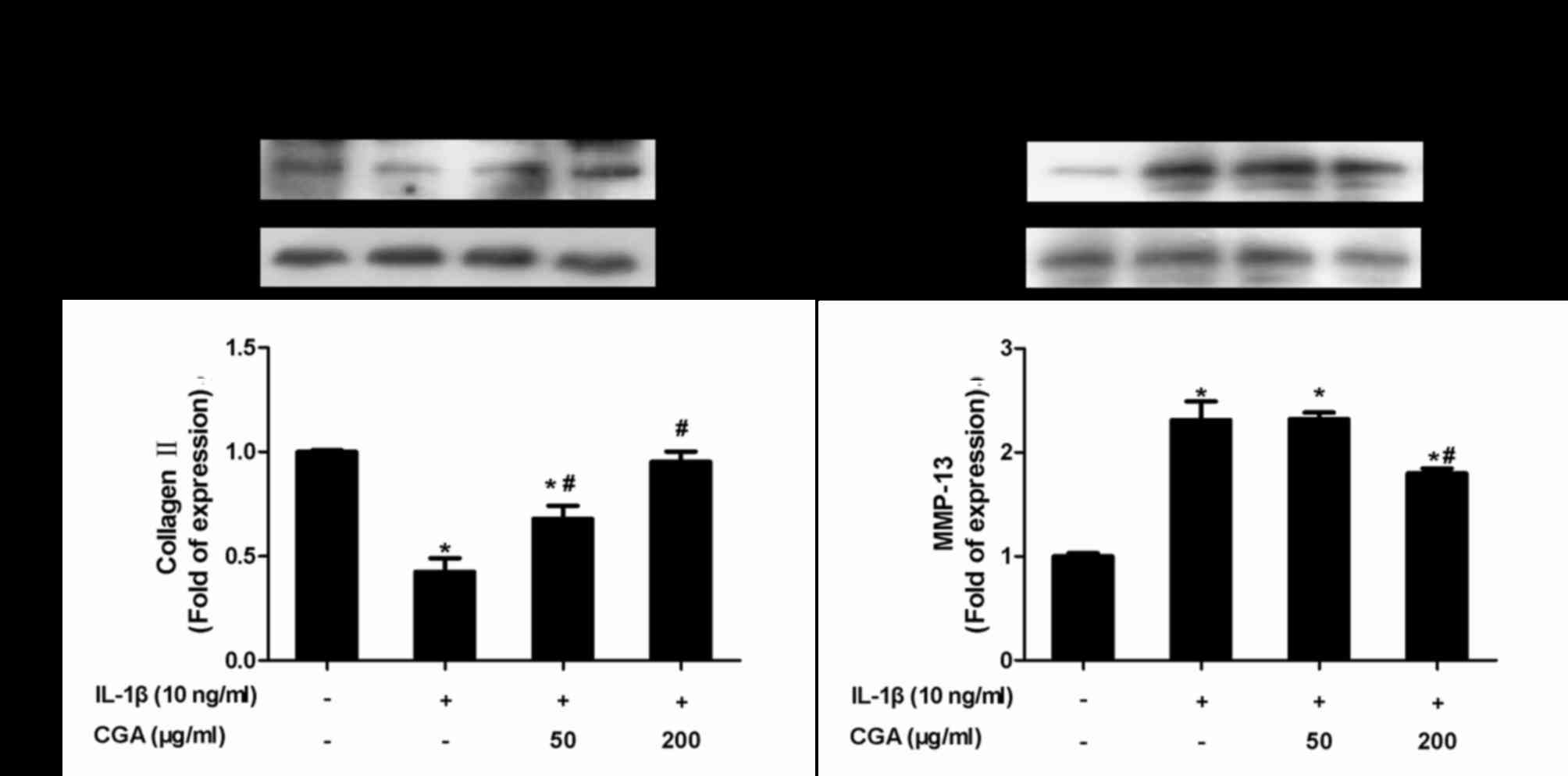
Inhibitory effects of CGA on collagen
II and MMP-13 expression in IL-1β-stimulated SW-1353
chondrocytes
Protein expression levels of collagen II and MMP-13
were examined using western blot analysis (Fig. 7). As expected, CGA significantly
diminished IL-1β-induced MMP-13 protein expression, and markedly
reversed the downregulation of collagen II expression in
IL-1β-stimulated SW-1353 chondrocytes.
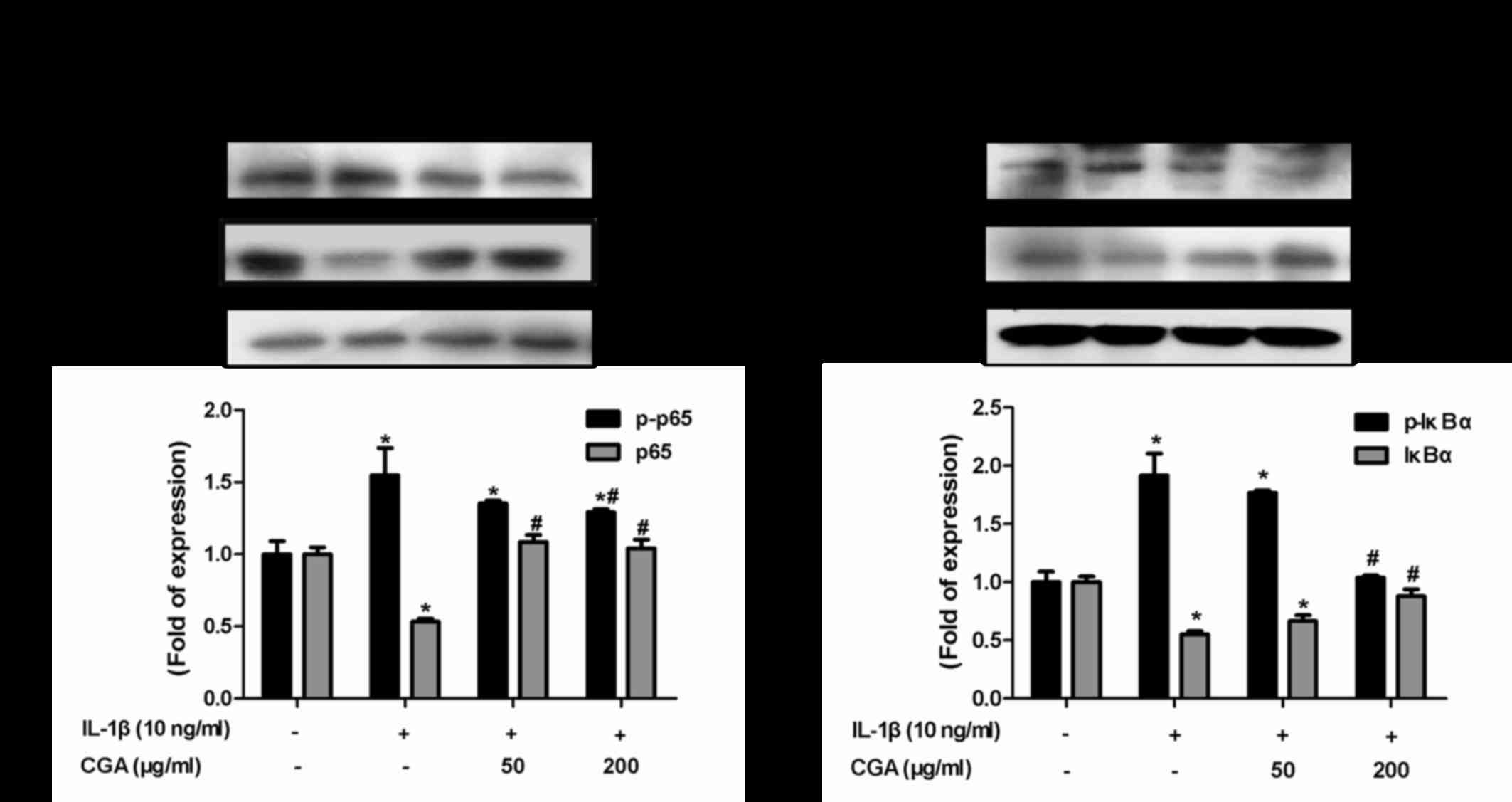
Effects of CGA on the IκBα/NF-κB
signaling pathway in IL-1β-stimulated SW-1353 chondrocytes
The NF-κB signal transduction pathway serves vital
roles in cartilage degradation and the inflammatory response. To
investigate the effects of CGA-mediated inflammatory response
inhibition on IL-1β-stimulated chondrocytes, NF-κB and its
inhibitory protein IκBα were detected. Western blot analysis was
used to detect the levels of NF-κB p65 phosphorylation and IκB
degradation. IL-1β stimulation resulted in the phosphorylation of
IκBα and NF-κB p65, whereas CGA reversed this effect (Fig. 8). These results indicated that the
NF-κB signal transduction pathway may be inhibited by CGA in
IL-1β-stimulated SW-1353 chondrocytes.
Discussion
OA is the most prevalent joint disease, and there
are currently no effective treatments to limit disease progression.
Inflammation has been recognized as an important process in the
pathogenesis of OA. Proinflammatory cytokines, including TNF-α and
IL-1, become activated during OA progression, and this induces
inflammatory responses and cartilage destruction (3). Commonly-used pharmacological
treatments, such as oral non-steroidal anti-inflammatory drugs, for
the treatment of patients with OA, are often associated with
serious adverse effects (22).
Therefore, plant-derived agents that possess anti-inflammatory
activity and low toxicity are more frequently being employed as a
therapeutic option in OA (23).
CGA, which is a bioactive phenolic acid component isolated from
plants, displays anti-inflammatory activity and has been
recommended for the treatment of OA (24). The present study aimed to determine
the anti-inflammatory and cartilage-protective effects of CGA on
IL-1β-stimulated SW-1353 chondrocytes.
IL-1β is an important proinflammatory cytokine
released by activated synoviocytes or chondrocytes, which serves an
important role in the progression of OA (25). Several studies have indicated that
IL-1β upregulates NO production via stimulation of iNOS activity,
and elevates the COX2-mediated expression of PGE2. In
addition, IL-1β induces the expression of other proinflammatory
cytokines, including IL-6 and IL-17, and stimulates matrix
remodeling via the regulation of MMPs, resulting in the degradation
of aggrecan and other matrix constituents (26,27).
Therefore, IL-1β-stimulated SW-1353 cells have been employed as an
in vitro cellular model of OA (8,28).
NO is produced from the amino acid L-arginine by the
enzymatic action of iNOS, which is induced by inflammatory stimuli,
indicating that this proinflammatory mediator could contribute to
OA disease (29). In the present
study, statistical analysis revealed that CGA markedly decreased
iNOS expression and NO production from IL-1β-stimulated SW-1353
chondrocytes in a concentration-dependent manner, thus suggesting
that CGA may possess an anti-inflammatory role via the regulation
of NO synthesis.
PGE2 is another key inflammatory
mediator; PGE2 is synthesized from arachidonic acid via
the stimulation of COX-2 during the inflammatory response. COX-2
has been recognized as an inducible or pathological enzyme that
participates in the inflammatory response by facilitating
PGE2 generation, and promotes the inflammatory
cytokine-induced metabolic imbalance of cartilage proteoglycans,
thereby advancing arthritis (30,31).
IL-6 has been considered as another crucial cytokine involved in
the inflammatory response. The present study explored the
anti-inflammatory role of CGA, and the results indicated that CGA
may inhibit the protein expression of PGE2 and COX-2,
and the secretion of IL-6, in human SW-1353 chondrocytes in a
concentration-dependent manner.
Cartilage destruction is a fundamental
disease-progression process that occurs in OA. Therefore, the role
CGA serves in abrogating this process was further explored.
Chondrocytes are the solitary cellular components of cartilage;
under normal conditions, these cells maintain the ECM, and balance
anabolic and catabolic metabolism (32). The human SW-1353 chondrosarcoma
line has been employed in several studies as an in vitro
model to investigate the molecular mechanisms and pharmacological
treatments for OA, due to its similarity with human OA chondrocytes
(7,33). MMPs are a family of zinc-dependent
proteases that serve important roles in cartilage ECM degradation
and maintenance, and are upregulated in pathophysiological
inflammatory conditions (34). MMP
upregulation results in the erosion of articular cartilage and thus
participates in OA progression. In particular, the collagenase
MMP-13 preferentially cleaves type II collagen, which is the
predominant collagen involved in the structural and functional
integrity of cartilage (35). The
present study explored whether CGA was able to exert
chondroprotective effects via MMP suppression in chondrocytes. The
results indicated that CGA reduced MMP-13 expression and reversed
the reduction of collagen II induced by IL-1β, compared with the
untreated group from human SW-1353 chondrocytes.
NF-κB is a cytokine-induced transcription factor
that serves an important role in regulating the expression of
various genes, including numerous proinflammatory cytokines,
adhesion molecules and proteases in arthritis, such as MMPs,
PGE2, NO and IL-6 (9);
several of the IL-1β-induced inflammatory mediators in chondrocytes
are regulated by the NF-κB signaling pathway (36). In light of the role of NF-κB in OA
progression, blocking its activity may have therapeutic advantages.
The p65 subunit of NF-κB is normally sequestered into the cytoplasm
under resting conditions, and is blocked by IκBα proteins (37). During inflammation, IκBα proteins
are phosphorylated and degraded by the proteasome, the NF-κB p65
subunit is released and phosphorylated and then translocated into
the nucleus, where it binds to DNA promoter regions and activates
the transcription of target genes (38). In the present study, CGA
intervention was able to reduce IκBα degradation and consequently
decrease phosphorylated-NF-κB p65 levels in human SW-1353
chondrocytes, and this occurred in a concentration-dependent
manner. These results indicated that the protective and
anti-inflammatory effects of CGA on human SW-1353 chondrocytes may
occur via the NF-κB pathway.
In conclusion, the present study demonstrated the
in vitro anti-inflammatory and cartilage-protective role of
CGA. The underlying mechanism appeared to involve the inhibition of
COX-2, PGE2, iNOS, NO, IL-6 and MMP-13. Furthermore,
IL-1β-induced collagen II destruction was abrogated via the
inhibition of IκBα/NF-κB activation. These results suggested that
CGA may be considered a novel therapeutic candidate against
arthritic diseases. Future studies should fully clarify the
molecular mechanisms underlying the effects of CGA on chondrocytes,
as there may be additional signaling pathways involved. In
vivo investigations of CGA in animals and humans are required
to further investigate the potential of CGA in the clinical
management of OA.
Acknowledgements
The present study was supported by the Research
Foundation of Xi'an Hong-Hui Hospital (grant no. YJ2014016).
References
|
1
|
Chang CC, Hsieh MS, Liao ST, Chen YH,
Cheng CW, Huang PT, Lin YF and Chen CH: Hyaluronan regulates PPARγ
and inflammatory responses in IL-1β-stimulated human chondrosarcoma
cells, a model for osteoarthritis. Carbohydr Polym. 90:1168–1175.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karlsen TA, de Souza GA, Ødegaard B,
Engebretsen L and Brinchmann JE: microRNA-140 inhibits inflammation
and stimulates chondrogenesis in a model of interleukin 1β-induced
osteoarthritis. Mol Ther Nucleic Acids. 5:e3732016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernandes JC, Martel-Pelletier J and
Pelletier JP: The role of cytokines in osteoarthritis
pathophysiology. Biorheology. 39:237–246. 2002.PubMed/NCBI
|
|
4
|
Wang K, Xu J, Hunter DJ and Ding C:
Investigational drugs for the treatment of osteoarthritis. Expert
Opin Investig Drugs. 24:1539–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siebuhr AS, Bay-Jensen AC, Jordan JM,
Kjelgaard-Petersen CF, Christiansen C, Abramson SB, Attur M,
Berenbaum F, Kraus V and Karsdal MA: Inflammation (or
synovitis)-driven osteoarthritis: An opportunity for personalizing
prognosis and treatment? Scand J Rheumatol. 45:87–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klatt AR, Klinger G, Neumüller O,
Eidenmuller B, Wagner I, Achenbach T, Aigner T and Bartnik E: TAK1
downregulation reduces IL-1beta induced expression of MMP13, MMP1
and TNF-alpha. Biomed Pharmacother. 60:55–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pattoli MA, MacMaster JF, Gregor KR and
Burke JR: Collagen and aggrecan degradation is blocked in
interleukin-1-treated cartilage explants by an inhibitor of IkappaB
kinase through suppression of metalloproteinase expression. J
Pharmacol Exp Ther. 315:382–388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Z, Wang Y, Piao T and Liu J:
Echinocystic acid inhibits IL-1β-induced COX-2 and iNOS expression
in human osteoarthritis chondrocytes. Inflammation. 39:543–549.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcu KB, Otero M, Olivotto E, Borzi RM
and Goldring MB: NF-kappaB signaling: Multiple angles to target OA.
Curr Drug Targets. 11:599–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Illing EA, Cho DY, Zhang S, Skinner DF,
Dunlap QA, Sorscher EJ and Woodworth BA: Chlorogenic acid activates
CFTR-mediated Cl- secretion in mice and humans: Therapeutic
implications for chronic rhinosinusitis. Otolaryngol Head Neck
Surg. 153:291–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu W, Guo T, Jiang WJ, Dong GL, Chen DW,
Yang SL and Li HR: Effects of ultrahigh pressure extraction on
yield and antioxidant activity of chlorogenic acid and cynaroside
extracted from flower buds of Lonicera japonica. Chin J Nat Med.
13:445–453. 2015.PubMed/NCBI
|
|
12
|
Chen X, Sang X, Li S, Zhang S and Bai L:
Studies on a chlorogenic acid-producing endophytic fungi isolated
from Eucommia ulmoides Oliver. J Ind Microbiol Biotechnol.
37:447–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pang C, Sheng YC, Jiang P, Wei H and Ji
LL: Chlorogenic acid prevents acetaminophen-induced liver injury:
The involvement of CYP450 metabolic enzymes and some antioxidant
signals. J Zhejiang Univ Sci B. 16:602–610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren S, Wu M, Guo J, Zhang W, Liu X, Sun L,
Holyst R, Hou S, Fang Y and Feng X: Sterilization of
polydimethylsiloxane surface with Chinese herb extract: A new
antibiotic mechanism of chlorogenic acid. Sci Rep. 5:104642015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao P, Zhang JF, Chen XX and Sun PL:
Microwave-assisted extraction and purification of chlorogenic acid
from by-products of Eucommia Ulmoides Oliver and its potential
anti-tumor activity. J Food Sci Technol. 52:4925–4934. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruifeng G, Yunhe F, Zhengkai W, Ershun Z,
Yimeng L, Minjun Y, Xiaojing S, Zhengtao Y and Naisheng Z:
Chlorogenic acid attenuates lipopolysaccharide-induced mice
mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur
J Pharmacol. 729:54–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang JW and Lee SM: Protective effects of
chlorogenic acid against experimental reflux esophagitis in rats.
Biomol Ther (Seoul). 22:420–425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Huang H, Yang T, Ye Y, Shan J,
Yin Z and Luo L: Chlorogenic acid protects mice against
lipopolysaccharide-induced acute lung injury. Injury. 41:746–752.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang SJ, Kim YW, Park Y, Lee HJ and Kim
KW: Anti-inflammatory effects of chlorogenic acid in
lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res.
63:81–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JH, Park JH, Kim YS and Han Y:
Chlorogenic acid, a polyphenolic compound, treats mice with septic
arthritis caused by Candida albicans. Int Immunopharmacol.
8:1681–1685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwak SC, Lee C, Kim JY, Oh HM, So HS, Lee
MS, Rho MC and Oh J: Chlorogenic acid inhibits osteoclast
differentiation and bone resorption by down-regulation of receptor
activator of nuclear factor kappa-B ligand-induced nuclear factor
of activated T cells c1 expression. Biol Pharm Bull. 36:1779–1786.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YJ, Tsai KS, Chan DC, Lan KC, Chen
CF, Yang RS and Liu SH: Honokiol, a low molecular weight natural
product, prevents inflammatory response and cartilage matrix
degradation in human osteoarthritis chondrocytes. J Orthop Res.
32:573–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai CF, Wang KT, Chen LG, Lee CJ, Tseng
SH and Wang CC: Anti-inflammatory effects of Vitis thunbergii var.
taiwaniana on knee damage associated with arthritis. J Med Food.
17:479–486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
dos Santos MD, Almeida MC, Lopes NP and de
Souza GE: Evaluation of the anti-inflammatory, analgesic and
antipyretic activities of the natural polyphenol chlorogenic acid.
Biol Pharm Bull. 29:2236–2240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi J, Schmitt-Talbot E, DiMattia DA and
Dullea RG: The differential effects of IL-1 and TNF-alpha on
proinflammatory cytokine and matrix metalloproteinase expression in
human chondrosarcoma cells. Inflamm Res. 53:377–389. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Y, Zhou L, Lv D, Liu H, He T and Wang
X: Poly(ADP-ribose) polymerase 1 inhibition prevents
interleukin-1β-induced inflammation in human osteoarthritic
chondrocytes. Acta Biochim Biophys Sin (Shanghai). 47:422–430.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vincenti MP and Brinckerhoff CE: Early
response genes induced in chondrocytes stimulated with the
inflammatory cytokine interleukin-1beta. Arthritis Res. 3:381–388.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Ding L, Zhang S, Jiang T, Yang Y
and Li R: Effects of icariin on the regulation of the
OPG-RANKL-RANK system are mediated through the MAPK pathways in
IL-1β-stimulated human SW1353 chondrosarcoma cells. Int J Mol Med.
34:1720–1726. 2014.PubMed/NCBI
|
|
29
|
Tao R, Wang S, Xia X, Wang Y, Cao Y, Huang
Y, Xu X, Liu Z, Liu P, Tang X, et al: Pyrroloquinoline quinone
slows down the progression of osteoarthritis by inhibiting nitric
oxide production and metalloproteinase synthesis. Inflammation.
38:1546–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan HW, Liu GY, Zhao CF, Li XF and Yang
XY: Differential expression of COX-2 in osteoarthritis and
rheumatoid arthritis. Genet Mol Res. 14:12872–12879. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu SM and Kim SJ: The thymoquinone-induced
production of reactive oxygen species promotes dedifferentiation
through the ERK pathway and inflammation through the p38 and PI3K
pathways in rabbit articular chondrocytes. Int J Mol Med.
35:325–332. 2015.PubMed/NCBI
|
|
32
|
Zhao L, Ye J, Wu GT, Peng XJ, Xia PF and
Ren Y: Gentiopicroside prevents interleukin-1 beta induced
inflammation response in rat articular chondrocyte. J
Ethnopharmacol. 172:100–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Petrella BL, Armstrong DA and Vincenti MP:
CCAAT-enhancer-binding protein beta activation of MMP-1 gene
expression in SW1353 cells: Independent roles of extracellular
signal-regulated and p90/ribosomal S6 kinases. J Cell Physiol.
226:3349–3354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu P, Chen W, Bao J, Jiang L and Wu L:
Cordycepin modulates inflammatory and catabolic gene expression in
interleukin-1beta-induced human chondrocytes from advanced-stage
osteoarthritis: An in vitro study. Int J Clin Exp Pathol.
7:6575–6584. 2014.PubMed/NCBI
|
|
35
|
Chen YJ, Chan DC, Lan KC, Wang CC, Chen
CM, Chao SC, Tsai KS, Yang RS and Liu SH: PPARγ is involved in the
hyperglycemia-induced inflammatory responses and collagen
degradation in human chondrocytes and diabetic mouse cartilages. J
Orthop Res. 33:373–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang SN, Xie GP, Qin CH, Chen YR, Zhang
KR, Li X, Wu Q, Dong WQ, Yang J and Yu B: Aucubin prevents
interleukin-1 beta induced inflammation and cartilage matrix
degradation via inhibition of NF-kB signaling pathway in rat
articular chondrocytes. Int Immunopharmacol. 24:408–415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiu J, Yu L, Zhang X, Wu Q, Wang D, Wang
X, Xia C and Feng H: Asiaticoside attenuates
lipopolysaccharide-induced acute lung injury via down-regulation of
NF-κB signaling pathway. Int Immunopharmacol. 26:181–187. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang QS, Xiang Y, Cui YL, Lin KM and Zhang
XF: Dietary blue pigments derived from genipin, attenuate
inflammation by inhibiting LPS-induced iNOS and COX-2 expression
via the NF-kB inactivation. PLoS One. 7:e341222012. View Article : Google Scholar : PubMed/NCBI
|